Abstract
This study aimed to determine the effects of different acute creatine loadings (ACRL) on repeated cycle sprints. Twenty-eight active subjects divided into the control (n=7) and the experimental (n=21) group. The exercise protocol comprised three 30s Anaerobic Wingate Tests (AWT) interspersed with six minutes recovery, without any supplements ingested and following placebo and creatine ingestion, according to each ACRL (40g, 100g and 135g throughout a four-day period). Blood and urinary creatine levels were also determined from the experimental group for each ACRL. Protein intake (across all groups) was held constant during the study. There were no changes in protein intake or performance of the control group. For the experimental group creatine supplementation produced significant (p<0.01) increases in body mass (82.5 ± 1.4kg pre vs 82.9 ± 1.2kg post), blood (0.21 ± 0.04mmol·l-1 pre vs 2.24 ± 0.98mmol·l-1 post), and urinary creatine (0.23 ± 0.09mmol·l-1 pre vs 4.29 ± 1.98mmol·l-1 post). No significant differences were found between the non-supplement and placebo condition. Creatine supplementation produced an average improvement of 0.7%, 11.8% and 11.1% for the 40g, 100g and 135g ACRL respectively. However, statistics revealed significant (p<0.01) differences only for the 100g and 135g ACRL. Mean ± SD values for the 100g ACRL for mean and minimum power were 612 ± 180W placebo vs 693 ± 221W creatine and 381 ± 35W placebo vs 415 ± 11W creatine accordingly. For the 135g ACRL the respective performance values were 722 ± 215W placebo vs 810 ± 240W creatine and 405 ± 59W placebo vs 436 ± 30W creatine. These data indicate that a 100g compared to 40g ACRL produces a greater potentiation of performance whilst, greater quantities of creatine ingestion (135g ACRL) can not provide a greater benefit.
Key words: Acute create loading, performance enhancement, dosage
Introduction
Currently, an increasing number of investigators (Balsom et al., 1993; Kamber et al., 1999; Rockwell et al., 2001) have investigated the potential benefit of creatine as an ergogenic aid for improving exercise performance. In the majority of the creatine supplementation studies the dosages used during an Acute Creatine Loading (ACRL) were 20g-25g day-1 for a period of 4-5 days (Greenhaff et al., 1994; Birch et al., 1994; Rockwell et al., 2001) totalling 100g of creatine. This supplementation regimen was based on the results of preliminary studies (Harris et al., 1992) which showed that dosages of 20g. day-1 and 30g. day-1 for a period of 3.5 days (n=1) and 4 days (n=3), 7 days (n=1) in group of five sedentary subjects led to an increase of 20% in total creatine pool. However, Harris et al. (1992) did not proceed to any comparisons in exercise performance data for each creatine dosage separately, in order to establish the most beneficial creatine loading regimen, possibly due to the small size of the sample group. Using a different experimental design Vandenberghe et al. (1999) reported that 25g day-1 for the first two days resulted an increase of 11% in muscle CP and 5-13% in muscle torque, whilst continuation of supplementation for another three days did not add to these effects. That report reverses the ‘‘traditional’’ 100g ACRL and there emerges the need for further research regarding creatine dosage optimisation and exercise potentiation. Such research will ensure the effectiveness of an ACRL, help avoid any overdose side effects, and present some reference creatine values for minimising urine creatine excretion alongside with the maximum beneficial effect on performance. Therefore, the aim of the present study was to establish an ACRL, which would cause the greatest benefit on repetitive all-out cycle performance on active individuals.
Methods
Subjects
Twenty eight active males with mean ± SD age 29.9 ± 4.6 years, body mass 81.6 ± 1.1 kg, height 1.79 ± 0.17m and % body fat 20.1 ± 5.1 participated in these tests. Twenty-one served as the experimental group, whilst seven served as the control group. All subjects were given an information protocol explaining potential risks of the experimental procedures. The present study had Ethical Approval from Ethics Committee of Local General Infirmary. The day of testing subjects were asked to attend the laboratory after a light breakfast (only toast with a little margarine and an orange juice). They were also asked not to participate in any kind of severe exercise 48hrs prior to the tests. However, they did not have to deviate from any of their normal patterns of eating or training during the supplementation period. For all subjects daily food intake was also monitored with the aid of dietary sheets and then analysed (for all nutrients) using a computer program (COMP-EAT4) prior to the initiation of the study (control week) and then, subjects were instructed to follow a protein, carbohydrate and caloric intake per day similar to that week throughout the next two supplementation periods (placebo and creatine week). All subjects were carefully selected based on their ability to comply with the study protocol and their incentive to explore the effects of creatine supplementation on their exercise performance. Therefore, subjects’ compliance in following the diet instructions was assumed to be excellent.
Experimental procedures
The present study consisted of testing over 12 weeks. Subjects were randomly divided into the experimental and control group with the first to comprise three subgroups. Each subgroup followed a different ACRL over four-day period. The dosages (in 5g doses) for subgroups 1, 2 and 3, were 10g day -1, 25g day-1 and 35g day-1 of placebo (POLYTHELENEGLYCOL 4000) or pure creatine monohydrate (CHEMIE-LINZ) respectively. The chemical composition of creatine supplements was assessed via repeated measures using a PYEUNICAM8 spectrophotometer from the laboratory of Leeds General Infirmary (Department of Surgery).
