Abstract
Myocardial ischemia/reperfusion (I/R) injury is partly mediated by thrombin. In support, the functional inhibition of thrombin has been shown to decrease infarct size after I/R. Several cellular responses to thrombin are mediated by a G-protein coupled protease-activated receptor 1 (PAR1). However, the role of PAR1 in myocardial I/R injury has not been well characterized. Therefore, we hypothesized that PAR1 inhibition will reduce the amount of myocardial I/R injury. After we detected the presence of PAR1 mRNA and protein in the rat heart by RT-PCR and immunoblot analysis, we assessed the potential protective role of SCH 79797, a selective PAR1 antagonist, in two rat models of myocardial I/R injury. SCH 79797 treatment immediately before or during ischemia reduced myocardial necrosis following I/R in the intact rat heart. This response was dose-dependent with the optimal dose being 25 μg/kg IV. Likewise, SCH 79797 treatment before ischemia in the isolated heart model reduced infarct size and increased ventricular recovery following I/R in the isolated heart model with an optimal concentration of 1 μM. This reduction was abolished by a PAR1 selective agonist. SCH 79797-induced resistance to myocardial ischemia was abolished by wortmannin, an inhibitor of PI3 kinase; L-NMA, a NOS inhibitor; and glibenclamide, a nonselective KATP channel blocker. PAR1 activating peptide, wortmannin, L-NMA and glibenclamide alone had no effect on functional recovery or infarct size. A single treatment of SCH 79797 administered prior to or during ischemia confers immediate cardioprotection suggesting a potential therapeutic role of PAR1 antagonist in the treatment of injury resulting from myocardial ischemia and reperfusion.
Keywords: myocardial ischemic reperfusion injury, protease-activated receptors, thrombin receptor antagonist
Introduction
The protease activated receptors (PARs) are comprised of four members belonging to a seven transmembrane G protein-coupled receptor family. PAR1, PAR2, PAR3, and PAR4 are encoded by different genes but are related by their unique mechanism of activation. Proteolytic cleavage of the N-terminus by thrombin or other serine proteases exposes a tethered ligand, which then transactivates the receptor [16, 26]. Thrombin is a non-selective activator of PAR1; therefore, in experimental systems, synthetic activating peptides (AP) containing 5–14 amino acid residues corresponding to the tethered ligand are used to selectively activate the receptor in the absence of thrombin [26]. The peptide TFLLR-amide is a PAR1 AP. PAR1 was originally detected in platelets where its activation causes platelet activation and aggregation [26]. In addition, PAR1 has been shown to be expressed in endothelial cells, vascular smooth muscle cells, and cardiomyocytes [10, 17, 23]. PARs modulate a variety of physiological processes depending on which PAR is expressed, which cell type it is acting on, and which serine protease activates it.
PAR1 has been implicated in a variety of cardiovascular pathologies. Its activation on platelets and endothelium contributes to platelet aggregation and thrombosis during acute coronary syndromes. Aberrant overexpression of PAR1 has been documented in the endothelium and vascular smooth muscle cells of human atherosclerotic arteries [20]. In addition, PAR1 activation is partly responsible for restenosis seen after balloon angioplasty [2]. Therefore, not surprisingly, PAR1 antagonists have been sought after to put into clinical use for the treatment of acute coronary syndromes and the prevention of in-stent restenosis [6].
PAR1 has been implicated in myocardial ischemia/reperfusion (I/R) injury as functional inhibition of thrombin was shown to decrease infarct size [7, 12]. More recently, PAR1 deficiency in a PAR1 knockout mouse has been demonstrated to be protective against renal failure in a model of renal I/R [24]. Even though PAR1 is expected to be activated by thrombin at the time of myocardial injury, little is known about its role in determining the extent of myocardial I/R injury. Therefore, we studied the ability of SCH 79797 (SCH), an antagonist of PAR1 [1], to protect the heart against injury from I/R. We hypothesized that SCH 79797, acting through PAR1, would be able to protect the heart against injury caused by I/R resulting in a decrease in infarct size and an increase in recovery of ventricular function after ischemia. We selected in vitro and in vivo models of regional myocardial ischemia and reperfusion to determine the ability of SCH to confer acute cardioprotection in the rat.
Materials and methods
Male Sprague Dawley (SD) rats at 8 weeks of age used in this study received humane care in compliance with the “Guide for the Care and Use of Laboratory Animals” formulated by the National Research Council, 1996. SCH (N –3-cyclopropyl-7-{[4-(1-methyl-ethyl)phenyl] methyl}-7H-pyrrolo{3,2-f]quinazoline-1,3-diamine) was obtained from Tocris Bioscience, Ellisville, MD.
RT-PCR
Total RNA was prepared from fresh heart tissues using TRIzol® Reagent according to the manufacturer's protocol (Invitrogen, Carlsbad, CA) and purified with RNeasy Mini Kit (Qiagen, Melbourne, Australia). cDNA was prepared from 1 μg total RNA using an iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA).
Forward and reverse primers used for amplifying human PAR1 were prepared commercially, based on published data [25]: sense 5′-CCTATGAGACAGCCA-GAATC-3′, antisense 5′-GCTTCTTGACCTTCATCC-3′ (PCR product 495 bp). The conditions for amplification were 95 °C for 2 min for one cycle, 95 °C for 30 s, 55 °C for 30 s, 72 °C for 60 s for 30 cycles and 72 °C for 1 min for 1 cycle. Two microliters of cDNA was used in each reaction along with iTaq DNA Polymerase and dNTP mix (Bio-Rad, Hercules, CA). Electrophoresis was conducted on a 4 % agarose gel stained with ethidium bromide.
