Abstract
Cocaine-induced psychomotor stimulation may be mediated by metabolic hypofrontality and modification of brain functional connectivity. Functional connectivity refers to the pattern of relationships among brain regions, and one way to evaluate this pattern is using interactivity correlations of the metabolic marker cytochrome oxidase among different regions. This is the first study of how repeated cocaine modifies: (1) mean cytochrome oxidase activity in neural areas using quantitative enzyme histochemistry, and (2) functional connectivity among brain regions using inter-correlations of cytochrome oxidase activity. Rats were injected with 15 mg/kg i.p. cocaine or saline for 5 days, which lead to cocaine-enhanced total locomotion. Mean cytochrome oxidase activity was significantly decreased in cocaine-treated animals in the superficial dorsal and lateral frontal cortical association areas Fr2 and Fr3 when compared to saline-treated animals. Functional connectivity showed that the cytochrome oxidase activity of the noradrenergic locus coeruleus and the infralimbic cortex were positively inter-correlated in cocaine but not in control rats. Positive cytochrome oxidase activity inter-correlations were also observed between the dopaminergic substantia nigra compacta and Fr2 and Fr3 areas and the lateral orbital cortex in cocaine-treated animals. In contrast, cytochrome oxidase activity in the interpeduncular nucleus was negatively correlated with that of Fr2, anterior insular cortex, and lateral orbital cortex in saline but not in cocaine groups. After repeated cocaine specific prefrontal areas became hypometabolic and their functional connectivity changed in networks involving noradrenergic and dopaminergic brainstem nuclei. We suggest that this pattern of hypofrontality and altered functional connectivity may contribute to cocaine-induced psychomotor stimulation.
Keywords: Functional connectivity, Cytochrome oxidase, Cocaine, Prefrontal networks, Hypofrontality
1. Introduction
Cocaine is a powerful psychomotor stimulant and its abuse and subsequent addiction are persistent public health problems. Human studies have shown a hypofrontality produced by chronic use of cocaine (Volkow et al., 1988; London et al., 1990; Matochik et al., 2003; Bolla et al., 2004). In animal models, repeated exposure to cocaine results in a progressive and enduring enhancement in locomotion (Post, 1980; Wise and Bozarth, 1987; Stewart and Badiani, 1993) and changes in various brain circuits, especially lower metabolic activity in frontal cortical areas and ventral striatum (Robinson and Berridge, 1993; Porrino et al., 2007). This study was conducted to investigate whether cocaine-enhanced locomotion may involve systems-level alterations in the interactivity or functional connectivity of specific prefrontal areas. While anatomical connectivity refers to patterns of structural relationships among brain regions, functional connectivity refers to patterns of relationships in metabolic activity among brain regions (McIntosh and Gonzalez-Lima, 1994a,b; Nair et al., 1999). If the enhancement of locomotion by repeated cocaine exposure is an emergent property of affected prefrontal areas interacting with subcortical regions, understanding it requires a network analysis of the patterns of interaction between brain regions.
Network functional connectivity uses covariance analyses that cannot determine directionality but can describe the patterns of interaction between brain regions, as has been evaluated by inter-regional correlation changes in cytochrome oxidase activity (Sakata et al., 2000; Padilla et al., 2011). This particular functional connectivity method using inter-correlations of cytochrome oxidase activity describes stable metabolic relationships among areas, and it also describes how the regions are modified across sustained behavioral paradigms (Puga et al., 2007; Conejo et al., 2010; Fidalgo et al., 2012) or drug treatments (Padilla et al., 2011; Riha et al., 2011). Characterizing which specific neural systems modify their metabolic capacity and functional connectivity as a result of repeated cocaine exposure may advance our understanding of cocaine-enhanced locomotion.
Cytochrome oxidase (also called cytochrome c oxidase, ferrocytochrome c: O2 oxidoreductase, EC 1.9.3.1, cytochrome aa3, or the respiratory enzyme) is a ubiquitous mitochondrial membrane integral protein responsible for the last step of the electron transport chain that catalyzes the transfer of electrons to oxygen, which serves to generate ATP via oxidative phosphorylation (Wong-Riley, 1989; Gonzalez-Lima and Garrosa, 1991). Neurons depend mostly on oxidative metabolism as an energy source. For this reason, the enzymatic activity of cytochrome oxidase is used as a metabolic marker for neuronal activity (Wong-Riley, 1989) and cytochrome oxidase enzyme histochemistry serves to map sustained changes in brain energy metabolism (Wong-Riley, 1989; Gonzalez-Lima and Garrosa, 1991; Hevner et al., 1993; Sakata et al., 2005). In particular, we have not seen acute effects on cytochrome oxidase histochemistry one hour after a single drug injection, but the longer-term oxidative capacity for energy metabolism (protein-synthesis-dependent enzyme induction over hours or days) of brain regions can be investigated using quantitative cytochrome oxidase histochemistry (Gonzalez-Lima and Cada, 1994; Padilla et al., 2011; Riha et al., 2011). However, to date, there has not been any cytochrome oxidase study in animals exposed to cocaine. Therefore, we were interested in using cytochrome oxidase to investigate altered relationships between neural areas after 5 days of cocaine exposure, rather than monitoring acute effects of cocaine exposure.
Quantitative enzyme histochemistry of cytochrome oxidase (Gonzalez-Lima and Cada, 1994; Gonzalez-Lima, 1998) has been used successfully in over a hundred previous studies to map alterations in brain oxidative metabolism in numerous learning tasks and drug treatments (Poremba et al., 1997, 1998; Villarreal et al., 2002; Hu et al., 2006; Gonzalez-Pardo et al., 2008; O’Reilly et al., 2009; Conejo et al., 2010; Padilla et al., 2011; Rojas et al., 2012). Analysis of inter-regional correlations of cytochrome oxidase activity (Sakata et al., 2000; Padilla et al., 2011) between cortical and subcortical regions after cocaine administration, especially between the prefrontal cortex and monoaminergic nuclei, may also identify underlying initial brain effects of repeated cocaine.
2. Results
2.1. Cocaine enhanced locomotion from days 1–5
The behavioral protocol showed that rats treated with cocaine (15 mg/kg i.p.) for five days had an increase in total locomotion relative to saline-injected rats, two way ANOVA F(9,110)=3.20 (p<0.001). Additionally on day 5, subjects injected with cocaine had a significantly (p<0.05) increased total locomotor activity (5621±533 pcc) when compared to day 1 (2966±552 pcc), two-way ANOVA F(9,110)=3.20 (p<0.05). There was no significant change in total locomotor activity between day 1 (655±88) and day 5 (542±82) in saline-treated rats (Fig. 1).
Fig. 1. Locomotor activity.
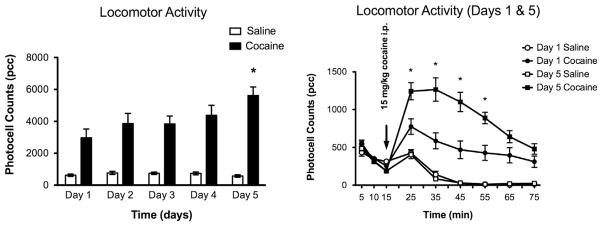
(A) Graph shows mean total locomotor activity (photocell counts/60 min, S.E.M. error bars) after 15 mg/kg cocaine (n=12) or saline (n=12) daily injections (i.p.) for days 1–5. (B) Graph shows comparison of day 1 and day 5 time course of total locomotor activity. Asterisks (*) denote a significant mean group difference as compared to day 1 (p<0.05).
2.2. Prefrontal regions became hypometabolic after repeated cocaine
Mean regional cytochrome oxidase effects of cocaine were focused on the prefrontal cortex. Cytochrome oxidase activity was significantly (p<0.05) decreased in cocaine-treated animals in the superficial layers of dorsal (Fr2) and lateral (Fr3) frontal cortex regions (DFS mean=217±8 and LFS mean=242±9) when compared to saline-treated animals (DFS mean=244±8 and LFS mean=265±6) (Fig. 2). Means and standard errors for all regions measured are reported in Table 1, which showed that the hypometabolic effect of repeated cocaine (15 mg/kg i.p. for 5 days) was specific to prefrontal cortical areas.
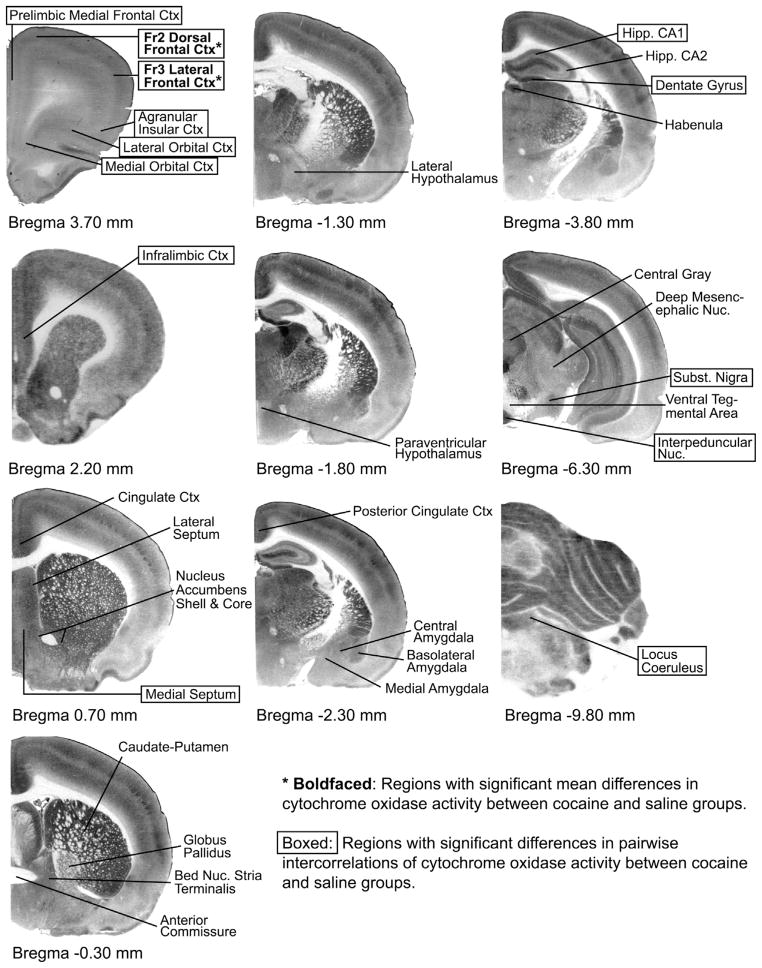
Fig. 2. Cytochrome-oxidase stained sections indicating regions of interest by Bregma level.
