Synopsis
There has been increasing evidence suggesting that vitamin D may play an important role in modifying risk of diabetes. In this regard, Vitamin D has both direct and indirect effects, the latter via regulation of calcium effects on various mechanisms related to the pathophysiology of type 2 diabetes, including pancreatic beta cell dysfunction, impaired insulin action and systemic inflammation. The human evidence comes primarily from many cross-sectional and prospective observational studies, most of which showed an inverse association between vitamin D status and prevalence or incidence of type 2 diabetes. The effect of vitamin D supplementation on glycemia or incident type 2 diabetes has been reported in several trials with mixed results. The present article describes the biological plausibility behind the potential association between vitamin D and type 2 diabetes and summarizes the current evidence supporting a relation between vitamin D and type 2 diabetes and briefly reports on the potential association between vitamin D and type 1 diabetes.
Keywords: vitamin D, type 2 diabetes, insulin resistance, insulin sensitivity, 25-hydroxyvitamin D
Introduction
Type 2 diabetes mellitus is a significant global health care problem and pharmacotherapies to treat the disease continue to emerge. However, the increasing burden of type 2 diabetes calls for an urgent need for innovative approaches to prevent its development. Recently, vitamin D has risen as a potential diabetes risk modifier.
The potentially significant extra-skeletal role of vitamin D is highlighted in several recently published studies, including the demonstration of the expression of the vitamin D receptor in a large number of non-skeletal cells, including pancreatic beta cells. Additional evidence has strongly suggested that vitamin D plays an important role in modifying the risk of type 2 diabetes, an effect which is likely mediated by an effect of vitamin D on beta cell function, insulin sensitivity and systemic inflammation. The evidence comes primarily from cross-sectional and longitudinal observational studies reporting on the association between vitamin D status and risk of type 2 diabetes or glycemia among patients with established type 2 diabetes. More recently, short-term, small randomized trials have reported the effect of vitamin D supplementation with or without calcium on diabetes risk and glycemia with mixed results.
The aims of the review are to: (1) describe the biological plausibility behind the potential association between vitamin D and diabetes, with emphasis on type 2 diabetes where most of the evidence exists and (2) summarize and synthesize the evidence from observational studies that report on the association of vitamin D status and risk of diabetes and from randomized trials that report on the effect of vitamin D supplementation on glycemia in patients with diabetes or at risk for diabetes.
Review of vitamin D physiology
Vitamin D exists in 2 forms: cholecalciferol (vitamin D3) and ergocalciferol (vitamin D2). Vitamin D3 is synthesized in the skin upon exposure to solar ultraviolet B radiation. During exposure to solar UVB radiation, 7-dehydrocholesterol in the skin is converted to pre-vitamin D3, which is immediately converted to vitamin D3 in a heat-dependent non-enzymatic process. Excessive exposure to sunlight degrades pre-vitamin D3 and vitamin D3 into inactive phyto-products (photo-degradation), avoiding vitamin D toxicity in the setting of excess sunlight. Vitamin D3 is also found is certain foods, such as fatty fish. Vitamin D2 is synthesized by plants and is found mostly in nutrients supplemented with vitamin D (e.g. milk) or dietary supplements. Whether endogenously synthesized or ingested through diet or supplements, vitamin D in the circulation is bound to the vitamin D-binding protein (DBP), which transports it to the liver, where vitamin D is converted by vitamin 25-hydroxylase to 25-hydroxyvitamin D [25OHD]. This form of vitamin D is biologically inactive and must be converted primarily in the kidneys by 25-hydroxyvitamin D-1alpha-hydroxylase to the biologically active form, 1,25-dihydroxyvitamin D [1,25(OH)2D]. The presence of 1-alpha-hydroxylase in extra-renal tissues suggest that vitamin D may have important role beyond the musculo-skeletal system. 25-hydroxyvitamin D is the major circulating form of vitamin D and is an excellent biomarker of exposure, either from cutaneous synthesis or dietary intake. Blood concentration of 25OHD is used by clinicians as a biomarker to determine vitamin D status.
Classification of vitamin D status
Clinicians and researchers use blood concentration of 25OHD as a biomarker to determine vitamin D status. However, there is no consensus on the 25OHD thresholds for vitamin D deficiency or insufficiency. The main guidelines by the Institute of Medicine (IOM) and the Endocrine Society differ on classification of vitamin D status, as shown in Table 1.[1] [2] The differences are explained by what populations were targeted by the guidelines and how the evidence was synthesized. The IOM guidelines concentrated on the general healthy population and placed emphasis on intervention studies. The IOM found no convincing evidence to link vitamin D with benefits for non-skeletal outcomes, such as diabetes. The IOM concluded that blood concentration of 25OHD > 20 ng/mL is consistent with favourable skeletal outcomes while there are only sparse data to support a higher level. The IOM also concluded that a level above 50 ng/mL should be a cause of concern about potential adverse events. In contrast, the Endocrine Society clinical practice guidelines concentrate on people at high risk for vitamin D deficiency and placed more emphasis on observational (epidemiologic) studies. Endocrine Society guidelines concluded that blood concentration of 25OHD > 30 ng/mL is desirable for optimal skeletal outcomes without any upper limit that would be concerning for safety. However, the Endocrine Society guidelines have been criticized by incorrectly characterizing several large population subgroups as at high risk and recommending widespread screening for vitamin D deficiency.[3] Both guidelines agreed that recommendations will require reconsideration in the future as additional data from ongoing randomized trials become available
Table 1.
Guidelines for vitamin D status by blood 25-hydroxyvitamin D concentration
| Cut-off, ng/mL1 | Institute of Medicine | Endocrine Society |
|---|---|---|
| <12 | Deficiency | Deficiency |
| 12 – 19 | Inadequacy | Deficiency |
| 20 – 29 | Sufficiency | Insufficiency |
| 30 – 49 | Sufficiency | Sufficiency |
| >50 | Reason for concern | Sufficiency |
To convert 25(OH)D concentration from ng/mL to nmol/L multiply by 2.459
Vitamin D intake requirements
The IOM report on dietary reference intakes for calcium and vitamin D recommends 600 international units per day of vitamin D for individuals 9–70 years and 800 international units for those older than 70 years as the recommended dietary allowance (RDA) (Table 2),[2] which is defined as the intake that meets the needs of 97.5% of the healthy population. The IOM report also concluded that the tolerable upper intake level (UL), above which the potential for adverse effects may increase with chronic use, is 4,000 IU/day. It is important to note that the UL amount is not intended as a target intake, rather, it is the upper limit for chronic intake of vitamin D above which toxicity may increase. In contrast, Endocrine Society clinical practice guidelines conclude that to raise the blood level of 25OHD consistently above 30 ng/mL, intakes of 1500 to 2000 IU/day may be required. The recommended intakes by the two guidelines differ for the same reasons as the recommendations for 25OHD levels. The IOM report clearly recognized the lack of long-term trials with vitamin D supplementation for non-skeletal outcomes as a major hurdle in establishing recommendation, while the Endocrine Society guidelines applied evidence from observational studies to develop its recommendations and considered 25OHD as a clinically important surrogate outcome that correlates with health and disease. The latter assumption should be approached with caution because although 25OHD is an excellent biomarker of exposure and correlates with outcomes, it is not a validated biomarker of effect that is causally related to health outcomes of interest. The evidence to support a causal association comes from long-term adequately powered randomized trials, which are lacking in relation to vitamin D and type 2 diabetes, as described below.
Table 2.
Vitamin D Recommended Intake*
| Institute of Medicine | Endocrine Society | |||
|---|---|---|---|---|
| RDA1 | UL2 | Daily requirement | UL | |
| 14–18 years | 600 IU | 4000 IU | 600–1000 IU | 4000 IU |
| 19–70 years | 600 IU | 4000 IU | 1500–2000 IU | 4000 IU |
| > 70 years | 800 IU | 4000 IU | 1500–2000 IU | 10000 IU |
RDA for skeletal outcomes (fractures and falls) only under conditions of minimal sun exposure. Applicable to normal healthy population groups
Recommended Dietary Allowance, intake that meets needs of 97.5% of healthy population
Tolerable Upper Intake Level, above which potential risk of adverse effects may increase with chronic use.
Biologic plausibility of an association between vitamin D and type 2 diabetes
Type 2 diabetes results from impaired beta cell function, increased insulin resistance and systems inflammation and there is evidence that vitamin D affects these pathways, as described next.
Vitamin D and insulin secretion
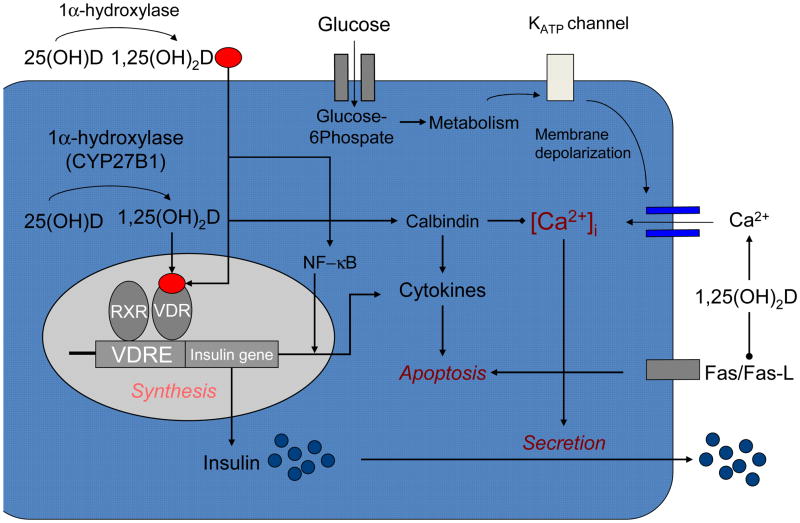
Based on pre-clinical studies, vitamin D seems to play a regulatory role in insulin secretion, beta-cell survival and calcium flux within beta-cells. A series of studies have shown that vitamin D deficiency impairs glucose-mediated insulin secretion in rat pancreatic beta cells [4–8], while vitamin D supplementation seems to restore such glucose-stimulated insulin secretion.[4, 7–11] Vitamin D may also have a direct effect on beta-cell function, which seems to be exerted by binding of its circulating active form to the vitamin D receptor (VDR) that is expressed in pancreatic beta-cells.[12] (Figure 1) Interestingly, mice lacking a functional VDR show an impaired insulin secretion following a glucose load. Such impairment appears associated with a decrease in insulin synthesis by the beta-cell resulting in a reduction in the amount of stored insulin [13]. Activation of vitamin D mediated by the 25(OH) D-1α-hydroxylase enzyme (CYP27B1) also occurs within the pancreatic beta cell allowing for an important paracrine effect of circulating 25-hydroxyvitamin D [14]. An additional effect of vitamin D on the pancreatic beta cell is the regulation of extracellular calcium concentration and flux through the beta cell. [15] Insulin secretion is a calcium-dependent process [16], therefore, alterations in calcium flux could have an effect on insulin secretion. [17–19] Vitamin D also regulates the function of calbindin, a cytosolic calcium-binding protein found in pancreatic beta cells[12, 20] and acts as a modulator of depolarization-stimulated insulin release via regulation of intracellular calcium. [21]
Figure 1.
