Abstract
Significance: Manganese superoxide dismutase (MnSOD) is a nuclear-encoded and mitochondria-matrix-localized oxidation-reduction (redox) enzyme that regulates cellular redox homeostasis. Cellular redox processes are known to regulate proliferative and quiescent growth states. Therefore, MnSOD and mitochondria-generated reactive oxygen species (ROS) are believed to be critical regulators of quiescent cells' entry into the cell cycle and exit from the proliferative cycle back to the quiescent state. Recent Advances/Critical Issues: Recent evidence suggests that the intracellular redox environment fluctuates during the cell cycle, shifting toward a more oxidized status during mitosis. MnSOD activity is higher in G0/G1 cells compared with S, G2 and M phases. After cell division, MnSOD activity increases in the G1 phase of the daughter generation. The periodic fluctuation in MnSOD activity during the cell cycle inversely correlates with cellular superoxide levels as well as glucose and oxygen consumption. Based on an inverse correlation between MnSOD activity and glucose consumption during the cell cycle, it is proposed that MnSOD is a central molecular player for the “Warburg effect.” Future Directions: In general, loss of MnSOD activity results in aberrant proliferation. A better understanding of the MnSOD and mitochondrial ROS-dependent cell cycle processes may lead to novel approaches to overcome aberrant proliferation. Since ROS have both deleterious (pathological) and beneficial (physiological) effects, it is proposed that “eustress” should be used when discussing ROS processes that regulate normal physiological functions, while “oxidative stress” should be used to discuss the deleterious effects of ROS. Antioxid. Redox Signal. 20, 1618–1627.
Introduction
The first evidence that intracellular oxidation-reduction (redox) reactions regulate cell division dates back to 1931 when Louis Rapkine demonstrated the cyclic pattern of intracellular soluble thiols during mitosis of sea urchin eggs (67). Subsequently, Kawamura and Dan (34) showed that the staining of protein thiols increased in the prophase of sea urchin eggs as the mitotic spindle was assembling. This increase in protein thiols remained high as cells moved into metaphase, followed by a gradual decrease in anaphase, and was almost undetectable in telophase. In synchronous populations of human adenocarcinoma (HeLa) cells, Mauro et al. (45) observed that the concentration of non-protein thiols increased approximately 3-fold during mitosis compared with interphase. Using an oxidation-sensitive chemical and flow cytometry measurements of cell cycle positions, we have demonstrated that the intracellular redox state shifts toward a more oxidized environment during mitosis compared with interphase in synchronized HeLa cell cultures (24). Furthermore, we observed a gradual increase in cellular glutathione (GSH) levels as the cells progressed through the cell cycle (Goswami and Spitz, unpublished observations), which was also consistent with a recent report from Conour et al. (13) demonstrating a significant increase in cellular GSH levels during S and G2 phases of the cell cycle compared with G1 phase. Moreover, we have shown a transient increase in pro-oxidant levels during the G1 phase that is required for the mouse embryonic fibroblasts (MEFs) to initiate DNA synthesis. Inhibiting this pro-oxidant event using an antioxidant (N-acetyl-L-cysteine [NAC]) significantly inhibited G1 cells' entry into S phase (49). Results from these previous studies have established the concept of a redox cycle (Fig. 1) within the mammalian cell cycle, coordinating cell cycle progression with cellular metabolism (47).