The purpose of this figure is to show representative control sections at the Bregma levels where the regions of interest were investigated in each brain and to illustrate schematically which were the affected regions. The quantitative densitometric cytochrome oxidase differences and functional connectivity changes cannot be seen with the naked eye by comparing sections, and are thus presented in Tables 1 and 2. To map these changes schematically in one figure, cocaine effects were illustrated so that regions appearing with boldfaced asterisks showed significant mean differences (p<0.05) in cytochrome oxidase (μmol/min/g tissue wet weight) between saline (n=12) and cocaine (n=12) groups (Table 1); and boxed regions showed significant differences (p<0.05) in pair-wise inter-regional correlations between saline and cocaine groups (Table 2).
Table 1.
Means and standard errors of cytochrome oxidase activity units (μmol/min/g tissue w/w) for all regions measured.
| Region | Bregma | Cocaine | Saline |
|---|---|---|---|
| Fr2 Dorsal Frontal Cortex Superficial Layer (DFS) | 3.7 | 216.8±8.3* | 243.5±8.4* |
| Fr3 Lateral Frontal Cortex Superficial Layer (LFS) | 241.5±8.7* | 264.8±6.3* | |
| Prelimbic Medial Frontal Cortex Superficial (MFS) | 227.3±4.6 | 225.7±4.7 | |
| Fr2 Dorsal Frontal Cortex Deep Layer (DFD) | 218.6±7.0 | 236.4±7.6 | |
| Prelimbic Medial Frontal Cortex Deep (MFD) | 221.8±4.3 | 228.0±4.7 | |
| Fr3 Lateral Frontal Cortex Deep Layer (LFD) | 242.4±7.8 | 259.0±5.9 | |
| Anterior Insular Cortex Superficial Layer (AIS) | 210.1±8.1 | 219.6±6.9 | |
| Anterior Insular Cortex Deep Layer (AID) | 231.9±7.8 | 248.0±6.4 | |
| Lateral Orbital Cortex Superficial Layer (LOS) | 238.3±6.9 | 239.6±5.2 | |
| Lateral Orbital Cortex Deep Layer (LOD) | 239.4±6.9 | 253.3±8.0 | |
| Medial Orbital Cortex Superficial Layer (MOS) | 226.7±7.2 | 230.2±6.0 | |
| Medial Orbital Cortex Deep Layer (MOD) | 235.6±6.3 | 244.4±8.9 | |
| Infralimbic Cortex Superficial Layer (ILS) | 2.2 | 229.8±6.5 | 226.4±6.9 |
| Infralimbic Cortex Deep Layer (ILD) | 232.5 ±7.1 | 233.8±7.1 | |
| Anterior Cingulate Cortex Superficial Layer (CGS) | 0.7 | 266.9±7.7 | 256.1 ±8.3 |
| Anterior Cingulate Cortex Deep Layer (CGD) | 257.5±8.7 | 247.4±7.9 | |
| Lateral Septum Nucleus (LS) | 245.8±6.1 | 242.4±3.1 | |
| Medial Septum Nucleus (MS) | 198.4±5.2 | 194.3 ±2.1 | |
| Accumbens Nucleus Shell (ACS) | 220.7±6.1 | 219.7±5.5 | |
| Accumbens Nucleus Core (ACC) | 245.0±7.9 | 246.7±6.4 | |
| Caudate Putamen (CPU) | −0.3 | 234.8±9.4 | 231.5±6.7 |
| Globus Pallidus (GP) | 158.5±5.2 | 150.7±3.2 | |
| Bed Nucleus Stria Terminalis (BST) | 213.2±5.7 | 214.4±2.4 | |
| Anterior Commissure (AC) | 55.6±3.6 | 53.4±3.9 | |
| Lateral Hypothalamic Area (LH) | −1.3 | 186.4±6.3 | 178.8±4.6 |
| Paraventricular Hypothalamic Nucleus (PVH) | −1.8 | 194.2±11.1 | 204.4±12.2 |
| Basolateral Amygdala (BLA) | −2.3 | 221.3±7.2 | 209.2±11.5 |
| Central Amygdala (CAM) | 228.5±6.8 | 234.3±8.4 | |
| Medial Amygdala (MAM) | 198.9±7.1 | 212.1±4.2 | |
| Posterior Cingulate/Retrosplenial Cortex (PCN) | 284.6±7.5 | 282.4±8.7 | |
| Field CA1 of Hippocampus (CA1) | −3.8 | 208.5±8.6 | 212.8±9.3 |
| Field CA2 of Hippocampus (CA2) | 226.0±11.3 | 225.0±12.1 | |
| Dentate Gyrus (DG) | 337.4±15.7 | 327.5±15.0 | |
| Medial Habenula (MHB) | 221.3±12.6 | 215.5±14.4 | |
| Lateral Habenula (LHB) | 293.8±13.4 | 288.6±17.9 | |
| Central Gray (CG) | −6.3 | 256.8±6.3 | 248.9±5.2 |
| Deep Mesencephalic Nucleus (DPME) | 168.0±5.8 | 183.7±13.0 | |
| Interpeduncular Nucleus (IP) | 378.4±13.3 | 353.5±20.6 | |
| Ventral Tegmental Area (VTA) | 102.8±9.8 | 95.9±12.3 | |
| Substantia Nigra Reticulata (SNR) | 234.7±7.4 | 234.3±11.3 | |
| Substantia Nigra Compacta (SNC) | 202.0±5.2 | 197.7±7.8 | |
| Locus Coeruleus (LC) | −9.8 | 250.4±19.3 | 267.3±10.5 |
Cytochrome oxidase activity was significantly (p<0.05) decreased in cocaine-treated animals (n=12) as compared to saline (n=12) in the superficial layers of dorsal (Fr2) and lateral (Fr3) frontal cortex regions.
2.3. Prefrontal regions increased their functional connectivity with noradrenergic and dopaminergic subcortical nuclei after repeated cocaine
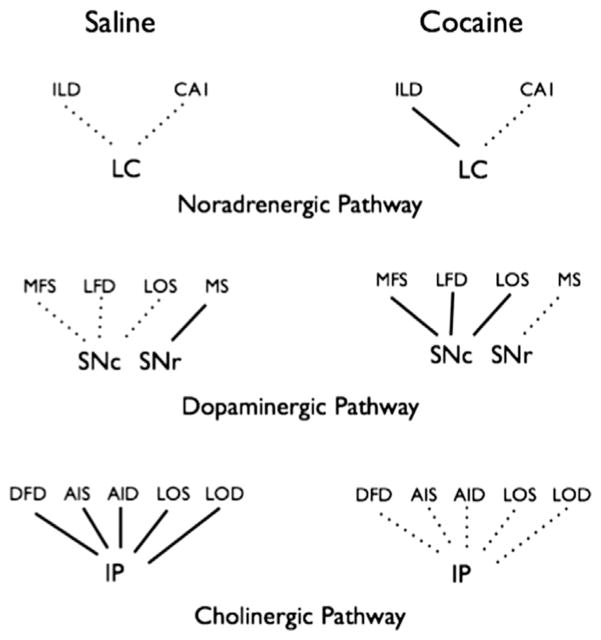
Specific prefrontal-subcortical nuclei inter-regional cytochrome oxidase correlations were significantly different between cocaine- and vehicle-treated animals (absolute value of Zabs>1.96, p<0.05), indicating that cocaine had significant effects on the functional connectivity of these regions, as illustrated in Fig. 3. Inter-regional correlations of cytochrome oxidase activity showed significant cocaine effects focused on prefrontal regions and noradrenergic and dopaminergic nuclei listed in Table 2. No significant cocaine-induced inter-correlations differences were found among other regions. For simplicity, correlations with no significant effects were not listed in Table 2. Three types of significant effects were found:
Fig. 3.
Schematic diagram illustrating the significant inter-regional correlations of cytochrome oxidase activity calculated in both saline (n=12) and cocaine (n=12) groups. Abbreviations are the same as in Table 1. Solid lines represent pair-wise Pearson’s correlations significantly different from zero (p<0.05) and dotted lines represent no significant correlation. The value and sign of the correlations with significant group differences are listed in Table 2.
Table 2.