Vitamin D and insulin secretion. Vitamin D can promote pancreatic beta cell function in several ways. The active form of vitamin D, (1,25OH2D), enters the beta cell from the circulation and interacts with the vitamin D receptor-retinoic acid x-receptor complex (VDR-RXR), which binds to the vitamin D response element (VDRE) found in the human insulin gene promoter, to enhance the transcriptional activation of the insulin gene and increase the synthesis of insulin. Vitamin D may promote beta-cell survival by modulating the generation (through inactivation of nuclear factor-kB [NF-kb]) and effects of cytokines. The anti-apoptotic effect of vitamin D may also be mediated by downregulating the Fas-related pathways (Fas/Fas-L). Activation of vitamin D also occurs intracellularly by 1-alpha hydroxylase, which is expressed in pancreatic beta cells. Vitamin D also regulates calbindin, a cytosolic calcium-binding protein found in beta cells, which acts as a modulator of depolarization-stimulated insulin release via regulatation of intracellular calcium. Calbindin may also protect against apoptotic cell death via its ability to buffer intracellular calcium. The effects of vitamin D may be mediated indirectly via its important and well-recognized role in regulating extracellular calcium (Ca2+), calcium flux through the beta cell and intracellular calcium (Ca2+)i. Alterations in calcium flux can directly influence insulin secretion, which is a calcium-dependent process.
Vitamin D and insulin sensitivity
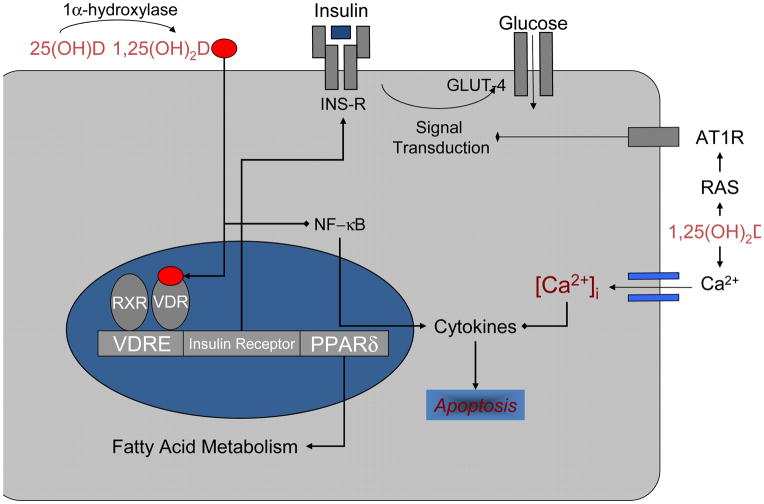
There are several ways in which vitamin D could affect insulin sensitivity. 1,25(OH)2D appears to stimulate the expression of insulin receptors, which in turn will affect insulin sensitivity. [22–25] 1,25(OH)2D enters insulin-responsive cells and interacts with the VDR activating the VDR-retinoic acid X-receptor (RXR) complex which binds to a vitamin D response element found in the human insulin receptor gene promoter region.( Figure 2) The result is an enhanced transcriptional activation of the insulin receptor gene increasing the total number of insulin receptors without altering their affinity. 1,25(OH)2D may also enhance insulin sensitivity by activating peroxisome proliferator-activated receptor delta (PPAR-δ), which is a transcription factor that regulates the metabolism of fatty acids in skeletal muscle and adipose tissue [26]. Vitamin D has also been found to improve muscle oxidative phosphorylation after exercise. Another potential effect of 1,25(OH)2D on insulin sensitivity might be exerted via its regulatory role in extracellular calcium concentration and flux through cell membranes. Calcium is essential for insulin-mediated intracellular processes in insulin-responsive tissues such as muscle and fat [27, 28], with a narrow range of intracellular calcium needed for optimal insulin-mediated functions [29]. Changes in intracellular calcium in insulin target tissues may contribute to peripheral insulin resistance [29–36] via an impaired insulin signal transduction [36, 37] leading to a decreased glucose transporter activity. [36–38] Hypovitaminosis D also leads to an increase in the levels of parathyroid hormone (PTH), which has been associated with insulin resistance. [39, 40] Vitamin D may also affect insulin resistance indirectly through the renin-angiotensin-aldosterone system (RAAS), as described below. Finally, vitamin D insufficiency has been associated with increased fat infiltration in skeletal muscle, which appears independent of body mass and is thought to contribute to a decreased insulin action. [41]
Figure 2.
Vitamin D and insulin action. In peripheral insulin-target cells, vitamin D may directly enhance insulin sensitivity by stimulating the expression of insulin receptors (INS-R) and/or by activating peroxisome proliferator-activated receptor (PPAR-δ), a transcription factor implicated in the regulation of fatty acid metabolism in skeletal muscle and adipose tissue. The effects of vitamin D may be mediated indirectly via its important and well-recognized role in regulating extracellular calcium (Ca2+), calcium flux through the cell and intracellular calcium (Ca2+)i. Vitamin D may promote beta-cell survival by modulating the generation (through inactivation of nuclear factor-kB [NF-kb]) and effects of cytokines. Vitamin D may also affect insulin resistance indirectly through the renin-angiotensin (AII)-aldosterone system.
Vitamin D and systemic inflammation
Vitamin D could directly and/or indirectly lessen the effects of systemic inflammation in patients with type 2 diabetes in several ways. For example, 1,25(OH)2D may protect against beta cell cytokine-induced apoptosis by directly modulating the expression and activity of cytokines, hence improving insulin sensitivity.[42–45] One such pathway may be through the down-regulation of NF-kB, a major transcription factor for TNF-alpha and other pro-inflammatory molecules.[46] Another pathway that may mediate the effect of 1,25(OH)2D on beta cell function is through counteracting cytokine-induced Fas expression, which in turn will have anti-apoptotic effects.[47] Several other immune-modulating effects of 1,25(OH)2D such as blockade of dendritic cell differentiation, inhibition of lymphocyte proliferation, inhibition of foam cell formation and cholesterol uptake by macrophages and enhanced regulatory T-lymphocyte development[43, 48] may provide additional protective pathways against beta cell destruction mediated by the systemic inflammation caused by type 2 diabetes.
Association between vitamin D status and type 2 diabetes
Cross sectional studies
There are many cross-sectional observational studies that have examined the association between vitamin D and type 2 diabetes and most have reported an inverse association between vitamin D status (25OHD concentration) and prevalent diabetes. One of the largest such cohorts is the National Health and Nutrition Examination Survey (NHANES) in United States, which reported an inverse association between 25OHD concentration and prevalence of diabetes in non-Hispanic whites and Mexican-Americans, but not African-Americans.[49] Similarly, this inverse association was seen in other large cohorts from the U.S.[40], Europe[50] and China.[51] The major limitation of cross-sectional studies is the potential of reverse causation; therefore, causality cannot be established.
Longitudinal studies
Longitudinal studies, where vitamin D status is assessed before the outcome (type 2 diabetes) is assessed have nearly universally shown an inverse association of vitamin D status and incident type 2 diabetes (Table 3).[52–67] In these studies, vitamin D status was assessed by self-reported vitamin D intake, predicted 25OHD concentration or measuring plasma or serum 25OHD concentration.
Table 3.