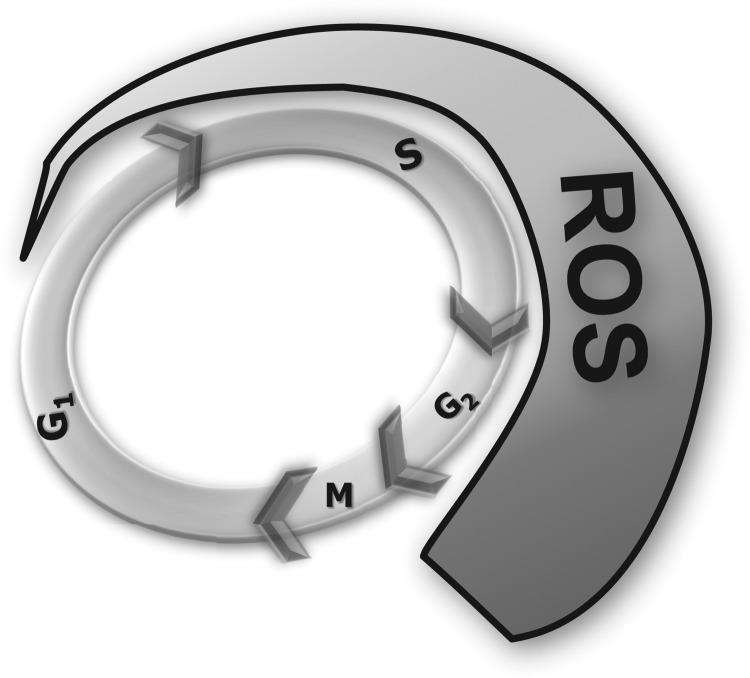
FIG. 1.
An illustration showing an increase in intracellular ROS levels during progression from G1 to S to G2 and M phases. ROS, reactive oxygen species.
Cell Cycle Regulatory Proteins
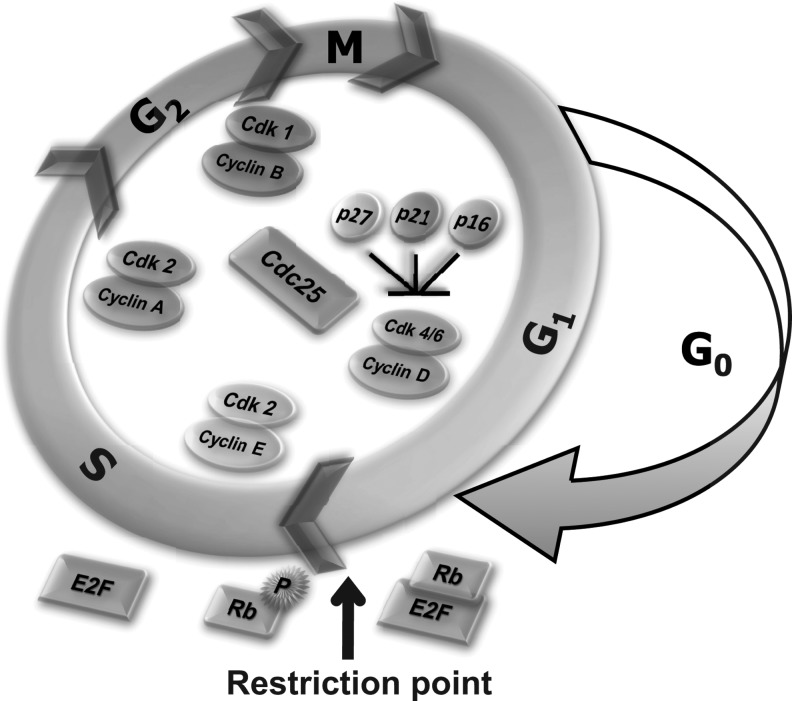
The sequential progression through the cell cycle is regulated by the periodic activation of cell cycle regulatory proteins (Fig. 2). The independent discoveries of cyclins in sea urchin oocytes, maturation-promoting factors in frog oocytes, and cell-division-cycle proteins in Saccharomyces cerevisiae corroborate to the present concept of cyclin and cyclin-dependent kinase (CDK) complexes regulating the cell cycle (18, 29, 44). The cyclin D family (D1, D2, and D3) in combination with CDK4 and CDK6 facilitates the cells' entry from the quiescent (G0) phase to the G1 phase of the proliferative cycle (80, 81). Cyclin D1 is the second most commonly amplified gene in the human cancer genome (3). Phosphorylation at Thr286 by glycogen synthase kinase (GSK-3β) activates proteasomal degradation of cyclin D1 (16). Phosphorylation of GSK-3β by protein kinase B (AKT) inactivates GSK-3β kinase activity, thereby stabilizing cyclin D1, which, in turn, facilitates the cells' entry from G0 to G1 phase (10). GSK-3β-independent and mirk/dyrk kinase-dependent phosphorylation at the Thr288 residue can also degrade cyclin D1 (92). Cyclin D1-CDK4/CDK6 phosphorylates the retinoblastoma (Rb) family of proteins (p110, p107, and p130), inactivating Rb and releasing E2F, a transcription factor that activates the transcription of several S-phase specific genes which are required for DNA synthesis (28, 52, 63). The transition from hypo- to hyper-phosphorylation of Rb with the subsequent release of E2F occurs at the “restriction point” (Fig. 2). Arthur B. Pardee defined the “restriction point” as the cells' duration in G1 phase after which the cells are committed to enter the S phase independent of the external conditions (65). Several recent studies indicate that cyclin D1 has a regulatory role in DNA repair as well as mitochondrial functions that are independent of its CDK4/CDK6-dependent cell cycle regulation (32, 71, 87).