Value and sign of pair-wise correlation coefficients (r) for the saline and cocaine groups and p values of Zabs for the group comparison of each regional pair. Abbreviations are the same as in Table 1.
| Saline (r) | Cocaine (r) | p value | |
|---|---|---|---|
| LC/ILD | −0.08 | 0.8 | 0.01 |
| LC/CA1 | −0.56 | 0.31 | 0.05 |
| SNC/MFS | 0.11 | 0.81 | 0.03 |
| SNC/LFD | −0.12 | 0.77 | 0.02 |
| SNC/LOS | −0.16 | 0.72 | 0.03 |
| SNR/MS | 0.6 | −0.3 | 0.03 |
| IP/DFD | −0.85 | 0 | 0.01 |
| IP/AIS | −0.67 | 0.16 | 0.03 |
| IP/AID | −0.95 | −0.17 | 0.001 |
| IP/LOS | −0.7 | 0.24 | 0.02 |
| IP/LOD | −0.85 | 0.3 | 0.001 |
| LFS/DG | 0 | 0.74 | 0.05 |
| MOS/AIS | 0.4 | 0.89 | 0.04 |
First, the noradrenergic locus coeruleus (LC) and deep layers of the infralimbic medial frontal cortex (ILD) were positively correlated in animals treated with cocaine (r=0.802) but not in saline-treated animals (r= −0.076, significant group difference p=0.013) (Fig. 4).
Fig. 4.
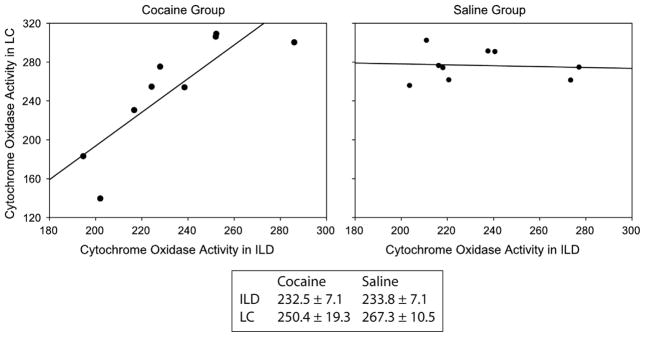
Inter-regional correlations patterns among nucleus locus coeruleus (LC) and deep infralimbic frontal cortex (ILD). Plots show significantly (p=0.01) different inter-regional correlations of cytochrome oxidase activity between the LC and the ILD in cocaine and saline treated animals. The line shows the best fit linear regression (r=0.80 in cocaine and r=−0.08 in saline treated animals). Box shows the mean cytochrome oxidase activity units (μmol/min/g tissue wet weight), which are not significantly different for both regions in saline (n=12) and cocaine treated rats (n=12).
Second, significant positive correlations were observed in cocaine subjects between the dopaminergic substantia nigra compacta (SNc) and the superficial layer of the prelimbic medial frontal cortex (MFS, r=0.809), the deep layer of the lateral (Fr3) frontal cortex (LFD, r=0.770) and the superficial layer of the lateral orbital cortex (LOS, r=0.717). These positive correlations were absent in saline-treated animals, revealing significant group differences (MFS, r=0.109 p=0.031, LFD, r= −0.118 p=0.015, and LOS r=−0.157 p=0.025). A positive correlation between the substantia nigra reticulata (SNr) and the medial septum (MS) was present in saline (r=0.602) but not in cocaine (r=−0.296 p=0.033) treated animals.
Third, in cholinergic pathways, interpeduncular nucleus (IP) cytochrome oxidase activity was negatively correlated with the activities of the deep layer of the dorsal (Fr2) frontal cortex (DFD, r=−0.851), the superficial and deep layers of the anterior insular cortex (AIS, r=−0.672 and AID, r=−0.954), the superficial and deep layers of the lateral orbital cortex (LOS, r=−0.697 and LOD, r=0.847) in saline treated animals but not in cocaine treated animals (DFD, r=0.000 p=0.008, AIS, r=0.164 p=0.032, AID, r=−0.173 p=0.0001, LOS, r=0.238 p=0.018, and LOD, r=0.300 p=0.001).
3. Discussion
Here we demonstrated two sets of metabolic changes in cytochrome oxidase activity in the brains of rats after a 5-day cocaine exposure paradigm. The rich datasets obtained from this mapping revealed not only mean cytochrome oxidase activity decreases in prefrontal regions (hypofrontality) but also modified functional connectivity between specific prefrontal regions and subcortical noradrenergic and dopaminergic nuclei. This cytochrome oxidase analysis demonstrated novel effects in subcortical brainstem nuclei that are too small to be reliably measured with noninvasive neuroimaging studies in humans. We speculate that these metabolic alterations produced by repeated cocaine impair prefrontal networks for inhibitory psychomotor control and might contribute to the observed enhanced locomotion.
3.1. Difference between mean activity and functional connectivity
Means provide measures of the average metabolic activity of individual regions (univariate measure), whereas functional connectivity provides measures of the interactivity among regions (covariance measure) (McIntosh and Gonzalez-Lima, 1994a, 1994b). These two statistical approaches have been embraced by functional neuroimaging studies because they are complementary, as the nervous system can be viewed as a complex network of interacting individual regions (Gonzalez-Lima and McIntosh, 1994). The analysis of regional means of cytochrome oxidase activity focuses on how the baseline metabolic activity of specific brain regions change after repeated cocaine, whereas the analysis of functional connectivity focuses on how baseline interactions among brain regions change after repeated cocaine. Two regions can have similar means across groups, but different functional connectivity across groups (Nair et al., 1999; Sakata et al., 2000). Fig. 4 helps to explain how two brain areas may have similar means across groups but different functional connectivity. For example, the mean cytochrome oxidase activity in the infralimbic deep (ILD) cortical area and the locus coeruleus (LC) nucleus are not different between cocaine and saline groups, but the functional connectivity between cocaine and saline groups is substantially different (LC-ILD inter-regional correlations=0.80 cocaine vs. −0.08 saline). Therefore, in addition to frontal hypometabolism, it is plausible that cocaine-enhanced locomotor behavior may be mediated by different patterns in the interactivity or functional connectivity among particular frontal-subcortical brain regions.
3.2. Cytochrome oxidase vs. other metabolic markers
Elegant studies with the 2-deoxyglucose (2-DG) method in rhesus monkeys have mapped regional decreases in metabolic activity of frontal cortical areas 32 (prelimbic), 25 (infralimbic) and 24 (anterior cingulate) and the ventral striatum after 5 days of cocaine (Porrino et al., 2007). Similar 2-DG studies in rats have shown glucose utilization decreases only in the nucleus accumbens after 5 days of cocaine self-administration, while there were decreases in infralimbic and prelimbic cortical regions after 30 days of cocaine (Macey et al., 2004).
Other studies using Fos immunohistochemistry and c-fos mRNA have been used to measure neuronal activity after repeated cocaine administration. These investigations differ in terms of number of injections given, doses and time of measurement after the last injection, and most importantly the fact that c-fos is a transient activity marker as opposed to cytochrome oxidase histochemistry that is a maker for chronic, sustained metabolic demand over days. Due to the transient effects inherent to immediate early gene expression, different results in c-Fos activity were found depending on whether repeated cocaine administration was given in the home cage or in the locomotor activity chambers. Home cage injections diminished Fos expression and c-Fos mRNA in the nAcc and caudate–putamen (Hope et al., 1992; Steiner and Gerfen, 1993). In contrast, repeated cocaine administration inside the activity chambers increased Fos expression in the nAcc without changes in the caudate–putamen (Crombag et al., 2002). Experiments using a similar cocaine administration paradigm to ours found a significant decrease in c-fos density in the medial prefrontal cortex and two subdivisions of the nAcc in animals challenged after a 2-days withdrawal period (Todtenkopf et al., 2002). These findings suggest that chronic cocaine administration induces a decrease in pre-frontal activity.
Interestingly, regions related to self-administration reward, such as the nucleus accumbens, showed no changes in mean cytochrome oxidase activity or in functional connectivity in our experimenter-administered cocaine paradigm (15 mg/kg i.p. for 5 days). It is possible that involvement of nucleus accumbens may require a different behavioral protocol such as self-administration to reveal self-stimulation effects of cocaine. But lack of effects on the midbrain ventral tegmental area (VTA) are consistent with the 2-DG mapping studies of cocaine-treated rats by Koch et al. (1997) and Porrino and Kornetsky (1988), although here we provide further alternative explanations (Porrino and Kornetsky, 1988; Koch et al., 1997). For example, after cocaine exposure the VTA changes from tonic to phasic neuronal firing (Porrino and Kornetsky, 1988; Koch et al., 1997; Schultz, 1998). This transition does not necessarily result in a mean change in neuronal metabolic profile. Thus, it is possible that total neuronal activity in the VTA is not changed, only the pattern of firing. Furthermore, we have recently shown that a decrease in VTA dopaminergic neuronal cell size occurs after cocaine sensitization (Arencibia-Albite et al., 2012). This diminution may compensate for the increase in metabolic activity that one may expect with the long-term potentiation known to occur after chronic cocaine treatment (Ungless et al., 2001). Therefore, some changes in neuronal activity patterns in areas like the VTA and the nucleus accumbens may not be detected with cytochrome oxidase histochemistry or with the cocaine protocol we used. These regions may show alterations only if a different methodology for mapping neuronal activity or a different behavioral protocol such as self-administration are used.