Since each subgroup was following a different ACRL (40g, 100g, 135g) they performed the exercise testing on separate weeks in order to make management of the experiment feasible. One reason was the required commitment and consistency of the subjects in consuming the beverage at a specific time each day was essential for the fulfilment of the purposes of the study. Therefore, subgroup 1, 2, and 3 performed the exercise testing in weeks 4-6, 7-9 and 10-12 respectively. For the subgroups 1, 2 and 3 the first, second and third week represented the baseline, placebo and creatine condition respectively. The control group performed the exercise testing in weeks 1-3 where the first week represented the baseline condition and the second and third the placebo condition. Since the kinetics of creatine washout has not been established yet, the investigator provided placebo and creatine supplements in the 1st and 2nd supplementation period respectively in order to avoid the carry-over effect. Even through placebo and creatine conditions were always administrated in this order they were conducted as a double blind design for the subjects and the test administrator.
All supplements were prepared by the investigators at University Campus, in powder form with similar texture, taste and appearance and were independently packaged in generic foil packets for double-blind administration. The supplement administrator who was not aware of the content of the packets dissolved placebo or creatine in 300ml of hot-warm water and hand over the solution to the subjects with morning, mid-day and evening meals. Two, five and seven solutions were prepared per day for the 40g, 100g and 135g ACRL respectively. Each solution was prepared by the supplement administrator immediately prior to the ingestion, and in the absence of the subjects.
The 4th day and one-hour after the last placebo dose all subjects performed three Anaerobic Wingate Tests (AWT) interspersed with 6 minutes active recovery (60rpm). A standardised warm-up was performed which involved 5 min of cycling at 60 Watts immediately followed by two 2 s sprints. The warm up was followed by a 5 min rest period where the subjects remained seated before the start of the AWT. The ergometer used during the AWT testing was a MONARK 814E (Sweden) brated pre, and post each test was bolted to the floor in order to provide greater stability during maximal cycling. Before starting the test a belt attached to the wall at one end, was passed around the subject’s waist in order to prevent the subject rising out of the saddle. Saddle height adjustments for each subject were made prior testing as well a series of 6 s cycle sprints as part of a habituation process with AWT (Havenetidis et al., 2003). The experimental conditions and procedures for performing the AWT were those described by Bar-Or (1987). The exercise protocol was repeated the following week with the use of creatine supplements under the same conditions and by following the same experimental procedures. The present exercise protocol is considered an ideal model for assessing the ergogenic properties of creatine as reported by other investigators (Dawson et al., 1995; Prevost et al., 1997). Repeated sprints are associated with cumulative fatigue, a condition, which is preferable in studies investigating the ergogenic effect of various supplements. Using a single exercise bout a less marked creatine phosphate depletion (a metabolic state which is linked with fatigue) would have been produced and consequently a failure to quantify the relationship between the elevated muscle creatine phosphate stores (following creatine supplementation) and the prevention of performance reduction. In contrast, the present exercise protocol has shown to maximally stress the creatine phosphate pathway as creatine phosphate concentration at the end of the 3rd AWT represented only 14% of the values measured at rest (Havenetidis et al., 2002).
Physiological Measurements
Measurements of body mass, height and estimation of percentage body fat (HARPENDEN, Durnin and Womersley, 1974) were made the day of testing, for baseline, placebo and creatine condition. For the three experimental groups blood samples were collected from an antecubital vein one-hour after the last dose on both occasions (placebo and creatine condition). All samples were centrifuged and the supernatant was stored, and analysed the following day via the enzymatic method (Harris et al., 1974). Urine samples were also collected each day of supplement ingestion (across a four-day period) during the placebo and the creatine condition at the end of five time intervals (11 a.m., 3 p.m., 7 p.m., 11 p.m. and 7a.m. representing periods I-V respectively) within a 24 hr period, after discarding the first sample (7 a.m. sample). Each 24-hr period consisted of 4 hr periods (periods I-IV) plus one 8 hr (period V). Immediately following each collection period the volume was measured, and 1% aliquot was transferred to a storage tube and frozen for future analysis. All samples were assayed in triplicate with a maximum 4% difference between triplicates accepted. Subjects collected the urine samples by themselves in bottles supplied by the investigators. Urinary creatine concentration was measured using the same enzymatic method for plasma creatine (Harris et al., 1974).
Performance Measurements
The indices measured during the AWT were: Mean power (MP) and Minimum Power (MIP). MP was calculated as the average mechanical power output during each 10 s interval (MP 0-10s, 10-20s, 20-30s), whilst MIP as the lowest power output during any 1s period during the whole 30s AWT. All AWT indices were measured using a computer package (Lakomy, 1986). Physiological and performance measurements were not made in the control group because the experimental group served as its own control throughout the first series of measurements which were carried out without any supplement administration (control week).
