Abstract
Aims: Fus1 has been established as mitochondrial tumor suppressor, immunomodulator, and antioxidant protein, but molecular mechanism of these activities remained to be identified. Based on putative calcium-binding and myristoyl-binding domains that we identified in Fus1, we explored our hypothesis that Fus1 regulates mitochondrial calcium handling and calcium-coupled processes. Results: Fus1 loss resulted in reduced rate of mitochondrial calcium uptake in calcium-loaded epithelial cells, splenocytes, and activated CD4+ T cells. The reduced rate of mitochondrial calcium uptake in Fus1-deficient cells correlated with cytosolic calcium increase and dysregulation of calcium-coupled mitochondrial parameters, such as reactive oxygen species production, ΔμH+, mitochondrial permeability transition pore opening, and GSH content. Inhibition of calcium efflux via mitochondria, Na+/Ca2+ exchanger significantly improved the mitochondrial calcium uptake in Fus1−/− cells. Ex vivo analysis of activated CD4+ T cells showed Fus1-dependent changes in calcium-regulated processes, such as surface expression of CD4 and PD1/PD-L1, proliferation, and Th polarization. Fus1−/− T cells showed increased basal expression of calcium-dependent NF-κB and NFAT targets but were unable to fully activate these pathways after stimulation. Innovation: Our results establish Fus1 as one of the few identified regulators of mitochondrial calcium handling. Our data support the idea that alterations in mitochondrial calcium dynamics could lead to the disruption of metabolic coupling in mitochondria that, in turn, may result in multiple cellular and systemic abnormalities. Conclusion: Our findings suggest that Fus1 achieves its protective role in inflammation, autoimmunity, and cancer via the regulation of mitochondrial calcium and calcium-coupled parameters. Antioxid. Redox Signal. 20, 1533–1547.
Introduction
Calcium is an ancient signal transduction element that acts as a ubiquitous second messenger in a wide range of different cells and tissues. Calcium handling (uptake and release) by mitochondria regulates basal mitochondrial activities, such as energy production, but is also critical for buffering and shaping of cytosolic calcium rises that in turn regulate a plethora of signaling pathways involved in contraction, proliferation, differentiation, secretion, metabolism, apoptosis, and gene expression (2, 47, 63). Only recently have proteins involved in mitochondrial calcium handling begun to emerge (4, 14, 40, 50, 66, 67), but full understanding of mitochondrial calcium handling is far from complete. Here, we identify that Fus1 plays a critical role in mitochondrial Ca2+ handling and that calcium-dependent mitochondrial and cellular functions are considerably altered in cells lacking Fus1.
Innovation.
Mitochondrial calcium handling (uptake and release) regulates not only basal mitochondrial activities such as energy production but is also critical for calcium rises that regulate contraction, proliferation, differentiation, secretion, metabolism, immune response, and gene expression. We establish Fus1 as novel regulator of calcium handling and metabolic coupling of mitochondrial parameters during T-cell activation. We found that Fus1 plays a major role in the regulation of multiple calcium-dependent T-cell effector functions and proper activation of NF-κB- and NFAT-dependent pathways that rely on intracellular Ca2+ oscillations and reactive oxygen species. Our findings suggest that Fus1 achieves its protective role in inflammation, autoimmunity, and cancers via the regulation of mitochondrial calcium and calcium-coupled parameters.
The 110 a.a. FUS1/TUSC2 protein is a tumor suppressor that we recently associated with mitochondrial activities. The Fus1 gene resides in the 3p21.3 chromosomal region that is frequently deleted in cancers (31, 51). Moreover, Fus1 expression is epigenetically suppressed by reactive oxygen species (ROS) in normal human and mouse cells (31, 62), suggesting that patients with chronically increased ROS (smoking) will have low Fus1 expression in exposed tissues and organs. Loss of Fus1 in mice results in an autoimmune-like syndrome with chronic inflammation and spontaneous formation of both vascular tumors and lymphomas (30). Following exposure to asbestos, Fus1 knockout (KO) mice have an altered acute inflammatory response, including unbalanced production of key pro- and anti-inflammatory cytokines (62). At the cellular level, Fus1 regulates mitochondrial homeostasis in both tumor and immune cells (62), but the molecular activities of Fus1 responsible for its systemic effects remained unclear. Here, based on protein context analysis, we suggested that Fus1 belongs to the Ca2+-myristoyl switch protein family and is involved in the regulation of Ca2+ handling. We report that Fus1 alters mitochondrial Ca2+ uptake and other parameters (ΔμH+ alteration, ROS and nitric oxide [NO] production, mitochondrial permeability transition pore [mPTP] opening, and GSH redox status) in normal epithelial and immune cells and explore possible mechanisms of the Fus1 activity. We show that Fus1 is required for coordinated and balanced mitochondrial changes during CD4+ T-cell activation, as well as for T-cell effector Ca2+-dependent activities. At the molecular level, we demonstrate that Fus1 loss in CD4+ T cells results in increased expression of Ca2+-regulated proteins at a steady state and underactivation of the NF-κB- and NFAT-dependent pathways after CD3/CD28 activation identifying Fus1 as a novel regulator of mitochondrial Ca2+ uptake and immune activities that rely on Ca2+ signaling.
Results
FUS1/TUSC2 is a potential Ca2+-binding protein
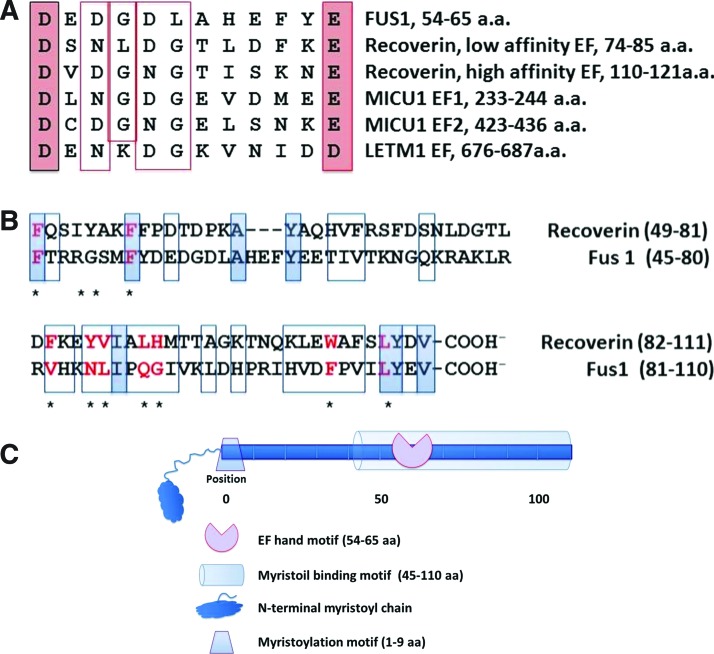
To gain insight into biological and molecular activities of Fus1, pairwise sequence alignment and analysis of Fus1 similarity with other proteins was performed using LALIGN at http://fasta.bioch.virginia.edu/fasta_www2/fasta_www.cgi?rm=lalign. A Fus1 protein fragment (pos. 54–65 a.a) was found to be highly homologous to the Ca2+-binding domains (EF-hands) of the canonical EF-hand protein, calmodulin, as well as to EF-hand domains of the mitochondrial proteins MICU1 (50) and LETM1 (32) (Fig. 1A).
FIG. 1.
Schematic diagram of the Fus1 protein domain structure deduced from multiple alignment shows key elements of the myristoyl-switch protein family. (A) The amino-terminal sequence of Fus1 fits consensus for EF-hand domains in recoverin and mitochondrial Ca2+-binding proteins. (B) Alignment of the Fus1 N-terminal fragment (pos. 45-110 a.a) and a myristoyl-binding domain of recoverin. Conserved positions are shown in shaded rectangles; chemically similar amino acids are indicated with open rectangles. Key amino acids that compose the myristoyl-binding hydrophobic pocket of recoverin are marked with asterisks and presented in red if they are identical or chemically similar to the corresponding residues in the Fus1 protein. (C) Schematic representation of the Fus1 protein domain structure. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Identification of a putative Ca2+-binding domain (this study) combined with a previously described N-myristoylation site crucial for the Fus1 activity (61) prompted the comparison of Fus1 structure to recoverin and other members of the Ca2+/myristoyl switch protein family (1, 57). A long N-terminal fragment of FUS1 (pos. 45–110 a.a.) has 53% homology to the myristoil-binding domain of recoverin (57) (Fig. 1C). Importantly, 9 of 11 key amino acids (82%) that form myristoyl-binding hydrophobic pocket of recoverin are identical or chemically similar in the Fus1 protein (Fig. 1C), suggesting that the 45–110 a.a. fragment of Fus1 may function as a myristoyl-binding site. Thus, our in silico analysis suggests that Fus1 may act as a Ca2+/myristoyl switch protein and its activity may be linked to mitochondrial Ca2+ handling. This hypothesis is supported by our data on Fus1 involvement in the regulation of mitochondrial membrane potential (MMP) and ROS production (62), two processes that rely on mitochondrial Ca2+ homeostasis (7, 8).