Immunoblot analysis
The free walls of the left ventricle were powdered in a liquid nitrogen cooled mortar and pestle. Ice-cold RIPA buffer (50 mM Tris-HCL, pH 7.5, 1% Nonidet P-40, 0.1mM EDTA, 0.1mM EGTA, 0.1% SDS, 0.1% deoxycholic acid) was added to powdered tissue, homogenized for 15 strokes and then centrifuged at 10,000xg at 4 °C for 10 min. Protein concentration was measured by the bicinchoninic acid protein assay. Aliquots of homogenates were mixed with sample buffer, boiled for 5 minutes, run on a 10 % polyacrylamide SDS gel and then transferred to a nitrocellulose membrane. After the membrane was blocked with 5 % non-fat milk in PBS, it was incubated with a 1:200 dilution of the primary antibody (Anti-Thrombin Receptor, Cat No. 611523BD, Bio-sciences, San Jose, CA) in PBS containing 0.1 % Tween-20, 3% bovine serum albumin and 0.1 % sodium azide. The blots were then incubated with the secondary antibody, followed with enhanced chemiluminescence solution (Amersham Life Science, Arlington Heights, IL) and then exposed to film for 1 minute.
SCH and cardioprotection studies in vivo
An in vivo anesthetized rat model was used for these experiments with the general surgical protocol and determination of infarct size described previously [22]. For infarct size studies, rats (n = 6) underwent 30 min of regional ischemia followed by 180 min of reperfusion. SCH was administered intravenously over 1 min starting 15 min prior to or 15 min after the onset of ischemia in a separate series of experiments (n = 6).
SCH and cardioprotection studies in vitro
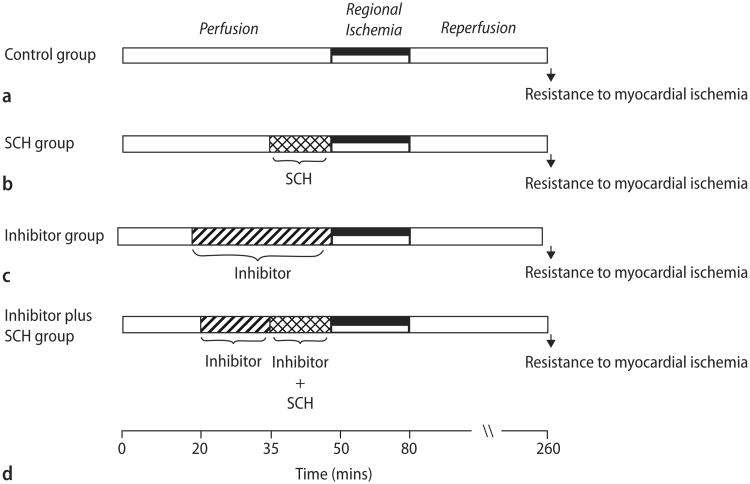
Rats were anesthetized with a mixture containing pentobarbital sodium (50mg/kg) and heparin (1000 IU/kg) i. p. Excised hearts were retrograde perfused through the aorta with a modified Krebs buffer and instrumented as previously described [4]. Briefly, coronary flow rate (CFR) was determined by timed collection of the coronary effluent. A saline-filled latex balloon connected to a pressure transducer was inserted into the left ventricle (LV), and baseline end-diastolic pressure was set at 5–10 mmHg. Heart rate, LV end-diastolic pressure, and LV developed pressures (LVDP) were recorded continuously. The measurements for post-ischemic recovery of LVDP used for comparison were taken at 180 min of reperfusion. After stabilization for 15–20 min, the hearts (n = 8 per group) were subjected to 30 min of regional ischemia, followed by 180 min of reperfusion. Group 1 received no treatment (Fig. 1A). The hearts in group 2 were infused continuously with different concentrations of SCH from 15 min prior to coronary occlusion until occlusion (Fig. 1B). The hearts in group 3 were continuously infused an inhibitor (lepirudin, PAR1-AP, wortmannin, L-NAME, or glibenclamide) starting at 30 min prior to occlusion (Fig. 1C). The hearts in group 4 were continuously infused with an inhibitor (lepirudin, PAR1-AP, wortmannin, L-NAME, or glibenclamide) starting 15 min prior to the commencement of SCH infusion (i. e. from 15 min prior to ischemia) for 30 min (Fig. 1D).
Fig. 1.
Schematic showing experimental protocols. All hearts were subjected to 30 min regional ischemia after a 50 min stabilization period and followed by 180 min of reperfusion. The control group (A) received no treatment. The SCH group (B) was continuously perfused with different concentrations of SCH 15 min prior to the onset of ischemia. The inhibitor groups were continuously perfused with an inhibitor (lepirudin, PAR1 AP, wortmannin, L-NMA, or glibenclamide) for the 30 min prior to ischemia without (C) or with (D) the addition of SCH 15 min prior to ischemia
Infarct induction and measurement
To induce ischemia, a ligature was positioned around the left main coronary artery and threaded through a plastic snare to permit reversible occlusion of the coronary artery. Coronary occlusion was induced for 30 min by clamping the snare onto the heart. Reperfusion was achieved by releasing the snare. At the end of 180 min reperfusion, the coronary artery was re-occluded, and the risk zone was delineated by perfusion 0.5 % Evans' blue into the aortic cannula. Hearts were sectioned and incubated in 1 % triphenyltetrazolium chloride in phosphate buffer (pH 7.4, 37 °C) for 15 min to define white necrotic tissue when fixed in 10 % formalin for 24 h. Area at risk (AAR) and infarct-to-risk ratios were determined by computerized planimetry using J-Image v 0.1.6 software (NIH, Bethesda, MD).