Observational longitudinal cohort studies of vitamin D status (plasma or serum 25[OH]D concentration, predicted 25[OH]D concentration or self-reported vitamin D intake) and incident type 2 diabetes
| Study, Year (reference) Cohort [Country] | Male, % | Mean baseline age (range), y | White, % | n*/N (incidence) | Vitamin D measure; comparison† | Mean follow-up, y (start-end) | Results, Adjusted RR, OR, or HR (95% CI) P for trend | Outcome (ascertainment method) | Adjustments |
|---|---|---|---|---|---|---|---|---|---|
| Liu et al, 2005 Women’s Health Study [US] | 0 | 52 (45–75) | 95 | 805/10,066 (8.0%) | Vitamin D intake (total); ≥511 vs. ≤159 IU/d | 9 (ND) | 0.73 (0.54, 0.99) § P=0.02 |
Type 2 diabetes (validated self-report) | Age |
| Pittas et al, 2006 [US] | 0 | 46 (30–55) | 98 | 4,843/83,779 (5.8%) | Vitamin D intake (total); >800 vs. ≤200 IU/d | 20 (1980–2000) | 0.87 (0.69, 1.09) P=0.67 |
Type 2 diabetes (validated self-report) | Age, BMI, exercise, residence, family history of diabetes, hypertension. calcium intake, smoking, alcohol, coffee, other diet |
| Knekt et al, 2008 Finnish Mobile Clinic Health Examination Survey [Finland] | 100 | ND (40–74) | 100 | 105/1,628 (6.4%); nested case-control study with 206 control participants | 25OHD concentration; 30 vs. 10 ng/mL (means) | 22 (1973–1994) | 0.49 (0.15, 1.64) P=0.06 |
Type 2 diabetes (medication-treated, registry-based) | Age, BMI, exercise, season, smoking, education, medications |
| 0 | ND (40–74) | 100 | 125/1,699 (7.4%); nested case-control study with 246 control participants | 25OHD concentration; 25 vs. 9 ng/mL (means) | 0.91 (0.37, 2.23) P=0.66 |
Age, BMI, exercise, season, smoking, education, medications | |||
| Knekt et al, 2008 Mini-Finland Health Survey [Finland] | 100 | 53 (40–69) | 100 | 83/1,948 (4.3%); nested case-control study with 245 control participants | 25OHD concentration; 31 vs. 9 ng/mL (means) | 17 (1978–1994) | 0.17 (0.05, 0.52) P<0.001 |
Type 2 diabetes (medication-treated, registry-based) | Age, BMI, exercise, season, smoking, education, medications |
| 0 | ND (40–69) | 100 | 99/2228 (4.4%); nested case-control study with 289 control participants | 25OHD concentration; 25 vs. 8 ng/mL (means) | 1.45 (0.58, 3.62) P=0.83 |
Age, BMI, exercise, season, smoking, education, medications | |||
| Kirii et al, 2009 Japan Public Health Center-based Prospective Study [Japan] | 100 | 57 (40–69) | NR (~100% Japanese) | 634/25,877 (2.4%) | Vitamin D intake (total); 720 vs. 188 IU/d (means) | 5 (1990–1998) | 0.96 (0.74, 1.23) P=0.35 |
Type 2 diabetes (validated self-report) | Age, BMI, exercise, family history of diabetes, smoking, diet, hypertension |
| 0 | 57 (40–69) | NR (~100% Japanese) | 480/33,919 (1.4%) | Vitamin D intake (total); 696 vs. 192 IU/d (means) | 5 (1990–1998) | 0.88 (0.67, 1.16) P=0.67 |
Type 2 diabetes (validated self-report) | ||
| Liu et al, 2010 Framingham Offspring Study [US] | 54 | 60 | ~100 | 133/2,956 (4.4%) | Predicted 25OHD score; 22 vs. 17 ng/mL (median) | 7 (1991–2001) | 0.60 (0.37, 0.97) P=0.03 |
Type 2 diabetes (medication-treated, laboratory-based) | Age, sex, waist circumference, ** family history of diabetes, hypertension, low HDL-cholesterol, high triglycerides, impaired fasting glucose, diet |
| Pittas et al, 2010 Nurses Health Study [US] | 0 | 46 (30–55) | 98 | 608/32,826 (1.8%); nested case-control study with 569 control participants | 25OHD concentration; 33 vs. 14 ng/mL (median) | 14 (1990– 2004) | 0.52 (0.33, 0.83) P=0.008 |
Type 2 diabetes (validated self-report) | Age, BMI, exercise, season, race, fasting status, latitude, hypercholesterolemia, hypertension, family history of diabetes, smoking, physical activity, alcohol, multivitamin use, diet |
| Anderson et al, 2010 Intermountain Healthcare system [US] | 25 | 55 | NR | NR/41,497 (NR); | 25OHD concentration; ≤15 vs. >30 ng/mL (median) | 1.3 (2000 –2009) | 1.89 (1.54, 2.33) P<0.001 |
Diabetes (physician-diagnosed based on ICD-9 code) | Age, gender, hypertension, hyperlipidemia, heart failure, infection, depression, renal failure |
| Grimnes et al, 2010 Tromso study [Norway] | NR NR |
60 (Non smokers) 57 (smokers) |
NR (majority Caucasians) NR (majority Caucasians) |
183/4157 64/1962 |
25OHD concentration; quartiles 25OHD concentration; quartiles |
11 (1994–2005) 11 (1194–2005) |
HR=0.95 (0.86–1.0) p NS HR=0.96(0.83–1.12) p NS |
Type 2 diabetes (self-report verified by A1C and hospital discharge diagnosis) Type 2 diabetes (self-report verified by A1C and hospital discharge diagnosis) |
Age, Sex, BMI, physical activity, month (stratified by smoking status) Age, Sex, BMI, physical activity, month (stratified by smoking status) |
| Bolland et al, 2010 Community dwelling women [Australia] | 0 | 74 (>55) | 100 | 15/1,471 | 25OHD concentration; <20 vs. ≥20 ng/mL | 5 (1998–2003) | 0.90 (0.4, 1.9) P=NS |
Type 2 diabetes (self-reported) | Age, weight, smoking, season, treatment allocation |
| Gagnon et al, 2011 [Australia] | 45 | 51 (xx) | 92 | 199/6,537 (3.8%); | 25OHD concentration; ≤19 vs. ≥32 ng/mL | 5 (1999, 2005) | 0.68 (0.43, 1.07) P=0.02 |
Type 2 diabetes (medication-treated, FPG or OGTT) | Age, gender, waist, exercise, race, |
| Robinson et al, 2011 WHI [US] | 0 | (50–79) | 317/5,140 (6.2%); nested case-control study | 25OHD concentration; < 20 vs. ≥30 ng/mL | 7.3 | 1.14 (0.68, 1.90) P=0.873 |
Diabetes (self-report, medication-treated) | Age, BMI, season, race, others | |
| Thorand et al, 2011 [Germany] | 53 | (35–74) | 100% | 416/1683 (25%) case-control | 25OHD concentration; 11 vs. 68 ng/mL (median) | 11 | 0.63 (0.44, 0.90) P=0.01 |
Type 2 diabetes (validated self-report) | Age, sex, survey, season, BMI, smoking, alcohol, physical activity, systolic blood pressure, total cholesterol/HDL cholesterol, parental history of DM. |
| Pittas et al, 2012 Diabetes Prevention Program [USA] | 33 | 51 | 57% | /2040 | 25OHD concentration; ≤13 vs. ≥30 ng/mL | 2.7 yr | 0.72 (0.56, 0.90) p=0.0054 |
Type 2 diabetes (OGTT) | Age, gender body mass index, race, family history of diabetes, personal history of hypertension at baseline, smoking status at baseline, alcohol consumption, C-reactive protein, kidney function, self-reported physical activity, calcium intake and treatment arm |
| Deleskog et al, 2012 [Denmark] | 100 | (35–56) | 100% | 145/1011 | 25OHD concentration; >28 vs. < 18 ng/mL (means) | 10 | 0.38 (0.21, 0.71) | Type 2 diabetes (OGTT) | Age, gender, BMI, exercise, season, BP, family history of diabetes |
| Forouhi et al, 2012 EPIC-Norfolk [Europe] | 52% | 58 | >90% | 621/826 nested case-control study | 25OHD concentration; >32 vs. <20 ng/mL (means) | 10 | 0.50 (0.32, 0.76) | Type 2 diabetes (validated self-report) | Age, gender, BMI, exercise, season, family history of diabetes, cholesterol, alcohol, smoking, education, supplement use |
| Afzal et al, 2013 Copenhagen City Heart Study [Europe] | 43% | 56 | 100% | 810/9841 | 25OHD concentration Quartiles value NR | 29 | 1.35 (1.09–1.66) | Type 2 diabetes (Self report, medication treated, non fasting glucose, registry based) | sex, age, smoking status (never/ever), BMI, income, and duration and intensity of leisure time physical activities. |
number of cases if nested case-control study
highest/lowest risk category versus reference category
estimated from reported data
25OHD, plasma or serum 25-hydroxyvitamin D; BMI, body mass index; BP, blood pressure; CVD, cardiovascular disease; HR, hazard ratio; IU, international units; ND, no data; OR, odds ratio; RR, relative risk
To convert 25OHD concentration from ng/mL to nmol/L multiply by 2.459
In one of the largest studies to date where vitamin D intake was the measure to assess vitamin D status, the Nurses’ Health Study, after multivariate adjustment for age, BMI, and non-dietary covariates, women who consumed more than 800 IU/day of vitamin D had a 23% lower risk for developing incident type 2 diabetes compared to women who consumed less than 200 IU/day (RR 0.77, 95%CI 0.63–0.94; p<0.01).[53] However, after adjusting for dietary factors, the association became non-significant. Similarly, in the Women’s Health Study, an intake of 511 IU/day or more of vitamin D was associated with 27% lower risk of developing type 2 diabetes compared with an intake of 159 IU/day or less. [52] The Women’s Health Study analysis is limited by the lack of adjustments for risk factors of type 2 diabetes other than age.
