Introduction
Hypertension (HTN) is present in more than 50% of patients with diabetes mellitus (DM) and contributes significantly to both micro and macrovascular disease in DM (1-4) (Fig 1). Indeed, the risk for cardiovascular disease (CVD) is four-fold higher in patients with both DM and HTN as compared to the normotensive non-diabetic controls (4, 5). To this point, a meta-analysis of 102 prospective studies involving 698,782 individuals found that DM is responsible for approximately a two-fold increased risk for coronary heart disease, stroke and deaths from cardiovascular cause, including heart failure, cardiac arrhythmia, sudden death, hypertensive disease, and aortic aneurysms (6). These data suggest that about 10% of vascular deaths in industrialized countries can be attributed to DM, and this burden will further increase as the incidence of diabetes continues to rise (6). In the Framingham Heart Study, DM was found to be associated with a 2-4 fold increased risk of myocardial infarction (MI), congestive heart failure, peripheral arterial disease, stroke, and death (7). Furthermore, a more recent analysis of the Framingham data showed that the population with HTN at the time of DM diagnosis had higher rates of mortality for all causes (32 versus 20 per 1000 person-years; P<0.001) and cardiovascular events (52 versus 31 per 1000 person-years; P<0.001) compared with normotensive subjects with DM, thus suggesting that much of this excess risk is attributable to coexistent HTN (8).
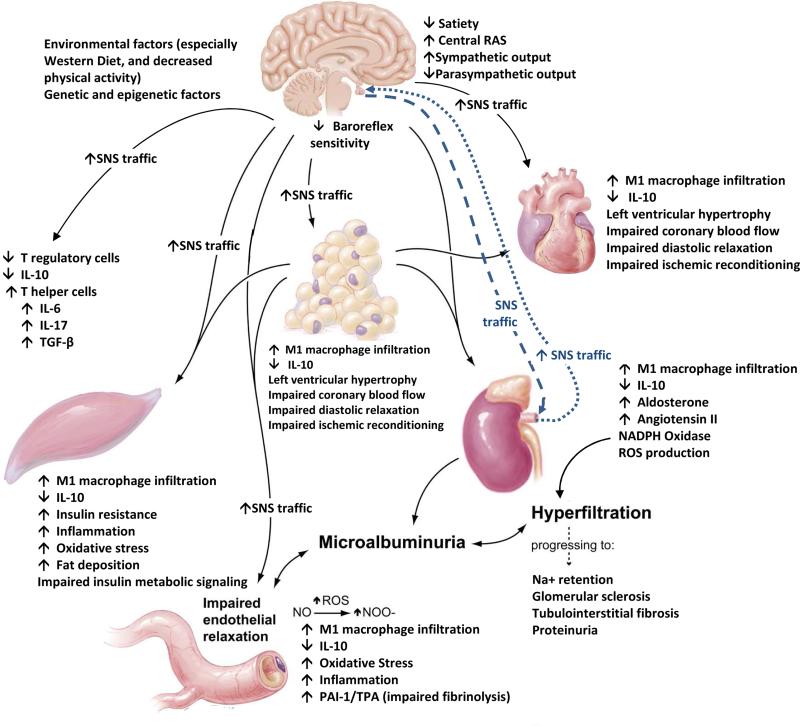
Figure 1.
Systemic and metabolic factors that promote coexistent diabetes mellitus, hypertension, cardiovascular, and chronic kidney disease. Adapted from Sowers JR. Recent Advances in Hypertension. J Am Heart Assoc 2013;61: 943-947; with permission.
The Burden
The National Health and Nutrition Examination Survey (NHANES) conducted from 2005 through 2008 estimated that HTN affects up to 65 million adults in the United States (9). Importantly, only 50% of hypertensive individuals have their blood pressure (BP) under control (10). The incidence of HTN is expected to increase further as the population ages and the frequency of obesity increases (10, 11). In a cross-sectional analysis of data from the Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes (SHIELD) comparing health outcomes between patients with DM, HTN and obesity relative to those with DM alone, obese patients with both DM and HTN exhibited greater healthcare resource utilization, higher incidence of depression, and lower quality of life (12). Another retrospective study assessed economic trends in patients with newly treated HTN-only, DM-only, and both newly treated HTN and DM for a period of time up to 24 months. Coexistent HTN and DM were associated with higher costs and resource utilization (13). Furthermore, the post-hoc analysis of CVD events found that the comorbid cohort had significantly more MIs and acute ischemic events, further rising the cost of care (13).
Epidemiology
In non-diabetic individuals, the prevalence of HTN is higher in men as compared to women until the age of 64 years when the gap closes and prevalence in females reaches that of males (8). Interestingly, women with impaired glucose tolerance (IGT) and DM have a higher incidence of HTN than men with equivalent impairment in glucose homeostasis (14). Alarmingly, diabetic women also have higher relative risk for death from CVD than diabetic men (15). The reason underlying the excess risk in diabetic women is still unclear. However, the increased risk of HTN in women with abnormal glucose tolerance may partially explain the high risk of CVD in this population.
The prevalence of HTN is different within various ethnic groups. In African-Americans, the incidence of HTN is higher when compared with Caucasians between the age of 45 and 75 years after which it is same in both ethnicities (16). Several mechanisms have been proposed to explain this finding, including higher rate of obesity, genetic predisposition, and environmental factors (17). Defects in renal sodium handling have also been observed more frequently in the African-American hypertensive populations, who have an increased prevalence of HTN and DM, than in other ethnic groups, further contributing to increased incidence of HTN (18). In contrast, a recent analysis of the NHANES 1999-2008 data revealed that the Mexican-American populations, who have a high prevalence of DM, has a lower risk of coexistent uncontrolled HTN and DM when compared with African-Americans and Caucasian participants (19). Currently, limited data is available on the incidence of coexistent HTN and DM among Asians in the United States.
There are several factors that contribute to increased coexistence of DM and HTN. The frequency of obesity in children and adolescents in industrialized countries has skyrocketed over the last several decades with an ominous parallel increment in the incidence of HTN and DM (2, 20). The multicenter Treatment Options for DM in Adolescents and Youth (TODAY) trial, which included 699 adolescents with DM aged 10–17 years, revealed that the prevalence of HTN increased from 11.6% at baseline to 33.8% by the end of study. Contrary to the adult data, the incidence of HTN was significantly increased in males versus females during the same period of time (21).
Pathophysiology: Converging pathways in coexisting DM and HTN
DM and HTN share several pathophysiologic mechanisms including: inappropriate activation of the renin angiotensin aldosterone system (RAAS), oxidative stress secondary to excessive production of reactive oxygen species (ROS), inflammation, impaired insulin-mediated vasodilatation, increased sympathetic nervous system (SNS) activation, dysfunctional innate and adaptive immune responses and abnormal renal handling of sodium (2,3). Obesity and increased visceral adiposity are key pathogenic factors behind the coexistence of both DM and HTN (3). Chronic low-grade inflammation and oxidative stress in the adipose tissue lead to increased production of angiotensinogen (AGT) and angiotensin II (Ang II) which consequent tissue RAAS activation (22, 23). Further, overexpression of AGT in the white adipose tissue results in elevated BP (22). Hence, AGT and Ang II have local as well as systemic effects on BP regulation (22,23). Ang II exerts many of its detrimental effects via activation of the Ang II type 1 receptor (AT1R) (24). The activation of AT1R in non-adrenal tissues results in multiple intracellular events, including production of ROS, reduced insulin metabolic signaling, and proliferative and inflammatory vascular responses resulting in endothelial dysfunction, insulin resistance and HTN (24). Thus, there is often an activated RAAS in coexistent DM and HTN.