Fus1 modulates Ca2+ uptake by mitochondria through Na+/Ca2+ exchanger
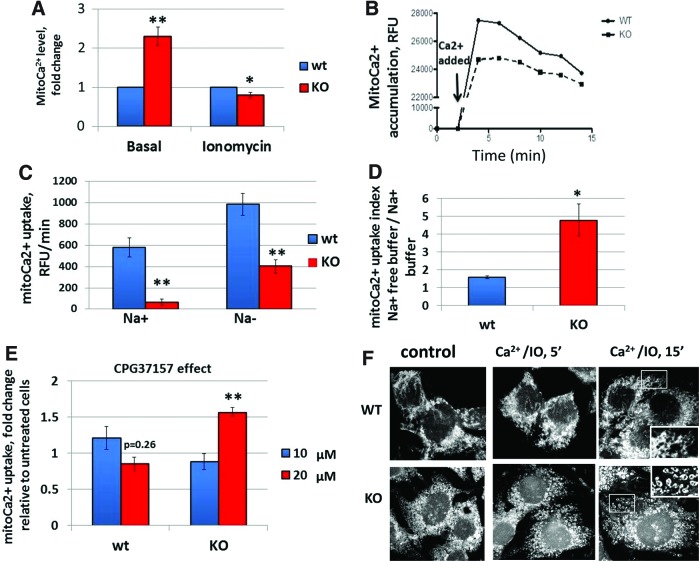
To establish the role of Fus1 in Ca2+ handling, mitochondrial calcium (mitoCa2+) accumulation was estimated in spontaneously immortalized Fus1 wild-type (WT) and Fus1 KO epithelial cells using fluorescence intensity changes of the mitoCa2+ sensor Rhod-2. Compared to WT cells, KO cells had significantly elevated basal mitoCa2+ levels but accumulated significantly less mitoCa2+ in response to the pretreatment with Ca2+ plus ionomycin (IO), a Ca2+ ionophore (Fig. 2A). To confirm these results, we measured Ca2+ uptake by energized mitochondria after a mild permeabilization of cells with digitonin that leaves intracellular ER and mitochondria membranes intact. Mitochondria of permeabilized WT cells took up added Ca2+ at an average rate of 580±91.2 RFU/min, whereas buffering by permeabilized Fus1 KO cells was approximately ninefold less at a rate of 64±29 RFU/min resulting in lower levels of accumulated Ca2+ (Fig. 2B).
FIG. 2.
Fus1 modulates mitochondrial Ca2+ uptake through Na+/Ca2+ exchanger and maintains basal and Ca2+-induced mitochondrial fusion in normal epithelial cells. (A) Relative basal and Ca2+/IO-induced levels of mitoCa2+ in Fus1 KO and WT immortalized epithelial cells. (B) Representative curve of mitochondrial Ca2+ dynamics in digitonin-permeabilized Fus1 KO and WT epithelial cells using Calcium Green-5N. Arrow denotes the addition of a pulse of CaCl2 (100 μM). (C) The absolute rates of mitoCa2+ uptake in digitonin-permeabilized Fus1 KO and Fus1 WT epithelial cells incubated in Na+-containing (Na+) and Na+-free buffer (Na−). (D) MitoCa2+ uptake indices (Ca2+ uptake in Na-free/Ca2+ uptake in Na+ buffer) in Fus1 KO and WT epithelial cells. (E) Effect of CGP37157 on mitochondrial Ca2+ uptake in digitonin-permeabilized epithelial cells. (F) Morphology of the mitochondria in immortalized kidney Fus1 KO and WT epithelial cells. Cells were preincubated with PBS/Ca2+ (control) and PBS/Ca2+/IO (Ca2+-loading conditions) for 5 and 15 min after staining with a mitochondria-specific dye Mitotracker Red. Detailed images of fusion (WT, upper panel) and fission (KO, lower panel) are shown in the insets. Data represent mean±SE; p-values are designated as *, <0.05; **, <0.01. IO, ionomycin; KO, knockout. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
mitoCa2+ accumulation is a dynamic process balanced by the uptake and release of Ca2+ ions. The major route of calcium efflux from mitochondria involves exchange of Na+ ions for Ca2+ ions through the mitochondrial Na+/Ca2+ exchanger (mNCX) (48) suggesting that the slower rate of mitoCa2+ accumulation by Fus1 KO cells may be associated with elevated activity of mNCX. To block mNCX, permeabilized epithelial cells were preincubated in Na+-free buffer and then loaded with Ca2+. The rate of mitoCa2+ accumulation in Na+-depleted media was increased both in WT (984±105.2 RFU/min) and KO cells (402±62.3 RFU/min) (Fig. 2C); however, in Fus1 KO cells, Ca2+ uptake index (rate in Na-free buffer/rate in Na+ buffer) was threefold higher (Fig. 2D). To confirm Fus1-dependent regulation of mitoCa2+ transport via mNCX, cells were treated with the specific mNCX inhibitor, benzothiazepine CGP37157. In WT cells, a maximal effect of mNCX inhibition was observed at 10 μM of CGP37157 reflected in 20% increase in mitoCa2+ uptake compared to untreated cells. In Fus1 KO cells, treatment with 20 μM CGP37157 resulted in the maximal mNCX inhibition that led to 60% increase in mitoCa2+ uptake (Fig. 2E). These data suggest that Fus1 is required for mitoCa2+ accumulation that may be modulated via mitochondrial Na+/Ca2+exchanger.
Prevalence of mitochondrial fission in Fus1 KO cells
Mitochondrial fission and fusion play critical roles in maintaining functional mitochondria when cells experience metabolic or environmental stresses. The balance of these processes is important for the maintenance of mitochondrial DNA, mitochondrial respiration, ROS and ATP production, Ca2+ uptake, cell proliferation, and autophagy (22, 49). We evaluated changes in mitochondrial morphology of Fus1 WT and Fus1 KO epithelial cells induced by Ca2+/IO treatment. As shown in Figure 2F, at a basal state, WT mitochondria formed filamentous/granular network while Fus1 KO mitochondria are represented by granular structures. Ca2+ overload induced mostly fusion in mitochondrial networks of WT cells with only minor signs of fission, whereas Fus1 KO cells exhibited profound fission with fragmentation into ring-shaped mitochondria (Fig. 2F). Thus, mitochondrial fission prevailed in Fus1 KO cells at a basal level and upon Ca2+ overload.
Fus1 maintains mitochondrial homeostasis in immune cells
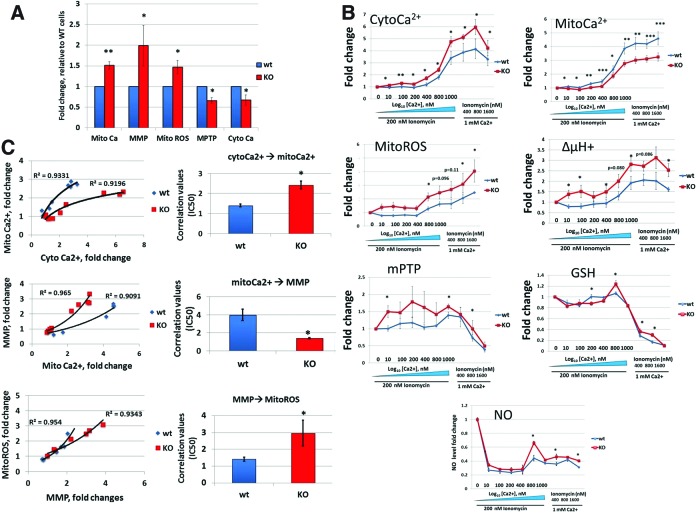
Calcium as a second messenger is involved in metabolic coupling. Ca2+ ions stimulate several dehydrogenases of the Krebs cycle and thus elevate MMP (ΔμH+) (24). In turn, elevated ΔμH+ induces ROS production (41). At the same time, during injury, alterations in ΔμH+, mitoCa2+, and mitoROS may promote opening of the mPTP, release of cytochrome c, and cell death (26). We performed a detailed comparative analysis of major Ca2+-dependent parameters, such as mitoCa2+, cytoCa2+ (measured with fluoro-3 acetoxymethyl ester [Fluo-3/AM]), ΔμH+ (measured with JC-1), mitoROS (measured with MitoSOX), and mPTP permeability (calcein) in Fus1 KO and WT splenocytes. We chose immune cells for this analysis since Fus1 plays a complex and not yet fully understood role in the immune system (30, 62). Relative to WT levels, the basal mitoCa2+ level was significantly higher in Fus1 KO cells, whereas the cytoCa2+ level was lower (Fig. 3A) suggesting that Fus1 negatively regulates steady-state Ca2+ handling by mitochondria. Accordingly, basal levels of Ca2+-dependent parameters ΔμH+, mitoROS, and mPTP permeability were higher in Fus1 KO cells (Fig. 3A). Overall, these results and our earlier findings (62) characterize Fus1 as a novel factor controlling steady-state mitochondrial Ca2+ homeostasis.
FIG. 3.
Fus1 is required for the maintenance of basal mitochondrial homeostasis and for mitochondrial response to Ca2+ overload in immune cells. (A) Fus1-dependent changes in basal mitochondrial parameters coupled with Ca2+ dynamics measured in splenocytes isolated from Fus1 WT and Fus1 KO mice (n=5). Results are expressed as fold changes relative to WT cells. (B) Dynamics of changes in major mitochondrial parameters upon increasing of calcium load in splenocytes of WT and Fus1 KO mice (n=5–6/group). Results are presented as fold changes in relative fluorescence (RF) values compared to control cells normalized to 1.0. (C) Analysis of the efficiency of transduction of coupled mitochondrial parameters in WT and KO splenocytes under conditions of increasing calcium load. Correlation values were calculated as IC50 and presented as mean±SE. IC50 was calculated in accordance with general rules applied for determination in values of effectiveness of parameters transformation restored from a series of dose–response data as described elsewhere (45). Data represent mean±SE; p-values are designated as *, <0.05; **, <0.01; ***, <0.001. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Fus1 is required for a measured response to Ca2+ agonist IO in splenocytes
Since Ca2+ transport plays an important role in the lymphocyte fate (25, 58, 60), changes in the levels of mitoCa2+, cytoCa2+, ΔμH+, nonprotein thiols, including glutathione (referred to thereafter as GSH, measured with Green CMFDA [5-chloromethylfluorescein diacetate]), NO (measured with DAF-FM), and mPTP permeability, were determined in WT and Fus1 KO splenocytes after IO treatment. Combination of IO and phorbol ester (e.g., PMA) allows to bypass signals from T-cell receptors resulting in Ca2+ elevation, T-cell activation, and production of IL-2, IL-4, IFN-γ, etc. (25).