In a nested case-control study conducted in Finland, which included 2 cohorts, participants in the highest quartile of 25OHD (mean 25OHD 27.6 ng/mL) had a 40% lower risk of developing incident type 2 diabetes, compared to those in the lowest quartile (mean 25OHD 8.9 ng/mL), after multivariate adjustment. However, the lower risk was only observed in men. [54] On the other hand, in the Nurse’s Health Study, the odds ratio for incident type 2 diabetes in the highest (mean 25OHD, 33.4 ng/ml) compared to the lowest quartile (mean 25OHD, 14.4ng/ml) was 0.52 (95% confidence interval, 0.33, 0.83) after multivariate adjustment. The inconsistency between the Finnish and the US female population could be secondary to different baseline mean 25OHD (15 ng/ml versus 23 ng/ml respectively) suggesting that a threshold 25OHD concentration above which the risk of type 2 diabetes declines. In the Diabetes Prevention Program, which included a much larger number of participants at high risk for developing diabetes, those in the highest tertile of 25-hydroxyvitamin D (median concentration 30.1 ng/mL) had a hazard ratio of 0.72 (95%CI, 0.56 to 0.90) for developing diabetes compared to participants in the lowest tertile (median concentration 12.8 ng/mL) after multivariate adjustment. [64]
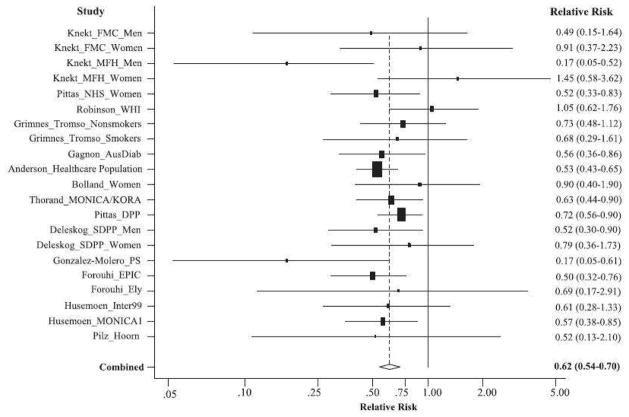
Recently, two meta-analyses of longitudinal observational studies have been reported with nearly identical results. Song et al included 21 studies and a total of 76,000 participants and calculated the risk of developing type 2 diabetes, according to baseline 25OHD level. There was a 38% lower risk of developing type 2 diabetes in the highest tertile of 25OHD compared to the lowest one (relative risk 0.62 [95% CI 0.54–0.70] (Figure 3), with little heterogeneity between studies.[68] The association was consistent regardless of diabetes diagnosis criteria, study size or follow-up duration and remained significant after adjustment for BMI and intermediate biomarkers. A linear trend analysis showed that a 4 ng/ml increment in 25OHD levels was associated with a 4% lower risk of type 2 diabetes (95% CI 3–6; P for linear trend, 0.0001). In another meta-analysis of 16 studies, Afzal et al estimated the odds ratio for type 2 diabetes to be 1.5 (95% CI, 1.33–1.70) for the bottom versus the top quartile of 25OHD concentration. [67]
Figure 3.
A random-effects meta-analysis of 21 independent prospective studies with adjusted RR and 95% CI of type 2 diabetes in relation to serum 25(OH)D levels (the highest category versus the lowest category).
Despite the consistency of these results, the observational nature of these studies precludes an assessment of cause and effect because residual confounding cannot be excluded.
The influence of vitamin D supplementation on type 2 diabetes
The effect of vitamin D supplementation on glycemia or incident type 2 diabetes has been reported in several trials with mixed results. (Table 4)
Table 4.
Randomized controlled trials of the effect of vitamin D (cholecalciferol [D3] or ergocalciferol [D2]) supplementation (with or without calcium) on glycemic measures
| Study, First author, Year [Country] | Men, % | Mean baseline age and/or range | BMI | Participants | Mean baseline 25(OH)D concentration, ng/mL calcium intake | Interventions (number of participants) | Study duration | Effect of vitamin D vs. placebo [p value] |
|---|---|---|---|---|---|---|---|---|
| Nilas and Christiansen, 1984 [Denmark] | 0 | 45–54 | ND | Postmenopausal healthy n=151 |
ND | D3, 2000 IU/d (n=25) vs. 1(OH)2D3 0.25 mcg/d (n=23) vs. placebo (n=103). All received calcium, 500 mg/d | 2 y | ↔FPG 2.16 vs. −5.94 vs. 2.34 mg/dL |
| Pittas et al., 2007 [US] | 38 | 71 (≥65) | 27 | Normal fasting glucose n=222 |
30 Calcium intake, 750 mg/d | D3, 700 IU/d plus calcium citrate, 500 mg/d (n=108) vs. placebo (n=114) | 3 y | ↔FPG change, 2.70 vs. 2.16 mg/dL [p=0.55] ↔ IRHOMA |
| 52 | 71 | Impaired fasting glucose n=92 |
30 Calcium intake, 680 mg/d | D3, 700 IU/d plus calcium citrate 500 mg/d (n=45) vs. placebo (n=47) | 3 y | ↓FPG change, 0.36 vs. 6.13 mg/dL [p=0.042] ↓ IRHOMA |
||
| De Boer et al., 2008 [US] | 0 | 62 (50–79) | Post-menopausal without diabetes | <32 (for 89% of participants) | D3, 400 IU/d plus calcium carbonate 1,000 mg/d (n=16,999) vs. placebo (n=16,952) | 7 y | ↔Incidence of Diabetes (self-reported), HR 1.01 (0.94 to 1.10) [p=0.95]; ↔Insulin secretion ↔ IRHOMA |
|
| 0 | 50–79 | Normal fasting glucose n=1,637 |
D3, 400 IU/d + calcium carbonate 1,000 mg/d (n=866) vs. placebo (n=771) | 6 y | ↔FPG change, 3.80 vs. 4.61mg/dL [p=0.32] | |||
| 0 | 50–79 | Impaired fasting glucose n=1,457 |
D3, 400 IU/d + calcium carbonate 1,000 mg/d (n=718) vs. placebo (n=739) | 6 y | ↔FPG change, 3.93 vs. 4.69 mg/dL [p=0.79] | |||
| Avenell et al., 2009 [UK] | 15 | 77 (≥70) | ND | History of fracture | ND | D3, 800 IU/d (n=2,649) vs. placebo (n=2,643) (2×2 factorial design with calcium carbonate 1,000 mg/d) |
2–5 y | ↔Incidence of Diabetes (self-reported), intention-to-treat HR 1.11 (0.77 to 1.62) [p=0.57] ↔Incidence of Diabetes (self-reported), compliant HR 0.68 (0.40 to 1.16) [p=0.16] |
| Zittermann et al., 2009 [Germany] | 33 | 48 (18–70) | 33 | Healthy; BMI>27 kg/m2 n=200 |
12 | D3, 3,332 IU/d (n=82) vs. placebo (n=83). All received weight reduction advice for 24 wk | 1 y | ↔Hemoglobin A1c change, −0.25% vs. −0.25% [p=0.96] ↔FPG change, −0.21 vs. − 0.27 mmol/L (−3.8 vs. −4.9 mg/dL) [p=0.39] |
| Von Hurst et al., 2010 [New Zealand] | 0 Indian (91%) |
42 (23–68) | 27.5 | Insulin resistance, no diabetes; 25(OH)D<20 ng/mL n=81 |
8 | D3, 4,000 IU/d (n=42) vs. placebo (n=39) | 26 wk | ↔FPG change, 0.1 vs. 0.1 mmol/L (1.8 vs. 1.8 mg/dL) [p=0.82]; ↓ IRHOMA (change from baseline) −0.2 vs. 0.2; p=0.02; (highest effect seen when final 25OHD>80 nmol/L) ↓ Insulin ↔ HOMA2%B; C-peptide |
| Jorde et al, 2010 [Norway] | 36 | 38 (21–70) | 35 | Overweight/Obe se; no diabetes n=438 |
23 | D3, 40,000 IU/wk (equivalent to 5,714 IU/d) (n=150) vs. D3, 20,000 IU/wk (equivalent to 2,857 IU/d) (n=139) vs. placebo (n=149). All received calcium 500 mg/d | 1 y | ↔Hemoglobin A1c change, 0.09% vs. 0.11% vs. 0.09% (0.4 vs. 1.4 vs. 1.4 mg/dL) [p=NS]; post-hoc ↔FPG change, 0.02 vs. 0.08 vs. 0.08 mmol/L [p=NS]; post-hoc ↔2hPG change, 0.15 vs. 0.36 vs. −0.02 mmol/L (2.7 vs. 6.5 vs. −0.4 mg/d) [p=NS]; post-hoc ↔ IRHOMA 0.23 vs −0.05 vs 0.36, p=NS ↔ QUICKI |
| Wood et al, 2012 [UK] | 0 | 64 | 26 | Healthy (NGT) post-menopausal women; n=305 | 13 | D3 1,000 IU/d orally (n=101) vs. D3 400 IU/d orally (n=102) vs. placebo (n=102) | 48 w | ↔FPG change, −1.1 vs. 0.9 vs. −2.3 mg/dL [p=0.23) ↔HOMA-IR change |
| Nagpal et al, 2009 [India] | 100 | 43 (>35) | 26 | Healthy; Central obesity; N=71 | 15 | D3 120,000 IU three times (equivalent to 8,571 IU/d) [N=35] vs. Placebo [N=36] | 6 w | ↑OGIS (change from baseline, ml/min*kg) 21.17 vs. −8.89 p=0.055 ↔HOMA ( change from baseline) 0.14 vs. 0.16 p=0.95 ↔QUICKI 0 vs 0 p= 0.9 |
| Mitri et al, 2011 [US] | 53 | 57 | 32 | Pre-diabetes; n=92 | 24 | D3 2000 IU/d vs. placebo-vitamin D (n=46); 2×2 factorial design with calcium 800 mg/d vs. placebo-calcium (n=46) | 16 w | ↔ A1c change, 0.06 vs. 0.14% [p=0.08] ↔ FPG change, 2.4 vs. 5.6 mg/dL [p=0.172) ↔ 2hPG change, −7.2 vs. 1.2 mg/dL [p=0.22] ↑ DI change 300 vs −126 in D3 vs non D3, [p=0.011] ↑ SI change −0.3 vs −0.9 in D3 vs non D3, [p=0.16] ↑ AIR change, 34 vs −53 in D3 vs non D3 [p=0.074] |
| Nazarian et al, 2011 [US] | 38 | 34–62 | 23–45 | Pre-diabetes (FPG 100–125 mg/dL); 25OHD≤30 ng/mL (n=8) | 20 | D3 10,000 IU/d for 4 weeks (n=8) | 4 w | No glycemic data ↔ DI (% from baseline) 21 [p=NS] ↑ SI (% from baseline) 42 [P=0.012] ↓ AIRg (% from baseline) 20 [P=0.011] |