Increased aldosterone production and augmented signaling through the mineralocorticoid receptor (MR) are also key events in the pathogenesis of HTN (25). Corticosteroids may also contribute to CVD in DM patients via actions mediated in part through activation of the MR (3). Adipose tissue is known to produce a lipid-soluble factor that stimulates aldosterone production from the adrenal zona glomerulosa (26, 27). Complement-C1q TNF-related protein 1 (CTRP1) is a novel adipokine that promotes aldosterone production in a rodent model of obesity and insulin resistance (28). Aldosterone activation of the MR in the renal distal tubule and collecting duct increases sodium retention leading to expansion of plasma volume and increased BP. In addition, aldosterone exerts non-genomic actions also likely through MR activation, which contribute to HTN by altering cellular redox state, signaling and endothelial-mediated vascular relaxation (25, 27). Thus, adipose tissue contributes to systemic elevations in BP, in part, through local production of components of the RAAS
Role of oxidative stress
Increased oxidative stress is a key pathogenic factor in the development of insulin resistance, DM and HTN (29). ROS can be produced in different vascular cell types, including endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) through activation of xanthine oxidase (XO), nitric oxide (NO) synthase, the mitochondrial respiratory chain, (30-33). In turn, ROS can lead to impaired endothelial function by direct tissue injury, reduction of bioavailable NO, and impaired NO-mediated vasodilation (30). One important additional source of ROS and endothelial dysfunction is endothelial nitric oxide synthase (eNOS) uncoupling. Under conditions of decreased availability of tetrahydrobiopterin (BH4, a cofactor in NO production) or the substrate L-arginine, eNOS switches from this coupled state to an uncoupled state resulting in production of superoxide (O2−) (31). Mitochondrial and XO mediated oxidative stress also contribute to this excess generation of ROS in coexistent DM and HTN (3). XO is also expressed in vascular endothelial and VSMC, and is another source of vascular oxidative stress, which generates O2− by catalyzing hypoxanthine and xanthine to uric acid (32). A major source of ROS is the membrane bound vascular-derived nicotinamide adenine dinucleotide phosphate-oxidase (NADPH), a protein enzyme composed of several subunits, including the membrane-bound subunits p22phox and Nox2, the cytosolic regulatory subunits p47phox, p67phox, p40phox and the small GTP-binding protein Rac1/Rac2 (34). Increased ROS production in turn results in cell as well as tissue damage by activating inflammatory pathways such as the NF-kB. Inflammation is characterized by increased activity of adhesion molecules, pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin (IL) 1 and 6, as well as acute phase reactants such as C Reactive Protein and molecules that promote fibrosis and remodeling, such as transforming growth factor beta (TGF-β) and plasminogen activator inhibitor 1 (PAI-1) (35). Interestingly, mechanical stretch (a characteristic phenomenon in HTN) can lead to membrane translocation and activation of p47phox and Rac1, thus leading to NADPH oxidase activation (32). Ang II and aldosterone can also directly activate NADPH oxidase and trigger oxidative stress (33).
Insulin resistance and hyperinsulinemia
Insulin resistance plays an important role in the development of both DM and HTN, as demonstrated by the fact that approximately 50% of hypertensive patients manifest systemic insulin resistance (3,36,37). Binding of insulin to its receptor (IR) triggers two major pathways. A metabolic signaling pathway mediated by phosphatidylinositol 3-kinase (PI3K), downstream protein kinase B signaling, ultimately results in translocation of glucose transporter 4 (GLUT-4) to plasma membrane, thus resulting in increased insulin mediated glucose transport in insulin sensitive tissues such as skeletal muscle (38). In addition, signaling through the PI3K/Akt pathway results in phosphorylation/activation of endothelial nitric oxide synthase (eNOS) and consequent NO production promotes endothelium mediated vasodilation (40). Insulin also signals through growth/ proliferative signaling pathway, which is mediated by mitogen-activated protein kinase (MAPK) (38,39). By activating MAPK dependent signaling pathways, insulin stimulates secretion of vasoconstrictor mediators, such as endothelin-1 (41,42), as well as increased expression of PAI-1, vascular cell adhesion molecule-1 (43). In conditions of normal insulin sensitivity, the balance between these vasoconstrictor and vasodilatory actions favors vasodilation). In insulin resistant states there is often deficient insulin metabolic signaling in concert with unchecked signaling through the growth pathway (1-3).
Maladaptive hyperinsulinemia/insulin resistance leads to abnormalities in vascular function, vascular stiffness, hypertrophy, fibrosis and remodeling. (44). Hyperinsulinemia also results in enhanced sympathetic output in humans through ventromedial hypothalamus mechanisms (45,46). Additionally leptin, which is an adipokine produced in adipose tissue and is increased in obese individuals, also increases sympathetic nerve activation likely through a central nervous system effect involving leptin receptor activation (47). Indeed, there is increasing evidence that increased afferent traffic from and efferent activity to the kidney plays an important role in development of hypertension associated with obesity and insulin resistance (3).
Importantly, insulin enhances sodium reabsorption in the diluting segment of the distal nephron, in part through increased expression of sodium transporters like the epithelial sodium channel (ENaC), with consequent decreases in sodium excretion (48). Hyperinsulinemia-mediated sodium retention could potentially contribute to the genesis of HTN via increased activation of sodium hydrogen exchanger activity in the proximal tubule as well as through the effects on ENAC more distally. While this is an attractive hypothesis, in an animal model of knockout of IR in the renal tubule epithelial cells, the absence of insulin action resulted in impaired natriuretic responses and HTN, likely due to reduce NO production (49). Because of these contradictory results, further studies are needed to clarify the physiological role of insulin on renal sodium handling.
Finally, sodium and uric acid are generally handled together; hence excess uric acid can increase along with sodium retention, thereby contributing to hyperuricemia, which is frequently seen in hypertensive patients (50). The propensity for increased uric acid levels is increased with our westernized diets that are high in fructose (3) (Fig 1).
Treatment of HTN: rationale, strategies and challenges in HTN
Impact of BP control
High BP is a strong independent risk factor for CVD and chronic kidney disease (CKD), and when HTN is associated with DM, the risk is increased even further (4,51). Although controversy exists regarding the optimal target for BP reduction (3,51,52), it remains clear that consistent control of BP in patients with DM is important for preventing and delaying both micro and macro vascular complications (53,54). Early data from landmark trials such as the United Kingdom Prospective Diabetes Study (UKPDS), Hypertension Optimal Treatment (HOT), Systolic Hypertension in the Elderly (SHEP), and Systolic Hypertension in Europe (Syst-Eur) showed that strict BP control was beneficial in hypertensive patients with diabetes. In a nine-year follow up of the UKPDS cohort, patients with both HTN and DM assigned to strict BP control (mean BP 144/82 mmHg) achieved a significant reduction in risk for all of the end-points related to DM, including death related to DM, and micro-vascular disease relative to patients who were treated conventionally (mean BP 154/87 mmHg) (54). The group allocated to tight BP control had a significant reduction in the risk of heart failure, with an additional non-significant reduction in the risk of MI. However, when all macro-vascular diseases were combined, including MI, sudden death, stroke, and peripheral vascular disease, the group assigned to tight BP control still exhibited a significant reduction in risk compared with the group assigned to less tight control (54).
The HOT study revealed that among the 1501 patients with DM at baseline, a stricter BP control (mean BP 140/81) halved the risk of major cardiovascular events when compared to the control group (mean BP 144/85) (55). The risk for stroke decreased significantly in individuals who reached the lower target BP. In participants reaching diastolic BP <80 mm Hg the risk was reduced roughly 30% relative to individuals who only reached a diastolic BP less than 90 mm Hg. In addition, cardiovascular mortality was also significantly lower in this group (diastolic BP less than 80 mm Hg) than in each of the other target groups. In addition, a non-significant decline was seen in the risk for all (MI) in the group with stricter BP control (55).
In the Syst-Eur trial, 492 patients (10.5%) had DM; after a two year follow up, the active treatment with antihypertensive drugs reduced overall mortality by 55%, mortality from cardiovascular causes by 76%, all cardiovascular events by 69%, fatal and nonfatal stroke by 73%, as well as all cardiac events by 63% (56). In the SHEP trial, DM patients randomized to active treatment with antihypertensive medications, exhibited lower frequency of stroke, nonfatal MI and fatal coronary heart disease (CHD), major coronary events, and all-cause mortality relative to patients treated with placebo (57). The Appropriate Blood Pressure Control in Diabetes (ABCD) trial demonstrated a significant decrease in all-cause stroke with intensive (mean BP 133/78 mmHg) versus moderate antihypertensive therapy (mean BP 139/86 mmHg) in patients with DM (58). This is important because of the increased risk of both fatal and non-fatal stroke in patients with coexistent DM and HTN. In summary, although specific targets are still controversial, control of HTN in the setting of DM is strongly supported by current evidence showing the critical impact that BP has on CVD in diabetic individuals (51,54, 58).
BP targets
Clinical management guidelines derived from the widely accepted Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) and the American Diabetes Association (ADA) have recommended strict treatment of HTN in the setting of DM, aiming at values <130 mm Hg for systolic BP and <80 mm Hg diastolic BP (53). Nonetheless, the additional beneficial effects of such lower BP targets remain unproven (59,60). Hence, the recently revised ADA guidelines suggest that the BP goal for people with DM and HTN should be <140/80 mmHg (61).