Statistics
Paired t-test comparisons were employed to detect differences in body mass, blood and urinary creatine pre and post supplementation. In all AWT indices and protein intake a two-way Analysis of Variance with repeated measures (ANOVA) was used on group (40g, 100g, and 135g ACRL) and condition (baseline, placebo and creatine) factors. A one-way ANOVA was also used to detect differences in blood creatine values in relation to the creatine dosage ingested.
Results
No significant differences in protein and carbohydrate intake were observed among the control, placebo and creatine weeks across all groups (Table 1).
Table 1.
Protein and carbohydrate intake (g) for each week across all groups. Values are Mean (SD).
| Acute Creatine Loading | Control week | Placebo week | Creatine week |
|---|---|---|---|
| Protein intake | |||
| 40g | 80.1 (8.3) | 76.4 (15.0) | 72.2 (25.0) |
| 100g | 86.9 (9.1) | 82.3 (10.7) | 83.8 (10.1) |
| 135g | 79.3 (11.9) | 87.1 (10.8) | 81.8 (15.3) |
| Control | 82.2 (12.2) | 84.3 (13.2) | 76.1 (16.2) |
| Carbohydrate intake | |||
| 40g | 295 (77) | 263 (47) | 285 (62) |
| 100g | 273 (79) | 271 (66) | 299 (68) |
| 135g | 328 (69) | 296 (70) | 303 (61) |
| Control | 291 (100) | 278(47) | 289 (62) |
In the current study body mass (Mean ± SD) increased (p<0.01) from 82.5 ± 11.7 kg, to 82.9 ± 11.7 kg, following creatine supplementation across the three supplementation groups (t-value –4.084). Body mass values at placebo vs creatine supplementation for the 40g, 100g and 135g ACRL were 84.8 ± 15.7 kg vs 85.0 ± 15.5 kg, 76.9 ± 6.4 kg vs 77.2 ± 6.5 kg and 85.6 ± 10.8 kg vs 86.3 ± 10.8 kg respectively. Details on experimental group anthropometry are shown in Table 2.
Table 2.
Experimental group anthropometry details.
| Subject | Body mass (kg) | Age (years) | Height (m) | Body fat (%) | Body mass post creatine (kg) | ACRL (g) |
|---|---|---|---|---|---|---|
| 1 | 72.5 | 26 | 1.87 | 17.84 | 73.0 | 40 |
| 2 | 71.8 | 22 | 1.77 | 15.77 | 72.0 | 40 |
| 3 | 63.5 | 28 | 1.76 | 10.40 | 63.5 | 40 |
| 4 | 96.0 | 30 | 1.76 | 28.15 | 96.1 | 40 |
| 5 | 106.6 | 21 | 1.85 | 21.13 | 105.9 | 40 |
| 6 | 93.3 | 32 | 1.88 | 26.33 | 93.5 | 40 |
| 7 | 90.6 | 25 | 1.73 | 20.95 | 90.8 | 40 |
| 8 | 70.0 | 23 | 1.75 | 11.87 | 70.2 | 100 |
| 9 | 74.0 | 22 | 1.84 | 9.75 | 74.0 | 100 |
| 10 | 84.0 | 28 | 1.82 | 10.24 | 84.3 | 100 |
| 11 | 76.7 | 25 | 1.77 | 12.90 | 76.9 | 100 |
| 12 | 87.0 | 25 | 1.80 | 19.49 | 87.3 | 100 |
| 13 | 70.9 | 30 | 1.70 | 14.08 | 70.9 | 100 |
| 14 | 76.1 | 22 | 1.86 | 7.24 | 76.9 | 100 |
| 15 | 75.5 | 20 | 1.73 | 20.27 | 75.9 | 135 |
| 16 | 76.0 | 28 | 1.80 | 16.86 | 76.9 | 135 |
| 17 | 75.0 | 19 | 1.73 | 19.26 | 75.9 | 135 |
| 18 | 89.0 | 22 | 1.79 | 19.90 | 89.9 | 135 |
| 19 | 86.0 | 20 | 1.91 | 15.15 | 86.6 | 135 |
| 20 | 94.5 | 21 | 1.94 | 13.84 | 95.1 | 135 |
| 21 | 103.0 | 24 | 1.94 | 22.84 | 103.9 | 135 |
Blood and urinary creatine were also significantly (p<0.01) increased and that increase was proportional to the creatine dosage ingested (Table 3).
Table 3.
Blood and urinary CR (mmol·l-1) response to different CR dosages. Values are Mean (SD).
| Blood creatine | Urinary creatine | |||
|---|---|---|---|---|
| Acute Creatine Loading | Pre Creatine | Post Creatine | Pre Creatine | Post Creatine |
| 40g | 0.17 (0.05) | 1.12 (0.08)** | 0.28 (0.05) | 2.07 (0.78)** |
| 100g | 0.24 (0.06) | 2.63 (0.66)** | 0.13(0.04) | 4.90 (2.10)** |
| 135g | 0.23 (0.06) | 2.97 (0.89)** | 0.28(0.04) | 5.97 (1.90)** |
** significantly different from the values pre creatine; p<0.01
Performance Data
On all occasions the highest power output was achieved during the first seconds of each AWT and then was followed by a steady decline. For the control group the statistics showed no significant differences in performance across the three weeks of testing, which indicates that repeated 30 s cycle sprints are not characterised by a significant learning effect (Figure 1). Average difference of performance between each AWT was ± 2.5% a value that was not statistically significant.