Statistical analysis
Data reported are mean ± SD. Statistical analysis was performed by use of repeated measures ANOVA with the Greenhouse-Geisser adjustment used to correct for the inflated risk of a Type I error [3]. If significant, the Mann-Whitney test was used as a second step to identify which groups were significantly different [3]. Significance was set at P < 0.05.
Results
Presence of PAR1 in the heart
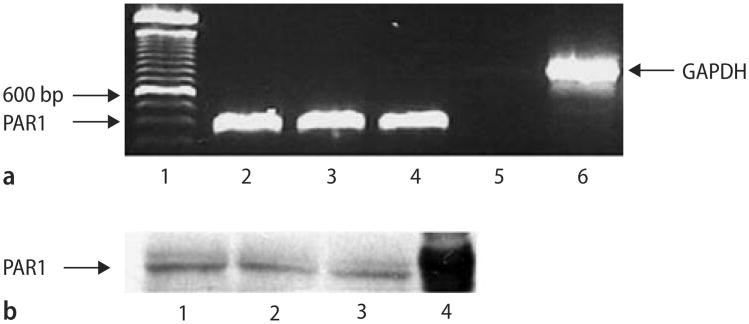
Isolated mRNA from whole heart homogenates was reverse transcribed and then amplified using primers based on the rat sequence for PAR1. PAR1 mRNA is constitutively expressed in the rat heart (Fig. 2A, lanes 2–4). The presence of PAR1 protein in whole heart homogenates obtained from SD rats was then detected by Western blotting (Fig. 2B, lanes 1–3). K-526 cells were used as a positive control (Fig.2B, lane 3). These observations indicate that the rat heart expresses PAR1 mRNA and protein. Despite several trials, we were not able to localize the cell types expressing PAR1 via immunohistochemistry of fixed and sectioned rat heart tissue although our positive control pancreas tissue stained with the PAR1 antibody (data not shown).
Fig. 2.
Analysis of PAR1 expression by RT-PCR and immunoblotting in fresh rat heart tissue. A Determination of PAR1 mRNA by RT-PCR. Total mRNA was amplified by RT-PCR with specific primers as described under Materials and methods. Products were separated on a 2 % (w/v) agarose gel, stained with ethidium bromide. PAR1 (355bp, lane 2–4) were detected in 3 different rat hearts analyzed. Lane 5 is a negative control that contains the PAR1 primers but no DNA. GAPDH (835bp, lane 6) is an internal control. Lane 1 represents a 100 bp ladder standard. B Determination of PAR1 protein by immunoblot analysis of rat heart ventricles using an anti-PAR1 antibody. Lanes 1–3 contains protein extract from 3 different rat ventricles. Lane 4 contains the positive control, protein extracts from the cell line K-562
SCH 79797 and cardioprotection in vivo
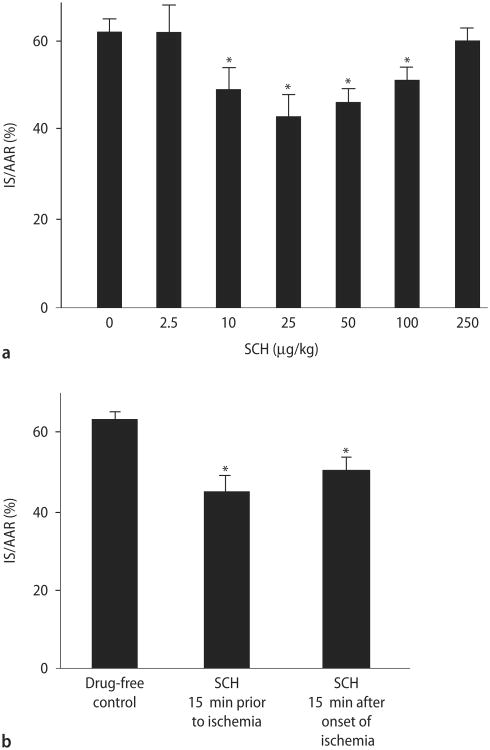
We then determined the ability of SCH to limit injury from myocardial I/R in vivo. We first performed a dose-response analysis, using 2.5, 10, 25, 50, 100, or 250 μg/kg doses, to determine the optimal protective dose. The principal endpoint of this study is infarct size, expressed as a percentage of the area at risk (AAR). Infarct size was 62 ± 3 % of the area at risk (AAR) in the control group. No significant change in infarct size was noted with the lowest (2.5 μg/kg) and highest (250 μg/kg) doses of SCH. However, a graded reduction was seen with the 10-100 μg/kg doses with 25 μg/kg being the optimally effective dose (Fig. 3A). These hearts had an infarct size of 43 ± 5 % of the AAR, which is approximately a 31 % reduction in infarct size compared to the control.
Fig. 3.
Analysis of SCH cardioprotective effects in vivo. A SCH dose-response study in vivo. Rats were treated intravenously with either saline or increasing doses of SCH (2.5, 10, 25, 50, 100, and 250 μg/kg) administered as an IV bolus at 15 min prior to ischemia, followed by 30 min of regional ischemia and 180 min reperfusion. Data are mean ± S. D., n = 6 hearts/group. *P < 0.05, SCH vs. drug-free control. B Phase of action of SCH. Infarct size was determined following 30 min of regional ischemia and 180 min of reperfusion. Data are mean ± SD, n = 6/group. IS infarct size, AAR area at risk. * = P < 0.05, SCH vs. drug-free control
Table 1 summarizes the hemodynamics between groups. There were no statistically significant differences in baseline parameters between the groups. Ischemia reduced mean arterial pressure (MAP) from baseline in all groups. Higher doses of SCH (100 and 250 μg/kg) had a larger reduction in MAP when compared to the control group or groups which received lower doses of SCH; however, this did not reach statistical significance. The reduction in mean arterial pressure continued in the drug treated groups during reperfusion but never reached significance. However, the changes in MAP did significantly affect the rate-pressure product (RPP), an indicator of myocardial oxygen demand, in the 100 μg/kg and 250 μg/kg dose groups. These doses reduced the RPP by 25% and 18%, respectively, (P < 0.05) without a change in heart rate.