The majority of the guidelines for management of HTN are based on the landmark UKPDS and HOT trials. However, the systolic BP achieved in the tight control arm in these trials was between 140 to 150 mm Hg. Only the intensive BP control groups in the hypertensive and normotensive ABCD studies reached the consensus JNC 7 goal of <130/80 mmHg. Recently, the results of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) BP study (59), showed that in patients with DM, targeting systolic BP to <120 mm Hg did not reduce the rate of CV events (nonfatal MI and death from cardiovascular causes), compared with subjects in whom the target was <140 mm Hg, except for fatal and non-fatal strokes. As expected, adverse events that were attributed to BP medication were more frequent in the intensive therapy group (59). Likewise, a post hoc analysis of the International Verapamil SR-Trandolapril (INVEST) study concluded that reducing systolic BP to <130 mm Hg in patients with DM and coronary artery disease was not associated with improved CVD outcomes compared with conventional BP control (systolic BP of 130- 139 mmHg) (60).
In another post-hoc analysis of the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET) study, the relationship between BP and overall cardiovascular risk followed a similar pattern in diabetic and non-diabetic patients. With the exception of stroke, reducing systolic BP below 130 mm Hg did not result in improvements in either fatal or nonfatal CVD outcomes (62). Furthermore, a recent meta-analysis of 13 major trials done in patients with DM or IGT showed that below a systolic BP of 130 mm Hg, there was only significant reduction in the rate of stroke, with no further reduction in cardiac, renal or retinal outcomes; however there was an increased incidence of major adverse events such as hyperkalemia, symptomatic hypotension, bradycardia, and cardiac arrhythmias (63). An additional concern about very strict BP targets is the possible deficiency in blood perfusion to the central nervous system in diabetic patients, who already have microvascular disease and impaired cerebrovascular autoregulation (64).
Certainly, the optimal BP goal for diabetic patients should be individualized. Nevertheless, available literature suggests that a maximal benefit of BP control in DM patient is attained with systolic BP between 130-135 mmHg and diastolic BP of 80 to 85 mm Hg, except in stoke prevention, where data suggests that further lowering BP may be beneficial.
Non-pharmacologic treatment: The role of therapeutic lifestyle intervention
Despite significant advances over the last several decades, the management of HTN is still far from ideal, and about 50% of hypertensive patients are still not optimally controlled. The reasons underlying these disappointing results appear to be multiple and include deficiencies in current both non-pharmacologic and pharmacologic management strategies. One of these issues, access to antihypertensive medications and BP control was studied in the cross-sectional Reasons for Geographic And Racial Differences in Stroke (REGARDS) cohort study (65). Although access to antihypertensive medications increased significantly from 66% in 2003 to 81% in 2007, this was not independently associated with improved BP control. African-American ethnicity, male sex, low income, and medication non-adherence were significant predictors of inadequate BP control, suggesting that poor BP control is multifactorial (65).
Non-pharmacological lifestyle interventions, which include dietary changes, low salt diet, weight loss, increased physical activity on a regular basis, and alcohol restriction, have shown to reduce BP in several controlled studies. Lifestyle changes including individualized counseling aimed at reducing total intake of fat, intake of saturated fat and increasing intake of fiber and physical activity result in significant improvements in BP and reduction in the incidence of DM (66).
The Dietary Approach to Stop Hypertension (DASH) is a nutritional strategy promoted by the United States National Heart, Lungs and Blood Institute (NHLBI) as a non-pharmacologic intervention to prevent and control HTN. The DASH diet includes foods rich in fruits, vegetables, whole grains, low-fat dairy products, and low in total fat and saturated fat, cholesterol, refined grains, as well as sweets, and has been shown to provide beneficial metabolic and cardiovascular effects in DM. Adherence to the DASH diet results in lower systolic as well as diastolic BP, body weight, waist circumference, blood glucose levels and A1C. It also has beneficial actions on lipid profile, and has shown to improve HDL cholesterol levels and lowers LDL cholesterol (67, 68).
The beneficial effects of DASH diet are probably due to its effect on some cardiovascular risk factors. After following the DASH diet for eight weeks, liver amino-transferase enzymes, plasma fibrinogen levels, and high sensitivity C-reactive protein (hs-CRP) all were reduced, suggesting that this type of diet can play an important role in reducing inflammation in DM (69). In the Lifestyle Changes Through the Use of Delivered Meals and Dietary Counseling in a Single-blind Study (STYLIST), the combination of dietary counseling by dietitians and delivery of calorie-controlled meals was effective in reducing body weight, BP and A1C, in patients with HTN and/or DM (70).
In addition, sodium intake per se has also been associated with CVD. Results from multiple trials have shown that reduction of dietary sodium (from a daily intake of 200 mmol [4,600 mg] to 100 mmol [2,300 mg] of sodium per day) lowers BP and may also reduce long term risk of cardiovascular events (71,72). However, results from a recent meta-analysis of seven randomized controlled trials (RCT) failed to provide strong evidence that salt reduction reduced all-cause mortality or CVD morbidity in normotensive or hypertensive individuals (73). The interventions used in this meta-analysis were capable of reducing urinary sodium excretion. Systolic and diastolic BPs were also reduced by an average of some 1 mm Hg in normotensives and by an average of 2–4 mm Hg in persons with hypertension and those with heart failure (73). However the methods of achieving salt reduction in the trials included in the review were relatively modest in their impact on sodium excretion and on BP levels, and would not be expected to have major impact on the burden of CVD. Sodium restriction has not been tested in the diabetic population in controlled clinical trials. However in a recent animal study the results are in favor of oxidative stress normalization as the beneficial influence of dietary sodium deprivation on cardiovascular remodeling in the model of insulin resistance in rats. Withdrawal of sodium from the fructose diet in these rats showed prevention of CVD effects of high fructose consumption, including production of superoxide anions/oxidative stress (74). In summary, the available literature suggests that reduction of dietary salt lowers BP and may also reduce long term risk of CVD events in hypertensive patients; however, the data in diabetic individuals are limited. Further, more studies need to be conducted to determine if increasing dietary potassium, calcium and magnesium may have beneficial effects on BP, CVD and metabolic control in patients with coexistent DM and HTN.
Finally, physical inactivity is a major underlying risk for CVD. In addition to changing the dietary patterns, increased aerobic physical activity on a regular basis, (such as brisk walking) is important in this population. Thirty to 45 minutes of brisk walking three to five days a week has demonstrated to improve lipid profiles, BP, as well as insulin resistance (75), and is currently recommended in most management guidelines (61). Increased physical activity may decrease the rapidity of development of both CVD and CKD events in persons with DM and HTN.
Pharmacological therapy
RAAS blockade
Use of Ang II converting enzyme inhibitors (ACEI) reduces the activity of Ang II, which results in vasodilatation, decreased BP and improvement in the deleterious effects of Ang II on cardiac, vascular and renal tissues (76,77). The Heart Outcomes Prevention Evaluation (HOPE) study compared the effects of the ACE inhibitor ramipril versus placebo on cardiovascular complications and showed 25% risk reduction in MI, stroke, or cardiovascular death after a median follow-up period of 4.5 years (78). A subgroup of 3,577 diabetic patients was analyzed in the MICRO-HOPE study, and demonstrated similar beneficial effects of ramipril on cardiovascular and all-cause mortality in patients with DM (79).
In contrast to ACEI, Ang II receptor blockers (ARBs) do not increase the levels of bradykinin, which can cause low patient adherence due to induction of cough. In a subset of the Losartan Intervention For Endpoint reduction in hypertension study (LIFE) including 1195 type 2 diabetic patients, a significant reduction in cardiovascular morbidity and mortality was reported in patients treated with losartan compared to individuals taking a β-blocker (atenolol). Importantly, a relative risk reduction of 24% for primary composite endpoint of cardiovascular morbidity and mortality (cardiovascular death, stroke, or MI) was seen in patients treated with losartan compared to atenolol despite almost similar BP reduction (80).