| Davidson et al, 2012 [US] | 32 | 52 (ND) | 32 | Pre-diabetes (FPG 110–125 mg/dL; 2hPG 140–199 mg/dL); 25OHD<30 ng/mL; Latino and African-American; n=117 | 22 | D3, orally weekly titrated to achieve 25OHD 65–90 ng/mL (mean dose required equivalent to 12,700 IU/d) (n=56) vs. Placebo (n=53) | 12 m | ↓ Hemoglobin A1c change, −0.1 vs. 0.1% [p=0.004] ↔FPG change, 1 vs. 4 mg/dL [p=0.27) ↔ 2hPG change, −11 vs. −9 mg/dL [p=0.64] ↔ DI1 change, 0.2 vs. 0 [p=0.39] ↔ DI2 change, −0.1 vs. 0 [p=0.32] ↔ Proportion with diabetes [p=NA] |
| Harris et al, 2012 [US] | 35 | 56 (NR) | 32 | Pre-diabetes/early diabetes (FPG>100 mg/dL; A1c 5.8–6.9%); Overweight; African-American; n=89 | D3, 4,000 IU/d orally (n=43) vs. Placebo (n=46) | 12 w | ↔Hemoglobin A1c change, −0.05 vs. 0.05% [p=0.97] ↔FPG change, −0.18 vs. − 0.54 mg/dL [p=0.81) ↔ 2hPG change, −7.2 vs. −6.5 mg/dL [p=0.98] ↓ Insulin sensitivity change (%),−4 vs. 12 [p=0.004] ↑ Insulin secretion change (%), 12 vs. 2 mg/dL [p=0.27) ↔ DI change, −11 vs. −9 mg/dL [p=0.64] |
|
| Witham et al, 2010 [United Kingdom] | ND | 65 (>18) | 31 | Type 2 diabetes; 25OHD<40 ng/mL n=61 |
18 | D3, 100,000 IU orally once (equivalent to 892 IU/d) (n=19) vs. D3, 200,000 IU orally once (equivalent to 1,785 IU/d) (n=20) vs. placebo (n=22) | 16 wk | ↔Hemoglobin A1c change, “no change” (data NR) ↔FPG change, “no change” (data NR) ↔IRHOMA change, “no change” (data NR) |
| Sugden et al, 2008 [UK] | 53 | 64 | 31 | Stable type 2 diabetes; 25(OH)D<20 ng/mL n=34 |
15 | D2, 100,000 IU once (equivalent to 1,785 IU/d) (n=17) vs. placebo (n=17) | 8 wk | ↔Hemoglobin A1c change, 0.01% vs. −0.05% [p=0.74] ↔IRHOMA change, −39.7 vs −25.6 [p=0.72] IRHOMA significantly improved if 25OHD rise >11 nmol/l |
| Jorde and Figenschau 2009 [Norway] | 50 | 56 (21–75) | Stable type 2 diabetes n=32 |
24 | D3, 40,000 IU/wk (equivalent to 5,714 IU/d) (n=16) vs. placebo (n=16) | 26 wk | ↔Hemoglobin A1c change, 0.2% vs. −0.2% [p=0.90] ↔FPG change, −0.2 vs. 0.4 mmol/L (−3.6 vs. 7.2 mg/dL) [p=0.43] ↔ IRHOMA 0.3 vs. −0.2, p=0.58 |
|
| Nikooyeh et al, 2011 [Iran] | 40 | 51 | 29 | Type 2 diabetes (FPG≥126 mg/dL) | 12 | D3 1000 IU/d in yogurt drink with 250 mg of calcium (n=30) vs. D3 1000 IU/d in yogurt drink with 500 mg of calcium (n=30) vs. placebo (plain yogurt drink; n=30) | 12 w | ↓ A1c change, −0.5 vs. 1.2% [p<0.01] ↓ FPG change, −9 vs. 16 mg/dL [p=0.01) |
| Soric et al, 2012 [Ohio, US] | 45 | 54 (21–75) | ND | Type 2 diabetes (A1c >7%); n=37 | ND | D3, 2,000 IU/d orally (n=19) vs. Vitamin C, 500 mg/d orally (n=18) | 12 w | ↔Hemoglobin A1c change, −0.4 vs. 0.1% [p=0.16] ↓ Hemoglobin A1c change, −1.4% vs. 0.2% [p=0.013] when baseline A1c>9% |
| Punthakee et al, 2012 [33 countries] | 60 | 66 | Type 2 diabetes (A1c 7.4%) [TIDE study) N=1,221 |
ND | D3, 1,000 IU/d orally (n=607) vs. placebo (n=614) | 40 m | ↔Hemoglobin A1c “NR ↔FPG change NR cancer; all-cause death, 0.3% vs. 0.5% |
|
| Heshmat, et al., 2012 [Iran] | 36 | 56.2(37–79) | 27.7 | Type 2 diabetes on diet or oral agents (A1c <7.5%) [TIDE study) N=42 |
46.9 | D3, 300,000 IU/d orally x1 dose (n=21) vs. placebo (n=21) | 3 m | ↔Hemoglobin A1c −0.05 % vs −0.2% p=0.495 ↑ FPG 16.2 vs −9.7 (p=0.007) ↑ HOMA 0.2 vs −0.9 p=0.017 |
25(OH)D, plasma or serum 25-hydroxyvitamin D; 2hPG, plasma glucose 2 hours after 75 gram glucose load; D3, cholecalciferol; D2, ergocalciferol; FPG, fasting plasma glucose; HR, hazard ratio; ND, no data;
GLUAUC, glucose area-under-the-curve after 75 gram glucose load; INSAUC, insulin area-under-the-curve after 75 gram glucose load; INS120, Insulin value at 120′ after glucose load is given; IR, insulin resistance; IRFI, Insulin resistance by fasting insulin; IRHOMA, Insulin resistance by homeostasis model assessment-; IRM, Insulin resistance after euglycemic hyperinsulinemic clamp; IRIVGTT, insulin resistance after intravenous glucose tolerance test;
↓ decreased (statistically significant), ↑ increased (statistically significant), ↔ no difference (no statistical significance);
To convert 25(OH)D concentration from ng/mL to nmol/L multiply by 2.459; to convert FPG form mg/dL to nmol/L, multiply by 0.0555
In trials that included participants with normal glucose tolerance at baseline, vitamin D supplementation had a neutral effect on measures of glycemia including fasting plasma glucose or hemoglobin A1c and insulin resistance measured by HOMA. [69–77] Similarly, vitamin D supplementation had no effect on incident type 2 diabetes in individuals with normal glucose tolerance at baseline. [71, 72] The major limitation in interpreting these results is that most were designed for non-glycemic outcomes and the analyses on vitamin D and type 2 diabetes were post-hoc. [69–72, 75] In addition, all trials with the exception of the Women’s Health Initiative trial [71] and the RECORD trial [72] were underpowered for glycemic outcomes. It is also important to note that adherence to the intervention would have played a major role in interpreting the results. For example, in a post-hoc analysis of the RECORD study, a community-based effectiveness trial designed for bone outcomes, [72] supplementation with 800 IU/day of vitamin D3 (given in a 2×2 factorial design with calcium carbonate) did not change the risk of self-reported type 2 diabetes; however, among study participants who were highly compliant with supplementation, there was a notable trend towards reduction in type 2 diabetes risk with vitamin D3 (OR 0.68; 95%CI 0.40–1.16).
The potential effect of vitamin D supplementation appears to be more prominent among persons who are at high risk for diabetes (e.g., pre-diabetes). In a post-hoc subgroup analysis conducted using data from a completed trial designed for fractures, combined vitamin D3 (700 IU/day) and calcium carbonate (500 mg/day) supplementation prevented the rise in insulin resistance (HOMA-IR) and fasting plasma glucose (FPG) in people with impaired fasting glucose, but not in individuals with normal fasting glucose at baseline,[70] suggesting that vitamin D may benefit only individuals at high risk for diabetes. In this study, the reduction in FPG over 3-years was similar to the reduction in FPG achieved with metformin or lifestyle, in the Diabetes Prevention Program, which was associated with a 31–58% decrease in incident diabetes.[78] In the Calcium and Vitamin D for type 2 Diabetes Mellitus (CaDDM) study, vitamin D supplementation (4,000 IU/day) in adults at risk for type 2 diabetes improved beta cell function and had a nearly statistically significant effect on the rise in A1c values. [79] Similarly, In another intervention study, where vitamin D was given without a placebo, insulin sensitivity improved after 4 weeks of vitamin D administration in persons with pre-diabetes.[80] In contrary, Davidson et al. found no effect of high dose vitamin D supplementation on insulin secretion, insulin sensitivity or incident diabetes in a population with impaired fasting glycemia or impaired glucose tolerance and low vitamin D levels. [81] In this study, the average daily dose of vitamin D supplementation was close to 12700 IU and the population was limited to non-Caucasians. According to the IOM, chronic administration of vitamin D in excess of 4,000 IU per day may not be beneficial. Therefore, the supra-physiologic dose of vitamin D supplemented in the study by Davidson et al and the difference in ethnicity could explain the discrepancy with other studies in persons with pre-diabetes.
In most trials that included participants with established type 2 diabetes, vitamin D supplementation had no effect on glycemic outcome measures after a follow-up period of 8–26 weeks.[82–89] However, these studies were underpowered and the effect of concurrent diabetes pharmacotherapy on the outcome measured was not reported.