Table 1.
Hemodynamic values for SCH 79797 dose-response studies in vivo.
| Baseline | 15min Ischemia | 3hr Reperfusion | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Heart rate, beats/min | MAP, mmHg | RPP mmHg/sec | Heart rate, beats/min | MAP, mmHg | RPP mmHg/sec | Heart rate, beats/min | MAP, mmHg | RPP mmHg/sec | |
| Drug-free control | 356±8 | 139±6 | 49±3 | 368±8 | 104±3 | 38±3 | 312±7 | 91±3 | 28±6 |
| SCH 79797 (2.5 μg/kg) | 370 ±10 | 122±4 | 45±3 | 370±11 | 114±9 | 42±6 | 340±20 | 78±4 | 27±3 |
| SCH 79797 (10 μg/kg) | 350 ± 15 | 124±5 | 44±3 | 358±14 | 106±8 | 38±7 | 338±13 | 71±6 | 24±3 |
| SCH 79797 (25 μg/kg) | 374±7 | 128±3 | 48±2 | 370±6 | 119±8 | 44±3 | 356±5 | 83±9 | 30±4 |
| SCH 79797 (50 μg/kg) | 355±15 | 128±5 | 45±1 | 340±11 | 97±9 | 34±8 | 335±5 | 79±9 | 26±1 |
| SCH 79797 (100 μg/kg) | 347±7 | 122±5 | 42±2 | 333 ±15 | 98±9 | 33±8 | 327±7 | 77±11 | 21±1* |
| SCH 79797 (250 μg/kg) | 376±7 | 119±3 | 45±2 | 344±6 | 87±7 | 30±3 | 320±10 | 71±5 | 23±2* |
Data are mean ± SE, n = 6/group,
P<0.05, SCH 79797 plus ischemia vs ischemia alone.
MAP mean arterial pressure
RPP rate pressure product
We then determined whether SCH reduces infarct size when given during ischemia. Rats were treated with an IV bolus of 25 μg/kg SCH 15 min after the onset of ischemia. SCH was able to reduce infarct size when administered during ischemia but to a lesser extent than administering SCH before ischemia (Fig. 3B).
SCH 79797 and cardioprotection studies in vitro
We then studied the effects of SCH in an isolated heart model. We first performed a concentration-response analysis, using 0.01, 0.1, 0.5, 1.0, 5.0, or 10.0 μM, to determine the optimal protective dose.
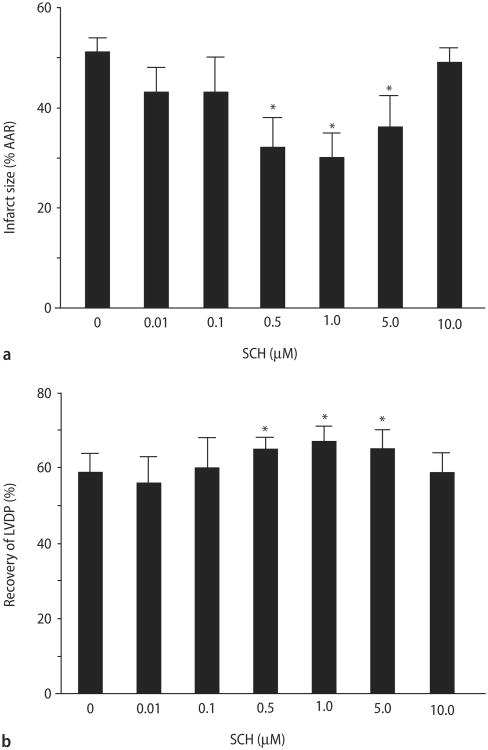
A similar reduction in infarct size was also seen in an isolated heart model of regional I/R injury (Fig. 4A). The isolated control hearts produced an infarct size of 51 ± 3 % of the AAR. Continuous administration of SCH for 15 min immediately before ischemia resulted in a concentration-dependent reduction of infarct size. One micromolar SCH led to the largest reduction in infarct size (30 ± 5 %), a 41 % decrease. SCH had no significant effect on infarct size when either a 10-fold higher or lower concentration was used.
Fig. 4.
SCH concentration-response study in vitro. Rat hearts were perfused for 15 min with SCH (0.01, 0.1, 0.5, 1, 5, 10 μM) prior to 30 min of regional ischemia and 180 min reperfusion. A Percent infarction of left ventricular after SCH treatment. B Recovery of left ventricular developed pressure. Data are mean ± S. D., n = 8 hearts/group. * = P < 0.05, SCH vs. drug-free control
Similarly, SCH increased recovery of left ventricular developed pressure (LVDP) in a concentration-dependent manner. Again, the optimal concentration was 1 μM (Fig. 4B). There were no differences in hemodynamics at any time points measured between control and SCH-treated groups (data not shown).
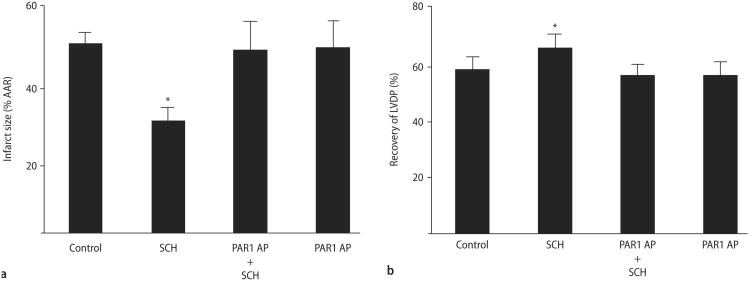
The limitation of infarct size of SCH was abolished by co-treatment with 20 μM PAR1 AP (49 ± 8%, Fig. 5A). PAR1 AP did not affect infarct size (50 ± 7 %) when given alone. Likewise, the co-treatment with PAR1 AP also blocked the recovery of LVDP (57 ± 4% pre-ischemic LVDP, Fig. 5B) and did not affect LVDP when given alone (57 ±5%).