The cytochrome oxidase technique, like 2-DG and fluorodeoxyglucose (FDG), offers a functional marker for neuronal energy metabolism (Gonzalez-Lima 1998). Cytochrome oxidase is the final step in the electron transport chain; therefore, its catalytic activity is critical for glucose oxidation and the creation of ATP (Wong-Riley, 1989; Wong-Riley et al., 1998). But cytochrome oxidase mapping was used to assess the cumulative effects of repeated drug manipulations over days (Padilla et al., 2011). While 2-DG/FDG and immediate early genes as c-fos evaluate on-going changes in neuronal activity during the period of tracer uptake or gene expression (usually 45–90 min), cytochrome oxidase measures longer-term alterations in enzyme levels that are induced by days of sustained metabolic changes on the implicated brain region (Gonzalez-Lima, 1998). These markers provide measures of neuronal metabolism, but some (2-DG, FDG, c-fos) measure more the on-going neuronal activation while the other (cytochrome oxidase) gives information on longer-term changes on neuronal oxidative metabolic capacity. The oxidative capacity for energy metabolism, as measured by quantitative cytochrome oxidase histochemistry, reflects the energy demand history of different brain regions after repeated cocaine exposure because cytochrome oxidase levels change by enzymatic induction (a process that requires protein synthesis) in a cumulative way after repeated daily changes in energy demand (Gonzalez-Lima and Cada, 1994). Thus, we found that cytochrome oxidase served to determine cumulative effects on prefrontal neural oxidative metabolic capacity and functional connectivity after a 5-day cocaine treatment, although it should be acknowledged that a more extended cocaine treatment or a different protocol using a withdrawal period could produce different changes.
3.3. Prefrontal cortical involvement
Mean cytochrome oxidase was decreased in cocaine treated animals when compared to saline injected rats in the superficial layers of dorsal (Fr2) and lateral (Fr3) frontal cortex regions (Table 1). Hypometabolic effects circumscribed to the superficial cortical layers of Fr2 and Fr3 imply an alteration of intercortical communication of association fibers between the superficial layers, as opposed to ascending or descending projection fibers that innervate deeper cortical layers. These Fr2 and Fr3 areas of Zilles and Wree shown in the atlas of Paxinos and Watson (1986), correspond to the most anterior secondary and tertiary association areas of the rat frontal cortex that are regarded as prefrontal cortical areas (Paxinos and Watson, 1986; Paxinos, 1995; Shumake and Gonzalez-Lima, 2003). They are not part of the rat primary motor cortex (Fr1) but may be prefrontal cortical areas involved in descending inhibitory control of psychomotor behavior. Therefore, cocaine-induced hypometabolism of Fr2 and Fr3 areas may lead to diminished inhibitory control that may manifest as enhanced locomotion.
Other prefrontal cortical areas in the rat showed cocaine-modified functional connectivity, including the prelimbic medial frontal cortex (area 32), the infralimbic medial frontal cortex (area 25), and the medial and lateral orbital frontal cortex (Fig. 2). These prefrontal regions receive projections from most of the mesocorticolimbic system such as the VTA, substantia nigra, amygdala, lateral hypothalamus, hippocampus, and other areas of the cortex (Groenewegen et al., 1997; Dalley et al., 2004b). They also project back to most of these regions (Groenewegen et al., 1997; Dalley et al., 2004a). But these mesocorticolimbic regions did not show significant changes in mean cytochrome oxidase or functional connectivity differences with the prefrontal areas after our cocaine paradigm. This may be related to the fact that cocaine was not self-administered by the rats and thus may not engage the mesocorticolimbic self-stimulation system.
As discussed below, there were only specific noradrenergic and dopaminergic brainstem nuclei that showed cytochrome oxidase inter-regional correlation differences with prefrontal areas when saline-treated animals are compared to cocaine-treated rats (Fig. 3). Since cocaine not only increases synaptic levels of noradrenaline and dopamine, but also that of serotonin and glutamate, simply increasing transmitter levels is unlikely to explain the specific cocaine-induced modification of functional connectivity observed in this study. We speculate that 5 days of cocaine leads to neuro-metabolic adaptations that are more specific for prefrontal cortical areas and their functional connectivity with noradre-nergic and dopaminergic brainstem nuclei. Future studies would be needed to further evaluate the mechanisms of these changes.
3.4. Noradrenergic pathways
Our studies found that the noradrenergic locus coeruleus (LC) and deep layers of the infralimbic cortex were positively correlated in cocaine-treated animals but not in control rats (Table 2 and Fig. 4). But inter-regional correlations do not provide information on the direction of the influence, so they need to be interpreted based on other known anatomical and functional data (Gonzalez-Lima and McIntosh, 1994). The noradrenergic system contributes to control stress responses, arousal, mood and alters learning and memory (Huether, 1996; Sved et al., 2001) and plays a key role in mediating reward (Poschel and Ninteman, 1963; Stein, 1964, 1975; Wise, 1978). The LC is the principal site for norepinephrine (NE) synthesis in the brain and it projects to extensive areas that may help modulate the observed cocaine-enhanced locomotion (projections reviewed by Foote et al. (1983), Grzanna and Fritschy (1991), Holstege and Bongers (1991), Jones (1991), Westlund et al. (1991) and Berridge and Waterhouse (2003)). It is also possible that a modified pattern of LC functional connectivity might underlie cocaine-induced behaviors not investigated in this study, such as self-stimulation, and future studies manipulating behavior or brain mechanisms would be important to test this possibility. Regions of the mesolimbic dopamine (DA) system, like the VTA, nucleus accumbens and amygdala receive noradrenergic inputs (Ungerstedt, 1971; Alheid and Heimer, 1988; Liprando et al., 2004; Mejias-Aponte et al., 2009). Also, noradrenergic neurons in the LC receive afferents from the infralimbic prefrontal cortex (Heidbreder and Groenewegen, 2003).
The links between the LC and the infralimbic cortex with the VTA are so strong that activation of alpha-1 receptors in the LC or in the infralimbic cortex by themselves increase VTA DA neuronal activity (Lategan et al., 1990; Blanc et al., 1994). In addition, our lab has shown that glutamate release onto VTA DA neurons is modulated by pre-synaptic alpha-1 receptors (Velasquez-Martinez et al., 2012); and systemic inhibition of alpha-1 and activation of alpha-2 receptors block the development and expression of cocaine sensitization (Jimenez-Rivera et al., 2006). Together with the observed change in functional connectivity between the LC and the infralimbic cortex, these data suggest the hypothesis that NE pathways to the infralimbic cortex play a critical role in the development of cocaine-induced neuroadaptations. Based on this hypothesis, the observed significant pair-wise positive correlation in cytochrome oxidase activity may be interpreted as repeated cocaine modifying the noradrenergic LC influence on the infralimbic cortex. This hypothesis deserves testing in future studies.
3.5. Dopaminergic pathways
There were significant positive correlations in cocaine-treated subjects between the substantia nigra compacta (SNc) and the superficial layer of the prelimbic medial frontal cortex (MFS), the deep layer of the Fr3 lateral frontal cortex (LFD) and the superficial layer of the lateral orbital cortex. These correlations were not present in saline-treated rats (Fig. 3). A positive correlation between the SN reticulata and the medial septum was present in saline and not cocaine treated animals. The SN is a predominantly dopaminergic area most commonly known for its involvement in Parkinson’s disease and the extra-pyramidal motor system linked to involuntary movement control. The presence of a positive correlation between the SN and specific prefrontal regions suggests a role of DA in the control of these areas after repeated cocaine exposure. Augmentation of DA release with repeated drug exposure is the basis of theories that suggest drugs of abuse impair adaptive circuitry to become hyper-responsive to drug stimuli (Berridge and Robinson, 1998; Goldstein and Volkow, 2002; Everitt and Robbins, 2005; Kalivas, 2008). In addition, there is a direct interaction from prefrontal cortex to SN (Watabe-Uchida et al., 2012).
The orbital frontal cortex projects to central parts of caudate–putamen and to the lateral part of nucleus accumbens shell (Schilman et al., 2008). This area also receives afferents from regions like the VTA, ventral pallidum and the medial temporal lobe (Krettek and Price, 1977; Groenewegen, 1988; Ray and Price, 1992) and it is thought to be important in drug vulnerability (Schoenbaum and Shaham, 2008). Hyper-activity of the orbitofrontal cortex in humans results in impulsive behavior (Baxter et al., 1987, 1989; Zametkin et al., 1990; Andreason et al., 1994) and this area compensates this hyperactivity by an inhibitory mechanism (Winstanley, 2007). Monkeys and humans show orbital cortex dysfunction after cocaine exposure (Franklin et al., 2002; Jentsch et al., 2002; Olausson et al., 2007). Increases in metabolic rates in the orbitofrontal cortex and basal ganglia in humans correlate negatively with the duration of abstinence (Volkow et al., 1991). Further studies suggest that orbital cortex abnormalities are a consequence of drug exposure and not a predisposing factor for drug addiction (Perry et al., 2011). The observed changes in cytochrome oxidase correlations between specific regions of the prefrontal cortex and the SNc may be relevant to a role of DA pathways in the loss of inhibitory control and increased locomotion seen after cocaine intake.