Splenocytes were gradually loaded with increased concentrations of Ca2+ or IO and rates of intracellular Ca2+ accumulation measured in WT and Fus1 KO splenocytes. Experimental conditions included gradually increased Ca2+ concentration (10 μM–1 mM) at a constant IO (200 nM) and gradually increased IO concentration (400–1600 nM) at a constant Ca2+ (1 mM).
Fold-change in Ca2+ accumulation and other parameters was calculated as a ratio of fluorescent dye intensities in Ca2+/IO-treated cells (F) to control cells treated with Ca2+ only (Fo). As shown in Figure 3B, alterations in cytoCa2+ level at IO presence resulted in changes of other mitochondrial parameters, such as mitoCa2+, ΔμH+, ROS, mPTP permeability, GSH, and NO.
At 1 mM extracellular Ca2+ concentration, the curves for cytoCa2+, mitoCa2+, and ΔμH+ (Fig. 3B) reached a plateau indicating that the system reached a saturation point that could not be changed even by increasing of IO concentration (up to 1600 nM).
Analysis of transduction of cytoCa2+ into mitoCa2+ showed that, in general, the accumulation of cytoCa2+ results in exponential increase in mitoCa2+, which was consistent with similar studies on Ca2+ accumulation (13). In WT cells, any small alterations of cytoCa2+ level resulted in significant changes in Ca2+ uptake by mitochondria reflected in a steep curve of mitoCa2+ accumulation (Fig. 3C). Fus1 loss led to a blunted Ca2+ response and a rightward shift of the mitoCa2+ accumulation curve suggesting suppression of Ca2+ uptake and/or elevated Ca2+ efflux by Fus1 KO mitochondria (Fig. 3C). Observed differences were calculated as EC50 values that were 60% higher for Fus1 KO cells compared to WT cells (Fig. 3C, bar graph). Calcium has a dual effect on ΔμH+: matrix Ca2+ elevates ΔμH+ due to its stimulatory effect on tricarbonic acid cycle and concomitant increase in proton pumping, whereas excess of Ca2+ uptake leads to ΔμH+ depolarization because of energy dissipation (13). Figure 3C depicts logistic curves describing the transmission of mitoCa2+ level into ΔμH+ changes. Fus1 loss results in a leftward shift of ΔμH+ curve reflecting more effective (2.6-fold) transduction of mitoCa2+ level into ΔμH+ at all used concentrations of Ca2+ and IO (Fig. 3C, left panel). Considering that upon increasing of Ca2+ load the rate of mitoCa2+ uptake in Fus1 KO cells is lower than in WT cells (Fig. 3C, bar graph), we suggest that the decreased mitoCa2+ uptake may prevent depolarizing effect of Ca2+ on ΔμH+ in Fus1 KO cells.
Mitochondria are a dominant cellular source of ROS under normal conditions. ROS production is determined by the ΔμH+ magnitude and is increased nonlinearly with ΔμH+ elevation (6). Indeed, production of ROS in WT and Fus1 KO splenocytes was increased upon treatment with Ca2+/IO in accordance with ΔμH+ changes (Fig. 3C). However, in Fus1 KO cells, transformation of ΔμH+ into ROS production was less efficient despite higher efficiency of transduction of mitoCa2+ into ΔμH+ (Fig. 3C).
mPTP opening triggered by the elevated Ca2+ in the mitochondrial matrix increases mitochondrial membrane permeability and causes ΔμH+ de-polarization, decrease in ATP, and increase in ROS productions. The fate of the cell after an insult depends on the extent of mPTP opening. If mPTP opens slightly, the cell may recover, whereas if it opens further, the cell may undergo apoptosis or necrosis dependent on the degree of opening (26). We showed that Ca2+-induced mPTP permeability was lower in Fus1 KO cells compared to Fus1 WT cells (Fig. 3C), suggesting that the Fus1 activity is involved in proper and timely mPTP opening under conditions resulting in Ca2+ overload.
GSH plays a central role in the removal of hydrogen peroxide produced by the respiratory chain and have the highest impact on the mitochondria redox status (68). GSH levels control mPTP permeability, ΔμH+, NO production, and a release of calcium to the cytosol (3, 68). In our Ca2+-loading experiments, GSH changes were reflected in a three-phase curve: (i) moderate drop at low Ca2+ concentrations (0–400 μM); (ii) moderate elevation induced by higher Ca2+ concentrations (800 μM), and (iii) dramatic drop at the highest Ca2+ concentration (1 mM) (Fig. 3B). Despite the fact that Ca2+-induced increase in the GSH level occurs earlier in Fus1 WT cells (Phase 1), the overall GSH levels at Ca2+-overloaded conditions (Phases 2 and 3) were higher in Fus1 KO cells (Fig. 3B).
NO is a signaling molecule produced in a Ca2+-dependent manner by NO synthases (NOS), including mitochondrial NOS (15). Elevated levels of NO inhibit mitochondrial Ca2+ uptake thus forming a negative feedback loop (59). We found that Ca2+ elevation results in changes in the NO production reflected in a three-phase curve similar to GSH alterations (Fig. 3B). Like GSH, overall synthesis of NO was elevated in Fus1 KO splenocytes (Fig. 3B).
Altogether, these data demonstrated that the Fus1 activity is required for mitoCa2+ uptake and for mitochondrial activities coupled with Ca2+ (ΔμH+ alteration, ROS and NO production, mPTP opening, and GSH redox status).
Fus1 is required for coordinated and balanced changes of mitochondrial parameters during CD3/CD28 activation of CD4+ T lymphocytes
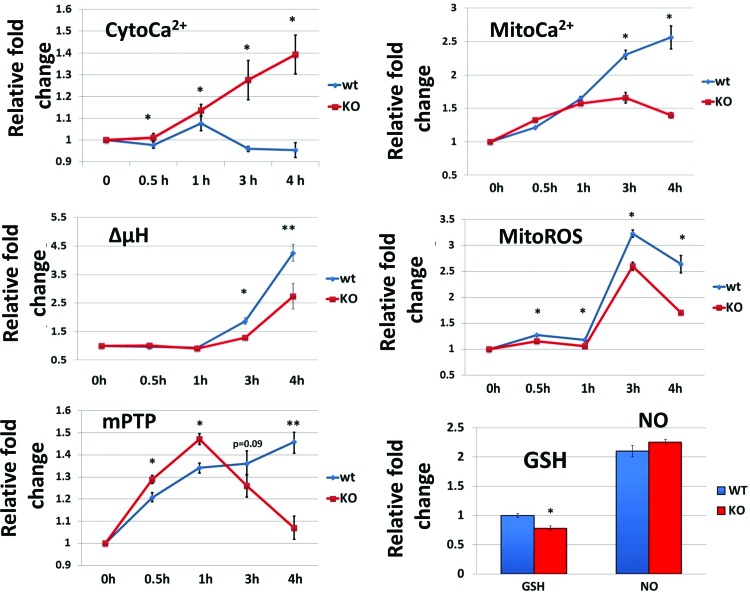
The signaling network that mediates the activation of T lymphocytes is tightly linked with the dynamic alterations in the levels of cytoCa2+, mitoCa2+, ΔμH+, ROS, and mPTP activity (20, 52). Given that Fus1 was required for normal mitochondrial response to Ca2+ and that Fus1 KO mice have immune abnormalities, the role of Fus1 in early mitochondrial changes upon T-lymphocyte activation was explored. CD3/CD28 activation of WT T cells resulted in minor deviations of cytoCa2+ from its basal level at 0.5, 1, 3, and 4 h of stimulation (0.98±0.02, 1.08±0.03, 0.96±0.01 and 0.95±0.03-fold changes, respectively) (Fig. 4). In contrast, a steady accumulation of cytoCa2+ was observed in Fus1 KO T lymphocytes at 0.5 h (1.01±0.02), 1 h (1.14±0.03), 3 h (1.28±0.09), and 4 h (1.39±0.09) after CD3/CD28 stimulation (Fig. 4). Parallel measurement of mitoCa2+ levels showed that mitochondria of WT T cells steadily accumulated Ca2+ during 4 h: 1.21±0.01-fold change at 0.5 h, 1.65±0.03-fold at 1 h, 2.30±0.07-fold at 3 h, and 2.6±0.2 at 4 h of stimulation (Fig. 4). In contrast, an initial rise of mitoCa2+ (1.33±0.01-fold) was observed in Fus1 KO T cells 0.5 h after stimulation, it plateaued between 1 and 3 h (1.57±0.02-fold; 1.66±0.08-fold) before decreasing at 4 h (1.40±0.04-fold) after stimulation (Fig. 4). Thus, CD3/CD28 activation of Fus1 KO CD4+ T cells led to a slower rate and shorter duration of mitoCa2+ accumulation. These results corroborate the results from Fus1 KO splenocytes after Ca2+ overload (Fig. 3C) or stimulation with IO (Fig. 3B) and suggest that Fus1 KO T cells' mitochondria are characterized by impaired Ca2+ uptake resulting in cytosolic accumulation of Ca2+.
FIG. 4.
Dynamic changes in Ca2+-coupled mitochondrial activities induced by CD3/CD28 stimulation of CD4+ T cells from Fus1 WT and Fus1 KO mice (n=5–6/group). Results are presented as fold changes in RF values compared to control cells normalized to 1.0. Data represent mean±SE; p-values are designated as *, <0.05; **, <0.01. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The ΔμH+ and mitoROS changes at 0.5 and 1 h of T-cell activation were similar in Fus1 WT and Fus1 KO T cells. However, stimulation of Fus1 KO T cells for 3 and 4 h resulted in significantly lower magnitude of ΔμH+ and mitoROS compared to WT T cells (Fig. 4).