Intriguingly, in the Antihypertensive Long-Term Use Evaluation (VALUE) and the Candesartan Antihypertensive Survival Evaluation in Japan (CASE-J) trials, cardiac morbidity and mortality were no different in patients treated with ARBs (valsartan and candesartan respectively) relative to patients treated with the long acting calcium channel blocker (CCB) amlodipine, although they did significantly reduce the incidence of DM (81,82). Similarly, its a subgroup analysis of the ONTARGET trial in 6391 patients with DM, telmisartan (ARB) and rampril (ACEI) had similar effects on cardiovascular morbidity and mortality (83). However, in this study the patients who received combination therapy with ACEI and ARB had an increased risk of adverse side effects including hypotension, syncope, renal dysfunction, and hyperkalemia. Importantly, the combination-therapy group had a significant increase in the relative risk of impairment of renal function (1.33, P<0.001). Also the risk of hypotension was higher in combination group with relative risk of 2.75 (p<0.001). The numbers of patients who had an increase in the potassium level of more than 5.5 mmol per liter were similar in the ramipril group (283 patients) and the telmisartan group (287 patients), but the number was significantly higher in the combination-therapy group (480 patients, P<0.001). Therefore, current literature does not recommend combined treatment with ARB and ACEI.
In addition to cardiovascular protection, available literature demonstrates that RAAS blockade provides renal protective effects. The Bergamo Nephrologic Diabetic complications Trial (BENEDICT) and the Randomized Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) trials found that in patients with DM, HTN and normoalbuminuria (<30 mg/gm of creatinine), RAAS blockade with an ACEI and an ARB respectively, delayed the onset of microalbuminuria (30-300 mg/gm) (84,85). Nevertheless, in the diabetic retinopathy candersartan trial (DIRECT) RAAS blockade failed to show prevention of microalbuminuria in normotensive patients with type 1 or type 2 DM (86).
Collectively, there is significant evidence to support RAAS blockade as the first line of therapy for HTN in DM to prevent or delay microalbuminuria; however evidence to sustain their use in normotensive diabetic patients (type 1 or 2) to prevent or delay the development of microalbuminuria is lacking.
Finally, RAAS blockade also has potential benefits beyond BP lowering effects, including improvements in insulin resistance, inflammation, oxidative stress and vascular function (87). In large clinical trials, the Randomized Aldactone Evaluation Study (RALES) and Eplerenone Post–Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) trials, MR blockade showed improvement in cardiovascular morbidity and mortality.
Improvement in endothelial dysfunction, decreased activation of matrix metalloproteinases, improved ventricular remodeling along with improvements in tissue fibrosis, inflammation, oxidative stress and insulin resistance have been postulated as the possible mechanisms responsible for these actions (87,88).
Calcium channel blockers (CCBs)
CCBs are very effective and well-tolerated antihypertensive therapy and have been extensively studied. In the ALLHAT study, treatment with amlodipine was associated with similar rates of coronary mortality and nonfatal MI as treatment with ACEI (lisinopril) and the diuretic chlorthalidone (77). However, the heart failure rate was higher in patients treated with CCBs as compared to chlorthalidone, which could be in part due to lower BP attained in the patients treated with the diuretic, or discontinuation of diuretic therapy in the CCB group patients.
In diabetic hypertensive patients, some trials have demonstrated that ACEI significantly reduced the risk of CVD compared with CCB (89,90) whereas another large-scale trial showed no difference (91). As described earlier, in the VALUE the CASE-J trials, no significant difference was seen in cardiac composite end points between ARBs and CCBs (92,93). In the Irbesartan Diabetic Nephropathy Trial (IDNT), this ARB showed better renal protection as compared to CCB , but failed to show any difference in reduction in CVD between the two (94).
In the large diabetic subgroup (5137 patients) in the BP-lowering arm of the Anglo– Scandinavian Cardiac Outcomes Trial (ASCOT), CCB (amlodipine) showed significant reduction in total cardiovascular events as compared to beta-blocker (95).
Diuretics
Thiazide-type diuretics have been the basis of antihypertensive therapy for long time. In the ALLHAT, the diuretic chlorthalidone was shown to be as effective as CCBs and ACEIs in reducing cardiovascular morbidity and mortality (77). In the Very Elderly Trial (HYVET), the thiazide-like diuretic indapamide reduced the rate of stroke, coronary heart disease, heart failure and all-cause mortality in very old hypertensive patients (96).
Thiazide diuretics do have some significant negative metabolic effects, in particular impaired glycemic control by impairment of insulin secretion and insulin sensitivity (97,98). They may also worsen insulin sensitivity and glucose tolerance by activation of the RAAS and the SNS, which can be attenuated by addition of MR blocker (99).
In high doses, diuretics can result in hypokalemia, hypomagnesemia, and/or hyperuricemia, all of which have been shown to worsen glucose control (97,98). Most of the adverse effects of thiazides are dose-dependent; hence using it in low dose combined with other medications will help avoid metabolic side effects.
Use of other agents like β-blockers remains controversial. There is some emerging evidence that β-blockers may be associated with weight gain which may further worsen glucose tolerance (97,100). However, in contrast to conventional β-blockers, nebivolol, a selective blocker of β1 adrenergic receptor with NO-potentiating vasodilatory action has not been associated with weight gain nor with worsened glucose tolerance (100).
β-blockers are generally not used as first line agents for hypertension in diabetic individuals, however, they are certainly considered as add-on therapy in patients with coronary artery disease and heart failure (61).
Incretin-based therapy and HTN: beyond glycemic control
A better understanding of the role of gut-derived hormones and their impact on carbohydrate homeostasis has been reached over the last decade, which has led to the development of incretin-based therapy. Glucagon like peptide 1 (GLP-1) and glucose dependent insulinotropic peptide(GIP) have been extensively studied in these regards, and are known to potentiate insulin secretion in a glucose-dependent manner, in response to presence of nutrients in the gut (i.e: the incretin effect). In addition, incretin hormones slow gastric emptying, thereby slowing the absorption of nutrients in the digestive tract, and suppressing glucagon production in pancreatic alpha cells (101).
The incretin effect is typically blunted in the setting of DM, and is restored by GLP-1 analogs such as exenatide or liraglutide, as well as inhibitors of the enzyme dipeptidyl peptidase 4 (DPP-4), which rapidly cleaves and inactivates GLP-1. In turn, reduced degradation of GLP-1 by DPP-4 inhibitors results in an enhanced incretin effect. GLP-1 action derives from activation of a highly specific G-protein coupled receptor (GLP-1R), which is expressed in several tissues, including pancreas, nervous system, kidney, cardiovascular tissue and immune cells (102).
In the clinical setting, GLP-1 analogs and DPP-4 inhibitors have proven to be efficacious for DM treatment as they contribute to improved beta cell function and glycemic control. In addition, there is mounting interest in the impact of incretin-based therapy on the cardiovascular and renal systems (103-111).
Both GLP-1 agonists and DPP-4 inhibitors have been shown to modulate BP, heart rate and contractility. Knock out (KO) of GLP-1R in mice results in impaired myocardial contractility and diastolic function (103). Acutely GLP-1 and DPP-4 inhibitors induce increases in BP and heart rate in rodents (104,105).
In humans, however, the actions of GLP-1 on BP and heart rate are less clear and experimental results have been more variable. Chronic treatment with GLP-1 analogs in type 2 diabetic patientss results in improvements in both systolic and diastolic BP without affecting heart rate (104,106). Similarly, in a study by Mistry et al, treatment with the DPP-4 inhibitor sitagliptin produced a modest reduction in BP (107). In young Zucker obese rats, treatment with linagliptin for eight weeks resulted in improved diastolic function, left ventricular hypertrophy and fibrosis. These changes occurred in concert with significant improvements in BP, eNOS, and calcium handling in cardiomyocytes (108). In the vasculature, DPP-4 inhibition also appears to affect endothelial function. Treatment for two-weeks with sitagliptin improved endothelium-dependent relaxation in renal arteries, restored renal blood flow, and reduced systolic BP in spontaneously hypertensive rats (SHR) (109). In addition, treatment with GLP-1 reduced the size of MI (23.2% to 14.1% of area at risk) in rat hearts (110).
Finally, the impact of incretin-based therapy on BP appears to be also related to activation of GLP-1R in renal tissue, which results in decreased expression of the sodium-hydrogen transporter type 3 (NH3) and leads to increased diuresis and sodium excretion in renal proximal tubules (111). DPP-4 inhibition results in lower mean BP in young SHR treated with sitagliptin for eight days, in concert with increased urinary flow and decreased NH3 expression and activity (112).