Fig. 5.
Inhibition of SCH cardioprotective effects by a PAR1 activation peptide. Rat hearts were perfused with saline, SCH, PAR1 AP or SCH plus PAR1 AP for 15 min prior to 30 min of regional ischemia and 180 min reperfusion. A Percent infarction. B Recovery of left ventricular developed pressure. Data are mean ± S. D. n = 6 hearts/group. * = P < 0.05, SCH vs. drug-free control
Thrombin is present and mediates ischemia/reperfusion injury in an isolated heart model
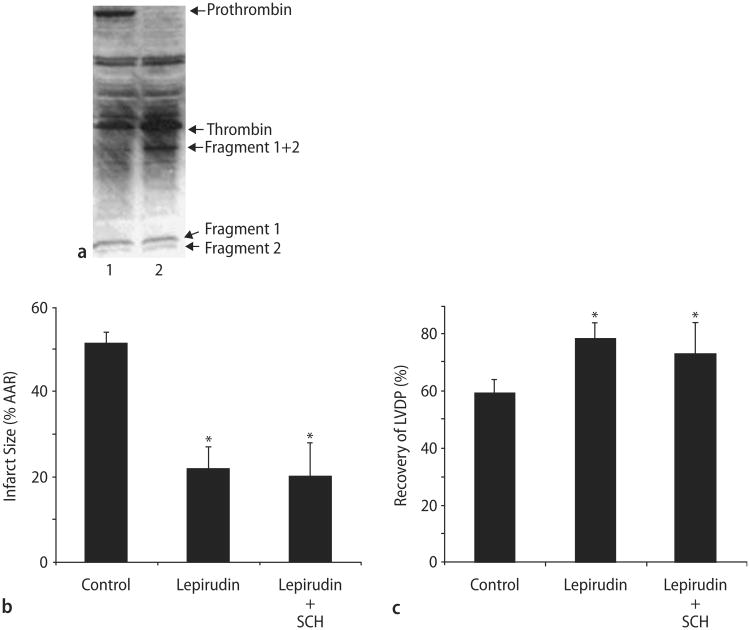
To verify that thrombin is present in the isolated heart model, we assayed for prothrombin and thrombin using immunoblot analysis. Not only was prothrombin present but also thrombin and the prothrombin cleavage products Fragment 1 + 2 (F1 + 2). Furthermore, Fragment 1 (F1) and Fragment 2 (F2) were also detected signifying that thrombin is active in our heart preparation (Fig. 6A).
Fig. 6.
Thrombin is present in isolated rat hearts during ischemia and its inhibition is cardioprotective. A Prothrombin is found in rat hearts during perfusion (lane 1) but not ischemia (lane 2). Thrombin along with other prothrombin cleavage products including fragments 1 and 2 are detected in heart lysates during perfusion and ischemia in buffer perfused isolated rat hearts. B Lepirudin with or without SCH decreases infarct size during I/R injury in isolated rat hearts. C Lepirudin with or without increases LVDP after I/R in isolated rat hearts. Data are means ± SD (n = 6 hearts/group). * = P < 0.05, Lepirudin or Lepirudin plus SCH vs. drug-free control
We then treated our isolated hearts with lepirudin, a direct thrombin inhibitor, with or without SCH. Thrombin inhibition was associated with a reduction in infarct size by 57% (Fig. 6B). SCH co-perfused with lepirudin did not significantly decrease infarct size any further than lepirudin alone. Likewise, lepirudin with or without SCH significantly increased LVDP when compared to the control (Fig. 6C).
Role of the PI3K/Akt pathway in SCH 79797-induced cardioprotection in vitro
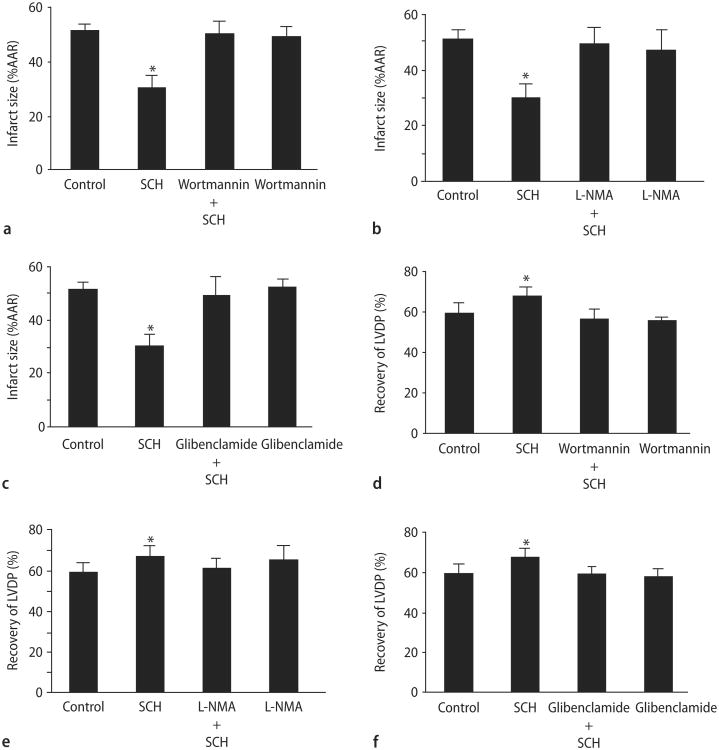
PI3K/Akt is an important mediator of cardioprotection [5]. To determine whether SCH-induced cardioprotection is mediated by PI3K/Akt, isolated hearts were perfused with the PI3K inhibitor, wortmannin, alone for 15 min or in combination with SCH (1 μM) for another 15 min prior to ischemia and reperfusion. Wortmannin (100 nM) abrogated the cardioprotective effect of SCH (Fig. 7A and D). Wortmannin alone has no effect on cardioprotection. Our data suggest that the cardioprotective effects of SCH are mediated by PI3K/Akt.