3.6. Cholinergic pathways
Cytochrome oxidase activity in the interpeduncular nucleus (IP) was negatively correlated with the activities of the deep layer of the Fr2 dorsal frontal cortex, the superficial and deep layers of the anterior insular cortex, the superficial and deep layers of the lateral orbital cortex in saline but not in cocaine-treated animals (Fig. 3). Although IP neurons are mainly GABAergic, its major inputs are cholinergic projections from the medial habenula. Indeed, the IP receives more acetylcholine input than any other region in the mammalian brain (Herkenham and Nauta, 1979; Villani et al., 1983; Artymyshyn and Murray, 1985; Contestabile et al., 1987; Eckenrode et al., 1987; Fasolo et al., 1992). Studies have shown that interpeduncular pathways (i.e. habenulaip) and the mesolimbic pathways are mutually inhibitory, (Sutherland and Nakajima, 1981; Nishikawa et al., 1986). In addition, it has been reported that cocaine injections decrease the extracellular levels of acetylcholine in the IP (Hussain et al., 2008). Nicotininc AChR subunits in the IP have been also been shown to play a crucial role in somatic withdrawal symptoms in drugs of abuse like nicotine (Salas et al., 2009). Acetylcholine is an integral component of the mesolimbic system (Hoebel et al., 2007). Cholinergic projections may be altered in learning and memory processes like those changed in drug abuse (Vorel et al., 2001; See et al., 2003). For example, nicotinic receptor inactivation decreases sensitivity to cocaine whereas nicotine exposure enhances cocaine seeking (Zachariou et al., 2001; Bechtholt and Mark, 2002). On the other hand, changes in the neurochemistry of the IP and its projections induce changes in motor behavior (Shannon and Peters, 1990; Salas et al., 2004; Taraschenko et al., 2007). For example, muscarinic antagonists enhance cocaine and amphetamine-induced locomotor effects (Shannon and Peters, 1990; Bymaster et al., 1993; Ichikawa et al., 2002), whereas mice lacking muscarinic M5 receptors self-administer less cocaine and show reduced conditioned place preference (Fink-Jensen et al., 2003). These studies suggest that cholinergic receptors mediate some component of cocaine-induced changes. Recent evidence has shown that blockade of nicotinic cholinergic receptors in the VTA prevents the cocaine-induced DA increase (Mark et al., 2011). There is also evidence that supports that disruption of IP signaling can lead to increased drug intake (Picciotto, 1998; Cui et al., 2003; Tapper et al., 2004; Fowler et al., 2011). The loss of connectivity of the IP observed in our experiments suggest that cocaine exposure produces an abnormality in acetylcholine signaling that may affect the connections between the IP and frontal cortical structures that could further enable cocaine sensitization.
3.7. Conclusion
First, the hypofrontality results presented here are in agreement with previous glucose metabolic mapping studies in cocaine-exposed animals (Porrino and Kornetsky, 1988; Koch et al., 1997). They also agree with previous human studies that suggest a hypofrontality produced by chronic use of drugs of abuse such as cocaine (Volkow et al., 1988; London et al., 1990; Matochik et al., 2003; Bolla et al., 2004). Second, our findings also indicate, for the first time, that repeated cocaine modifies the functional connectivity between specific prefrontal regions and subcortical noradrenergic, dopaminergic and cholinergic pathways. Taken together these patterns of functional connectivity suggest the general hypothesis that prefrontal networks change from cholinergic influences to networks driven by noradrenergic and dopaminergic nuclei after repeated cocaine administration. Finally, we speculate that when these prefrontal networks for inhibitory control are modified by repeated cocaine, enhanced locomotion may arise, which suggests that treatments that increase the metabolic capacity of these prefrontal networks may antagonize the psychomotor effects of cocaine (Chen et al., 2013).
4. Experimental procedures
4.1. Subjects
All procedures were performed according to the US Public Health Service publication “Guide for the Care and Use of Laboratory Animals” and were approved by the Animal Care and Use Committee at the University of Puerto Rico Medical Sciences Campus. Twenty-four Sprague-Dawley male rats (Taconic Farms) weighing between 250–300 g were housed two per cage and maintained at constant temperature and humidity with a 12-h light/dark cycle. All behavioral experiments were carried out during the light period. Water and food were provided ad libitum.
4.2. Cocaine protocol and behavior
Animals were randomly divided into two groups (saline n=12 and cocaine n=12). Prior to any drug treatment, all animals (n=24) were habituated to locomotor chambers (AccuScan Instruments Inc., Columbus, OH) for two daily 1 h sessions. Each cage frame houses sensor panels and a row of 16 infrared beams. When a rodent intersects the beams, the software detects a photocell count. On experimental day 1, animals were placed for 15 min in the photocell box. After 15-min habituation, animals were treated with either 15 mg/kg i.p. of cocaine (Sigma, St. Louis, MO, USA) or isovolumetric saline injections. This particular dose repeated for five injections was chosen because it is a well-established protocol to induce progressive cocaine-enhanced locomotion that is commonly used in our laboratory (Jimenez-Rivera et al., 2006; Arencibia-Albite et al., 2012; Santos-Vera et al., 2013) and others (Pierce et al., 1996; Robinson and Berridge, 2001; Yoon et al., 2007). Therefore, using this protocol makes this study more relevant to compare with similar cocaine rat studies in the literature. Immediately after injections, total locomotor activity was assessed for 1 h. The total number of beam breaks (photocell counts) determined total locomotion. This procedure was repeated for 5 consecutive days. On day 5, after locomotor activity was assessed for 1 h, rats’ brains were collected for tissue processing.
4.3. Tissue processing and cytochrome oxidase staining
Brains were quickly removed and frozen in isopentane. Using a cryostat microtome (Leica CM3000, Germany) at −20 °C, brains were sectioned at 40 μm, mounted on slides and kept frozen at −40 °C until they were processed using quantitative cytochrome oxidase enzyme histochemistry (Gonzalez-Lima, 1998). Series of coronal sections from each brain were used to perform cytochrome oxidase histochemistry following the validated quantitative protocol described by Gonzalez-Lima and collaborators (Gonzalez-Lima and Cada, 1994; Gonzalez-Lima and Jones, 1994) that has been used in hundreds of other cytochrome oxidase studies. The staining reaction signal is produced when diaminobenzidine is oxidized to a visible indamine polymer. The reaction product is further intensified by the addition of cobalt to the preincubation solution. Since continuous reoxidation of cytochrome c by cytochrome oxidase is needed for the accumulation of the visible product, this reaction under linear conditions serves to visualize cytochrome oxidase reactivity. Enzymatic activity units are calculated using calibration standards made of brain paste which showed a linear relationship (r=0.99) between cytochrome oxidase activity units measured spectrophotometrically and cytochrome oxidase reactivity measured with densitometry. Briefly, frozen slides were fixed for 5 min using a 10% sucrose phosphate buffer (0.1 M pH 7.6) containing 0.5% glutaraldehyde to adhere the sections to the slides. Next, slides were rinsed in three changes of a 10% sucrose phosphate buffer (0.1 M) for 5 min each to remove red blood cells and warm tissue to room temperature. Then the slides were pre-incubated in 0.05 M Tris buffer (pH 7.6), with 275-mg/l cobalt chloride, 10% sucrose, and 0.5% dimethylsulfoxide, for 10 min to enhance staining contrast (metal intensification). Slides were then rinsed for 5 min in phosphate buffer and incubated in 700 ml of oxygen-saturated 0.1 M phosphate buffer (350 mg diaminobenzidine tetrahydrochloride, 52.5 mg cytochrome c, 35 g sucrose, 14 mg catalase, and 1.75 ml dimethylsulfoxide) at 37 °C for 1 h. The tissue was fixed in buffered formalin to stop the last reaction (for 30 min at room temperature with 10% sucrose and 4% formalin). Finally, the slides were dehydrated in a series of ethanol baths (increasing from 30% to 100% ethanol), cleared by immersion in xylene, and cover slipped with Permount (Fisher Scientific, Pittsburgh, PA, USA).
Each batch of slides was accompanied by a set of brain paste standards to quantify enzymatic activity and to control for staining variability across batches. For these standards, the brains from 12 additional Sprague-Dawley male rats were removed after decapitation, stored at 4 °C (in sodium phosphate buffer, pH 7.6), and then homogenized at 4 °C (Gonzalez-Lima, 1998). Cytochrome oxidase activity of the brain paste was spectrophotometrically assessed as described by Gonzalez-Lima and Cada (1998), and activity units were defined at pH 7 and 37 °C, where 1 unit oxidizes 1 μmol of reduced cytochrome c per min (μmol/min/g tissue wet weight). Remaining paste was frozen in the same manner as the experimental brains and stored at −40 °C. Immediately prior to each cytochrome oxidase staining procedure, cryostat sections of different thickness (10, 20, 40, 60 and 80 μm) were obtained from the rat brain paste and mounted on a slide. These sets of sections of known cytochrome oxidase activity were used as calibration standards in each cytochrome oxidase staining bath.
4.4. Cytochrome oxidase activity mapping
Using a stereotaxic atlas of the rat brain (Paxinos and Watson, 1986) as well as a cytochrome oxidase atlas of the rat brain (Gonzalez-Lima, 1998), cytochrome oxidase-stained sections were carefully selected for both the appropriate levels of brain regions of interest and the integrity of the sections. The regions of interest examined are illustrated by Bregma level in Fig. 2. An image-processing system consisting of a high-gain video camera, Targa-M8 image capture board, Everex computer, Sony color monitor, DC-powered illuminator, and JAVA software (Jandel Scientific, San Rafael, CA, USA) was used to sample optical density (OD) from each ROI. This system was calibrated before each measurement session using an OD tablet (Kodak, Rochester, NY, USA). The film had a known set of absolute OD units in seven standards ranging from 0 to 0.92 OD units. Background subtraction of the clear part of the slide without sections was used to correct possible optical artifacts from the camera. The histochemical reaction product from cytochrome oxidase staining was measured in OD units. In each measured region, four readings of each section were taken on each of three adjacent sections to yield 12 readings per region per brain. For each region measured, size of the square-shaped sampling window was adjusted so that it was as large as possible while still allowing two, non-overlapping readings to be taken bilaterally (four total). The size of the window was held identical across subjects, as was the number of readings for each ROI. The OD values of these readings were then converted to cytochrome oxidase activity units (μmol/min/g tissue w/w) using a regression curve (r2>0.90) that was obtained from the mean OD values and enzymatic activity of the tissue standards stained in the same batch and imaged in the same measurement session (Gonzalez-Lima, 1998).