FIG. 2.
Cell cycle machinery regulating progression from G1 to S to G2 and M phases. Cyclins and CDKs are the positive regulators of the cell cycle. CKIs (p16, p21, and p27) are the negative regulators of the cell cycle. Hypo-phosphorylated Rb sequesters E2F, which, in turn, restricts entry into S phase. Cyclin D/CDK 4,6 phosphorylates Rb, and this phosphorylation of Rb releases E2F. E2F is a transcription factor that is required for the transcription of multiple S-phase specific genes. Restriction point refers to duration in the G1 phase, beyond which cells will continue to S phase and cell division independent of the external environment. CDK, cyclin-dependent kinases; CKIs, cyclin-dependent kinase inhibitors; Rb, retinoblastoma.
Cyclin E is the next cell cycle regulatory protein that is activated late in the G1 phase. Cyclin E in association with CDK2 further phosphorylates Rb and inactivates its function (70). Cyclin E is degraded once cells enter S phase. Recent evidence suggests that cyclin E integrates the mitochondrial fusion process to an increase in ATP production and the initiation of DNA synthesis at the G1/S border (21, 51). The cyclin A/CDK2 kinase complex is activated during S phase, while transit through G2 phase of the cell cycle is associated with the activation of the cyclin A/CDK1 and cyclin B1/CDK1 kinase complexes. Progression through M phase requires the kinase activity of cyclin B1/CDK1 (66). It is interesting to note that cyclin B1/CDK1-dependent phosphorylation of dynamin-related protein-1 (Ser-585) regulates mitochondrial fission during mitosis (82). These recent reports further suggest the existence of a cross-talk between mitochondrial function and cell cycle regulatory machinery.
The complicated regulation of the cell cycle machinery is further evident from the observations that the kinase activity of cyclin/CDK complexes is regulated by a family of dual specific phosphatases. CDC25 (A, B, and C) dephosphorylates pThr14 and pTyr15 on CDKs, which leads to the activation of cyclin/CDK activity (79). While cyclin/CDKs are the positive regulators of the cell cycle, CDK inhibitors (CKIs) are the negative regulators of the cell cycle (25). CKIs regulate the assembly and activity of the cyclin/CDK complexes. They are broadly classified into two families: INK4 (inhibitors of CDK4) and CIP/KIP (CDK inhibitory protein)/(Kinase inhibitory protein). The INK4-family of proteins (p16 [INK4A], p15 [INK4B], p18 [INK4C], and p19 [INK4D]) bind CDK4/CDK6 and inhibit their kinase activities. The KIP family (p27 and p57) inhibits mainly cyclin E/CDK2 kinase complexes. The inhibitory effect of p21 has a broader specificity, and it can inhibit all cyclin/CDK activities. Thus, redox control of this complex cell cycle molecular network can influence progression from G0/G1 to S to G2 and M phases.