The dynamics of activation-induced mPTP changes was drastically different between WT and Fus1 KO CD4+ T cells at all analyzed time points. In WT T cells, activation resulted in a gradual closing of mPTP between 0 and 4 h (Fig. 4). In Fus1 KO T cells, after initial pronounced closing of mPTP between 30 and 60 min, mPTP converted to an open state between 2 and 4 h post-activation (Fig. 4). At 24 h post-activation, CD4+ T cells of both genotypes demonstrated an open mPTP state (data not shown).
Analysis of GSH levels at 4 h post-activation, which represents a peak time point for a CD3/CD28-induced Ca2+ uptake, showed no GSH changes in WT T cells, but a significant GSH decrease in Fus1 KO cells (Fig. 4, bar graph). At the same time, no differences in NO production were noted between Fus1 WT and Fus1 KO T cells (Fig. 4, bar graph). Thus, alterations in GSH content may represent one of the Fus1-dependent factors affecting the activity of T cells. Taken together, our data strongly suggest that Fus1 is involved in the regulation of mitoCa2+ uptake capacity and alterations of Ca2+-dependent mitochondrial parameters (ΔμH+, ROS production, mPTP opening, and GSH oxidation–reduction) during early stages of CD4+ T-cell activation.
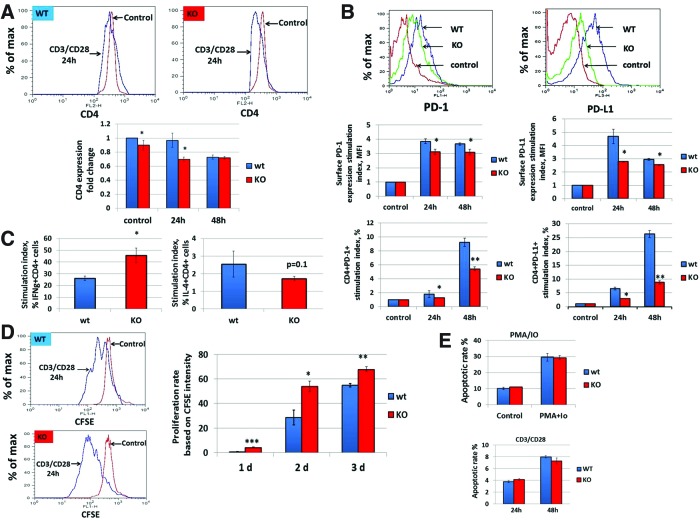
Fus1 regulates expression of surface molecules in CD4+ T cells
Previously, we reported that Fus1-deficient mice develop autoimmune syndrome resembling systemic lupus erythematosus (SLE) (30). SLE is characterized by a specific pattern of cellular and molecular Ca2+-dependent changes in T cells, including increased endocytic recycling of CD4 molecule (20) and increased expression of immunoregulatory molecules, such as PD-1, PD-L1, CTLA-4, and BTLA. (34). PD-1 and PD-L1 are transcriptional targets of the NF-κB complex (35, 37), whose activity is strictly dependent on the communication between mitochondria and nucleus mediated by ROS and Ca2+ oscillations (17, 33). Here, we compared basal surface levels of CD4 on CD4+ T cells and found that Fus1 KO T cells have a slight but significantly lower basal CD4 level (Fig. 5A). CD3/CD28 activation profoundly decreased surface CD4 expression on Fus1 KO T cells after 24 h of stimulation while no changes were observed in Fus1 WT T cells at this time. Only 48 h of activation produced a similar level of CD4 decrease in both Fus1 KO and WT cells (Fig. 5A, bar graph). Thus, the dynamics of the surface CD4 expression is dependent on Fus1 activities.
FIG. 5.
Fus1 modulates Ca2+ mobilization-dependent processes during CD4+ T- cell activation. (A, B) Effect of Fus1 loss on the dynamic changes in surface levels of CD4 and (B) PD-1/PD-L1 molecules during CD3/CD28 stimulation of WT and Fus1 KO CD4+ T lymphocytes. (C–E) Fus1-dependent changes in the effector functions of activated CD4+ T cells, such as Th differentiation, which was defined based on preferential expression of IFNγ (Th1 cell marker) or IL-4 (Th2 cell marker) (C), proliferation (D), and apoptosis (E). Data represent mean±SE; p-values are designated as *, <0.05; **, <0.01; ***, <0.001. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Comparison of the basal level of PD-1 and its ligand PD-L1 in T cells showed no difference between genotypes (data not shown); however, upon CD3/CD28 stimulation, upregulation of PD-1 and PD-L1 was significantly attenuated in Fus1 KO T cells (Fig. 5B) suggesting that poor immunosuppressive mechanisms in Fus1 KO animals may contribute to their autoimmune phenotype (30).
Fus1 regulates CD4+ T-cell effector functions
Accumulation of mitoCa2+ triggers T-cell processes including Th differentiation (60), proliferation (47, 63), and apoptosis (20). To determine if Fus1 regulates T-cell differentiation, CD4+ T cells were stimulated with CD3/CD28 for 36 h followed by the assessment of IFNγ and IL-4, the hallmark cytokines of Th1 and Th2 cells, respectively. Absence of Fus1 significantly increased Th1 differentiation (stimulation index or IFNγ-fold increase, WT vs. KO: 26.2 vs. 45.7-fold, p<0.05) (Fig. 5D). Concomitantly, the stimulation index for IL-4 production was lower in Fus1 KO CD4+ T cells although the difference did not reach statistical significance (WT vs. KO: 2.55 vs. 1.72, p=0.1) (Fig. 5D). These data suggest that Fus1 inhibits Th1 differentiation following CD3/CD28 stimulation and thus affects the Th1/Th2 cytokine axis. To assess the proliferative capacity of CD4+ T cells under conditions simulating a strong antigen signal, purified T cells were labeled with CFSE and stimulated with CD3/CD28 antibodies. As shown in Figure 5D, Fus1 WT CD4+ T cells had little to no proliferative response to the stimulation at day 1 with a marked increase in the proliferation rate at day 2 and robust proliferation at day 3 post-activation. In contrast, proliferation of Fus1 KO CD4+ T cells was detectable at day 1 and remained higher throughout the experimental time course of stimulation (Fig. 5E). These results indicate that Fus1 suppresses proliferative capacity of CD4+ T cells following short-term (1 day) and extended stimulation.
Data presented here support Fus1 as a regulator of mitoCa2+ uptake, ΔμH+, ROS production, and mPTP permeability after IO and CD3/CD28 stimulation. In addition, Fus1 loss results in an autoimmune-like syndrome and regulated Th proliferation after stimulation. To determine if Fus1 also alters primary Th-cell apoptosis, T cells were activated with PMA/IO or CD3/CD28 and apoptosis was measured by annexin and PI staining after 9 h (9) and 24–48 h of stimulation (42), correspondingly. We were not able to detect Fus1-dependent differences in PMA+IO (Fig. 5E) or CD3/CD28-induced (Fig. 5E) apoptosis in CD4+ T cells, suggesting that Fus1 activities may not be involved in T-cell apoptosis under analyzed stimulatory conditions; however, additional experiments are required to make a definitive conclusion on the role of Fus1 in T-cell apoptosis.
Fus1 KO T cells overexpress NF-κB/NFAT- and Ca2+-binding proteins and Ca2+-regulated genes at a steady state
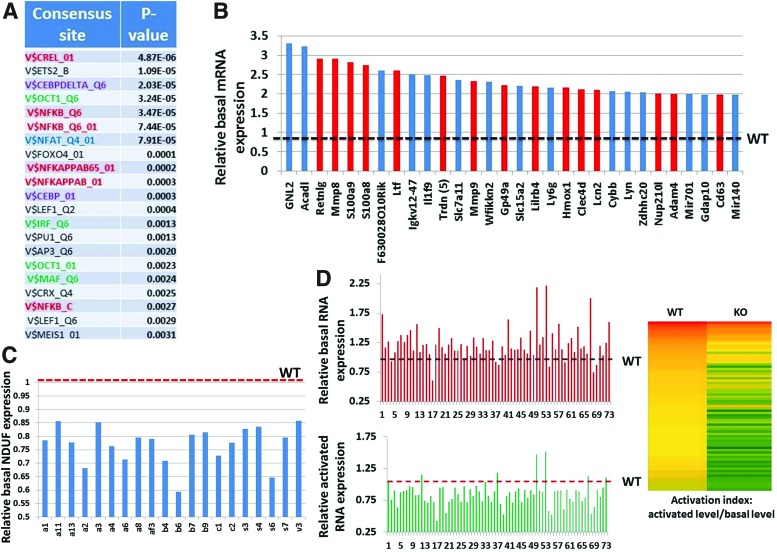
To understand the mechanisms through which Fus1 deficiency affects diverse molecular pathways in T cells, transcriptional profiles of isolated WT and Fus1 KO CD4+ T cells were compared. Basal expression of 222 GO-annotated genes was at least 1.5-fold higher in Fus1 KO than in WT T cells. Enrichment of canonical pathways and processes affected by Fus1 loss were identified using Genecodis and GSEA analyses (http://genecodis.cnb.csic.es/analysis and www.broadinstitute.org/gsea). Promoters of genes suppressed by Fus1 (upregulated in Fus1 KO T cells) were highly enriched in binding sites for Ca2+-regulated transcription factors (TFs) NFAT, NF-κB, and C/EBP as well as for TF binding sites that synergistically cooperate with NFAT (MAF, IRF, and OCT1) (28) (Fig 6A). Activation of these TFs depends on Ca2+ levels and oscillations (17), consistent with a role of Fus1 as a regulator of subcellular Ca2+ pools. Further support of Fus1 in Ca2+-dependent signaling was provided by the upregulation of a large set of Ca2+-associated genes in Fus1 KO T cells (Fig. 6B). Of interest, Retnlg (resistin), a proinflammatory cytokine, which induces a rapid increase in intracellular Ca2+ concentration and NF-κB activation (5), was increased approximately threefold in Fus1 KO cells. Other highly expressed genes in the absence of Fus1 were the Ca2+ binding and EF-hand domain-containing proteins, S100a8 and S100a9, which function mainly as a S100A8/A9 heterotetramer. S100A8/A9 expression is a hallmark of inflammatory conditions, such as rheumatoid arthritis, inflammatory bowel disease, and multiple sclerosis. (54). As both proteins are also known to induce multiple endopeptidases (64), increased expression of Mmp8, Mmp9, Adam4, Adam8, and Adam19 observed in CD4+ Fus1 KO cells provided additional support for S100a8/S100a9 activation. Among the top 20 genes whose expression was inhibited by Fus1 was Triadin (Trdn, six probes), a protein of the Ca2+ release complex that modulates Ca2+ homeostasis (55); two members of Ca2+-dependent C-type lectin family, Clec4d and Clec4n, and Lipocalin 2, a neutrophil Ca2+-induced gene (36) (Fig. 6B). The complete list of Fus1 targets upregulated in Fus1 KO T cells is shown in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/ars). Interestingly, no significant enrichment of TF sites in the promoters of the genes downregulated in Fus1 KO cells could be found; however, there was a strong enrichment for genes encoding mitochondrial proteins involved in oxidation–reduction and in electron transport (p=0.0006 and p=0.0008, respectively), including NDUF proteins (Fig. 6C), supporting the idea of mitochondrial dysfunction in Fus1-deficient cells.