Fig. 7.
SCH-induced cardioprotection in vitro is mediated through PI3K/Akt, NOS, and KATP channels. Percent infarction with a PI3K/Akt (A), NOS (B), and a KATP channel (C) inhibitor with or without prior treatment with SCH and recovery of left ventricular developed pressure with a PI3K (D), NOS (E), or a KATP channel (F) inhibitor with or without prior treatment with SCH. The PI3K inhibitor was wortmannin (100 nM). The NOS inhibitor was L-NMA (100 μM). The potassium channel blocker was glibenclamide (3 μM). Data are means ± SD (n = 8 hearts/gp). * = P < 0.05, SCH vs. drug-free control
Role of nitric oxide synthase in SCH-induced cardioprotection in vitro
Increased NO produced by activating NOS protects the heart against injury from I/R [18]. To determine whether SCH protects the heart by a mechanism involving nitric oxide synthase, hearts were perfused with L-NMA (100 μM), a general NOS inhibitor alone for 15 min prior to ischemia or in combination with SCH (1 μM) for a further 15 min prior to ischemia. L-NMA abolished the cardioprotective effect of SCH but had no effect alone (Fig. 7B and E). These data suggest that NOS mediates the cardioprotective effects of SCH.
Role of KATP channels in SCH 79797-induced cardioprotection in vitro
KATP channels, highly expressed in myocardial sarcolemma and thought to be expressed in myocardial mitochondria, have been found to serve as triggers and mediators of cardioprotection [8, 13, 19, 21]. To investigate a role for KATP channels in mediating SCH-induced cardioprotection, hearts were perfused with the non-selective KATP channel blocker, glibenclamide, alone for 15 min or in combination with SCH (1 μM) for 15 min prior to ischemia. Glibenclamide (3 μM) completely abolished the cardioprotective effect of SCH (Fig. 7C and F). Glibenclamide alone had no effect on infarct size. Our data suggest that the cardioprotective effects of SCH are mediated by a KATP channel.
Discussion
The major finding of this study is that a single treatment with SCH, a selective PAR1 antagonist, before or during ischemia significantly reduces infarct size associated with I/R injury. In addition, we have demonstrated that inhibition of PI3K/Akt, NOS, and KATP channels abrogates the cardioprotection induced by inhibition of PAR1. Collectively, these data suggest that inhibition of PAR1 protects against I/R injury via a mechanism that involves the activation of PI3K/Akt, NOS, and KATP channels.
Although PAR1 mRNA has been detected in rat cardiomyocyte cell cultures [23], neither the mRNA nor the protein has been shown to be present in the rat heart. Since mRNA expression does not always correlate with protein production and because culturing cells in vitro may change gene expression patterns, we extended these findings and show that both PAR1 mRNA and protein are found in intact rat hearts. We were not able to confirm these findings in fixed sectioned rat heart tissue by immunohistochemistry although our positive control pancreas tissue stained with the PAR1 antibody. This is most likely due to low expression levels of PAR1 in the rat heart. Our finding correlates with the weak signal we detected by immunoblot analysis.
Since PAR1 is activated by thrombin, which is generated from circulating prothrombin, we first used an in vivo model of I/R injury to study the effects of PAR1 inhibition. We found that a single treatment with SCH before or during ischemia reduces cardiac necrosis and increases the recovery of ventricular function following reperfusion in vivo. This effect of SCH on infarct size reduction is dose-dependent with optimal protection occurring at 25 μg/kg IV.
Platelet PAR1 activation causes platelet aggregation, endothelial PAR1 activation causes vasodilation and vascular smooth muscle PAR1 activation causes vasoconstriction [9]. Furthermore, it causes the up-regulation of PECAM and ICAM on the endothelium and facilitates the influx of inflammatory mediators to the site of injury. Therefore, to eliminate hemodynamic and blood-borne cellular influences, we employed an isolated heart preparation. We confirmed that thrombin is still present and active in this model; thereby maintaining the potential to cleave and activate PAR1. Furthermore, the direct inhibition of thrombin with lepirudin is cardioprotective; thus, implicating thrombin in I/R injury in the isolated heart model. We then found that a treatment with SCH before ischemia reduces infarct size and increases the recovery of ventricular function following reperfusion in a concentration dependent manner in vitro. One micromolar SCH was found to be the optimal concentration to confer cardioprotection in this model system. Increased resistance to myocardial ischemia conferred by SCH was blocked by the PAR1 activating peptide TFLLR-amide indicating an obligatory role for the PAR1 receptor in cardioprotection. We believe our study is the first to demonstrate the cardioprotective effects of SCH and PAR1 blockade on I/R injury in the heart.