Cellular Antioxidant Network
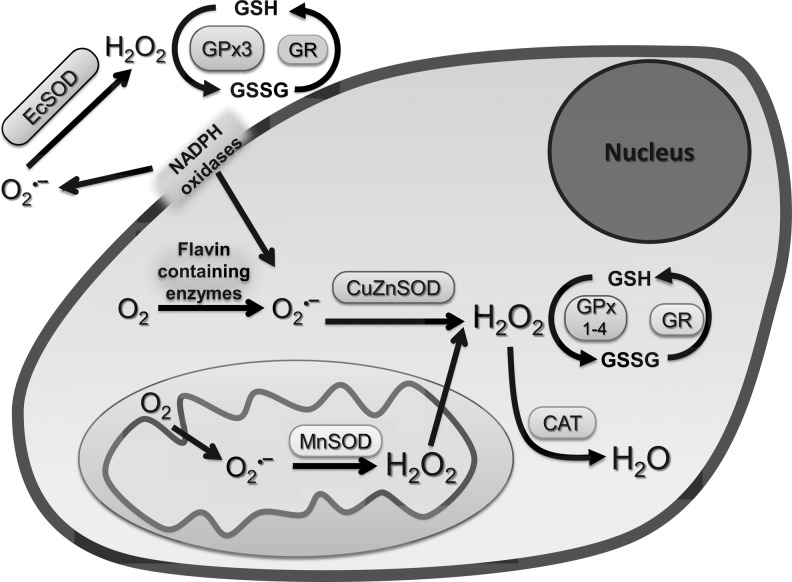
The intracellular redox environment is influenced by the production of reactive oxygen species (ROS: superoxide and hydrogen peroxide [H2O2]) produced during metabolism and the antioxidant network that removes ROS (Fig. 3). The production of superoxide (O2•−) and H2O2 are the consequence of the incomplete reduction of molecular oxygen (27). Intracellular production of ROS results primarily from the mitochondrial electron transport chain as well as from oxygen-metabolizing flavin-containing proteins, for example, xanthine oxidases, cytochrome P450, nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, and myeloperoxidase (5, 27, 37, 43, 69, 78, 91).
FIG. 3.
An illustration showing the intracellular prooxidant and antioxidant network. SOD, superoxide dismutase; Ec, extracellular; GPx, glutathione peroxidase; CAT, catalase; GSH, glutathione; GSSG, glutathione disulfide; GR, glutathione reductase.
The cellular antioxidant network includes both the antioxidant enzyme systems and small-molecular-weight antioxidants. Small-molecular-weight antioxidants include GSH, cysteine, ascorbic acid, and α-tocopherol. The antioxidant enzyme network includes superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), glutaredoxin, thioredoxin, and the six-member family of peroxiredoxin (15, 19, 68, 85). SOD converts superoxide to H2O2. There are three SOD enzymes in mammalian cells (Fig. 3). MnSOD is a nuclear-encoded and mitochondria-matrix-localized homotetrameric enzyme that converts mitochondrially generated superoxide to H2O2 (46). Copper zinc SOD (CuZnSOD) is localized in the cytoplasm, nucleus, and mitochondria inter-membrane space (20). The third member of the SOD family, extracellular SOD (EcSOD) is present in the plasma membrane (23). Catalase and GPx 1–4 neutralize H2O2 to water. Catalase is localized primarily in the peroxisomes, and different isozymes of GPx are found in most sub-cellular compartments (59, 90). Therefore, changes in the antioxidant network can significantly impact cellular ROS levels, which can then influence various biological endpoints such as cell proliferation, differentiation, and cell death (75).