Figure 1.
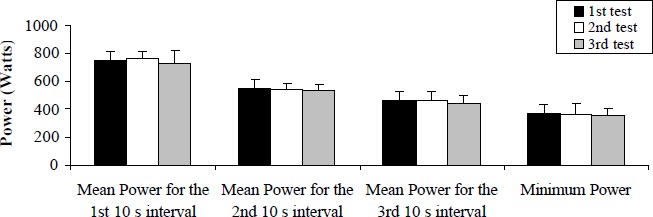
Power output response across repeated tests for the control group. Values are Mean (SD) combining the three 30 s sprints in each condition.
In contrast, among the three experimental groups significant differences were observed on condition (placebo vs creatine) and group by condition interaction (40g ACRL creatine condition versus 100g ACRL creatine condition, p<0.01 and 40g ACRL creatine condition versus 135g ACRL creatine condition, p<0.01). A summary of AWT data for all groups during baseline, placebo and creatine condition is presented in Table 3.
The present data showed that performance potentiation was influenced by the creatine dosage used. Figure 2 shows the percentage difference between creatine and placebo condition across all AWT indices for each supplementation group.
Figure 2.
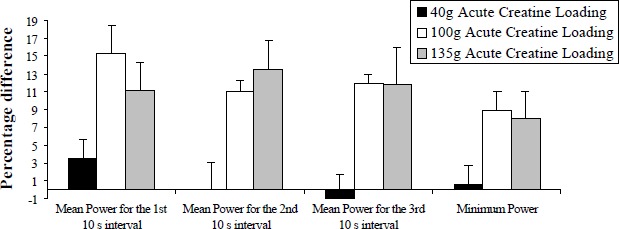
Average performance potentiation (%) using three different Acute Creatine Loadings. Values are Mean (SD) combining the three 30 s sprints in each condition.
The present data also indicates that performance potentiation was not consistent for all supplementation groups and throughout the 30s period. This is more profound when power output expressed per second (Figures 3 - 5).
Figure 3.
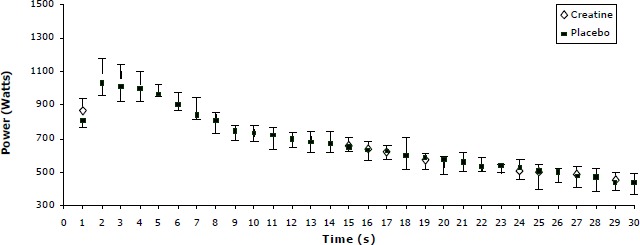
Power decline pattern for the placebo and creatine condition for the 40g Acute Creatine Loading. Values are Mean (SD) combining the three 30s sprints in each condition.
Figure 5.
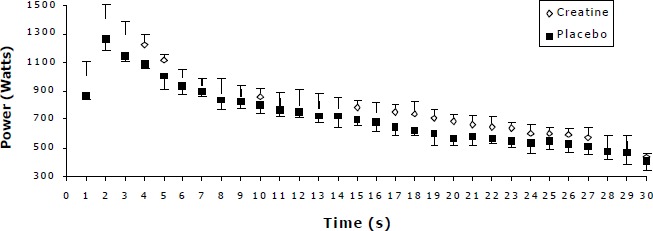
Power decline pattern for the placebo and creatine condition for the 135g Acute Creatine Loading. Values are Mean (SD) combining the three 30s sprints in each condition.
As shown by Figures 3 to 5 there is an increase in power output throughout the 30 s period for the 100g and 135g ACRL in creatine to the placebo condition. In contrast, for the 40g ACRL the power slopes, after the 4th second, for the placebo and creatine conditions were almost identical.
Discussion
The present study showed that creatine supplementation per se does not necessarily improve exercise performance, but it is influenced by the amount of creatine ingested during a four-day ACRL. The amount of creatine ingested in the present ACRL was 40g, 100g and 135g and led to an average (across all AWT indices) improvement of 0.7%, 11.8% and 11.1% respectively. This difference in performance potentiation could be related to differences in blood creatine concentration (post supplementation) which influences creatine muscle uptake (Harris et al., 1992). Fitch and Shields (1966) who developed a model for creatine entry into muscle based on results with the use of guinea pigs showed that the facilitation of creatine entry is much greater when blood creatine concentration exceeds 1 mmol·l-1. Based on the short time period that plasma creatine remains elevated (half-life 1-1.5 hours) it is possible that the effect of creatine ingestion on muscle entry did not last long, especially if the plasma levels (1.1 ± 0.1 mmol·l-1) for the 40g ACRL were just above the threshold. The latter, together with an initial high muscle total creatine concentration could have resulted a decreased creatine uptake by the muscle. Alternatively, for the 100g and 135g ACRL significantly higher blood creatine concentrations were presented, alongside with a greater potentiation of performance compared to the 40g ACRL, which seems that is not sufficient to achieve the highest values of muscle creatine uptake. In contrast, creatine dosages totaling 100g or greater (135g) produce a blood creatine concentration far beyond the threshold of 1 mmol·l-1, therefore facilitating creatine entry into the muscle. It is noteworthy that blood creatine values at placebo were similar among groups and within the normal values reported by other investigators (Tortora and Anagnostakos, 1990; Engelhardt et al., 1998) therefore, no initial differences existed between groups prior supplementation.