Vitamin D and type 1 diabetes
Type 1 diabetes is characterized by autoimmune destruction of pancreatic islet beta cells, leading to absolute insulin deficiency. Many effects of vitamin D on the pathophysiology of type 1 diabetes have been described, including changes in the immune-mediated destruction, [90] but also the beta-cell itself. The latter effect may, at least in part, be mediated indirectly by the effect of vitamin D on calcium homeostasis. It has also been reported that specific vitamin D receptor polymorphisms interact with the HLADRB1 allele, which predisposes to type 1 diabetes.[91]. Evidence from animal studies in non-obese diabetic mice (NOD), which undergo destruction of pancreatic beta cells that mimics the pathogenesis of type 1 diabetes in humans, suggests that vitamin D deficiency is associated with development of diabetes while administration of 1,25-dihydroxyvitamin D to these mice prevented the development of diabetes. [92]
In humans, the prevalence of type 1 diabetes has been inversely correlated with ultra violet B radiation and altitude, suggesting that low vitamin D synthesis may be important in the pathogenesis of type 1 diabetes. Lack of vitamin D supplementation in infancy has been associated with increased risk of type 1 diabetes later in life. In the Finnish birth cohort study, children who regularly took the recommended dose of 2,000 IU/day of vitamin D had lower risk of developing diabetes compared with those who regularly received less than the recommended amount.[93] A meta-analysis based on five observational studies concluded that vitamin D supplementation in early childhood is associated with decreased diabetes risk.[94] Recently, Sorensen et al reported that lower maternal serum concentration of 25OHD during pregnancy was associated with an increased risk of childhood-onset type 1 diabetes, suggesting that in utero exposure to vitamin D may also be important. There are limited data from intervention studies with vitamin D in patients with type 1 diabetes. In patients with new onset type 1 diabetes, Gabbat et al reported that supplementation with 2000 IU per day of cholecalciferol over 18 months resulted in a favorable immunologic effect and a slower decline of residual beta-cell function but without any change in glycemia. [95] Two earlier studies of calcitriol supplementation in type 1 diabetes did not show a positive effect on beta cell residual function. [96, 97]
Although the data from animal and epidemiological studies seem promising, large trials evaluating the efficacy and safety of vitamin D supplementation in prevention or treatment of type 1 diabetes are lacking.
Summary
Findings from basic science suggest that vitamin D may play a significant role in both types of diabetes. In human studies, the evidence for a potential association is stronger for vitamin D and type 2 diabetes with much less data on type 1 diabetes. However, the evidence about type 2 diabetes in humans comes almost exclusively from observational studies, which may be confounded by a variety of factors and, therefore, these studies preclude an assessment of cause and effect. There are no published trials specifically designed to test the safety and efficacy of long-term vitamin D administration to reduce the risk of developing type 2 diabetes; therefore, firm conclusions cannot be drawn regarding the role of vitamin D for prevention or treatment of diabetes. On numerous occasions, encouraging findings from observational studies were not confirmed by well-designed clinical trials (e.g. hormone replacement therapy, vitamin E and other supplements) [98, 99] and prevailing clinical practice was overturned. Therefore, evidence from randomized controlled trials is needed to address the issue of causality and to rigorously assess the protective effect of vitamin D on type 2 diabetes.
There are several ongoing randomized trials to test the hypothesis that vitamin D supplementation lowers type 2 diabetes risk. The vitamin D and omega-3 trial (VITAL study, www.vitalstudy.org) is a large community-based 2×2 factorial trial that is testing the effectiveness of 2,000 IU/day of vitamin D3 vs. less than 800 IU/day (the other factor is omega-3 fatty acids vs. placebo) in primary prevention of cancer, cardiovascular disease and stroke. An ancillary study to VITAL will evaluate the effect of vitamin D supplementation on diabetes incidence, based on self-reported data among those with normal glucose tolerance at baseline. The vitamin D and type 2 diabetes study (D2d, www.d2dstudy.org) is a large multi-center clinical trial conducted in twenty cities around the United States, specifically designed to test whether vitamin D supplementation reduces risk of incident diabetes in patients with pre-diabetes. The D2d study will enroll approximately 2,400 participants will be followed for up to 4 years for development of diabetes.
If the results of these larger trials, and other ongoing studies, confirm a favorable benefit/harm ratio of vitamin D supplementation, vitamin D would likely be integrated into contemporary strategies for the prevention of type 2 diabetes in the more than 79 million Americans at risk of developing diabetes and to treatment in the more than 10 million Americans with established diabetes. Until then, vitamin D is a promising, yet unproven dietary intervention for type 2 diabetes.
Key points.
Observational studies suggest a link between vitamin D and diabetes
The potential effect of vitamin D appears to be more prominent among persons at risk for diabetes.
The optimal blood 25-hydroxyvitamin D concentration associated with reduced risk of type 2 diabetes is not clear.
The evidence from randomized controlled trials to support the hypothesis that vitamin D supplementation prevents type 2 diabetes is lacking.
Acknowledgments
Source of funding: By research grants R01DK76092, U34DK091958 and U01DK098245 (to AGP) from the National Institute of Diabetes and Digestive and Kidney Disease, the Office Of The Director - National Institutes of Health, and the National Institutes of Health Office of Dietary Supplements
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine Dietary Reference Intakes for Calcium and Vitamin D. The National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- 3.Rosen CJ, Abrams SA, Aloia JF, et al. IOM committee members respond to endocrine society vitamin D guideline. J Clin Endocrinol Metab. 2012;97:1146–1152. doi: 10.1210/jc.2011-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norman AW, Frankel JB, Heldt AM, et al. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980;209:823–825. doi: 10.1126/science.6250216. [DOI] [PubMed] [Google Scholar]
- 5.Chertow BS, Sivitz WI, Baranetsky NG, et al. Cellular mechanisms of insulin release: the effects of vitamin D deficiency and repletion on rat insulin secretion. Endocrinology. 1983;113:1511–1518. doi: 10.1210/endo-113-4-1511. [DOI] [PubMed] [Google Scholar]
- 6.Kadowaki S, Norman AW. Dietary vitamin D is essential for normal insulin secretion from the perfused rat pancreas. J Clin Invest. 1984;73:759–766. doi: 10.1172/JCI111269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka Y, Seino Y, Ishida M, et al. Effect of vitamin D3 on the pancreatic secretion of insulin and somatostatin. Acta Endocrinol (Copenh) 1984;105:528–533. doi: 10.1530/acta.0.1050528. [DOI] [PubMed] [Google Scholar]
- 8.Cade C, Norman AW. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology. 1986;119:84–90. doi: 10.1210/endo-119-1-84. [DOI] [PubMed] [Google Scholar]
- 9.Bourlon PM, Faure-Dussert A, Billaudel B. The de novo synthesis of numerous proteins is decreased during vitamin D3 deficiency and is gradually restored by 1, 25-dihydroxyvitamin D3 repletion in the islets of langerhans of rats. J Endocrinol. 1999;162:101–109. doi: 10.1677/joe.0.1620101. [DOI] [PubMed] [Google Scholar]
- 10.Cade C, Norman AW. Rapid normalization/stimulation by 1,25-dihydroxyvitamin D3 of insulin secretion and glucose tolerance in the vitamin D-deficient rat. Endocrinology. 1987;120:1490–1497. doi: 10.1210/endo-120-4-1490. [DOI] [PubMed] [Google Scholar]
- 11.Clark SA, Stumpf WE, Sar M. Effect of 1,25 dihydroxyvitamin D3 on insulin secretion. Diabetes. 1981;30:382–386. doi: 10.2337/diab.30.5.382. [DOI] [PubMed] [Google Scholar]
- 12.Johnson JA, Grande JP, Roche PC, et al. Immunohistochemical localization of the 1,25(OH)2D3 receptor and calbindin D28k in human and rat pancreas. Am J Physiol. 1994;267:E356–360. doi: 10.1152/ajpendo.1994.267.3.E356. [DOI] [PubMed] [Google Scholar]
- 13.Zeitz U, Weber K, Soegiarto DW, et al. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. Faseb J. 2003;17:509–511. doi: 10.1096/fj.02-0424fje. [DOI] [PubMed] [Google Scholar]
- 14.Bland R, Markovic D, Hills CE, et al. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in pancreatic islets. J Steroid Biochem Mol Biol. 2004;89–90:121–125. doi: 10.1016/j.jsbmb.2004.03.115. [DOI] [PubMed] [Google Scholar]
- 15.Sergeev IN, Rhoten WB. 1,25-Dihydroxyvitamin D3 evokes oscillations of intracellular calcium in a pancreatic beta-cell line. Endocrinology. 1995;136:2852–2861. doi: 10.1210/endo.136.7.7789310. [DOI] [PubMed] [Google Scholar]
- 16.Milner RD, Hales CN. The role of calcium and magnesium in insulin secretion from rabbit pancreas studied in vitro. Diabetologia. 1967;3:47–49. doi: 10.1007/BF01269910. [DOI] [PubMed] [Google Scholar]
- 17.Yasuda K, Hurukawa Y, Okuyama M, et al. Glucose tolerance and insulin secretion in patients with parathyroid disorders. Effect of serum calcium on insulin release. N Engl J Med. 1975;292:501–504. doi: 10.1056/NEJM197503062921003. [DOI] [PubMed] [Google Scholar]