Combined pharmacologic therapy
Although treatment for HTN is often initiated with a single agent, typically a majority of diabetic patients will require combination therapy to control their BP. In a randomized, parallel-group, double-blind international trial comparing the once daily single-pill combination of telmisartan 80 mg and amlodipine 10 mg (Telmisartan/Amlodipine) (T/A) with once-daily amlodipine 10 mg (Amlodipine) (A) in patients with DM and HTN, T/A provided prompt and greater BP decreases compared to A monotherapy, with the majority of patients achieving the BP goal (< 140/90 mm Hg) (113).
The Avoiding Cardiovascular Events Through Combination Therapy in Patients Living With Systolic Hypertension (ACCOMPLISH) trial, which included 6,946 patients with diabetes, compared the outcome effects of the ACEI benazepril (20-40 mg/day) combined with amlodipine (5-10mg/day) (B+A) or hydrochlorothiazide (12.5-25 mg/day). Benazepril combined with amlodipine (B+A) was superior to the benazepril hydrochlorothiazide (B+H) combination, as there was 20% reduction in the primary endpoint (CV death, stroke, MI, revascularization, hospitalization for unstable angina or resuscitated cardiac arrest) in the (B+A) arm compared with the (B+H) arm (p=0.0002) despite a similar drop in BP in both groups. The mean value of BP after the treatment adjustments were 131.5/72.6 mm Hg in the B+A arm and 132.7/73.7 mm Hg in the B+H arm (114). The study was stopped early because of a difference in outcomes favoring amlodipine. The data from this study are consistent with the ASCOT study (115), which also demonstrated the cardiovascular benefits of the ACEI/CCB combination.
Fixed-dose combinations in a single tablet may increase compliance compared with corresponding free-drug components given separately, as it simplifies treatment and thereby can improve adherence on the part of the patients (116).
The escalation of double-drug treatment to triple-drug therapy may improve BP control in the clinical practice. A subgroup analysis in African-Americans and non-African-Americans with HTN, compared triple-combination treatment with olmesartan 40 mg, amlodipine 10 mg, and hydrochlorothiazide 25 mg with the component dual-combination treatments. Triple-combination treatment resulted in significant and similar mean reductions in diastolic and systolic BP relative to dual-combination treatment. Indeed a greater proportion of participants on triple combination reached the target BP, when compared with dual-combination treatments at the end of 12 weeks regardless of ethnicity (117). Because HTN and DM generally require multiple antihypertensive agents to achieve a goal BP, triple-combination therapy may represent an important treatment option to improve BP control in this patient population.
Perspectives
Telehealth
As can be inferred from previous sections, the treatment of HTN remains challenging and demands constant reshaping. Currently newer strategies are being tried for optimal control of HTN in diabetic patients, including the use of remote services like TeleHealth. TeleHealth encompasses the use of medical information exchange remotely via electronic communications to improve a patient's clinical health status. The use of TeleHealth for transmission of education and advice to the patient on an ongoing basis with close surveillance by nurses and or physicians improves clinical outcomes (118). A randomized controlled trial conducted at the Iowa City VA Medical Center (ICVAMC), evaluated the efficacy of nurse managed home Telehealth intervention to improve outcomes in veterans with comorbid DM and HTN. Intervention subjects experienced a significant decrease in systolic BP compared with the other groups at six months and this pattern was maintained at 12 months (118).
Renal Denervation
Activation of renal sympathetic nerves plays an important role in the pathogenesis of HTN (119). Renal denervation (RDN) is a percutaneous catheter-based renal sympathetic denervation procedure to disrupt renal afferent and efferent nerves using radiofrequency ablation (120, 123, 124). In a multicenter, prospective, randomized trial (Symplicity HTN-2) evaluating the role of RDN in patients with resistant HTN, office-based BP measurements in the group assigned to the procedure (n=52) decreased by 32/12 mm hg (baseline of 178/96 mm Hg, p<0·0001). This reduction persisted after six months (120). However, the study did not include ambulatory BP monitoring (ABPM) in the analysis, which has shown to be more closely related to cardiovascular morbidity and mortality than office BP (121,122).
Another study of 50 patients investigated the effect of RDN on glucose homeostasis and BP control in patients with resistant HTN. Systolic and diastolic BP, fasting glucose, insulin, C peptide, hemoglobin A1c and insulin sensitivity were measured before, one and three months after treatment. At one and three months, office BP was reduced by 28/10 mm Hg (P<0.001) and 32/12 mm Hg (P<0.001) respectively in the treatment group, without changes in concurrent antihypertensive treatment. Interestingly, there were also significant improvements in fasting glucose, insulin, C-peptide levels and markers of insulin resistance (123). In a recent multi-center study RDN demonstrated significant reductions in systolic and diastolic BP, taken both in-office and through 24-hour ABPM (124). In-office systolic and diastolic BP changes were significantly more pronounced than changes in 24-hour continuous measurements. Furthermore, there was no effect on ABPM in pseudo-resistant patients, whereas in-office BP was reduced to a similar extent. Thus, RDN may represent an important and novel approach for selective reduction of renal sympathetic drive which results in improvement in both insulin resistance and resistant HTN. Certainly this strategy deserves additional clinical research.
Key Points.
Patients with hypertension and type 2 diabetes are at increased risk of cardiovascular and chronic renal disease.
Factors involved in the pathogenesis of both hypertension and type 2 diabetes include inappropriate activation of the renin angiotensin aldosterone system, oxidative stress, inflammation, impaired insulin-mediated vasodilatation, augmented sympathetic nervous system activation, altered innate and adaptive immunity and abnormal sodium handling by the kidney.
Renin angiotensin aldosterone system blockade is a key therapeutic strategy in the treatment of hypertension in type 2 diabetes.
Interesting emerging therapies include renal denervation and carotid body denervation.
Acknowledgment
The authors wish to thank Brenda Hunter for editorial assistance.
Funding Sources:
Dr. Sowers: NIH (R01 HL73101-01A1 & R01 HL107910-01) Veterans Affairs Merit System 0018
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have nothing to disclose.
Conflict of Interest:
Dr. Sowers: Merck Pharmaceuticals Advisory Board
References
- 1.Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001;37:1053–1059. doi: 10.1161/01.hyp.37.4.1053. [DOI] [PubMed] [Google Scholar]
- 2.Sowers JR, Whaley-Connell A, Hayden M. The role of overweight and obesity in the cardiorenal syndrome. Cardiorenal Med. 2011;1:5–12. doi: 10.1159/000322822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sowers JR. Diabetes mellitus and vascular disease. Hypertension. 2013;61(5):943–7. doi: 10.1161/HYPERTENSIONAHA.111.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stamler J, Vaccaro O, Neaton JD, et al. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 5.Hu G, Jousilahti P, Tuomilehto J. Joint effects of history of hypertension at baseline and Type 2 diabetes at baseline and during follow-up on the risk of coronary heart disease. Eur Heart J. 2007;28:3059–3066. doi: 10.1093/eurheartj/ehm501. [DOI] [PubMed] [Google Scholar]
- 6.Sarwar N, Gao P, Seshasai SR, et al. Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox CS. Cardiovascular disease risk factors, type 2 diabetes mellitus, and the Framingham Heart Study. Trends Cardiovasc Med. 2010;20:90–95. doi: 10.1016/j.tcm.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen G, McAlister FA, Walker RL, et al. Cardiovascular outcomes in Framingham participants with diabetes: the importance of blood pressure. Hypertension. 2011;57:891–897. doi: 10.1161/HYPERTENSIONAHA.110.162446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010;303:2043. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 10.Wright JD, Hughes JP, Ostchega Y, et al. Mean systolic and diastolic blood pressure in adults aged 18 and over in the United States, 2001-2008. Natl Health Stat Report. 2011;35:1–22. 24. [PubMed] [Google Scholar]