The present results do not support those reported by Vandenberghe et al. (1999), who measured nine males performing five bouts of 30 dynamic maximal voluntary contractions of the knee extension muscles separated by 2-min rest intervals. In that study a 50g ACRL has shown to be equally beneficial to exercise performance, compared compared to a 125g ACRL. This inconsistency between Vandenberghe et al’ s. (1999) findings and those of this study could be related to a number of factors. Firstly, there is a possibility the subjects in that study to have reached fatigue since that maximal repeated knee extension protocol was performed twice and within such a small time period (days 1, 3 and 6) and consequently not be able to show further improvement with the continuation of creatine supplementation. Secondly, the average potentiation across the first two sets of knee extensions were 6.6% compared to the 3.6% for the 50g and 125g ACRL which indicates a further 3% improvement alongside with a 5% increase in creatine phosphate concentration. These findings show that continuation of creatine supplementation provided more benefit to those subjects at least at the initial phase of exercise which was noted by the investigators themselves (Vandenberghe et al., 1999). Alternatively, in the light of the 40g ACRL present data, the significant performance improvement shown in Vandenberghe et al’s. (1999) data could be due to differences in the administration protocol (supplements and placebo were 2,5g Isostar® tablets and Maltodextrine respectively) which might have provided more carbohydrate in favour of creatine uptake in the muscle (Green et al., 1996). As a general rule, differences in sample groups and exercise protocols are the main factors which lead to differences among creatine supplementation studies and this is proven to be correct in this case.
The present findings are in agreement with studies where a 40g or lower ACRL were used (Peyrebrune et al., 1998 ; Preen et al., 2002). These studies showed no improvement in swimming and cycle ergometry (Peyrebrune et al., 1998; Preen et al., 2002, respectively), compared to the respective values of 22% (p<0.01), and 2% (p<0.05) for a 100g ACRL (Jones et al., 1999; Theodorou et al., 1999). Similar results to a 40g ACRL were also reported by other research groups employing creatine regimens 60g-80g (Earnest et al., 1997; Odland et al., 1997; Rockwell et al., 2001) where there was a trend for performance enhancement but it was not significant. These reports strengthen the present speculation that the 100g ACRL consists a ‘‘threshold’’ where the occurrence of the ergogenic effects of creatine can be significantly increased. However, other studies showed no increase in exercise performance even with the use of dosages totaling 100g (Mujika et al., 1996; Cooke and Barnes, 1997; Finn et al, 2001). It is likely differences in subjects and exercise protocol in the above studies explain this apparent contradiction with the present results. For example, Finn et al. (2001) utilised endurance trained athletes (triathletes), whilst the sample group used in the present study were active subjects however, they were participating in multiple sprint events (rugby, soccer) in a recreational way. It has been suggested (Greenhaff et al., 1994; Engelhardt et al, 1998; Kamber et al., 1999) that the magnitude of improvement following creatine supplementation varies considerably for endurance and sprint trained groups due to differences related to the metabolic and mechanical properties of their fibre recruitment pattern.
Another finding of the present study was that using a greater than 100g ACRL (135g) no further improvement was observed. Despite the fact that few direct comparisons between different ACRL have been reported in the literature, in studies where dosages totaling 135g or more were used, performance potentiation was similar (Scneider et al., 1997; Volek et al., 1997) to those utilised the ‘‘traditional’’ 100g ACRL (Birch et al., 1994; Izquierdo et al., 2002). Additionally, in studies (Mujika et al., 2000; Yquel et al., 2002) where the ACRL was between (120g) the dosages used in this study (100g and 135g), again exercise improvement was not elevated beyond the values observed for the 100g ACRL.
Á possible explanation for the lack of further improvement with the use of a greater than 100g ACRL might be an increased creatine excretion. Considering that the greater blood creatine concentrations were accompanied by a greater 24hr urinary creatine excretion, there is a possibility that increased blood creatine levels did not necessarily lead to a more efficient muscle creatine entry, and consequently to a greater facilitation of performance. In support of this mechanism when blood creatine expressed in relation of the dosage ingested (mmoles·g-1 of dosage) the 135g ACRL showed significantly (p<0.05) lower values, an indication of greater excretion (Figure 6). It is worth mentioning that urinary creatine values at placebo and following supplementation coincide with those reported by other investigators (Poortmans, et al., 1997; Vandenberghe et al., 1997; Engelhardt et al, 1998) thus, it is unlikely fluctuations among groups to be attributed to kidney malfunction or/and muscle injury.
Figure 6.