4.5. Statistical analysis of locomotion, mean cytochrome oxidase activity and functional connectivity
Total locomotor activity, expressed as photocell counts, between groups were analyzed using Two-way ANOVA for repeated measures followed by Bonferroni post-test in order to establish behavioral locomotor effects (numbers are presented as mean ± standard error). A significant day 1 to day 5 difference in mean photocell counts at p<0.05 was considered a successful enhanced locomotor effect. Group differences in mean cytochrome oxidase activity measured in each brain region were evaluated by one-way ANOVA. Functional connectivity was assessed by computing separate pair-wise Pearson correlation matrices of cytochrome oxidase activity across all regions of interest for each group (within-group analysis) (Puga et al., 2007). In this data-driven approach, all the brain regions are evaluated and the brain effects determine which are the relevant inter-regional correlations, as opposed to using a more restricted theory-driven or arbitrary selection of regions for analysis (McIntosh and Gonzalez-Lima, 1994a, 1994b). A “jackknife” procedure was used to ensure the reliability of significant correlations and to protect against the effects of outliers to avoid inflated correlation estimates which sometimes result from small sample sizes. In this procedure, an individual is removed from the data set and correlations are computed on the data set with n−1 subjects. The individual is then replaced into the data set, and another individual is removed. Correlations and significance tests are recomputed on the data set with n−1 subjects. This procedure is repeated until all individuals have been removed once. Based on the calculation of all possible pairwise correlations resulting from removing one subject at a time, this procedure takes into consideration only those correlations that remain significant (p<0.01) across all possible combinations. These correlations were then tested for significant differences between groups (between-group analysis). The Fisher Z transformation was used to convert each correlation to a Z score to test differences in inter-regional correlations between groups (Jones and Gonzalez-Lima, 2001; Bruchey and Gonzalez-Lima, 2006; Puga et al., 2007). Significant group differences of pair-wise inter-regional correlations between cocaine- and vehicle-treated animals were calculated as absolute value of Zabs>1.96 (p<0.05). As with all functional connectivity methods, inter-regional correlations do not provide information on the direction of the influence, so they need to be interpreted based on known anatomical pathways. For example, if region A that influences region B via an anatomical path showed a significant pair-wise positive correlation in cytochrome oxidase activity after treatment, it may be inferred that a change in activity in region A was functionally related to a corresponding alteration in region B (Puga et al., 2007). The term functional connection was used to refer to jackknife-tested reliably significant cytochrome oxidase activity correlations between two brain regions (Padilla et al., 2011).
Acknowledgments
This work was supported by grants from NIGMS SC1GM084854 to CAJR, MH65728 to FGL and MBRS-RISE R25-GM061838 to MVH. The authors thank Maria C. Velazquez, Bermary Santos and Rafael Vazquez for their help during brain collection. We also thank Dr. Jason Shumake for his help with statistical analysis and Dr. Douglas Barrett for his assistance with figure making. Dr. Vélez-Hernández conducted this study in partial fulfillment of the requirements for the Ph.D. degree at the University of Puerto Rico, Medical Sciences Campus.
References
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Andreason PJ, Zametkin AJ, Guo AC, Baldwin P, Cohen RM. Gender-related differences in regional cerebral glucose metabolism in normal volunteers. Psychiatry Res. 1994;51:175–183. doi: 10.1016/0165-1781(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Arencibia-Albite F, Vazquez R, Velasquez-Martinez MC, Jimenez-Rivera CA. Cocaine sensitization inhibits the hyperpolarization-activated cation current Ih and reduces cell size in dopamine neurons of the ventral tegmental area. J Neurophysiol. 2012;107:2271–2282. doi: 10.1152/jn.00818.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artymyshyn R, Murray M. Substance P in the interpeduncular nucleus of the rat: normal distribution and the effects of deafferentation. J Comp Neurol. 1985;231:78–90. doi: 10.1002/cne.902310107. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Jr, Phelps ME, Mazziotta JC, Guze BH, Schwartz JM, Selin CE. Local cerebral glucose metabolic rates in obsessive-compulsive disorder. A comparison with rates in unipolar depression and in normal controls. Arch Gen Psychiatry. 1987;44:211–218. doi: 10.1001/archpsyc.1987.01800150017003. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Jr, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, Gerner RH, Sumida RM. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry. 1989;46:243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- Bechtholt AJ, Mark GP. Enhancement of cocaine-seeking behavior by repeated nicotine exposure in rats. Psychopharmacology (Berl) 2002;162:178–185. doi: 10.1007/s00213-002-1079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Blanc G, Trovero F, Vezina P, Herve D, Godeheu AM, Glowinski J, Tassin JP. Blockade of prefronto-cortical alpha 1-adrenergic receptors prevents locomotor hyperactivity induced by subcortical D-amphetamine injection. Eur J Neurosci. 1994;6:293–298. doi: 10.1111/j.1460-9568.1994.tb00272.x. [DOI] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchey AK, Gonzalez-Lima F. Brain activity associated with fear renewal. Eur J Neurosci. 2006;24:3567–3577. doi: 10.1111/j.1460-9568.2006.05229.x. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Heath I, Hendrix JC, Shannon HE. Comparative behavioral and neurochemical activities of cholinergic antagonists in rats. J Pharmacol Exp Ther. 1993;267:16–24. [PubMed] [Google Scholar]
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–362. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- Conejo NM, Gonzalez-Pardo H, Gonzalez-Lima F, Arias JL. Spatial learning of the water maze: progression of brain circuits mapped with cytochrome oxidase histochemistry. Neurobiol Learn Mem. 2010;93:362–371. doi: 10.1016/j.nlm.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Contestabile A, Villani L, Fasolo A, Franzoni MF, Gribaudo L, Oktedalen O, Fonnum F. Topography of cholinergic and substance P pathways in the habenulo-interpeduncular system of the rat. An immunocytochemical and microchemical approach. Neuroscience. 1987;21:253–270. doi: 10.1016/0306-4522(87)90337-x. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Jedynak JP, Redmond K, Robinson TE, Hope BT. Locomotor sensitization to cocaine is associated with increased Fos expression in the accumbens, but not in the caudate. Behav Brain Res. 2002;136:455–462. doi: 10.1016/s0166-4328(02)00196-1. [DOI] [PubMed] [Google Scholar]
- Cui C, Booker TK, Allen RS, Grady SR, Whiteaker P, Marks MJ, Salminen O, Tritto T, Butt CM, Allen WR, Stitzel JA, McIntosh JM, Boulter J, Collins AC, Heinemann SF. The beta3 nicotinic receptor subunit: a component of alpha-conotoxin MII-binding nicotinic acetylcholine receptors that modulate dopamine release and related behaviors. J Neurosci. 2003;23:11045–11053. doi: 10.1523/JNEUROSCI.23-35-11045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004a;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Bouger P, Chudasama Y, Cardinal RN, Robbins TW. Cortical cholinergic function and deficits in visual attentional performance in rats following 192 IgG-saporin-induced lesions of the medial prefrontal cortex. Cereb Cortex. 2004b;14:922–932. doi: 10.1093/cercor/bhh052. [DOI] [PubMed] [Google Scholar]
- Eckenrode TC, Barr GA, Battisti WP, Murray M. Acetylcholine in the interpeduncular nucleus of the rat: normal distribution and effects of deafferentation. Brain Res. 1987;418:273–286. doi: 10.1016/0006-8993(87)90095-3. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fasolo A, Virgili M, Panzica GC, Contestabile A. Immunohistochemistry and neurochemistry of the habenulo-interpeduncular connection after partial developmental depletion of habenular cholinergic neurons in the rat. Exp Brain Res. 1992;90:297–301. doi: 10.1007/BF00227241. [DOI] [PubMed] [Google Scholar]
- Fidalgo C, Conejo NM, Gonzalez-Pardo H, Arias JL. Functional interaction between the dorsal hippocampus and the striatum in visual discrimination learning. J Neurosci Res. 2012;90:715–720. doi: 10.1002/jnr.22774. [DOI] [PubMed] [Google Scholar]
- Fink-Jensen A, Fedorova I, Wortwein G, Woldbye DP, Rasmussen T, Thomsen M, Bolwig TG, Knitowski KM, McKinzie DL, Yamada M, Wess J, Basile A. Role for M5 muscarinic acetylcholine receptors in cocaine addiction. J Neurosci Res. 2003;74:91–96. doi: 10.1002/jnr.10728. [DOI] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Lima F. Cytochrome oxidase in neuronal metabolism and Alzheimer’s disease. Plenum Press; New York: 1998. [Google Scholar]