- 11.Kaplan NM, Victor RG. Kaplan's Clinical Hypertension. 10th ed Wolter's Kluwer; Philadelphia: 2010. Hypertension in the population at large. p. 1. [Google Scholar]
- 12.Green AJ, Bazata DD, Fox KM, et al. Quality of life, depression, and healthcare resource utilization among adults with type 2 diabetes mellitus and concomitant hypertension and obesity: a prospective survey. Cardiol Res Pract. 2012 doi: 10.1155/2012/404107. article ID 404107:doi:10.1155/2012/404107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eaddy MT, Shah M, Lunacsek O, et al. The burden of illness of hypertension and comorbid diabetes. Curr Med Res Opin. 2008;24:2501–2507. doi: 10.1185/03007990802297529. [DOI] [PubMed] [Google Scholar]
- 14.Haffner SM, Valdez R, Morales PA, et al. Greater effect of glycemia on incidence of hypertension in women than in men. Diabetes Care. 1992;15:1277–1284. doi: 10.2337/diacare.15.10.1277. [DOI] [PubMed] [Google Scholar]
- 15.Hu G, DECODE Study Group Gender difference in all-cause and cardiovascular mortality related to hyperglycaemia and newly-diagnosed diabetes. Diabetologia. 2003;46:608–617. doi: 10.1007/s00125-003-1096-6. [DOI] [PubMed] [Google Scholar]
- 16.Carson AP, Howard G, Burke GL, et al. Ethnic differences in hypertension incidence among middle-aged and older adults: the multi-ethnic study of atherosclerosis. Hypertension. 2011;57:1101–1107. doi: 10.1161/HYPERTENSIONAHA.110.168005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dyer AR, Liu K, Walsh M, et al. Ten-year incidence of elevated blood pressure and its predictors: the CARDIA Study, Coronary Artery Risk Development in (Young) Adults. J Hum Hypertens. 1999;13:13–21. doi: 10.1038/sj.jhh.1000740. [DOI] [PubMed] [Google Scholar]
- 18.Etkin H, Mahoney J, Forsthoefel M, et al. Racial differences in hypertension associated with cell sodium permeability. Nature (Lond) 1982;297:588–89. doi: 10.1038/297588a0. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Song P. Is the association of diabetes with uncontrolled blood pressure stronger in Mexican Americans and Blacks than in Whites among diagnosed hypertensive patients? Am J Hypertens. 2013 Jul 17; doi: 10.1093/ajh/hpt109. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.TODAY study group Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care. 2013;36:1735–1741. doi: 10.2337/dc12-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massiera F, Bloch-Faure M, Ceiler D, et al. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J. 2001;15:2727–2729. doi: 10.1096/fj.01-0457fje. [DOI] [PubMed] [Google Scholar]
- 23.Boustany CM, Bharadwaj K, Daugherty A, et al. Activation of the systemic and adipose reninangiotensin system in rats with diet-induced obesity and hypertension. Am J Physiol Regul Integr Comp Physiol. 2004;287:R943–R949. doi: 10.1152/ajpregu.00265.2004. [DOI] [PubMed] [Google Scholar]
- 24.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 25.Williams JS, Williams GH. 50th anniversary of aldosterone. J Clin Endocrinol Metab. 2003;88:2364–2372. doi: 10.1210/jc.2003-030490. [DOI] [PubMed] [Google Scholar]
- 26.Whaley-Connell A, Johnson MS, Sowers JR. Aldosterone: role in the cardiometabolic syndrome and resistant hypertension. Prog Cardiovasc Dis. 2010;52:401–409. doi: 10.1016/j.pcad.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caprio M, Feve B, Claes A, et al. Pivotal role of the mineralocorticoid receptor in corticosteroid-induced adipogenesis. FASEB J. 2007;21:2185–94. doi: 10.1096/fj.06-7970com. [DOI] [PubMed] [Google Scholar]
- 28.Jeon JH, Kim KY, Kim JH, Baek A, Cho H, Lee YH, Kim JW, Kim D, Han SH, Lim JS, Kim KI, Yoon do Y, Kim SH, Oh GT, Kim E, Yang Y. A novel adipokine CTRP1 stimulates aldosterone production. FASEB J. 2008;22:1502–1511. doi: 10.1096/fj.07-9412com. [DOI] [PubMed] [Google Scholar]
- 29.Cooper SA, Whaley-Connell A, Habibi J, et al. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol. 2007;293:H2009–H2023. doi: 10.1152/ajpheart.00522.2007. [DOI] [PubMed] [Google Scholar]
- 30.Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42:1075–81. doi: 10.1161/01.HYP.0000100443.09293.4F. [DOI] [PubMed] [Google Scholar]
- 31.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 32.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 33.Johar S, Cave AC, Narayanapanicker A, et al. Aldosterone mediates angiotensin II-induced interstitialcardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J. 2006;20:1546–1548. doi: 10.1096/fj.05-4642fje. [DOI] [PubMed] [Google Scholar]
- 34.Barhoumi T, Kasal DA, Li MW, et al. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension. 2011;57:469–476. doi: 10.1161/HYPERTENSIONAHA.110.162941. [DOI] [PubMed] [Google Scholar]
- 35.Brown NJ. Aldosterone and vascular inflammation. Hypertension. 2008;51:161–167. doi: 10.1161/HYPERTENSIONAHA.107.095489. [DOI] [PubMed] [Google Scholar]
- 36.Ferrannini E, Buzzigoli G, Bonadonna R, et al. Insulin resistance in essential hypertension. N Engl J Med. 1987;317:350–357. doi: 10.1056/NEJM198708063170605. [DOI] [PubMed] [Google Scholar]
- 37.Bonora E, Capaldo B, Perin PC, et al. Hyperinsulinemia and insulin resistance are independently associated with plasma lipids, uric acid and blood pressure in non-diabetic subjects. The GISIR database. Nutr Metab Cardiovasc Dis. 2008;18:624–631. doi: 10.1016/j.numecd.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Muniyappa R, Quon MJ. Insulin action and insulin resistance in vascular endothelium. Curr Opin Clin Nutr Metab Care. 2007;10:523–530. doi: 10.1097/MCO.0b013e32819f8ecd. [DOI] [PubMed] [Google Scholar]
- 39.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 40.Vincent MA, Montagnani M, Quon MJ. Molecular and physiologic actions of insulin related to production of nitric oxide in vascular endothelium. Curr Diab Rep. 2003;3:279–288. doi: 10.1007/s11892-003-0018-9. [DOI] [PubMed] [Google Scholar]
- 41.Potenza MA, Marasciulo FL, Chieppa DM, et al. Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am J Physiol Heart Circ Physiol. 2005;289:H813–H822. doi: 10.1152/ajpheart.00092.2005. [DOI] [PubMed] [Google Scholar]
- 42.Formoso G, Chen H, Kim JA, et al. Dehydroepiandrosterone mimics acute actions of insulin to stimulate production of both nitric oxide and endothelin 1 via distinct phosphatidylinositol 3-kinase- and mitogen-activated protein kinase-dependent pathways in vascular endothelium. Mol Endocrinol. 2006;20:1153–1163. doi: 10.1210/me.2005-0266. [DOI] [PubMed] [Google Scholar]
- 43.Mukai Y, Wang CY, Rikitake Y, et al. Phosphatidylinositol 3-kinase/protein kinase Akt negatively regulates plasminogen activator inhibitor type-1 expression in vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2006;292:H1937–H1942. doi: 10.1152/ajpheart.00868.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim J, Montagnani M, Kwaug KK, et al. Reciprocal relationship between insulin resitance and endothelial dysfunction. Circulation. 2006:113;1888–1904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 45.Heagerty AM, Heerkens EH, Izzard AS. Small artery structure and function in hypertension. J Cell Mol Med. 2010;14:1037–1043. doi: 10.1111/j.1582-4934.2010.01080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson EA, Hoffman RP, Balon TW, et al. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991;87:2246–52. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landsberg L. Insulin-mediated sympathetic stimulation: role in the pathogenesis of obesity-related hypertension (or, how insulin affects blood pressure, and why). J Hypertens. 2001;19:523–528. doi: 10.1097/00004872-200103001-00001. [DOI] [PubMed] [Google Scholar]
- 43.Haynes WG, Morgan DA, Walsh SA, et al. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100:270–278. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song J, Hu X, Riazi S, et al. Regulation of blood pressure, the epithelial sodium channel (ENaC), andother key renal sodium transporters by chronic insulin infusion in rats. Am J Physiol Renal Physiol. 2006;290:F1055–F1064. doi: 10.1152/ajprenal.00108.2005. [DOI] [PubMed] [Google Scholar]