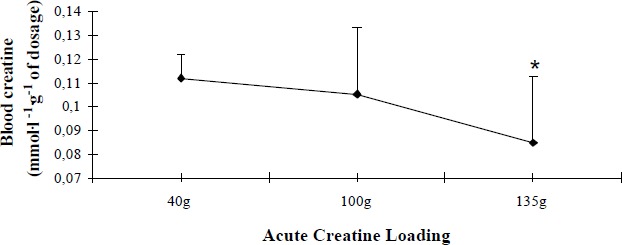
Blood creatine concentration in relation to different creatine dosages. Values are Mean (SD). *significantly different from 40g and 100g Acute Creatine Loading; p<0.05.
Despite the fact that the highest creatine uptake is accomplished only when creatine is ingested mixed with carbohydrates (Green et al., 1996), since all groups in the present study did not ingest carbohydrates, any differences in performance would be possibly related to the amount of creatine ingested. Additionally, carbohydrate intake, as shown by the dietary analysis (Table 1), was similar across conditions and groups. The authors have chosen not to use carbohydrates mixed with creatine in order to assess initially the effect of different creatine regimens on exercise performance and then to proceed to the addition of carbohydrates on future studies. However, it must be emphasized that the homogeneity of the subjects, in terms of the initial total creatine concentration in the muscle was not known, a factor which could have influence creatine uptake and consequently performance potentiation in the present study.
Another interesting finding of the present study was that subjects’ power output was facilitated in a different way during the 40g compared to the 100g and 135g ACRL. In more detail, power increase following creatine supplementation was evident in the first seconds of cycling for all groups, but it was still present until the end of the 30 s period only for the 100g and 135g ACRL. With the use of a 40g ACRL the beneficial effect of creatine disappeared after the first four seconds of cycling (Figure 3) and that phenomenon was reflected upon all AWT indices. The highest improvement in performance was shown in MP0-10s (3.5%) and then became negligible for the MP10-20s (0%), MP20-30s (-1%) and MIP (0.5%). In contrast, power output (compared to placebo) was consistently higher (Figures 4 - 5 ) for the 100g and 135g ACRL throughout the whole 30 s period and consequently led to significantly higher power values for the 1st (13.2%) 2nd (12.2%) and 3rd (11.9%) MP interval. The above observations give an indication of the existence of two mechanisms that may operate with the use of the present creatine dosages. Firstly, a likely elevated pre exercise creatine phosphate concentration which is suggested (Harris et al., 1992; Greenhaff et al., 1994) that delay the depletion of creatine phosphate stores during exercise and extends the time period that the adenosine triphosphate-creatine phosphate system is predominant, providing an increased power output within the first 10 s. Evidently, the greatest improvement (across all groups) was presented in the first seconds of exercise (10%) and gradually reduced to the end of the 30s period (5.8%). Even for the 40g ACRL the improvement in the first seconds of cycling was such (3.5%) that almost reached the significance level (p=0.06).
Figure 4.
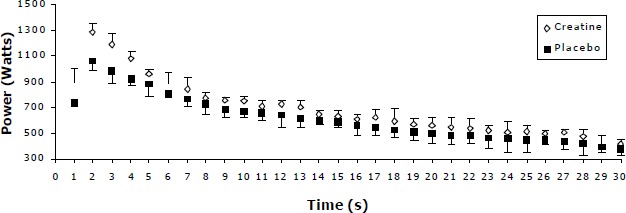
Power decline pattern for the placebo and creatine condition for the 100g Acute Creatine Loading. Values are Mean (SD) combining the three 30s sprints in each condition.
A second mechanism that seems to operate was a more efficient buffering capacity of the creatine phosphate-creatine system following creatine supplementation (Birch et al., 1994; Greenhaff et al., 1994). However, this second mechanism operated only for the 100g and 135g ACRL, as power output was sustained higher beyond the 10 s period, suggesting that with these dosages it was possible there was accelerated hydrogen ion elimination. Additionally, a greater depletion of creatine phosphate stores and/or metabolic acidosis caused by use of this exercise protocol (three Wingate Tests) might have served as a facilitating factor, which highlighted any beneficial effects of creatine supplementation. The findings of Balsom et al. (1993) support the aforementioned theory since he has showed that performance potentiation (using a similar dosage) during ten 6s cycle sprints became significant only towards the end of the exercise protocol and beyond the 4s period. However, the absence of muscle data in this study does not give the opportunity to support the existence of these mechanisms with biochemical data.
Conclusion
The use of a 100g compared to a 40g acute creatine loading produced a greater and constant potentiation of sprint cycle performance, whilst no significantly greater benefit occurred with the use of a greater dosage (135g). These findings support previous reports that the use of the commonly accepted creatine-loading regimen of 100g may provide ergogenic benefit. Performance potentiation is greater in the first seconds of repeated sprint cycling (even for the low creatine dosage) and progressively diminishes towards the end of a 30s period. This ergogenic pattern could be attributed primary to an elevated pre exercise creatine phosphate concentration and to a lesser extent to a more efficient buffering capacity. Additional research however, should evaluate the use of varying creatine dosages, in relation to body mass, as studies show that sample group specificity seems to affect the magnitude of performance potentiation.