FIG. 6.
Fus1-dependent expression changes observed in CD4+ T cells suggest dysregulation of the Ca2+-dependent NF-κB and NFAT-pathways at basal and activated conditions and mitochondrial dysfunction in Fus1 KO lymphocytes. (A) Enrichment with consensus sites for Ca2+-regulated TFs (NFAT [blue], NF-κB [red], and CEBP [violet]) and TFs that synergistically cooperate with NFAT (green) in the promoters of genes upregulated at basal levels (×1.5-fold and higher) in Fus1 KO lymphocytes (B). The list of top 30 genes overexpressed in unstimulated Fus1 KO T cells is enriched with genes that encode Ca2+-binding and Ca2+-regulated proteins (red bars). Overexpression is shown as fold change over WT expression (1.0) that is marked with dotted black line. (C) Fus1 KO T cells show global underexpression of mitochondrial proteins including a large cluster of genes that encode enzymes involved in oxidative phosphorylation (NDUF family; symbols of individual NDUF proteins are shown under X axis). Relative WT level (1.0) is marked with the dotted red line (D). CD3/CD28-stimulated Fus1 KO T cells demonstrate global underexpression of activation response genes compared to WT cells. Bottom bar graph shows relative expression of 73 genes in Fus1 KO cells that were found activated at highest levels in WT cells. Relative WT level (WT=1.0) is marked with the dotted red line. Upper bar graph shows global overexpression of the same set of genes in Fus1 KO T cells at basal conditions. Activation indices for these genes in WT and Fus1 KO T cells are presented as a color-coded diagram where highest values are shown in red and lowest values in green. For the entire list of genes, see Supplementary Table S2. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Fus1 deficiency in CD4+ T cells inhibits the activation of CD3/CD28-dependent genes
Given that calcium uptake by mitochondria plays a crucial role during T-cell activation (52), effects of Fus1 loss on gene expression in stimulated CD4+ T cells were examined. Comparison of stimulated to unstimulated WT T cells revealed upregulation of a number of well-characterized genes (e.g., IRF4, IL2, TNFα) that served as positive internal controls of CD3/CD28-induced activation. Of the genes upregulated in stimulated WT cells, 119 genes that were increased ≥1.5-fold were considered to be induced by CD3/CD28 T-cell activation (Supplementary Table S2). Activation indices for these genes (activated level/basal level) were significantly higher in WT cells than in KO cells (Fig. 6D, left panel). Remarkably, 39% of genes upregulated in WT cells failed to be activated at all in Fus1 KO T cells (Fig. 6D and Supplementary Table S2). Comparison of the absolute expression levels of these genes at basal and activated states revealed that despite the higher basal expression of these genes in Fus1 KO T cells (Fig. 6D), the level of these genes after activation was generally lower in Fus1 KO cells than in WT cells. These results suggest that loss of Fus1 in T cells impairs proper upregulation of genes involved in response to activation.
Discussion
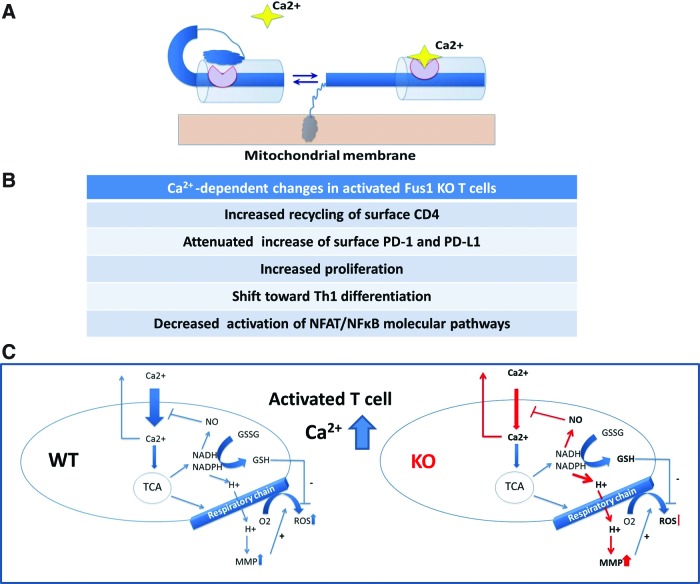
We previously reported that Fus1 links inflammatory response and mitochondrial homeostasis affecting ΔμH+, ROS, and cytokine production by activated T cells (62); however, the molecular function(s) of Fus1 in mitochondria remained unknown. Based on pairwise alignment and context analysis combined with a Fus1 N-terminal myristoylation site identified earlier (61), we explored the hypothesis that Fus1 is a Ca2+/myristoyl switch protein. The members of this family change conformation and membrane attachment depending on Ca2+ level (1, 57, 69), thus helping to localize proteins to distinct signaling compartments and coordinate regulation of Ca2+ signaling cascades. Our hypothetical scheme of Fus1 Ca2+-dependent conformations based on its domain structure is depicted in Figure 7A.
FIG. 7.
Hypothetical schemes of Fus1 protein Ca2+-dependent conformation changes and Ca2+ uptake-coupled mitochondrial events that are regulated by Fus1 activities. (A) Schematic diagram of proposed calcium-myristoyl switch in Fus1. The binding of one Ca2+ ion promotes the release of the myristoyl group from the myristoyl-binding hydrophobic pocket and anchoring of the protein in membrane structures. (B) The list of the Ca2+-dependent processes in activated T cells affected by Fus1 loss. (C) Schematic representation of interactions and transitions between different mitochondrial processes and their regulation by cytosolic Ca2+ elevation in WT and Fus1 KO immune cells. Parameters shown in bold were tested in our study. Processes affected in Fus1 KO cells are shown with red arrows. For detailed description of the presented model, see Supplementary Data. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Here, Fus1 was shown to be involved in mitochondrial Ca2+ handling, as well as Ca2+-dependent processes and pathways (Fig. 7B). Studies presented here contribute to a better understanding of Fus1 activities with mechanistic insight. A number of conclusions have emerged from this study. First, Fus1 regulates subcellular Ca2+ pools via mitochondrial Ca2+ handling linked to the activity of the Na+/Ca2+ exchanger (mNCX). Second, Fus1 controls metabolic coupling of mitochondrial parameters and efficiency of their sequential transduction via the regulation of mitoCa2+ handling. Third, Fus1 activities at basal and Ca2+-overload conditions are coupled with mitochondrial dynamics (fission/fusion). Fourth, in agreement with the crucial role of calcium signals in the regulation of multiple lymphocyte functions (2, 47, 52, 63), Fus1 is required for coordinated and balanced changes in mitochondrial parameters in T cells, regulation of surface markers, T-cell proliferation, and Th1/Th2 differentiation during the activation of CD4+ T cells. Fifth, in CD4+ T cells, Fus1 plays a major role in the proper and timely activation of NF-κB- and NFAT-dependent pathways regulated via intracellular Ca2+ oscillations (17). Each of these points as well as our model of coupling of Ca2+-dependent mitochondrial processes in Fus1 WT and Fus1 KO mitochondria are discussed below.
The maintenance of Ca2+ homeostasis in cells involves Ca2+ uptake by mitochondria regulated by the mitochondrial calcium uniporter (MCU) and Ca2+ efflux regulated via NCX. In this study, we identified Fus1 as a novel regulator of mitoCa2+ handling at both steady-state and Ca2+ overload conditions. Our data suggest that Fus1 modulates mitoCa2+, at least, in part, via the regulation of NCX that exports one Ca2+ ion for every three imported Na+ ions (11, 27, 39). Since NCX inhibition resulted in mitoCa2+ accumulation in Fus1 KO cells, NCX inhibitors could be considered therapeutics for treating diseases associated with Fus1 deficiency, such as cancer and autoimmunity (30).
Disrupted mitochondrial dynamics results in major cellular dysfunction that precipitates in various diseases (12, 16). Our data (Fig. 2F) suggest that Fus1 activity maintains mitochondrial fusion, which may stem from the Fus1 effect on mitoCa2+ handling. Mitochondrial fission/fusion cycles are balanced by a master regulator of mitochondrial biogenesis PGC-1α that is itself regulated via Ca2+ (65). Alternatively, the observed Fus1 effect on mitochondrial dynamics may come from the regulation of Ca2+-dependent CaMK1a and calcineurin involved in the phosphorylation/dephosphorylation of DRP1, the main regulator of mitochondrial fragmentation (10). Further work is required to identify the important details of Fus1 involvement in this process.