- 49.Tiwari S, Sharma N, Gill PS, et al. Impaired sodium excretion and increased blood pressure in mice with targeted deletion of renal epithelial insulin receptor. Proc Natl Acad Sci USA. 2008;105:6469–6774. doi: 10.1073/pnas.0711283105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muscelli E, Natali A, Bianchi S, et al. Effect of insulin on renal sodium and uric acid handling in essential hypertension. Am J Hypertens. 1996;9:746–752. doi: 10.1016/0895-7061(96)00098-2. [DOI] [PubMed] [Google Scholar]
- 51.Garcia-Touza M, Sowers JR. Evidence-based hypertension treatment in patients with diabetes. J Clin Hypertens (Greenwich. 2012;14:97–102. doi: 10.1111/j.1751-7176.2011.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cushman WC, Evans GW, Byington RP, et al. ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.American Diabetes Association Standards of medical care in diabetes --2011. Diabetes Care. 2011;34:S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 55.Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension OptimalTreatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755–62. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 56.Tuomilehto J, Rastenyte D, Birkenhäger WH, et al. Effects of calcium-channel blockade in older patients with diabetes and systolic hypertension. Systolic Hypertension in Europe Trial Investigators. N Engl J Med. 1999;340:677–684. doi: 10.1056/NEJM199903043400902. [DOI] [PubMed] [Google Scholar]
- 57.Curb JD, Pressel SL, Cutler JA, Savage PJ, Applegate WB, Black H, et al. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. JAMA. 1996;276:1886–92. [PubMed] [Google Scholar]
- 58.Schrier RW, Estacio RO, Jeffers B. Appropriate blood pressure control in NIDDM (ABCD) trial. Diabetologia. 1996;39:1646–1654. doi: 10.1007/s001250050629. [DOI] [PubMed] [Google Scholar]
- 59.ACCORD Study Group. Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cooper-DeHoff RM, Gong Y, Handberg EM, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA. 2010;304:61–68. doi: 10.1001/jama.2010.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.American Diabetes Association Standards of medical care in diabetes-2013. Diabetes Care. 2013;36:S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Redon J, Mancia G, Sleight P, et al. ONTARGET Investigators. Safety and efficacy of low blood pressures among patients with diabetes: subgroup analyses from the ONTARGET (Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial). J Am Coll Cardiol. 2012;59:74–83. doi: 10.1016/j.jacc.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 63.Bangalore S, Kumar S, Lobach I, et al. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and bayesian random-effects meta-analyses of randomized trials. Circulation. 2011;123:2799–2810. doi: 10.1161/CIRCULATIONAHA.110.016337. [DOI] [PubMed] [Google Scholar]
- 64.Kim YS, Davis SC, Truijen J, et al. Intensive blood pressure control affects cerebral blood flow in type 2 diabetes mellitus patients. Hypertension. 2011;57:738–745. doi: 10.1161/HYPERTENSIONAHA.110.160523. [DOI] [PubMed] [Google Scholar]
- 65.Cummings DM, Letter AJ, Howard G, et al. Generic medications and blood pressure control in diabetic hypertensive subjects: results from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Diabetes Care. 2013;36:591–597. doi: 10.2337/dc12-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tuomilehto J, Lindström J, Eriksson JG, et al. Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 67.Sacks FM, Svetky LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 68.Azadbakht L, Fard NR, Karimi M, et al. Effects of the dietary approaches to stop hypertension (DASH) eating plan on cardiovascular risks among type 2 diabetic patients. Diabetes Care. 2011;34:55–57. doi: 10.2337/dc10-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Azadbakht L, Surkan PJ, Esmaillzadeh A, et al. The dietary approaches to stop hypertension eating plan affects C-reactive protein, coagulation abnormalities, and hepatic function tests among type 2 diabetic patients. J Nutr. 2011;141:1083–1088. doi: 10.3945/jn.110.136739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Noda K, Zhang B, Iwata A, et al. STYLIST Study Investigators. Lifestyle changes through the use of delivered meals and dietary counseling in a single-blind study. The STYLIST study. Circ J. 2012;76:1335–1344. doi: 10.1253/circj.cj-12-0164. [DOI] [PubMed] [Google Scholar]
- 71.Cook NR, Cutler JA, Obarzanek E, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ. 2007;334:885–888. doi: 10.1136/bmj.39147.604896.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cook NR, Kumanyika SK, Cutler JA. Effect of change in sodium excretion on change in blood pressure corrected for measurement error. The Trials of Hypertension Prevention, Phase I. Am J Epidemiol. 1998;148:431–444. doi: 10.1093/oxfordjournals.aje.a009668. [DOI] [PubMed] [Google Scholar]
- 73.Taylor RS, Ashton KE, Moxham T, et al. Reduced dietary salt for the prevention of cardiovascular disease: a meta-analysis of randomized controlled trials (Cochrane review) Am J Hypertens. 2011;24:843–53. doi: 10.1038/ajh.2011.115. [DOI] [PubMed] [Google Scholar]
- 74.Rugale C, Oudot C, Desmetz C, et al. Sodium restriction prevents cardiovascular remodeling associated with insulin-resistance in the rat. Ann Cardiol Angeiol (Paris) 2013;62:139–143. doi: 10.1016/j.ancard.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 75.Whelton SP, Chin A, Xin X, et al. Effect of aerobic exercise on blood pressure: a metaanalysis of randomized, controlled trials. Ann Intern Med. 2002;136:493–503. doi: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- 76.Hansson L, Lindholm LH, Niskanen L, et al. Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomized trial. Lancet. 1999:353:611–616. doi: 10.1016/s0140-6736(98)05012-0. [DOI] [PubMed] [Google Scholar]
- 77.ALLHAT Collaborative Research Group Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 78.The Heart Outcomes Prevention Evaluation Study Investigators Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high risk patients. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 79.Heart Outcomes Prevention Evaluation (HOPE) Study Investigators Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253–259. [PubMed] [Google Scholar]
- 80.Lindholm LH, Ibsen H, Dahlöf B, et al. LIFE Study Group Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:1004–1010. doi: 10.1016/S0140-6736(02)08090-X. [DOI] [PubMed] [Google Scholar]
- 81.Ogihara T, Nakao K, Fukui T, et al. Candesartan Antihypertensive Survival Evaluation in Japan Trial Group Effects of candesartan compared with amlodipine in hypertensive patients with high cardiovascular risks: candesartan antihypertensive survival evaluation in Japan trial. Hypertension. 2008;51:393–398. doi: 10.1161/HYPERTENSIONAHA.107.098475. [DOI] [PubMed] [Google Scholar]
- 82.Fretheim A. VALUE: analysis of results. Lancet. 2004;364:934–935. doi: 10.1016/S0140-6736(04)17015-3. [DOI] [PubMed] [Google Scholar]
- 83.Yusuf S, Teo KK, Pogue J, et al. ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 84.Ruggenenti P, Perna A, Ganeva M, et al. Impact of blood pressure control and angiotensinconverting enzyme inhibitor therapy on new-onset microalbuminuria in type 2 diabetes: a post hoc analysis of the BENEDICT trial. J Am Soc Nephrol. 2006;17:3472–3481. doi: 10.1681/ASN.2006060560. [DOI] [PubMed] [Google Scholar]
- 85.Haller H, Ito S, Izzo J, et al. Olmesartan for delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364:907–917. doi: 10.1056/NEJMoa1007994. [DOI] [PubMed] [Google Scholar]
- 86.Bilous R, Chatuverdi N, Sjolie AK, et al. Effect of candesartan on microalbuminuria and albumin excretion rate in diabetes: three randomized trials. Ann Intern Med. 2009;5:11–20. doi: 10.7326/0003-4819-151-1-200907070-00120. [DOI] [PubMed] [Google Scholar]
- 87.Lastra G, Whaley-Connell A, Manrique C, et al. Low-dose spironolactone reduces reactive oxygen species generation and improves insulin-stimulated glucose transport in skeletal muscle in the TG(mRen2)27 rat. Am J Physiol Endocrinol Metab. 2008;295:E110–116. doi: 10.1152/ajpendo.00258.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lastra-Lastra G, Sowers JR, Restrepo-Erazo K, et al. Role of aldosterone and angiotensin II in insulin resistance: an update. Clin Endocrinol. 2009;71:1–6. doi: 10.1111/j.1365-2265.2008.03498.x. [DOI] [PubMed] [Google Scholar]
- 89.Estacio RO, Jeffers BW, Hiatt WR, et al. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non-insulin-dependent diabetes andhypertension. N Engl J Med. 1998;338:645–652. doi: 10.1056/NEJM199803053381003. [DOI] [PubMed] [Google Scholar]