- 18.Gedik O, Zileli MS. Effects of hypocalcemia and theophylline on glucose tolerance and insulin release in human beings. Diabetes. 1977;26:813–819. doi: 10.2337/diab.26.9.813. [DOI] [PubMed] [Google Scholar]
- 19.Fujita T, Sakagami Y, Tomita T, et al. Insulin secretion after oral calcium load. Endocrinol Jpn. 1978;25:645–648. doi: 10.1507/endocrj1954.25.645. [DOI] [PubMed] [Google Scholar]
- 20.Kadowaki S, Norman AW. Pancreatic vitamin D-dependent calcium binding protein: biochemical properties and response to vitamin D. Arch Biochem Biophys. 1984;233:228–236. doi: 10.1016/0003-9861(84)90621-0. [DOI] [PubMed] [Google Scholar]
- 21.Sooy K, Schermerhorn T, Noda M, et al. Calbindin-D(28k) controls [Ca(2+)](i) and insulin release. Evidence obtained from calbindin-d(28k) knockout mice and beta cell lines. J Biol Chem. 1999;274:34343–34349. doi: 10.1074/jbc.274.48.34343. [DOI] [PubMed] [Google Scholar]
- 22.Leal MA, Aller P, Mas A, et al. The effect of 1,25-dihydroxyvitamin D3 on insulin binding, insulin receptor mRNA levels, and isotype RNA pattern in U-937 human promonocytic cells. Exp Cell Res. 1995;217:189–194. doi: 10.1006/excr.1995.1078. [DOI] [PubMed] [Google Scholar]
- 23.Maestro B, Campion J, Davila N, et al. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr J. 2000;47:383–391. doi: 10.1507/endocrj.47.383. [DOI] [PubMed] [Google Scholar]
- 24.Maestro B, Molero S, Bajo S, et al. Transcriptional activation of the human insulin receptor gene by 1,25-dihydroxyvitamin D(3) Cell Biochem Funct. 2002;20:227–232. doi: 10.1002/cbf.951. [DOI] [PubMed] [Google Scholar]
- 25.Maestro B, Davila N, Carranza MC, et al. Identification of a Vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol. 2003;84:223–230. doi: 10.1016/s0960-0760(03)00032-3. [DOI] [PubMed] [Google Scholar]
- 26.Dunlop TW, Vaisanen S, Frank C, et al. The human peroxisome proliferator-activated receptor delta gene is a primary target of 1alpha,25-dihydroxyvitamin D3 and its nuclear receptor. J Mol Biol. 2005;349:248–260. doi: 10.1016/j.jmb.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 27.Ojuka EO. Role of calcium and AMP kinase in the regulation of mitochondrial biogenesis and GLUT4 levels in muscle. Proc Nutr Soc. 2004;63:275–278. doi: 10.1079/PNS2004339. [DOI] [PubMed] [Google Scholar]
- 28.Wright DC, Hucker KA, Holloszy JO, et al. Ca2+ and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes. 2004;53:330–335. doi: 10.2337/diabetes.53.2.330. [DOI] [PubMed] [Google Scholar]
- 29.Draznin B, Sussman K, Kao M, et al. The existence of an optimal range of cytosolic free calcium for insulin-stimulated glucose transport in rat adipocytes. J Biol Chem. 1987;262:14385–14388. [PubMed] [Google Scholar]
- 30.Byyny RL, LoVerde M, Lloyd S, et al. Cytosolic calcium and insulin resistance in elderly patients with essential hypertension. Am J Hypertens. 1992;5:459–464. doi: 10.1093/ajh/5.7.459. [DOI] [PubMed] [Google Scholar]
- 31.Draznin B, Lewis D, Houlder N, et al. Mechanism of insulin resistance induced by sustained levels of cytosolic free calcium in rat adipocytes. Endocrinology. 1989;125:2341–2349. doi: 10.1210/endo-125-5-2341. [DOI] [PubMed] [Google Scholar]
- 32.Draznin B, Sussman KE, Eckel RH, et al. Possible role of cytosolic free calcium concentrations in mediating insulin resistance of obesity and hyperinsulinemia. J Clin Invest. 1988;82:1848–1852. doi: 10.1172/JCI113801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Draznin B, Sussman KE, Kao M, et al. Relationship between cytosolic free calcium concentration and 2-deoxyglucose uptake in adipocytes isolated from 2- and 12-month-old rats. Endocrinology. 1988;122:2578–2583. doi: 10.1210/endo-122-6-2578. [DOI] [PubMed] [Google Scholar]
- 34.Ohno Y, Suzuki H, Yamakawa H, et al. Impaired insulin sensitivity in young, lean normotensive offspring of essential hypertensives: possible role of disturbed calcium metabolism. J Hypertens. 1993;11:421–426. doi: 10.1097/00004872-199304000-00013. [DOI] [PubMed] [Google Scholar]
- 35.Segal S, Lloyd S, Sherman N, et al. Postprandial changes in cytosolic free calcium and glucose uptake in adipocytes in obesity and non-insulin-dependent diabetes mellitus. Horm Res. 1990;34:39–44. doi: 10.1159/000181793. [DOI] [PubMed] [Google Scholar]
- 36.Zemel MB. Nutritional and endocrine modulation of intracellular calcium: implications in obesity, insulin resistance and hypertension. Mol Cell Biochem. 1998;188:129–136. [PubMed] [Google Scholar]
- 37.Williams PF, Caterson ID, Cooney GJ, et al. High affinity insulin binding and insulin receptor-effector coupling: modulation by Ca2+ Cell Calcium. 1990;11:547–556. doi: 10.1016/0143-4160(90)90031-o. [DOI] [PubMed] [Google Scholar]
- 38.Reusch JE, Begum N, Sussman KE, et al. Regulation of GLUT-4 phosphorylation by intracellular calcium in adipocytes. Endocrinology. 1991;129:3269–3273. doi: 10.1210/endo-129-6-3269. [DOI] [PubMed] [Google Scholar]
- 39.Chiu KC, Chuang LM, Lee NP, et al. Insulin sensitivity is inversely correlated with plasma intact parathyroid hormone level. Metabolism. 2000;49:1501–1505. doi: 10.1053/meta.2000.17708. [DOI] [PubMed] [Google Scholar]
- 40.Reis JP, von Muhlen D, Kritz-Silverstein D, et al. Vitamin D, parathyroid hormone levels, and the prevalence of metabolic syndrome in community-dwelling older adults. Diabetes Care. 2007;30:1549–1555. doi: 10.2337/dc06-2438. [DOI] [PubMed] [Google Scholar]
- 41.Gilsanz V, Kremer A, Mo AO, et al. Vitamin D status and its relation to muscle mass and muscle fat in young women. J Clin Endocrinol Metab. 2010;95:1595–1601. doi: 10.1210/jc.2009-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riachy R, Vandewalle B, Kerr Conte J, et al. 1,25-dihydroxyvitamin D3 protects RINm5F and human islet cells against cytokine-induced apoptosis: implication of the antiapoptotic protein A20. Endocrinology. 2002;143:4809–4819. doi: 10.1210/en.2002-220449. [DOI] [PubMed] [Google Scholar]
- 43.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Giulietti A, van Etten E, Overbergh L, et al. Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-Dihydroxyvitamin D(3) works as anti-inflammatory. Diabetes Res Clin Pract. 2007;77:47–57. doi: 10.1016/j.diabres.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Gysemans CA, Cardozo AK, Callewaert H, et al. 1,25-Dihydroxyvitamin D3 modulates expression of chemokines and cytokines in pancreatic islets: implications for prevention of diabetes in nonobese diabetic mice. Endocrinology. 2005;146:1956–1964. doi: 10.1210/en.2004-1322. [DOI] [PubMed] [Google Scholar]
- 46.Cohen-Lahav M, Douvdevani A, Chaimovitz C, et al. The anti-inflammatory activity of 1,25-dihydroxyvitamin D3 in macrophages. J Steroid Biochem Mol Biol. 2007;103:558–562. doi: 10.1016/j.jsbmb.2006.12.093. [DOI] [PubMed] [Google Scholar]
- 47.Riachy R, Vandewalle B, Moerman E, et al. 1,25-Dihydroxyvitamin D3 protects human pancreatic islets against cytokine-induced apoptosis via down-regulation of the Fas receptor. Apoptosis. 2006;11:151–159. doi: 10.1007/s10495-006-3558-z. [DOI] [PubMed] [Google Scholar]
- 48.Oh J, Weng S, Felton SK, et al. 1,25(OH)2 vitamin d inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009;120:687–698. doi: 10.1161/CIRCULATIONAHA.109.856070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 50.Hypponen E, Boucher BJ, Berry DJ, et al. 25-hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: a cross-sectional study in the 1958 British Birth Cohort. Diabetes. 2008;57:298–305. doi: 10.2337/db07-1122. [DOI] [PubMed] [Google Scholar]
- 51.Lu L, Yu Z, Pan A, et al. Plasma 25-hydroxyvitamin D concentration and metabolic syndrome among middle-aged and elderly Chinese individuals. Diabetes Care. 2009;32:1278–1283. doi: 10.2337/dc09-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu S, Song Y, Ford ES, et al. Dietary calcium, vitamin D, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care. 2005;28:2926–2932. doi: 10.2337/diacare.28.12.2926. [DOI] [PubMed] [Google Scholar]
- 53.Pittas AG, Dawson-Hughes B, Li T, et al. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care. 2006;29:650–656. doi: 10.2337/diacare.29.03.06.dc05-1961. [DOI] [PubMed] [Google Scholar]
- 54.Knekt P, Laaksonen M, Mattila C, et al. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology. 2008;19:666–671. doi: 10.1097/EDE.0b013e318176b8ad. [DOI] [PubMed] [Google Scholar]
- 55.Kirii K, Mizoue T, Iso H, et al. Calcium, vitamin D and dairy intake in relation to type 2 diabetes risk in a Japanese cohort. Diabetologia. 2009;52:2542–2550. doi: 10.1007/s00125-009-1554-x. [DOI] [PubMed] [Google Scholar]
- 56.Liu E, Meigs JB, Pittas AG, et al. Predicted 25-hydroxyvitamin D score and incident type 2 diabetes in the Framingham Offspring Study. Am J Clin Nutr. 2010;91:1627–1633. doi: 10.3945/ajcn.2009.28441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pittas AG, Sun Q, Manson JE, et al. Plasma 25-hydroxyvitamin D concentration and risk of incident type 2 diabetes in women. Diabetes Care. 2010;33:2021–2023. doi: 10.2337/dc10-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anderson JL, May HT, Horne BD, et al. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. 2010;106:963–968. doi: 10.1016/j.amjcard.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 59.Grimnes G, Emaus N, Joakimsen RM, et al. Baseline serum 25-hydroxyvitamin D concentrations in the Tromso Study 1994–95 and risk of developing type 2 diabetes mellitus during 11 years of follow-up. Diabet Med. 2010;27:1107–1115. doi: 10.1111/j.1464-5491.2010.03092.x. [DOI] [PubMed] [Google Scholar]