Ca2+ influx is crucial for T-cell activation and differentiation (20, 21, 46). Using two models of lymphocyte activation, we showed that loss of Fus1 in immune cells consistently resulted in reduced mitoCa2+ uptake and coordinate increase of Ca2+ in the cytosol (Figs. 3B, C and 4A, B). Changes in subcellular Ca2+ pools in Fus1 KO cells were accompanied by altered dynamics of mitochondrial parameters (ΔμH+, mitoROS, mPTP permeability, NO synthesis, and GSH level), dependent on the nature of stimuli and/or duration of stimulation (Figs. 3D–H and 4C–G). These observations suggest that Fus1 is involved in fine-tuning of mitochondrial activities during T-cell activation.
Analysis of the efficiency of transduction of coupled mitochondrial parameters is a yet another way to assess the nature of Fus1-dependent pathological mitochondrial changes in immune cells. Thus, transduction of MMP into ROS in Fus1 KO cells was less efficient despite higher efficiency of transduction of mitoCa2+ into MMP in these cells (Fig. 3C). We explain it by compromised antioxidative cellular mechanisms, such as NADH, NADPH, or GSH oxidation/reduction. The use of the same source (NADH) for the generation of MMP and reduction of GSH leads to a shift in MMP if reduction equivalents are redirected toward GSH synthesis (3). At the same time, reduction in mitoCa2+ uptake would decrease Ca2+-dependent ROS generation, shift GSH oxidation, and maintain MMP elevation. Indeed, we showed that reduced mitoCa2+ uptake in Ca2+/IO-treated Fus1 KO cells correlated with higher levels of GSH synthesis (Fig. 3C). Unbalanced Ca2+-dependent ROS production and GSH synthesis is the most likely reason why transformation of MMP→ROS in Fus1 KO splenocytes is distorted. The hypothetical scheme of Fus1-dependent changes in coupled mitochondrial parameters is shown in Figure 7C.
In this study, we demonstrate that Ca2+-dependent processes are also altered in Fus1 KO T cells. We found that Fus1 KO CD4+ T cells have (i) a reduced basal level of the surface CD4 expression and (ii) accelerated decrease of CD4 expression following CD3/CD28 activation (Fig. 5A) that is known to be dependent on protein kinase C and NF-κB activities (53). We previously reported that Fus1 KO mice develop autoimmune syndrome resembling SLE (30). SLE is characterized by a specific pattern of changes in T cells including increased endocytic recycling of CD4 molecules through the NO→mTOR axis (20). We found earlier that CD3+CD4−CD8− (double negative) T cells selectively accumulated in inflammatory milieu of Fus1 KO mice are concomitant with the decrease in CD4+ cell number (62). Combined, these data suggest that Fus1 is required for the maintenance of CD4 levels on unstimulated T cells, thus attenuating CD4 loss from the cell surface upon T-cell stimulation.
The PD-1/PD-L1 regulatory axis plays a central role in the suppression of autoimmune response (34). Both PD-1 and PD-L1 are transcriptional targets of NF-κB (35, 37), whose activity is strictly dependent on mitochondria-mediated ROS production (29) and Ca2+ oscillations (17). We showed here that Fus1 loss attenuates the upregulation of these molecules in activated T cells (Fig. 5B, C). Since PD-1 and PD-L1 are major players in the autoimmune defense, we speculate that their altered expression may precipitate the autoimmune phenotype of Fus1 KO mice (30).
It is now commonly accepted that mitochondria regulate cell cycle progression and proliferation of lymphocytes and other cell types via ROS production, Ca2+ uptake, and changes in Ca2+-dependent parameters (i.e., MMP de- or hyperpolarization) (47, 63). Our data linking Fus1 with intracellular Ca2+ regulation led us to determine if Fus1 was required for proper regulation of T cells proliferation. Indeed, Fus1 loss robustly stimulated proliferation of CD4+ T cells upon activation (Fig. 5E), thus establishing dependence of one of the crucial T-cell effector functions on the Fus1 activity.
One of our most intriguing observations is a significant CD3/CD28-induced Th1 shift in Fus1 KO CD4+ T cells (Fig. 5C). The relative proportion of generated Th1 versus Th2 cells plays a critical role in determining the outcome of the host immune response to an infection. Identification of the various factors involved in regulating this balance remains an active area of investigation (56). There is currently only limited data linking mitochondrial activity and cell polarization. The mitochondrial PRELI protein inhibits Th2 differentiation (56), and lower MCU activity is linked to a lower activation of Th2 cells compared to Th1 cells upon identical stimulation (60). Although these data support a role for mitochondria in Th-cell polarization, important details of this regulation have been elusive. Data presented here are consistent with augmented Th1 differentiation in the absence of Fus1 resulting from impaired mitoCa2+ uptake during early activation steps. Murine Th1 and Th2 lymphocytes differ in the dynamics of Ca2+ response since Th2 cells have more efficient mechanisms of cytoCa2+ clearance (18). We showed that cytoCa2+ level was higher in Fus1 KO CD4+ T cells correlating with the slower mitoCa2+ uptake during first 4 h of activation (Fig. 4). Further experiments will be needed to determine if reduced clearance of cytoCa2+ in the absence of Fus1 stimulates Th1 differentiation at the expense of Th2.
To better understand how decreased Fus1 expression globally alters a variety of immune responses, we have explored molecular pathways dependent on Fus1. It was shown that mitoCa2+ uptake and release regulate the frequency of spontaneous and induced cytoCa2+ oscillations, which, in turn, determine the transcriptional activity of NF-κB and NFAT, two main TFs that regulate T-cell activation (17). Since NF-κB is a redox-sensitive TF (29) and ROS production is regulated by mitoCa2+ uptake (19), changes in mitoCa2+ shift the redox balance, which will further alter NF-κB transcriptional activity. Indeed, comparison of expression profiles of Fus1 WT and Fus1 KO CD4+ T cells revealed enrichment with consensus sites for binding of NF-κB, NFAT, and other calcium-regulated TFs in the promoters of genes upregulated in Fus1 KO cells at the basal level. Also, higher basal expression of Ca2+-binding and Ca2+-regulated proteins in Fus1 KO CD4+ T cells compared to WT cells (Supplementary Table S1) is consistent with higher cytoCa2+ levels in Fus1 KO T cells. Remarkably, stimulated Fus1 KO CD4+ T cells showed compromised activation of the NF-κB and NFAT pathways in agreement with the slower dynamics and lower amplitudes of CD3/CD28-induced mitochondrial changes (ROS, MMP, and mPTP) that we observed in these cells (Fig. 4C–E). These changes may have resulted in insufficient and (or) distorted activation of the NF-κB and NFAT proteins and aberrant expression of their targets.
Thus, correction of mitoCa2+ uptake in Fus1-deficient tissues may significantly improve a variety of biological Ca2+-dependent processes. These data create a basis for designing therapeutic approaches for the treatment of Fus1-deficiency-based diseases, such as autoimmunity, cancer, and inflammation.
Materials and Methods
Flow cytometric analysis of cytoCa2+, mitoCa2+, MMP, mitoROS, and mPTP
Cytoplasmic calcium levels were measured by loading the cells with 1 μM Fluo-3/AM (excitation, 506 nm; emission, 526 nm; recorded in FL-1; Molecular Probes). Mitochondrial calcium level was estimated by loading the cells with 4 μM Rhod2/AM, which is compartmentalized into the mitochondria (46). Production of mitochondrial ROS (superoxide anion) was assessed fluorometrically using oxidation-sensitive fluorescent probes MitoSOX (Molecular Probes) as described elsewhere (43). Δψm was quantitated using a potential-dependent J-aggregate-forming lipophilic cation JC-1, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine iodide (Molecular Probes). JC-1 selectively incorporates into mitochondria, where it forms monomers (fluorescence in green, 527 nm) or aggregates, at high transmembrane potentials (fluorescence in red, 590 nm) (46). Cells were incubated with 0.5 μM JC-1 for 15–45 min at 37°C before flow cytometry. Co-treatment with a protonophore, 5 μM carbonyl cyanide m-chlorophenylhydrazone (Molecular Probes), for 15 min at 37°C resulted in decreased JC-1 fluorescence and served as a positive control for the disruption of Δψm (46). mPTP permeability was monitored through a release of fluorescent calcein from mitochondria via mPTP. The fluorescence from cytosolic calcein was quenched by the addition of CoCl2, whereas the fluorescence from the mitochondrial calcein was maintained. Production of NO was estimated with dye 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM; Molecular Probes). Inside the cell, DAF-FM is deacetylated and retained in the cell. Nonfluorescent DAF-FM after reaction with NO forms fluorescent benzotriazole. For NO level estimation, cells were incubated with 1–5 μM DAF-FM for 1 h at 37°C. Excitation and emission maximum of DAF-FM are 495 and 515 nm, respectively (45). Oxidation of intracellular nonprotein thiols (primarily GSH) was measured using the Cell Tracker Green CMFDA probe, which has a chloromethyl group that, when reacting with thiols, gets converted into a fluorescent adduct. For GSH measurement, cells were incubated with 1 μM CMDFA for 30–60 min at 37°C. Excitation and emission maximum of CMDFA are 492 and 517 nm, respectively (23).
Antibodies and reagents
Conjugated mouse monoclonal antibodies, CD4-FITC, CD4-PE, IFNgamma-PE, IL-4-FITC, PD-1-FITC, and PD-L1-PE (Biolegend), were used for FACS. Mouse CD4+ T-cell isolation kit II was purchased from Miltenyi Biotec. Probes for the measurement of cytosolic (Fluo-3) and mitochondrial Ca2+ (Rhod-2) were purchased from AnaSpec Inc. Probes for other mitochondrial parameters, such as membrane potential (JC-1), reactive oxygen species (MitoSOX), permeability transition pore assay, and mitochondrial morphology (MitoTracker Red), were all from Molecular Probes (Invitrogen). CGP37157, inhibitor of mitochondrial NCX, was obtained from Sigma. Calcium Green-5N for the measurement of Ca2+ uptake by mitochondria in digitonin-permeabilized cells was purchased from Molecular Probes (Invitrogen).