The cardioprotective effect of SCH is observed immediately after treatment indicating that rather than producing the induction of new genes, post-translational modification of existing proteins and subsequent activation of cell survival mediators is necessary to manifest its cardioprotective effect. Indeed, the signaling pathways by which SCH protects against myocardial I/R injury are mediated in part by the activation of PI3K/Akt, NOS, and KATP channels. PI3K/Akt activation has been shown to be an important component which results in a decrease in apoptosis which occurs after reperfusion. NOS acts as a mediator against myocardial stunning and infarction. Activation of KATP channels is thought to serve as both a trigger and an end effector in the signal transduction pathway of acute preconditioning. Currently there are two KATP channels thought to be present in the cardiac myocyte, one in the sarcolemma (sarc KATP channel) and one in the inner mitochondrial membrane (mito KATP channel). There is evidence to suggest that opening either channel may be important in producing cardioprotection although the bulk of evidence suggests that it is the mito KATP channel that is responsible for mediating the protective effect of ischemic preconditioning [14, 15]. The role of additional components in the signaling pathway by which the heart is protected by SCH against injury remains unknown.
Study limitations and scope for further work
The mechanism behind SCH's ability to up-regulate the cardioprotection pathway is currently unknown. SCH may be able to activate PI3K/Akt, NOS, and KATP channels directly or indirectly. A direct activation is unlikely since specific activation of PAR1 with PAR1 activating peptides abolished the cardioprotective effect of SCH indicating that SCH works exclusively through the PAR1 pathway. This finding correlates with the published specificity of SCH in platelet activation [1]. Therefore, most likely SCH induces the activation of PI3K/Akt, NOS and KATP channels indirectly. It is unclear what role PAR1 plays in the activation of PI3K/Akt, NOS and KATP channels. Therefore, we propose that blocking the induction of the PAR1 signaling pathway, allows for another signaling pathway to become dominant and thereby the activation of PI3K/Akt, NOS, and KATP channels is increased. We speculate that this is possible by SCH uncoupling G proteins from PAR1 and making them available to other receptors involved in cardioprotection.
Conclusion
In conclusion, we have demonstrated for the first time that SCH attenuates myocardial injury and dysfunction related to myocardial I/R injury when given before or during ischemia. These cardioprotective effects appear to be related to the inhibition of PAR1. The results of this study provide strong evidence that a PAR1 antagonist has important beneficial effects during ischemia and reperfusion and represents a novel cardioprotective strategy in the clinical setting of myocardial ischemia and reperfusion as encountered during cardiac surgery or during myocardial infarction.
Acknowledgments
The secretarial assistance of Mary Lynne Koenig is gratefully acknowledged.
The studies described were supported in part by NIH grants HL54075 (JEB) and HL0831 (GJG). JLS is supported by an NIH training grant (HL07792) awarded to the Medical College of Wisconsin.
Contributor Information
Jennifer L. Strande, Email: jstrande@mcw.edu, Division of Cardiovascular Medicine, Medical College of Wisconsin, 8701 Watertown Plank Rd., Milwaukee (WI) 53226, USA, Tel.: +1-414/456-8895, Fax: + 1-414/453-9700.
Anna Hsu, Dept. of Pharmacology and Toxicology, Medical College of Wisconsin, Milwaukee (WI), USA.
Jidong Su, Division of Cardiothoracic Surgery, Medical College of Wisconsin, Milwaukee (WI), USA.
Xiangping Fu, Division of Cardiothoracic Surgery, Medical College of Wisconsin, Milwaukee (WI), USA.
Garrett J. Gross, Dept. of Pharmacology and Toxicology, Medical College of Wisconsin, Milwaukee (WI), USA
John E. Baker, Dept. of Pharmacology and Toxicology, Medical College of Wisconsin, Milwaukee (WI), USA, Division of Cardiothoracic Surgery, Medical College of Wisconsin, Milwaukee (WI), USA, Children's Research Institute, Children's Hospital of Wisconsin, Milwaukee (WI), USA
References
- 1.Ahn HS, Foster C, Boykow G, Stamford A, Manna M, Graziano M. Inhibition of cellular action of thrombin by N3-cyclopropyl-7-[[4-(1-methylethyl) phenyl]methyl]-7H-pyrrolo [3, 2-f] quinazoline-1,3-diamine (SCH 79797), a nonpeptide thrombin receptor antagonist. Biochem Pharmacol. 2000;60:1425–1434. doi: 10.1016/s0006-2952(00)00460-3. [DOI] [PubMed] [Google Scholar]
- 2.Andrade-Gordon P, Derian CK, Maryanoff BE, Zhang HC, Addo MF, Cheung W, Damiano BP, D'Andrea MR, Darrow AL, de Garavilla L, Eckardt AJ, Giardino EC, Haertlein BJ, McComsey DF. Administration of a potent antagonist of protease-activated receptor-1 (PAR-1) attenuates vascular restenosis following balloon angioplasty in rats. J Pharmacol Exp Ther. 2001;298:34–42. [PubMed] [Google Scholar]
- 3.Baker JE, Holman P, Gross GJ. Preconditioning in immature rabbit hearts: role of KATP channels. Circulation. 1999;99:1249–1254. doi: 10.1161/01.cir.99.9.1249. [DOI] [PubMed] [Google Scholar]