- Gonzalez-Lima F, Cada A. Cytochrome oxidase activity in the auditory system of the mouse: a qualitative and quantitative histochemical study. Neuroscience. 1994;63:559–578. doi: 10.1016/0306-4522(94)90550-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Garrosa M. Quantitative histochemistry of cytochrome oxidase in rat brain. Neurosci Lett. 1991;123:251–253. doi: 10.1016/0304-3940(91)90943-n. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Jones D. Quantitative mapping of cytochrome oxidase activity in the central auditory system of the gerbil: a study with calibrated activity standards and metal-intensified histochemistry. Brain Res. 1994;660:34–49. doi: 10.1016/0006-8993(94)90836-2. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima FA, McIntosh AR. Neural network interactions related to auditory learning analyzed with structural equation modeling. Hum Brain Mapp. 1994;2:23–44. [Google Scholar]
- Gonzalez-Lima F, Cada A. Quantitative histochemistry of cytochrome oxidase activity: theory, methods and regional brain vulnerability. In: Gonzalez-Lima F, editor. Cytochrome Oxidase in Neuronal Metabolism and Alzheimer’s Disease. 1998. pp. 55–90. [Google Scholar]
- Gonzalez-Pardo H, Conejo NM, Arias JL, Monleon S, Vinader-Caerols C, Parra A. Changes in brain oxidative metabolism induced by inhibitory avoidance learning and acute administration of amitriptyline. Pharmacol Biochem Behav. 2008;89:456–462. doi: 10.1016/j.pbb.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ. Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience. 1988;24:379–431. doi: 10.1016/0306-4522(88)90339-9. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Uylings HB. The anatomical relationships of the prefrontal cortex with limbic structures and the basal ganglia. J Psychopharmacol. 1997;11:99–106. doi: 10.1177/026988119701100202. [DOI] [PubMed] [Google Scholar]
- Grzanna R, Fritschy JM. Efferent projections of different subpopulations of central noradrenaline neurons. Prog Brain Res. 1991;88:89–101. doi: 10.1016/s0079-6123(08)63801-7. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. J Comp Neurol. 1979;187:19–47. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Liu S, Wong-Riley MT. An optimized method for determining cytochrome oxidase activity in brain tissue homogenates. J Neurosci Methods. 1993;50:309–319. doi: 10.1016/0165-0270(93)90038-s. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Avena NM, Rada P. Accumbens dopamine-acetylcholine balance in approach and avoidance. Curr Opin Pharmacol. 2007;7:617–627. doi: 10.1016/j.coph.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege JC, Bongers CM. Ultrastructural aspects of the coeruleo-spinal projection. Prog Brain Res. 1991;88:143–156. doi: 10.1016/s0079-6123(08)63804-2. [DOI] [PubMed] [Google Scholar]
- Hope B, Kosofsky B, Hyman SE, Nestler EJ. Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc Natl Acad Sci USA. 1992;89:5764–5768. doi: 10.1073/pnas.89.13.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Xu X, Gonzalez-Lima F. Vicarious trial-and-error behavior and hippocampal cytochrome oxidase activity during Y-maze discrimination learning in the rat. Int J Neurosci. 2006;116:265–280. doi: 10.1080/00207450500403108. [DOI] [PubMed] [Google Scholar]
- Huether G. The central adaptation syndrome: psychosocial stress as a trigger for adaptive modifications of brain structure and brain function. Prog Neurobiol. 1996;48:569–612. doi: 10.1016/0301-0082(96)00003-2. [DOI] [PubMed] [Google Scholar]
- Hussain RJ, Taraschenko OD, Glick SD. Effects of nicotine, methamphetamine and cocaine on extracellular levels of acetylcholine in the interpeduncular nucleus of rats. Neurosci Lett. 2008;440:270–274. doi: 10.1016/j.neulet.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa J, Chung YC, Li Z, Dai J, Meltzer HY. Cholinergic modulation of basal and amphetamine-induced dopamine release in rat medial prefrontal cortex and nucleus accumbens. Brain Res. 2002;958:176–184. doi: 10.1016/s0006-8993(02)03692-2. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De La Garza R, 2nd, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26:183–190. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- Jimenez-Rivera CA, Feliu-Mojer M, Vazquez-Torres R. Alpha-noradrenergic receptors modulate the development and expression of cocaine sensitization. Ann NY Acad Sci. 2006;1074:390–402. doi: 10.1196/annals.1369.039. [DOI] [PubMed] [Google Scholar]
- Jones BE. Noradrenergic locus coeruleus neurons: their distant connections and their relationship to neighboring (including cholinergic and GABAergic) neurons of the central gray and reticular formation. Prog Brain Res. 1991;88:15–30. doi: 10.1016/s0079-6123(08)63797-8. [DOI] [PubMed] [Google Scholar]
- Jones D, Gonzalez-Lima F. Associative effects of Pavlovian differential inhibition of behaviour. Eur J Neurosci. 2001;14:1915–1927. doi: 10.1046/j.0953-816x.2001.01810.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox Res. 2008;14:185–189. doi: 10.1007/BF03033809. [DOI] [PubMed] [Google Scholar]
- Koch S, Piercey MF, Galloway MP, Svensson KA. Interactions between cocaine and (−)-DS 121: studies with 2-deoxyglucose autoradiography and microdialysis in the rat brain. Eur J Pharmacol. 1997;319:173–180. doi: 10.1016/s0014-2999(96)00852-7. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol. 1977;171:157–191. doi: 10.1002/cne.901710204. [DOI] [PubMed] [Google Scholar]
- Lategan AJ, Marien MR, Colpaert FC. Effects of locus coeruleus lesions on the release of endogenous dopamine in the rat nucleus accumbens and caudate nucleus as determined by intracerebral microdialysis. Brain Res. 1990;523:134–138. doi: 10.1016/0006-8993(90)91646-x. [DOI] [PubMed] [Google Scholar]
- Liprando LA, Miner LH, Blakely RD, Lewis DA, Sesack SR. Ultrastructural interactions between terminals expressing the norepinephrine transporter and dopamine neurons in the rat and monkey ventral tegmental area. Synapse. 2004;52:233–244. doi: 10.1002/syn.20023. [DOI] [PubMed] [Google Scholar]
- London ED, Cascella NG, Wong DF, Phillips RL, Dannals RF, Links JM, Herning R, Grayson R, Jaffe JH, Wagner HN., Jr Cocaine-induced reduction of glucose utilization in human brain. A study using positron emission tomography and [fluorine 18]-fl uorodeoxyglucose. Arch Gen Psychiatry. 1990;47:567–574. doi: 10.1001/archpsyc.1990.01810180067010. [DOI] [PubMed] [Google Scholar]
- Macey DJ, Rice WN, Freedland CS, Whitlow CT, Porrino LJ. Patterns of functional activity associated with cocaine self-administration in the rat change over time. Psychopharmacology (Berl) 2004;172:384–392. doi: 10.1007/s00213-003-1676-7. [DOI] [PubMed] [Google Scholar]
- Mark GP, Shabani S, Dobbs LK, Hansen ST. Cholinergic modulation of mesolimbic dopamine function and reward. Physiol Behav. 2011;104:76–81. doi: 10.1016/j.physbeh.2011.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F. Network interactions among limbic cortices, basal forebrain, and cerebellum differentiate a tone conditioned as a Pavlovian excitor or inhibitor: fluorodeoxyglucose mapping and covariance structural modeling. J Neurophysiol. 1994a;72:1717–1733. doi: 10.1152/jn.1994.72.4.1717. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F. Structural equation modeling and its application to network analysis in functional brain imaging. Hum Brain Mapp. 1994b;2:2–22. [Google Scholar]
- Mejias-Aponte CA, Drouin C, Aston-Jones G. Adrenergic and noradrenergic innervation of the midbrain ventral tegmental area and retrorubral field: prominent inputs from medullary homeostatic centers. J Neurosci. 2009;29:3613–3626. doi: 10.1523/JNEUROSCI.4632-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair HP, Collisson T, Gonzalez-Lima F. Postnatal development of cytochrome oxidase activity in fiber tracts of the rat brain. Brain Res Dev Brain Res. 1999;118:197–203. doi: 10.1016/s0165-3806(99)00149-2. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Fage D, Scatton B. Evidence for, and nature of, the tonic inhibitory influence of habenulointerpeduncular pathways upon cerebral dopaminergic transmission in the rat. Brain Res. 1986;373:324–336. doi: 10.1016/0006-8993(86)90347-1. [DOI] [PubMed] [Google Scholar]
- O’Reilly KC, Shumake J, Bailey SJ, Gonzalez-Lima F, Lane MA. Chronic 13-cis-retinoic acid administration disrupts network interactions between the raphe nuclei and the hippocampal system in young adult mice. Eur J Pharmacol. 2009;605:68–77. doi: 10.1016/j.ejphar.2008.12.037. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Krueger DD, Tronson NC, Nairn AC, Taylor JR. Orbitofrontal cortex and cognitive-motivational impairments in psychostimulant addiction: evidence from experiments in the non-human primate. Ann NY Acad Sci. 2007;1121:610–638. doi: 10.1196/annals.1401.016. [DOI] [PubMed] [Google Scholar]
- Padilla E, Shumake J, Barrett DW, Sheridan EC, Gonzalez-Lima F. Mesolimbic effects of the antidepressant fluoxetine in Holtzman rats, a genetic strain with increased vulnerability to stress. Brain Res. 2011;1387:71–84. doi: 10.1016/j.brainres.2011.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G. The Rat Nervous System. Academic Press; San Diego: 1995. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Sydney, Orlando: 1986. [Google Scholar]
- Perry JL, Joseph JE, Jiang Y, Zimmerman RS, Kelly TH, Darna M, Huettl P, Dwoskin LP, Bardo MT. Prefrontal cortex and drug abuse vulnerability: translation to prevention and treatment interventions. Brain Res Rev. 2011;65:124–149. doi: 10.1016/j.brainresrev.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR. Common aspects of the action of nicotine and other drugs of abuse. Drug Alcohol Depend. 1998;51:165–172. doi: 10.1016/s0376-8716(98)00074-x. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poremba A, Jones D, Gonzalez-Lima F. Metabolic effects of blocking tone conditioning on the rat auditory system. Neurobiol Learn Mem. 1997;68:154–171. doi: 10.1006/nlme.1997.3792. [DOI] [PubMed] [Google Scholar]