Earlier research presented ROS as toxic byproducts of living in an aerobic environment. ROS damage cellular macromolecules (nucleic acids, proteins, and lipids), leading to the activation of the cell death processes (apoptosis, necrosis, mitotic catastrophe, etc.). However, both our research and that of others has shown that ROS have normal physiological functions, where they can act as signaling molecules (second messengers) regulating numerous cellular processes, including cellular proliferation (2, 9, 47–49). The mechanisms regulating receptor-ligand and ROS-signaling are distinct. Receptor-ligand signaling involves non-covalent bond formation and complementarity in molecular shape. In contrast, ROS signaling involves covalent bond formation. The second-messenger properties of ROS are believed to be carried out by the thiol-disulfide exchange reactions at specific cysteine residues that are present in various signaling proteins, for example, tyrosine kinases, tyrosine phosphatases, mitogen-activated protein (MAP) kinases, or ion channels (64). The dual function of ROS (signaling molecules and toxins) could result from the differences in their concentrations (threshold), pulse duration (flux), and sub-cellular localization. This hypothesis is consistent with an earlier report by Laurent et al. (39), where both the mitogenic and toxic effects of H2O2 were clearly demonstrated in NIH 3T3 mouse fibroblasts cultured in vitro. H2O2 (0.02–0.13 μM) enhanced proliferation, while treatment with 0.25–2 μM H2O2 resulted in cell death. Therefore, while higher levels of ROS can be toxic, low levels of ROS may serve as signaling molecules regulating numerous cellular processes, including proliferation. Since SODs convert superoxide to H2O2, it is reasonable to postulate that SOD can regulate a redox cycle within the cell cycle, thereby influencing redox processes that facilitate progression from G0/G1 to S to G2 and M phases.
MnSOD and the Cell Cycle
MnSOD and cell cycle progression
MnSOD (sod2; EC1.15.1.1) is a nuclear-encoded and mitochondria-matrix localized antioxidant enzyme that converts mitochondria-generated superoxide to H2O2 (35). Human MnSOD is present in chromosome 6, and it has five exons and two transcripts (1.5 and 4.2 kb) that have the same open reading frame but which differ in the length of their 3′-untranslated region. MnSOD is a homotetramer with a subunit molecular mass of 22 kDa and one Mn3+ per monomer. A five-coordinate bipyramidal geometry in each subunit is provided by His-26 and His-74 from the helical domain and Asp-159 and His-163 from the β-sheet domain along with a water molecule (6). Since mitochondria are the major source of intracellular ROS generation, MnSOD is believed to be an important regulator of numerous cellular processes that include cell cycle progression.
In a series of elegant papers, Oberley et al. first hypothesized the involvement of MnSOD and ROS during proliferation of normal and cancer cells, cell differentiation, and aging (55, 57, 58). According to the “unified theory” proposed by Oberley et al., a cell division signal is initiated in the plasma membrane, leading to the production of H2O2. The membrane-originated signal alters the ratio of cyclic nucleotide levels, and it inactivates catalase and peroxidase, resulting in the accumulation of H2O2. Signal-initiated accumulation of H2O2 stimulates glucose uptake, which then facilitates cellular proliferation. The authors proposed that increased levels of oxygen will halt cellular proliferation, while lower fluxes of superoxide will support proliferation. Furthermore, they proposed that a loss (or decreased levels) of MnSOD combined with increases in superoxide levels will inhibit differentiation and support proliferation (58).
The authors went on to discuss that such a hypothesis would also account for increased glycolysis in cancer cells. Warburg (88) proposed that the increased glycolysis (“Warburg effect”) in cancer cells is caused by damage to the respiratory system (i.e., mitochondria). Oberley et al. (58) proposed that the damage in cancer cell mitochondria could be caused due to a loss (or decrease) in MnSOD expression. It is anticipated that a decrease in MnSOD expression will increase superoxide (ROS) production, which will lead to an increase in glycolysis and cancer cell proliferation. Although both theories (“Warburg effect” and “unified theory”) of cancer cell proliferation have their own merits and limitations, these theories stimulated research efforts to examine a causal relationship between mitochondrial function and cellular proliferation.