- 60.Bolland MJ, Bacon CJ, Horne AM, et al. Vitamin D insufficiency and health outcomes over 5 y in older women. Am J Clin Nutr. 2010;91:82–89. doi: 10.3945/ajcn.2009.28424. [DOI] [PubMed] [Google Scholar]
- 61.Gagnon C, Lu ZX, Magliano DJ, et al. Serum 25-hydroxyvitamin D, calcium intake, and risk of type 2 diabetes after 5 years: results from a national, population-based prospective study (the Australian Diabetes, Obesity and Lifestyle study) Diabetes Care. 2011;34:1133–1138. doi: 10.2337/dc10-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robinson JG, Manson JE, Larson J, et al. Lack of association between 25(OH)D levels and incident type 2 diabetes in older women. Diabetes Care. 2011;34:628–634. doi: 10.2337/dc10-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thorand B, Zierer A, Huth C, et al. Effect of serum 25-hydroxyvitamin D on risk for type 2 diabetes may be partially mediated by subclinical inflammation: results from the MONICA/KORA Augsburg study. Diabetes Care. 2011;34:2320–2322. doi: 10.2337/dc11-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pittas AG, Nelson J, Mitri J, et al. Plasma 25-hydroxyvitamin D and progression to diabetes in patients at risk for diabetes: an ancillary analysis in the Diabetes Prevention Program. Diabetes Care. 2012;35:565–573. doi: 10.2337/dc11-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deleskog A, Hilding A, Brismar K, et al. Low serum 25-hydroxyvitamin D level predicts progression to type 2 diabetes in individuals with prediabetes but not with normal glucose tolerance. Diabetologia. 2012;55:1668–1678. doi: 10.1007/s00125-012-2529-x. [DOI] [PubMed] [Google Scholar]
- 66.Forouhi NG, Ye Z, Rickard AP, et al. Circulating 25-hydroxyvitamin D concentration and the risk of type 2 diabetes: results from the European Prospective Investigation into Cancer (EPIC)-Norfolk cohort and updated meta-analysis of prospective studies. Diabetologia. 2012 doi: 10.1007/s00125-012-2544-y. [DOI] [PubMed] [Google Scholar]
- 67.Afzal S, Bojesen SE, Nordestgaard BG. Low 25-hydroxyvitamin D and risk of type 2 diabetes: a prospective cohort study and metaanalysis. Clin Chem. 2013;59:381–391. doi: 10.1373/clinchem.2012.193003. [DOI] [PubMed] [Google Scholar]
- 68.Song Y, Wang L, Pittas AG, et al. Blood 25-Hydroxy Vitamin D Levels and Incident Type 2 Diabetes: A meta-analysis of prospective studies. Diabetes Care. 2013;36:1422–1428. doi: 10.2337/dc12-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nilas L, Christiansen C. Treatment with vitamin D or its analogues does not change body weight or blood glucose level in postmenopausal women. Int J Obes. 1984;8:407–411. [PubMed] [Google Scholar]
- 70.Pittas AG, Harris SS, Stark PC, et al. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30:980–986. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 71.de Boer IH, Tinker LF, Connelly S, et al. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women’s Health Initiative. Diabetes Care. 2008;31:701–707. doi: 10.2337/dc07-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Avenell A, Cook JA, MacLennan GS, et al. Vitamin D supplementation and type 2 diabetes: a substudy of a randomised placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438) Age Ageing. 2009;38:606–609. doi: 10.1093/ageing/afp109. [DOI] [PubMed] [Google Scholar]
- 73.Zittermann A, Frisch S, Berthold HK, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr. 2009;89:1321–1327. doi: 10.3945/ajcn.2008.27004. [DOI] [PubMed] [Google Scholar]
- 74.von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient - a randomised, placebo-controlled trial. Br J Nutr. 2010;103:549–555. doi: 10.1017/S0007114509992017. [DOI] [PubMed] [Google Scholar]
- 75.Jorde R, Sneve M, Torjesen P, et al. No improvement in cardiovascular risk factors in overweight and obese subjects after supplementation with vitamin D3 for 1 year. J Intern Med. 2010;267:462–472. doi: 10.1111/j.1365-2796.2009.02181.x. [DOI] [PubMed] [Google Scholar]
- 76.Wood AD, Secombes KR, Thies F, et al. Vitamin D3 Supplementation Has No Effect on Conventional Cardiovascular Risk Factors: A Parallel-Group, Double-Blind, Placebo-Controlled RCT. J Clin Endocrinol Metab. 2012;97:3557–3568. doi: 10.1210/jc.2012-2126. [DOI] [PubMed] [Google Scholar]
- 77.Nagpal J, Pande JN, Bhartia A. A double-blind, randomized, placebo-controlled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle-aged, centrally obese men. Diabet Med. 2009;26:19–27. doi: 10.1111/j.1464-5491.2008.02636.x. [DOI] [PubMed] [Google Scholar]
- 78.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mitri J, Dawson-Hughes B, Hu FB, et al. Effects of vitamin D and calcium supplementation on pancreatic beta cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: the Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am J Clin Nutr. 2011;94:486–494. doi: 10.3945/ajcn.111.011684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nazarian S, St Peter JV, Boston RC, et al. Vitamin D3 supplementation improves insulin sensitivity in subjects with impaired fasting glucose. Transl Res. 2011;158:276–281. doi: 10.1016/j.trsl.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davidson MB, Duran P, Lee ML, et al. High-dose vitamin D supplementation in people with prediabetes and hypovitaminosis D. Diabetes Care. 2013;36:260–266. doi: 10.2337/dc12-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sugden JA, Davies JI, Witham MD, et al. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25:320–325. doi: 10.1111/j.1464-5491.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- 83.Jorde R, Figenschau Y. Supplementation with cholecalciferol does not improve glycaemic control in diabetic subjects with normal serum 25-hydroxyvitamin D levels. Eur J Nutr. 2009;48:349–354. doi: 10.1007/s00394-009-0020-3. [DOI] [PubMed] [Google Scholar]
- 84.Witham MD, Dove FJ, Dryburgh M, et al. The effect of different doses of vitamin D(3) on markers of vascular health in patients with type 2 diabetes: a randomised controlled trial. Diabetologia. 2010 doi: 10.1007/s00125-010-1838-1. [DOI] [PubMed] [Google Scholar]
- 85.Nikooyeh B, Neyestani TR, Farvid M, et al. Daily consumption of vitamin D- or vitamin D + calcium-fortified yogurt drink improved glycemic control in patients with type 2 diabetes: a randomized clinical trial. Am J Clin Nutr. 2011;93:764–771. doi: 10.3945/ajcn.110.007336. [DOI] [PubMed] [Google Scholar]
- 86.Soric MM, Renner ET, Smith SR. Effect of daily vitamin D supplementation on HbA1c in patients with uncontrolled type 2 diabetes mellitus: A pilot study*. J Diabetes. 2012;4:104–105. doi: 10.1111/j.1753-0407.2011.00164.x. [DOI] [PubMed] [Google Scholar]
- 87.Punthakee Z, Bosch J, Dagenais G, et al. Design, history and results of the Thiazolidinedione Intervention with vitamin D Evaluation (TIDE) randomised controlled trial. Diabetologia. 2012;55:36–45. doi: 10.1007/s00125-011-2357-4. [DOI] [PubMed] [Google Scholar]
- 88.Heshmat R, Tabatabaei-Malazy O, Abbaszadeh-Ahranjani S, et al. Effect of vitamin D on insulin resistance and anthropometric parameters in Type 2 diabetes; a randomized double-blind clinical trial. Daru: journal of Faculty of Pharmacy, Tehran University of Medical Sciences. 2012;20:10. doi: 10.1186/2008-2231-20-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Witham MD, Dove FJ, Dryburgh M, et al. The effect of different doses of vitamin D(3) on markers of vascular health in patients with type 2 diabetes: a randomised controlled trial. Diabetologia. 2010;53:2112–2119. doi: 10.1007/s00125-010-1838-1. [DOI] [PubMed] [Google Scholar]
- 90.Takiishi T, Gysemans C, Bouillon R, et al. Vitamin D and diabetes. Rheumatic diseases clinics of North America. 2012;38:179–206. doi: 10.1016/j.rdc.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 91.Israni N, Goswami R, Kumar A, et al. Interaction of vitamin D receptor with HLA DRB1 0301 in type 1 diabetes patients from North India. PloS one. 2009;4:e8023. doi: 10.1371/journal.pone.0008023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mathieu C, Waer M, Laureys J, et al. Prevention of autoimmune diabetes in NOD mice by 1,25 dihydroxyvitamin D3. Diabetologia. 1994;37:552–558. doi: 10.1007/BF00403372. [DOI] [PubMed] [Google Scholar]
- 93.Hypponen E, Laara E, Reunanen A, et al. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 94.Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child. 2008;93:512–517. doi: 10.1136/adc.2007.128579. [DOI] [PubMed] [Google Scholar]
- 95.Gabbay MA, Sato MN, Finazzo C, et al. Effect of cholecalciferol as adjunctive therapy with insulin on protective immunologic profile and decline of residual beta-cell function in new-onset type 1 diabetes mellitus. Arch Pediatr Adolesc Med. 2012;166:601–607. doi: 10.1001/archpediatrics.2012.164. [DOI] [PubMed] [Google Scholar]
- 96.Walter M, Kaupper T, Adler K, et al. No effect of the 1alpha,25-dihydroxyvitamin D3 on beta-cell residual function and insulin requirement in adults with new-onset type 1 diabetes. Diabetes Care. 2010;33:1443–1448. doi: 10.2337/dc09-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bizzarri C, Pitocco D, Napoli N, et al. No protective effect of calcitriol on beta-cell function in recent-onset type 1 diabetes: the IMDIAB XIII trial. Diabetes Care. 2010;33:1962–1963. doi: 10.2337/dc10-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 99.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. Jama. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 100.Harris SS, Pittas AG, Palermo NJ. A randomized, placebo-controlled trial of vitamin D supplementation to improve glycaemia in overweight and obese African Americans. Diabetes Obes Metab. 2012;14:789–794. doi: 10.1111/j.1463-1326.2012.01605.x. [DOI] [PubMed] [Google Scholar]