Acknowledgement
The authors would like to thank the State Scholarship Foundation of Greece for the financial support of the present study. Our gratitude is extended to R.F.G.J. King, Research Fellow in Leeds General Infirmary, U.K. and R. Butterly, Senior Lecturer in Leeds Metropolitan University, for their advice and guidance throughout the period of this study. Many thanks also to all the staff in the Department of Chemical Pathology (Leeds General Infirmary) for their precious help in the blood and urine sample collection.
Biographies
Konstantinos HAVENETIDIS
Employment:
Leeds Metropolitan University, School of Leisure and Sports Studies, Leeds, UK
Degrees:
MSc, PhD
Research interest:
Sports nutrition and evaluation of sporting performance
E-mail: kohave1968@yahoo.com
Ourania MATSOUKA
Employment:
A member of staff in Democritus University of Thrace, Greece.
Degrees:
MSc, PhD
Research interest:
Outdoor activities, motor skill learning, and handicap individuals’ exercise ability
E-mail: ramatsou@hotmail.com
Carlton Brian COOKE
Employment:
Professor in Sports and Exercise Science at Leeds Metropolitan University, U.K.
Degrees:
BSc, PhD
Research interest:
Children’s and adult’s activity, fitness and health, biomechanics of sports technique and physiology of elite performance.
E-mail: c.cooke@lmu.ac.uk
Apostolos THEODOROU
Employment:
Lecturer in the College of Sports Science, Greece
Degrees:
MSc, PhD
Research interest:
Sports coaching and exercise physiology
E-mail: tolislaura@teledomenet.gr
References
- Bar-Or O. (1987) Wingate test: update on reliability and validity. Sports Medicine 4, 381-394 [DOI] [PubMed] [Google Scholar]
- Balsom P.D., Ekblom B., Soderlund K., Sjodin B., Hultman E. (1993) Creatine Supplementation and dynamic high-intensity intermittent exercise. Scandinavian Journal of Medicine and Science in Sports 3, 143-149 [Google Scholar]
- Birch R., Noble D., Greenhaff P.L. (1994) The influence of dietary creatine Supplementation on performance during repeated bouts of maximal isokinetic cycling in man. European Journal of Applied Physiology 69, 253-259 [DOI] [PubMed] [Google Scholar]
- Cooke W., Barnes W.S. (1997) The influence of recovery duration on high-intensity exercise performance after oral creatine supplementation. Canadian Journal of Applied Physiology 22, 454-467 [DOI] [PubMed] [Google Scholar]
- Dwason B., Cutler M., Moody A., Lawerence S., Goodman C., Randall N. (1995) Effects of oral creatine loading on single and repeated maximal short sprints. Australian Journal of Science and Medicine in Sport 27(3), 56-61 [PubMed] [Google Scholar]
- Durnin J.V.G.A., Womersley J. (1974) Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. British Journal of Nutrition 32, 77-97 [DOI] [PubMed] [Google Scholar]
- Earnest C.P., Almanda A.L., Mitchell T.L. (1997) Effects of creatine monohydrate ingestion on intermediate duration anaerobic treadmill running to exhaustion. Journal of Strength and Conditioning Research 11, 234-238 [Google Scholar]
- Engelhardt M., Neumann G., Berbalk A., Reuter I. (1998) Creatine supplementation in endurance sports. Medicine and Science in Sports and Exercise 30, 1123-1129 [DOI] [PubMed] [Google Scholar]
- Finn J.P., Ebert T.R., Withers R.T., Carey M.F., Mackay M., Phillips J.W., Febbraio M.A. (2001). Effect of creatine supplementation on metabolism and performance in humans during intermittent sprint cycling. European Journal of Applied Physiology 84, 238-243 [DOI] [PubMed] [Google Scholar]
- Fitch C.D., Shields R.P. (1966) Creatine metabolism in skeletal muscle. Creatine movement across muscle membranes. Journal of Biological Chemistry 241, 3611-3614 [PubMed] [Google Scholar]
- Green A.L., Hultman E., Macdonald I.A., Sewell D.A., Greenhaff P.L. (1996). Carbohydrate ingestion augments skeletal muscle creatine accumulation during creatine supplementation in humans. American Journal of Physiology 271, E821-826 [DOI] [PubMed] [Google Scholar]
- Greenhaff P.L., Bodin K. G., Soderlund K., Hultman E. (1994) Effect of oral creatine Supplementation on skeletal muscle phosphocreatine resynthesis. American Journal of Physiology 266, E725-730 [DOI] [PubMed] [Google Scholar]
- Harris R.C., Hultman E., Nordesjo L.O. (1974) Glycogen, glycolytic intermediates and high energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scandinavian Journal of Clinical and Laboratory Investigations 33, 109-120 [PubMed] [Google Scholar]
- Harris R.C., Soderlund K., Hultman E. (1992) Elevation of creatine in resting and exercised muscle of normal subjects by creatine Supplementation. Clinical Science 32, 367-374 [DOI] [PubMed] [Google Scholar]