- Poremba A, Jones D, Gonzalez-Lima F. Classical conditioning modifies cytochrome oxidase activity in the auditory system. Eur J Neurosci. 1998;10:3035–3043. doi: 10.1046/j.1460-9568.1998.00304.x. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Kornetsky C. The effects of cocaine on local cerebral metabolic activity. NIDA Res Monogr. 1988;88:92–106. [PubMed] [Google Scholar]
- Porrino LJ, Smith HR, Nader MA, Beveridge TJ. The effects of cocaine: a shifting target over the course of addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1593–1600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poschel BP, Ninteman FW. Norepinephrine: a possible excitatory neurohormone of the reward system. Life Sci. 1963;10:782–788. doi: 10.1016/0024-3205(63)90087-0. [DOI] [PubMed] [Google Scholar]
- Post RM. Intermittent versus continuous stimulation: effect of time interval on the development of sensitization or tolerance. Life Sci. 1980;26:1275–1282. doi: 10.1016/0024-3205(80)90085-5. [DOI] [PubMed] [Google Scholar]
- Puga F, Barrett DW, Bastida CC, Gonzalez-Lima F. Functional networks underlying latent inhibition learning in the mouse brain. Neuroimage. 2007;38:171–183. doi: 10.1016/j.neuroimage.2007.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray JP, Price JL. The organization of the thalamocortical connections of the mediodorsal thalamic nucleus in the rat, related to the ventral forebrain-prefrontal cortex topography. J Comp Neurol. 1992;323:167–197. doi: 10.1002/cne.903230204. [DOI] [PubMed] [Google Scholar]
- Riha PD, Rojas JC, Gonzalez-Lima F. Beneficial network effects of methylene blue in an amnestic model. Neuroimage. 2011;54:2623–2634. doi: 10.1016/j.neuroimage.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Rojas JC, Bruchey AK, Gonzalez-Lima F. Low-level light therapy improves cortical metabolic capacity and memory retention. J Alzheimers Dis. 2012;32:741–752. doi: 10.3233/JAD-2012-120817. [DOI] [PubMed] [Google Scholar]
- Sakata JT, Coomber P, Gonzalez-Lima F, Crews D. Functional connectivity among limbic brain areas: differential effects of incubation temperature and gonadal sex in the leopard gecko, Eublepharis macularius. Brain Behav Evol. 2000;55:139–151. doi: 10.1159/000006648. [DOI] [PubMed] [Google Scholar]
- Sakata JT, Crews D, Gonzalez-Lima F. Behavioral correlates of differences in neural metabolic capacity. Brain Res Brain Res Rev. 2005;48:1–15. doi: 10.1016/j.brainresrev.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Salas R, Cook KD, Bassetto L, De Biasi M. The alpha3 and beta4 nicotinic acetylcholine receptor subunits are necessary for nicotine-induced seizures and hypolocomotion in mice. Neuropharmacology. 2004;47:401–407. doi: 10.1016/j.neuropharm.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Salas R, Sturm R, Boulter J, De Biasi M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J Neurosci. 2009;29:3014–3018. doi: 10.1523/JNEUROSCI.4934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Vera B, Vazquez-Torres R, Marrero HG, Acevedo JM, Arencibia-Albite F, Velez-Hernandez ME, Miranda JD, Jimenez-Rivera CA. Cocaine sensitization increases I h current channel subunit 2 (HCN 2) protein expression in structures of the mesocorticolimbic system. J Mol Neurosci. 2013;50:234–245. doi: 10.1007/s12031-012-9920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilman EA, Uylings HB, Galis-de Graaf Y, Joel D, Groenewegen HJ. The orbital cortex in rats topographically projects to central parts of the caudate-putamen complex. Neurosci Lett. 2008;432:40–45. doi: 10.1016/j.neulet.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Shaham Y. The role of orbitofrontal cortex in drug addiction: a review of preclinical studies. Biol Psychiatry. 2008;63:256–262. doi: 10.1016/j.biopsych.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- See RE, McLaughlin J, Fuchs RA. Muscarinic receptor antagonism in the basolateral amygdala blocks acquisition of cocaine-stimulus association in a model of relapse to cocaine-seeking behavior in rats. Neuroscience. 2003;117:477–483. doi: 10.1016/s0306-4522(02)00665-6. [DOI] [PubMed] [Google Scholar]
- Shannon HE, Peters SC. A comparison of the effects of cholinergic and dopaminergic agents on scopolamine-induced hyperactivity in mice. J Pharmacol Exp Ther. 1990;255:549–553. [PubMed] [Google Scholar]
- Shumake J, Gonzalez-Lima F. Brain systems underlying susceptibility to helplessness and depression. Behav Cogn Neurosci Rev. 2003;2:198–221. doi: 10.1177/1534582303259057. [DOI] [PubMed] [Google Scholar]
- Stein L. Self-stimulation of the brain and the central stimulant action of amphetamine. Fed Proc. 1964;23:836–850. [PubMed] [Google Scholar]
- Stein L. Norepinephrine reward pathways: role of self-stimulation, memory consolidation, and schizophrenia. Nebr Symp Motiv. 1975;22:113–159. [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Cocaine-induced c-fos messenger RNA is inversely related to dynorphin expression in striatum. J Neurosci. 1993;13:5066–5081. doi: 10.1523/JNEUROSCI.13-12-05066.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J, Badiani A. Tolerance and sensitization to the behavioral effects of drugs. Behav Pharmacol. 1993;4:289–312. [PubMed] [Google Scholar]
- Sutherland RJ, Nakajima S. Self-stimulation of the habenular complex in the rat. J Comp Physiol Psychol. 1981;95:781–791. doi: 10.1037/h0077833. [DOI] [PubMed] [Google Scholar]
- Sved AF, Cano G, Card JP. Neuroanatomical specificity of the circuits controlling sympathetic outflow to different targets. Clin Exp Pharmacol Physiol. 2001;28:115–119. doi: 10.1046/j.1440-1681.2001.03403.x. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Taraschenko OD, Rubbinaccio HY, Shulan JM, Glick SD, Maisonneuve IM. Morphine-induced changes in acetylcholine release in the interpeduncular nucleus and relationship to changes in motor behavior in rats. Neuropharmacology. 2007;53:18–26. doi: 10.1016/j.neuropharm.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todtenkopf MS, Mihalakopoulos A, Stellar JR. Withdrawal duration differentially affects c-fos expression in the medial prefrontal cortex and discrete subregions of the nucleus accumbens in cocaine-sensitized rats. Neuroscience. 2002;114:1061–1069. doi: 10.1016/s0306-4522(02)00272-5. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand. 1971;367 (Suppl):1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Velasquez-Martinez MC, Vazquez-Torres R, Jimenez-Rivera CA. Activation of alpha1-adrenoceptors enhances glutamate release onto ventral tegmental area dopamine cells. Neuroscience. 2012;216:18–30. doi: 10.1016/j.neuroscience.2012.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani L, Contestabile A, Fonnum F. Autoradiographic labeling of the cholinergic habenulo-interpeduncular projection. Neurosci Lett. 1983;42:261–266. doi: 10.1016/0304-3940(83)90272-0. [DOI] [PubMed] [Google Scholar]
- Villarreal JS, Gonzalez-Lima F, Berndt J, Barea-Rodriguez EJ. Water maze training in aged rats: effects on brain metabolic capacity and behavior. Brain Res. 2002;939:43–51. doi: 10.1016/s0006-8993(02)02545-3. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, Alpert R, Hoff A. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry. 1991;148:621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Mullani N, Gould KL, Adler S, Krajewski K. Cerebral blood flow in chronic cocaine users: a study with positron emission tomography. Br J Psychiatry. 1988;152:641–648. doi: 10.1192/bjp.152.5.641. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Zhang D, Carlton SM, Sorkin LS, Willis WD. Noradrenergic innervation of somatosensory thalamus and spinal cord. Prog Brain Res. 1991;88:77–88. doi: 10.1016/s0079-6123(08)63800-5. [DOI] [PubMed] [Google Scholar]
- Winstanley CA. The orbitofrontal cortex, impulsivity, and addiction: probing orbitofrontal dysfunction at the neural, neurochemical, and molecular level. Ann NY Acad Sci. 2007;1121:639–655. doi: 10.1196/annals.1401.024. [DOI] [PubMed] [Google Scholar]
- Wise RA. Catecholamine theories of reward: a critical review. Brain Res. 1978;152:215–247. doi: 10.1016/0006-8993(78)90253-6. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Wong-Riley MT. Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends Neurosci. 1989;12:94–101. doi: 10.1016/0166-2236(89)90165-3. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT, Huang Z, Liebl W, Nie F, Xu H, Zhang C. Neurochemical organization of the macaque retina: effect of TTX on levels and gene expression of cytochrome oxidase and nitric oxide synthase and on the immunoreactivity of Na+ K+ ATPase and NMDA receptor subunit I. Vision Res. 1998;38:1455–1477. doi: 10.1016/s0042-6989(98)00001-7. [DOI] [PubMed] [Google Scholar]
- Yoon HS, Kim S, Park HK, Kim JH. Microinjection of CART peptide 55–102 into the nucleus accumbens blocks both the expression of behavioral sensitization and ERK phosphorylation by cocaine. Neuropharmacology. 2007;53:344–351. doi: 10.1016/j.neuropharm.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Caldarone BJ, Weathers-Lowin A, George TP, Elsworth JD, Roth RH, Changeux JP, Picciotto MR. Nicotine receptor inactivation decreases sensitivity to cocaine. Neuropsychopharmacology. 2001;24:576–589. doi: 10.1016/S0893-133X(00)00224-4. [DOI] [PubMed] [Google Scholar]
- Zametkin AJ, Nordahl TE, Gross M, King AC, Semple WE, Rumsey J, Hamburger S, Cohen RM. Cerebral glucose metabolism in adults with hyperactivity of childhood onset. N Engl J Med. 1990;323:1361–1366. doi: 10.1056/NEJM199011153232001. [DOI] [PubMed] [Google Scholar]