- 90.Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21:597–603. doi: 10.2337/diacare.21.4.597. [DOI] [PubMed] [Google Scholar]
- 91.Leenen FH, Nwachuku CE, Black HR, et al. Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group. Clinical events in high-risk hypertensive patients randomly assigned to calcium channel blocker versus angiotensin-converting enzyme inhibitor in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Hypertension. 2006;48:374–384. doi: 10.1161/01.HYP.0000231662.77359.de. [DOI] [PubMed] [Google Scholar]
- 92.Julius S, Kjeldsen SE, Weber M, et al. VALUE trial group. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 93.Ogihara T, Nakao K, Fukui T, et al. Candesartan Antihypertensive Survival Evaluation in Japan Trial Group. Effects of candesartan compared with amlodipine in hypertensive patients with high cardiovascular risks: candesartan antihypertensive survival evaluation in Japan trial. Hypertension. 2008;51:393–398. doi: 10.1161/HYPERTENSIONAHA.107.098475. [DOI] [PubMed] [Google Scholar]
- 94.Berl T, Hunsicker LG, Lewis JB, et al. Irbesartan Diabetic Nephropathy Trial. Collaborative Study Group Cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial of patients with type 2 diabetes and overt nephropathy. Ann Intern Med. 2003;138:542–549. doi: 10.7326/0003-4819-138-7-200304010-00010. [DOI] [PubMed] [Google Scholar]
- 95.Ostergren J, Poulter NR, Sever PS, et al. ASCOT investigators The Anglo–Scandinavian Cardiac Outcomes Trial: blood pressure-lowering limb: effects in patients with Type II diabetes. J Hypertens. 2008;26:2103–2111. doi: 10.1097/HJH.0b013e328310e0d9. [DOI] [PubMed] [Google Scholar]
- 96.Beckett NS, Peters R, Fletcher AE, et al. HYVET Study Group Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 58:1887–1898. doi: 10.1056/NEJMoa0801369. 32008. [DOI] [PubMed] [Google Scholar]
- 97.Manrique C, Johnson M, Sowers JR. Thiazide diuretics alone or with beta-blockers impair glucose metabolism in hypertensive patients with abdominal obesity. Hypertension. 2010;55:15–17. doi: 10.1161/HYPERTENSIONAHA.109.142620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cooper-DeHoff RM, Wen S, Beitelshees AL, et al. Impact of abdominal obesity on incidence of adverse metabolic effects associated with antihypertensive medications. Hypertension. 2010;55:61–68. doi: 10.1161/HYPERTENSIONAHA.109.139592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Raheja P, Price A, Wang Z, et al. Spironolactone prevents chlorthalidone-induced sympathetic activation and insulin resistance in hypertensive patients. Hypertension. 2012;60:319–325. doi: 10.1161/HYPERTENSIONAHA.112.194787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhou X, Ma L, Habibi J, et al. Nebivolol improves diastolic dysfunction and myocardial remodeling through reductions in oxidative stress in the Zucker obese rat. Hypertension. 2010;55:880–888. doi: 10.1161/HYPERTENSIONAHA.109.145136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 102.Thorens B, Porret A, Buhler L, et al. Cloning and functional expression of the human islet GLP-1 receptor: demonstration that exendin-4 is an agonist and exendin-(9–39) an antagonist of the receptor. Diabetes. 1993;42:1678–1682. doi: 10.2337/diab.42.11.1678. [DOI] [PubMed] [Google Scholar]
- 103.Gros R, You X, Baggio LL, et al. Cardiac function in mice lacking the glucagon-like peptide-1 receptor. Endocrinology. 2003;144:2242–2252. doi: 10.1210/en.2003-0007. [DOI] [PubMed] [Google Scholar]
- 104.Grieve DJ, Cassidy RS, Green BD. Emerging cardiovascular actions of the incretin hormone glucagon-like peptide-1: potential therapeutic benefits beyond glycaemic control? Br J Pharmacol. 2009;157:1340–1351. doi: 10.1111/j.1476-5381.2009.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev. 2012;33:187–215. doi: 10.1210/er.2011-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Horton ES, Silberman C, Davis KL, et al. Weight loss, glycemic control, and changes in cardiovascular biomarkers in patients with type 2 diabetes receiving incretin therapies or insulin in a large cohort database. Diabetes Care. 2010;33:1759–1765. doi: 10.2337/dc09-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mistry GC, Maes AL, Lasseter KC. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on blood pressure in nondiabetic patients with mild to moderate hypertension. J Clin Pharmacol. 2008;48:592–598. doi: 10.1177/0091270008316885. [DOI] [PubMed] [Google Scholar]
- 108.Aroor AR, Sowers JR, Bender SB, et al. Dipeptidylpeptidase inhibition is associated with improvement in blood pressure and diastolic function in insulin-resistant male zucker obese rats. Endocrinology. 2013;154:2501–2513. doi: 10.1210/en.2013-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu L, Liu J, Wong WT, et al. Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon-like peptide 1-dependent mechanism. Hypertension. 2012;60:833–841. doi: 10.1161/HYPERTENSIONAHA.112.195115. [DOI] [PubMed] [Google Scholar]
- 110.Ossum A, van Deurs U, Engstrøm T, et al. The cardioprotective and inotropic components of the postconditioning effects of GLP-1 and GLP-1(9-36)a in an isolated rat heart. Pharmacol Res. 2009;60:411–417. doi: 10.1016/j.phrs.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 111.Girardi AC, Fukuda LE, Rossoni LV, et al. Dipeptidyl peptidase IV inhibition downregulates Na+ - H+ exchanger NHE3 in rat renal proximal tubule. Am J Physiol Renal Physiol. 2008;294:F414–F422. doi: 10.1152/ajprenal.00174.2007. [DOI] [PubMed] [Google Scholar]
- 112.Pacheco BP, Crajoinas RO, Couto GK, et al. Dipeptidyl peptidase IV inhibition attenuates blood pressure rising in young spontaneously hypertensive rats. J Hypertens. 2011;29:520–528. doi: 10.1097/HJH.0b013e328341939d. [DOI] [PubMed] [Google Scholar]
- 113.Sharma AM, Bakris G, Neutel JM, et al. Single-pill combination of telmisartan/amlodipine versus amlodipine monotherapy in diabetic hypertensive patients:an 8-week randomized, parallel-group, double-blind trial. Clin Ther. 2012;34:537–551. doi: 10.1016/j.clinthera.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 114.Weber MA, Bakris GL, Jamerson K, et al. ACCOMPLISH Investigators. Cardiovascular events during differing hypertension therapies in patients with diabetes. J Am Coll Cardiol. 2010;56:77–85. doi: 10.1016/j.jacc.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 115.Dahlof B, Swever PS, Poulter NR, et al. ASCOT Investigators. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA); a multicenter randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 116.Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysis. Hypertension. 2010;55:399–407. doi: 10.1161/HYPERTENSIONAHA.109.139816. [DOI] [PubMed] [Google Scholar]
- 117.Chrysant SG, Littlejohn T, 3rd, Izzo JL, Jr, et al. Triple-Combination therapy with olmesartan, amlodipine, and hydrochlorothiazide in black and non-black study participants with hypertension: the TRINITY randomized, double-blind, 12-week, parallel-group study. Am J Cardiovasc Drugs. 2012;12:233–243. doi: 10.1007/BF03261832. [DOI] [PubMed] [Google Scholar]
- 118.Wakefield BJ, Holman JE, Ray A, et al. Effectiveness of home telehealth in comorbid diabetes and hypertension: a randomized, controlled trial. Telemed J E Health. 2011;17:254–261. doi: 10.1089/tmj.2010.0176. [DOI] [PubMed] [Google Scholar]
- 119.Johns EJ, Abdulla MH. Renal nerves in blood pressure regulation. Curr Opin Nephrol Hypertens. 2013;22:504–510. doi: 10.1097/MNH.0b013e3283641a89. [DOI] [PubMed] [Google Scholar]
- 120.Symplicity HTN-2 Investigators. Esler MD, Krum H, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 121.Fagard RH, Celis H, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008;51:55. doi: 10.1161/HYPERTENSIONAHA.107.100727. [DOI] [PubMed] [Google Scholar]
- 122.Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med. 2006;354:2368–2374. doi: 10.1056/NEJMra060433. [DOI] [PubMed] [Google Scholar]
- 123.Mahfoud F, Schlaich M, Kindermann I, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation. 2011;123:1940–1946. doi: 10.1161/CIRCULATIONAHA.110.991869. [DOI] [PubMed] [Google Scholar]
- 124.Mahfoud F, Ukena C, Schmieder RE, et al. Ambulatory blood pressure changes after renal sympathetic denervation in patients with resistant hypertension. Circulation. 2013;128:132–140. doi: 10.1161/CIRCULATIONAHA.112.000949. [DOI] [PubMed] [Google Scholar]