- 4.Baker JE, Konorev EA, Gross GJ, Chilian WM, Jacob HJ. Resistance to myocardial ischemia in five rat strains: is there a genetic component of cardio-protection? Am J Physiol Heart Circ Physiol. 2000;278:H1395–H1400. doi: 10.1152/ajpheart.2000.278.4.H1395. [DOI] [PubMed] [Google Scholar]
- 5.Cai Z, Semenza GL. Phosphatidylinositol-3-kinase signaling is required for erythropoietin-mediated acute protection against myocardial ischemia/reperfusion injury. Circulation. 2004;109:2050–2053. doi: 10.1161/01.CIR.0000127954.98131.23. [DOI] [PubMed] [Google Scholar]
- 6.Chackalamannil S. G-protein coupled receptor antagonists-1: protease activated receptor-1 (PAR-1) antagonists as novel cardiovascular therapeutic agents. Curr Top Med Chem. 2003;3:1115–1123. doi: 10.2174/1568026033452122. [DOI] [PubMed] [Google Scholar]
- 7.Chong AJ, Pohlman TH, Hampton CR, Shimamoto A, Mackman N, Verrier ED. Tissue factor and thrombin mediate myocardial ischemia-reperfusion injury. Ann Thorac Surg. 2003;75:S649–S655. doi: 10.1016/s0003-4975(02)04691-x. [DOI] [PubMed] [Google Scholar]
- 8.Cole WC, McPherson CD, Sontag D. ATP-regulated K+ channels protect the myocardium against ischemia/reperfusion damage. Circ Res. 1991;69:571–581. doi: 10.1161/01.res.69.3.571. [DOI] [PubMed] [Google Scholar]
- 9.Coughlin SR. Protease-activated receptors in the cardiovascular system. Cold Spring Harb Symp Quant Biol. 2002;67:197–208. doi: 10.1101/sqb.2002.67.197. [DOI] [PubMed] [Google Scholar]
- 10.D'Andrea MR, Derian CK, Leturcq D, Baker SM, Brunmark A, Ling P, Darrow AL, Santulli RJ, Brass LF, Andrade-Gordon P. Characterization of protease-activated receptor-2 immunore-activity in normal human tissues. J Histochem Cytochem. 1998;46:157–164. doi: 10.1177/002215549804600204. [DOI] [PubMed] [Google Scholar]
- 11.Erlich JH, Boyle EM, Labriola J, Kovacich JC, Santucci RA, Fearns C, Morgan EN, Yun W, Luther T, Kojikawa O, Martin TR, Pohlman TH, Verrier ED, Mackman N. Inhibition of the tissue factor-thrombin pathway limits infarct size after myocardial ischemia-reperfusion injury by reducing inflammation. Am J Pathol. 2000;157:1849–1862. doi: 10.1016/S0002-9440(10)64824-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garlid KD, Paucek P, Yarov-Yarovoy V, Murray HN, Darbenzio RB, DiAlonzo AJ, Lodge NJ, Smith MA, Grover GJ. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Potential mechanism of cardioprotection. Circ Res. 1997;81:1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- 13.Gross GJ, Fryer RM. Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ Res. 1999;84:973–979. doi: 10.1161/01.res.84.9.973. [DOI] [PubMed] [Google Scholar]
- 14.Hanley PJ, Daut J. K(ATP) channels and preconditioning: a re-examination of the role of mitochondrial K(ATP) channels and an overview of alternative mechanisms. J Mol Cell Cardiol. 2005;39:17–50. doi: 10.1016/j.yjmcc.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Hollenberg MD. Protease-mediated signalling: new paradigms for cell regulation and drug development. Trends Pharmacol Sci. 1996;17:3–6. doi: 10.1016/0165-6147(96)81562-8. [DOI] [PubMed] [Google Scholar]
- 16.Mirza H, Yatsula V, Bahou WF. The proteinase activated receptor-2 (PAR-2) mediates mitogenic responses in human vascular endothelial cells. J Clin Invest. 1996;97:1705–1714. doi: 10.1172/JCI118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyuki K, Tetsuya T, Mitsuo T, Akiko M, Satoshi Y, Jun S, Natsuya K, Satoaki M, Jun A, Masao N. The dual effects of nitric oxide synthase inhibitors on ischemia-reperfusion injury in rat hearts. Basic Research in Cardiology. 2003;98:319–328. doi: 10.1007/s00395-003-0423-x. [DOI] [PubMed] [Google Scholar]
- 18.Much-Ellingsen J, Bugge E, Ytrehus K. Blockade of the KATP-channel by glibenclamide aggravates ischemic injury, and counteracts ischemic preconditioning. Basic Research in Cardiology. 1996;91:382–388. doi: 10.1007/BF00788718. [DOI] [PubMed] [Google Scholar]
- 19.Nelken NA, Soifer SJ, O'Keefe J, Vu TK, Charo IF, Coughlin SR. Thrombin receptor expression in normal and atherosclerotic human arteries. J Clin Invest. 1992;90:1614–1621. doi: 10.1172/JCI116031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 21.Patel HH, Fryer RM, Gross ER, Bundey RA, Hsu AK, Isbell M, Eusebi LO, Jensen RV, Gullans SR, Insel PA, Nithipatikom K, Gross GJ. 12-lipoxygenase in opioid-induced delayed cardioprotection: gene array, mass spectrometric, and pharmacological analyses. Circ Res. 2003;92:676–682. doi: 10.1161/01.RES.0000065167.52922.F6. [DOI] [PubMed] [Google Scholar]
- 22.Sabri A, Muske G, Zhang H, Pak E, Darrow A, Andrade-Gordon P, Steinberg SF. Signaling properties and functions of two distinct cardiomyocyte protease-activated receptors. Circ Res. 2000;86:1054–1061. doi: 10.1161/01.res.86.10.1054. [DOI] [PubMed] [Google Scholar]
- 23.Sevastos J, Kennedy SE, Davis DR, Sam M, Peake PW, Charlesworth JA, Mackman N, Erlich JH. Tissue factor deficiency and PAR-1 deficiency are protective against renal ischemia reperfusion injury. Blood. 2007;109:577–583. doi: 10.1182/blood-2006-03-008870. [DOI] [PubMed] [Google Scholar]
- 24.Rohatgi T, Henrick-Noack P, Sedehizade F, Goertler M, Wallesch CW, Reyman KG, Reiser G. Transient focal ischemia in rat brain differentially regulates mRNA expression of protease-activated receptors 1 to 4. J Neuroscience Res. 2004;75:273–279. doi: 10.1002/jnr.10847. [DOI] [PubMed] [Google Scholar]
- 25.Vu TK, H D, Wheaten VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]