- Havenetidis K., Matsouka R., Konstadinou V. (2003) Establishment of the highest peak anaerobic power prior to the commencement of the Anaerobic Wingate Test. Journal of Human Movement Studies 44, 479-487 [Google Scholar]
- Havenetidis K., Theodorou A., Cooke C.B., King R.F.G.J. (2002) Effects of an acute cr loading on muscle metabolites and anaerobic performance. In : Proceedings of the 7th annual congress of the European College of Sport Science. Ed: Koskolou M., Geladas N., Klissouras V.2, 593 [Google Scholar]
- Jones A.M., Atter T., Georg K.P. (1999) Oral creatine supplementation improves multiple sprint performance in elite ice hockey players. Journal of Sport Medicine and Physical Fitness 39, 189-196 [PubMed] [Google Scholar]
- Kamber M., Koster M., Kreis R., Walker G., Boesch C., Hoppeler H. (1999) Creatine supplementation-Part I: performance, clinical chemistry and muscle volume. Medicine and Science in Sports and Exercise 31, 1763-1769 [DOI] [PubMed] [Google Scholar]
- LaKomy H.K.A. (1986) Measurement of work and power output using friction-loaded cycle ergometers. Ergonomics 29, 509-517 [DOI] [PubMed] [Google Scholar]
- Mujika I., Padilla S., Ibanez J., Izquierdo M., Gorostiaga E. (2000) Creatine supplementation and sprint performance in soccer players. Medicine and Science in Sports and Exercise 32, 518-525 [DOI] [PubMed] [Google Scholar]
- Mujika I., Chatard J.C., Lacoste L., Barale F., Geys,ant A. (1996) Creatine supplementation does not improve sprint performance in competitive swimmers. Medicine and Science in Sports and Exercise 28, 1435-1441 [DOI] [PubMed] [Google Scholar]
- Odland L.M., MacDougall J.D., Tarnopolsky M., Elorriaga A., Borgmann A. (1997) Effect of oral creatine supplementation on muscle [PCr] and short-term maximum power output. Medicine and Science in Sports and Exercise 29, 216-219 [DOI] [PubMed] [Google Scholar]
- Peyrebrune M.C., Nevill M.E., Donaldson F.J., Cosford D.J. (1998) The effects of oral creatine supplementation on performance in single and repeated sprint swimming. Journal Sports Sciences 16, 217-279 [DOI] [PubMed] [Google Scholar]
- Poortmans J.R., Auquier H., Renaut V., Durussel A., Saugy M., Brisson G.R. (1997) Effect of short-term creatine supplementation on renal responses in men. European Journal of Applied Physiology 76, 566-567 [DOI] [PubMed] [Google Scholar]
- Preen D., Dawson B., Goodman C., Lawrence S., Beilby J., Ching S. (2002) Pre-exercise oral creatine ingestion does not improve prolonged intermittent sprint exercise in humans. Journal of Sport Medicine and Physical Fitness 42, 320-329 [PubMed] [Google Scholar]
- Prevost M.C., Nelson, A G., Morris G.S. (1997) Creatine supplementation enhances intermittent work performance. Research Quarterly for Exercise and Sport 68, 61-68 [DOI] [PubMed] [Google Scholar]
- Rockwell J.A., Rankin J.W., Toderico B. (2001) Creatine supplementation affects muscle creatine during energy restriction. Medicine and Science in Sports and Exercise 33, 61-68 [DOI] [PubMed] [Google Scholar]
- Schneider D.A., McDonough P.J., Fadel P.J., Berwick J. (1997) Creatine supplementation and the total work performed during 15-s and 1-min bouts of maximal cycling. Australian Journal of Science and Medicine in Sport 29, 65-68 [PubMed] [Google Scholar]
- Theodorou A., Cooke C.B., King R.F.G.J., Hood C., Denison T., Wainwright B.G., Havenetidis K. (1999) The effect of longer-term creatine supplementation on elite swimming performance after an acute creatine loading. Journal of Sports Sciences 17, 853-859 [DOI] [PubMed] [Google Scholar]
- Tortora A., Anagnostakos G. (1990). Normal values for selected blood and urine tests. : Principles of Anatomy and Physiology. Ed: Tortora A., Anagnostakos G.6th edition Harper and Row Publishers; 715-722 [Google Scholar]
- Vandenberghe K., Van-Hecke P. Van-Leemputte M. Vanstapel F. Hespel P (1999) Phosphocreatine resynthesis is not affected by creatine loading. Medicine and Science in Sports and Exercise 31, 236-242 [DOI] [PubMed] [Google Scholar]
- Vandenberghe K., Goris M, Van-Hecke P., Van-Leemputte M., Vangerven L., Hespel P. (1997) Long-term creatine intake is beneficial to muscle performance during resistance training. Journal of Applied Physiology 83, 2055-2063 [DOI] [PubMed] [Google Scholar]
- Volek J.S., Kraemer W.J., Bush J.A., Boetes M., Incledon T., Clark K.L., Lynch J.M. (1997) Creatine supplementation enhances muscular performance during high-intensity resistance exercise. Journal of American Diet Association 97, 765-770 [DOI] [PubMed] [Google Scholar]
- Yquel R.J., Arsac L.M., Thiaudiere E., Canioni P., Manier G. (2002) Effect of creatine supplementation on phosphocreatine resynthesis, inorganic phosphate accumulation and pH during intermittent maximal exercise. Journal of Sports Sciences 20, 427-437 [DOI] [PubMed] [Google Scholar]