Cell culture
Mouse immortalized kidney epithelial cells were maintained in DMEM/10% FBS medium with 100 μg/ml Anti-Anti mixture (all from Gibco, Inc.) at 37°C and 5% CO2.
Splenocyte isolation, CD4+ T-cell purification and activation
CD4+ cells were purified from the whole splenocyte fraction using CD4+ isolation kit II (Miltenyi Biotec). CD4+ cells were activated, unless otherwise indicated, with plate-bound α-CD3 (1.0 μg/ml) and soluble α-CD28 (1.0 μg/ml; both from Biolegend) and cultured at a density of ∼1×106 cells/ml in 24-well plates in RPMI-1640 medium supplemented with Anti-Anti mixture and 10% FBS.
Mitochondrial morphology imaging
Epithelial cells at ∼70%–80% confluency were treated with Ca2+ and Ca2+/ionomysin for 15 mins and with Mitotracker Red. Cells were then washed and fixed in 3.7% formaldehyde for 15 mins in the dark, permeabilized, and mounted with DAPI-containing mounting media. Zeiss LSM 510 META Upright Confocal Microscope was used for analyzing mitochondrial morphology and making images.
Measurement of mitochondrial Ca2+ uptake capacity of digitonin-permeabilized cells
Extramitochondrial free Ca2+ was monitored in the presence of digitonin-permeabilized cells as described in (44, 50). Briefly, cells (2×106 cells/ml) were resuspended in KCl medium (125 mM KCl, 2 mM K2HPO4, 1 mM MgCl2, 20 mM Hepes, pH 7.0) containing 5 mM glutamate, 5 mM malate, and 5 mM succinate as oxidable substrates and 0.5 μM Ca2+ green-5N. The plasma membranes were then selectively permeabilized with digitonin (0.01%wt/vol final). Fluorescence (Ex506/Em531 nm) was monitored at room temperature using a SynergyMX (BioTek) fluorescence spectrophotometer. The involvement of mNCX in the Ca2+ homeostasis was measured by the replacement of Na-containing buffer with Na-free buffer or by the addition of benzodiazepine CGP37157 (10–20 μM), a blocker of mNCX, to challenged cells.
Flow cytometric analysis of cytoCa2+, mitoCa2+, MMP, mitoROS, mPTP, NO, and GSH was performed as described in detail in Supplementary Data.
CD4+ T-cell proliferation assay
Proliferation of CD4+ T cells was measured by flow cytometry after a CFSE dilution assay as described elsewhere (38). Splenocytes were stained with CFSE (1 μM), stimulated with CD3/CD28 (1 μg/ml of each) or left unstimulated, harvested after 1, 2, and 3 days, stained for a surface CD4 expression with anti-mouse CD4 antibodies labeled with PerCP-Cy5.5 (Biolegend), and acquired on FACS Calibur flow cytometer (Beckman Coulter).
Intracellular cytokine staining
Cytokine staining and analysis was performed as described elsewhere (56). The antibodies used were anti-mouse IFNγ-FITC (1/100 μl permeabilization buffer) and α-IL4-PE (1/100 μl permeabilization buffer) (Biolegend).
Apoptosis T-cell assays
Apoptosis was monitored by flow cytometry after concurrent staining with FITC-conjugated Annexin V (Annexin V-FITC; R&D Systems) (FL-1) and 7-AAD (FL-3) as described elsewhere (46). Apoptosis rates were expressed as a shift in Annexin V binding in PI-negative cells.
CD4+ T cells RNA isolation and microarray analysis
RNA from unstimulated and stimulated for 12 h with CD3/CD28 antibodies CD4+ T cells collected from five animals per group was isolated with the RNAeasy mini RNA isolation kit (Qiagen), mixed together to obtain sufficient for hybridization RNA amount, and subjected to differential expression analysis using gene 1.0 microarray platform, which was performed in the Genomic Core Facility at the Vanderbilt University.
Statistical analysis
Results are presented as mean±SE. Comparisons between the two groups were performed using the Student's t-test. When analyzing statistical differences between the KO and WT mice, p<0.05 was considered significant.
Supplementary Material
Abbreviations Used
- Ca2+
calcium
- cyto
cytoplasmic
- DAF-FM
4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate
- Fluo-3/AM
fluoro-3 acetoxymethyl ester
- GSH
nonprotein thiols including glutathione
- IO
ionomycin
- KO
knockout
- MCU
mitochondrial calcium uniporter
- mito
mitochondrial
- MMP or ΔμH+
mitochondrial membrane potential
- mNCX
mitochondrial sodium–calcium exchanger
- mPTP
mitochondrial permeability transition pore
- Na+
sodium
- NO
nitric oxide
- ROS
reactive oxygen species
- SLE
systemic lupus erythematosus
- WT
wild type
Acknowledgments
I wish to thank Dr. N. Issaeva for help with confocal microscopy analysis. I apologize to those colleagues whose work I could not cite owing to space limitations. This work was supported by grant 1R21ES017496-01A1 to A.V.I. and 1 SC1 CA182843-01A1 to A.S., both from the National Institutes of Health, USA.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ames JB, Ishima R, Tanaka T, Gordon JI, Stryer L, and Ikura M. Molecular mechanics of calcium-myristoyl switches. Nature 389: 198–202, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Antico Arciuch VG, Elguero ME, Poderoso JJ, and Carreras MC. Mitochondrial regulation of cell cycle and proliferation. Antioxid Redox Signal 16: 1150–1180, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aon MA, Cortassa S, Maack C, and O'Rourke B. Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J Biol Chem 282: 21889–21900, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, and Mootha VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476: 341–345, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertolani C, Sancho-Bru P, Failli P, Bataller R, Aleffi S, DeFranco R, Mazzinghi B, Romagnani P, Milani S, Gines P, Colmenero J, Parola M, Gelmini S, Tarquini R, Laffi G, Pinzani M, and Marra F. Resistin as an intrahepatic cytokine: overexpression during chronic injury and induction of proinflammatory actions in hepatic stellate cells. Am J Pathol 169: 2042–2053, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brookes PS. Mitochondrial H(+) leak and ROS generation: an odd couple. Free Radic Biol Med 38: 12–23, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Brookes PS, Yoon Y, Robotham JL, Anders MW, and Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 287: C817–C833, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Camello-Almaraz MC, Pozo MJ, Murphy MP, and Camello PJ. Mitochondrial production of oxidants is necessary for physiological calcium oscillations. J Cell Physiol 206: 487–494, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Catala-Rabasa A, Ndagire D, Sabio JM, Fedetz M, Matesanz F, and Alcina A. High ACSL5 transcript levels associate with systemic lupus erythematosus and apoptosis in Jurkat T lymphocytes and peripheral blood cells. PLoS One 6: e28591, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cereghetti GM, Stangherlin A, Martins de Brito O, Chang CR, Blackstone C, Bernardi P, and Scorrano L. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci U S A 105: 15803–15808, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaptal V, Besserer GM, Ottolia M, Nicoll DA, Cascio D, Philipson KD, and Abramson J. How does regulatory Ca2+ regulate the Na+-Ca2+ exchanger? Channels (Austin) 1: 397–399, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Chen H. and Chan DC. Mitochondrial dynamics—fusion, fission, movement, and mitophagy—in neurodegenerative diseases. Hum Mol Genet 18: R169–R176, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortassa S, Aon MA, Marban E, Winslow RL, and O'Rourke B. An integrated model of cardiac mitochondrial energy metabolism and calcium dynamics. Biophys J 84: 2734–2755, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Stefani D, Raffaello A, Teardo E, Szabo I, and Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476: 336–340, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dedkova EN. and Blatter LA. Modulation of mitochondrial Ca2+ by nitric oxide in cultured bovine vascular endothelial cells. Am J Physiol Cell Physiol 289: C836–C845, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Detmer SA. and Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol 8: 870–879, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Dolmetsch RE, Lewis RS, Goodnow CC, and Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386: 855–858, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Fanger CM, Neben AL, and Cahalan MD. Differential Ca2+ influx, KCa channel activity, and Ca2+ clearance distinguish Th1 and Th2 lymphocytes. J Immunol 164: 1153–1160, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Feissner RF, Skalska J, Gaum WE, and Sheu SS. Crosstalk signaling between mitochondrial Ca2+ and ROS. Front Biosci (Landmark Ed) 14: 1197–1218, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez D. and Perl A. Metabolic control of T cell activation and death in SLE. Autoimmun Rev 8: 184–189, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol 7: 690–702, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Fulop L, Szanda G, Enyedi B, Varnai P, and Spat A. The effect of OPA1 on mitochondrial Ca(2)(+) signaling. PLoS One 6: e25199, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillis C, Haegerstrand A, Ragnarson B, and Bengtsson L. Rapid visualization of viable and nonviable endothelium on cardiovascular prosthetic surfaces by means of fluorescent dyes. J Thorac Cardiovasc Surg 108: 1043–1048, 1994 [PubMed] [Google Scholar]
- 24.Glancy B. and Balaban RS. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry 51: 2959–2973, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo L, Urban JF, Zhu J, and Paul WE. Elevating calcium in Th2 cells activates multiple pathways to induce IL-4 transcription and mRNA stabilization. J Immunol 181: 3984–3993, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol 46: 821–831, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Hernandez-SanMiguel E, Vay L, Santo-Domingo J, Lobaton CD, Moreno A, Montero M, and Alvarez J. The mitochondrial Na+/Ca2+ exchanger plays a key role in the control of cytosolic Ca2+ oscillations. Cell Calcium 40: 53–61, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Hogan PG, Chen L, Nardone J, and Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev 17: 2205–2232, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Hughes G, Murphy MP, and Ledgerwood EC. Mitochondrial reactive oxygen species regulate the temporal activation of nuclear factor kappaB to modulate tumour necrosis factor-induced apoptosis: evidence from mitochondria-targeted antioxidants. Biochem J 389: 83–89, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivanova AV, Ivanov SV, Pascal V, Lumsden JM, Ward JM, Morris N, Tessarolo L, Anderson SK, and Lerman MI. Autoimmunity, spontaneous tumourigenesis, and IL-15 insufficiency in mice with a targeted disruption of the tumour suppressor gene Fus1. J Pathol 211: 591–601, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Ivanova AV, Ivanov SV, Prudkin L, Nonaka D, Liu Z, Tsao A, Wistuba I, Roth J, and Pass HI. Mechanisms of FUS1/TUSC2 deficiency in mesothelioma and its tumorigenic transcriptional effects. Mol Cancer 8: 91, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang D, Zhao L, and Clapham DE. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science 326: 144–147, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaminski MM, Roth D, Sass S, Sauer SW, Krammer PH, and Gulow K. Manganese superoxide dismutase: a regulator of T cell activation-induced oxidative signaling and cell death. Biochim Biophys Acta 1823: 1041–1052, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Kim HK, Guan H, Zu G, Li H, Wu L, Feng X, Elmets C, Fu Y, and Xu H. High-level expression of B7-H1 molecules by dendritic cells suppresses the function of activated T cells and desensitizes allergen-primed animals. J Leukoc Biol 79: 686–695, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Kondo A, Yamashita T, Tamura H, Zhao W, Tsuji T, Shimizu M, Shinya E, Takahashi H, Tamada K, Chen L, Dan K, and Ogata K. Interferon-gamma and tumor necrosis factor-alpha induce an immunoinhibitory molecule, B7-H1, via nuclear factor-kappaB activation in blasts in myelodysplastic syndromes. Blood 116: 1124–1131, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JH, Kye KC, Seo EY, Lee K, Lee SK, Lim JS, Seo YJ, Kim CD, and Park JK. Expression of neutrophil gelatinase-associated lipocalin in calcium-induced keratinocyte differentiation. J Korean Med Sci 23: 302–306, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, and Sharpe AH. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol 33: 2706–2716, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Lyons AB. and Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods 171: 131–137, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, and O'Rourke B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res 99: 172–182, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mallilankaraman K, Cardenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenar T, Csordas G, Madireddi P, Yang J, Muller M, Miller R, Kolesar JE, Molgo J, Kaufman B, Hajnoczky G, Foskett JK, and Madesh M. MCUR1 is an essential component of mitochondrial Ca(2+) uptake that regulates cellular metabolism. Nat Cell Biol 15: 123, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marchi S, Giorgi C, Suski JM, Agnoletto C, Bononi A, Bonora M, De Marchi E, Missiroli S, Patergnani S, Poletti F, Rimessi A, Duszynski J, Wieckowski MR, and Pinton P. Mitochondria-ros crosstalk in the control of cell death and aging. J Signal Transduct 2012: 329635, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mukherjee P, Sen PC, and Ghose AC. Lymph node cells from BALB/c mice with chronic visceral leishmaniasis exhibiting cellular anergy and apoptosis: involvement of Ser/Thr phosphatase. Apoptosis 11: 2013–2029, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, and Horng T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci U S A 109: 11282–11287, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy AN, Bredesen DE, Cortopassi G, Wang E, and Fiskum G. Bcl-2 potentiates the maximal calcium uptake capacity of neural cell mitochondria. Proc Natl Acad Sci U S A 93: 9893–9898, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagy G, Barcza M, Gonchoroff N, Phillips PE, and Perl A. Nitric oxide-dependent mitochondrial biogenesis generates Ca2+ signaling profile of lupus T cells. J Immunol 173: 3676–3683, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagy G, Koncz A, and Perl A. T cell activation-induced mitochondrial hyperpolarization is mediated by Ca2+- and redox-dependent production of nitric oxide. J Immunol 171: 5188–5197, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nunez L, Valero RA, Senovilla L, Sanz-Blasco S, Garcia-Sancho J, and Villalobos C. Cell proliferation depends on mitochondrial Ca2+ uptake: inhibition by salicylate. J Physiol 571: 57–73, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palty R. and Sekler I. The mitochondrial Na(+)/Ca(2+) exchanger. Cell Calcium 52: 9–15, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Parone PA, Da Cruz S, Tondera D, Mattenberger Y, James DI, Maechler P, Barja F, and Martinou JC. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS One 3: e3257, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, and Mootha VK. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature 467: 291–296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prudkin L, Behrens C, Liu DD, Zhou X, Ozburn NC, Bekele BN, Minna JD, Moran C, Roth JA, Ji L, and Wistuba II. Loss and reduction of FUS1 protein expression is a frequent phenomenon in the pathogenesis of lung cancer. Clin Cancer Res 14: 41–47, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quintana A, Pasche M, Junker C, Al-Ansary D, Rieger H, Kummerow C, Nunez L, Villalobos C, Meraner P, Becherer U, Rettig J, Niemeyer BA, and Hoth M. Calcium microdomains at the immunological synapse: how ORAI channels, mitochondria and calcium pumps generate local calcium signals for efficient T-cell activation. EMBO J 30: 3895–3912, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raposo RA, Trudgian DC, Thomas B, van Wilgenburg B, Cowley SA, and James W. Protein kinase C and NF-kappaB-dependent CD4 downregulation in macrophages induced by T cell-derived soluble factors: consequences for HIV-1 infection. J Immunol 187: 748–759, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roth J, Goebeler M, and Sorg C. S100A8 and S100A9 in inflammatory diseases. Lancet 357: 1041, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Shen X, Franzini-Armstrong C, Lopez JR, Jones LR, Kobayashi YM, Wang Y, Kerrick WG, Caswell AH, Potter JD, Miller T, Allen PD, and Perez CF. Triadins modulate intracellular Ca(2+) homeostasis but are not essential for excitation-contraction coupling in skeletal muscle. J Biol Chem 282: 37864–37874, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Tahvanainen J, Kallonen T, Lahteenmaki H, Heiskanen KM, Westermarck J, Rao KV, and Lahesmaa R. PRELI is a mitochondrial regulator of human primary T-helper cell apoptosis, STAT6, and Th2-cell differentiation. Blood 113: 1268–1277, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Tanaka T, Ames JB, Harvey TS, Stryer L, and Ikura M. Sequestration of the membrane-targeting myristoyl group of recoverin in the calcium-free state. Nature 376: 444–447, 1995 [DOI] [PubMed] [Google Scholar]
- 58.Thiel M, Wolfs MJ, Bauer S, Wenning AS, Burckhart T, Schwarz EC, Scott AM, Renner C, and Hoth M. Efficiency of T-cell costimulation by CD80 and CD86 cross-linking correlates with calcium entry. Immunology 129: 28–40, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thyagarajan B, Malli R, Schmidt K, Graier WF, and Groschner K. Nitric oxide inhibits capacitative Ca2+ entry by suppression of mitochondrial Ca2+ handling. Br J Pharmacol 137: 821–830, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toldi G, Kaposi A, Zsembery A, Treszl A, Tulassay T, and Vasarhelyi B. Human Th1 and Th2 lymphocytes are distinguished by calcium flux regulation during the first 10 min of lymphocyte activation. Immunobiology 217: 37–43, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Uno F, Sasaki J, Nishizaki M, Carboni G, Xu K, Atkinson EN, Kondo M, Minna JD, Roth JA, and Ji L. Myristoylation of the fus1 protein is required for tumor suppression in human lung cancer cells. Cancer Res 64: 2969–2976, 2004 [DOI] [PubMed] [Google Scholar]
- 62.Uzhachenko R, Issaeva N, Boyd K, Ivanov SV, Carbone DP, and Ivanova AV. Tumour suppressor Fus1 provides a molecular link between inflammatory response and mitochondrial homeostasis. J Pathol 227: 456–469, 2012 [DOI] [PubMed] [Google Scholar]
- 63.Valero RA, Senovilla L, Nunez L, and Villalobos C. The role of mitochondrial potential in control of calcium signals involved in cell proliferation. Cell Calcium 44: 259–269, 2008 [DOI] [PubMed] [Google Scholar]
- 64.van Lent PL, Grevers LC, Blom AB, Arntz OJ, van de Loo FA, van der Kraan P, Abdollahi-Roodsaz S, Srikrishna G, Freeze H, Sloetjes A, Nacken W, Vogl T, Roth J, and van den Berg WB. Stimulation of chondrocyte-mediated cartilage destruction by S100A8 in experimental murine arthritis. Arthritis Rheum 58: 3776–3787, 2008 [DOI] [PubMed] [Google Scholar]
- 65.Ventura-Clapier R, Garnier A, and Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res 79: 208–217, 2008 [DOI] [PubMed] [Google Scholar]
- 66.Waldeck-Weiermair M, Duan X, Naghdi S, Khan MJ, Trenker M, Malli R, and Graier WF. Uncoupling protein 3 adjusts mitochondrial Ca(2+) uptake to high and low Ca(2+) signals. Cell Calcium 48: 288–301, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waldeck-Weiermair M, Jean-Quartier C, Rost R, Khan MJ, Vishnu N, Bondarenko AI, Imamura H, Malli R, and Graier WF. Leucine zipper EF hand-containing transmembrane protein 1 (Letm1) and uncoupling proteins 2 and 3 (UCP2/3) contribute to two distinct mitochondrial Ca2+ uptake pathways. J Biol Chem 286: 28444–28455, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yap LP, Garcia JV, Han D, and Cadenas E. The energy-redox axis in aging and age-related neurodegeneration. Adv Drug Deliv Rev 61: 1283–1298, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zozulya S. and Stryer L. Calcium-myristoyl protein switch. Proc Natl Acad Sci U S A 89: 11569–11573, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.