MnSOD protein levels and activity were found to be higher in quiescent (G0) compared with proliferating NIH 3T3 mouse fibroblasts and WI38 human lung fibroblasts (40, 56, 60). NIH 3T3 fibroblasts synchronized to quiescence by serum starvation showed a significant increase in MnSOD expression, which decreased substantially during S phase after the synchronized cells had been allowed to enter the proliferative cycle by adding serum to the culture medium (40). We have shown that MnSOD activity in MCF-10A human mammary epithelial cells decreased from 120 U/mg in quiescent state to 30 U/mg in proliferating cells (74). In synchronized cell populations of MB231 human mammary epithelial cancer cells, MnSOD activity showed a cyclic pattern: G1/S phase, 40 U/mg; G2+M phases, 26 U/mg; and G1 phase of the daughter generation, 63 U/mg (74). It is interesting to note that MnSOD activity in the G1 phase of the daughter generation is comparable to MnSOD activity in the G1 phase of the parental generation, indicating that the cyclic pattern of MnSOD activity during the parental generation is faithfully preserved in the daughter generation. Overexpression of MnSOD delays cell cycle progression in NIH 3T3 fibroblasts by extending the transit time of G1 and S phases without any change in G2 transit time (36).
A regulatory role of MnSOD during the cell cycle is also evident from our studies using both genetic and pharmacological manipulations of MnSOD expression (72, 73, 76, 77). MEFs with wild-type MnSOD showed a typical growth curve consisting of a lag, exponential, and plateau phase (77). Exit from the exponential to plateau phase was delayed in MnSOD heterozygous MEFs, while MnSOD homozygous knockout MEFs failed to exit the proliferative cycle. The inhibition in cellular proliferation of MnSOD heterozygous MEFs was associated with a delayed transit through G2+M phases. Overexpression of MnSOD facilitated exit of heterozygous MEFs from the proliferative to the quiescent state. Furthermore, results from our recent study also showed an inverse correlation between MnSOD activity and the percentage of S-phase cells (12).
A lower MnSOD activity was also observed in highly proliferating cancer cells (14, 30, 54, 61, 86, 89). As anticipated, overexpression of MnSOD suppressed cancer cell proliferation in both cells cultured in vitro and mouse xenograft of human cancers (14, 30, 54, 61, 86, 89). MnSOD overexpression-induced suppression of proliferation in androgen-independent human prostate cancer (PC-3) cells was associated with a delay in progression from G1 to S phase (86). Thus, a lower MnSOD activity is associated with cellular proliferation, whereas a higher MnSOD activity supports cellular quiescence. Furthermore, a cyclic pattern of MnSOD activity during the cell cycle of the parental generation is faithfully replicated in the daughter generation.
The periodic change in MnSOD activity during the cell cycle regulating cell cycle phase-specific response is also evident under conditions of oxidative stress. Ionizing radiation (IR)-induced chromosomal aberration in human lymphocytes was higher in G2 and M phases, correlating with a decrease in MnSOD activity; while G0 cells with a higher MnSOD activity correlated with a lower incidence of chromosomal aberration (53). Furthermore, IR-induced chromosomal aberration in G2 and M phases was suppressed in SOD-treated human lymphocytes (11). High and low LET radiation-induced cell cycle phase-specific toxicity also correlated with the periodic changes in MnSOD activity during the cell cycle. A higher MnSOD activity during G1 phase was associated with radioresistance, while radiosensitization of G2 cells correlated with a lower MnSOD activity (4). Consistent with these results, we reported radioresistance of MnSOD overexpressing SCC25 human oral squamous carcinoma cells and MIA PaCa-2 human pancreatic cancer cells (22, 33). MnSOD overexpressing irradiated cells showed a higher percentage of G2 cells, which was associated with a decrease in phosphorylated ATM and cyclin B1 protein levels. Furthermore, IR-induced G2-checkpoint activation was absent in MnSOD homozygous knockout MEFs (17, 26, 41, 62, 83). Inhibition of MnSOD expression in MCF7 human breast cancer cells adapted to low-dose IR decreased cyclin B1, cyclin D1, and p21 expression (26, 41, 62). These results suggest that the cell cycle phase-specific radiation response is regulated via MnSOD activity-dependent control of cell cycle gene expression.
Regulation of MnSOD activity during the cell cycle
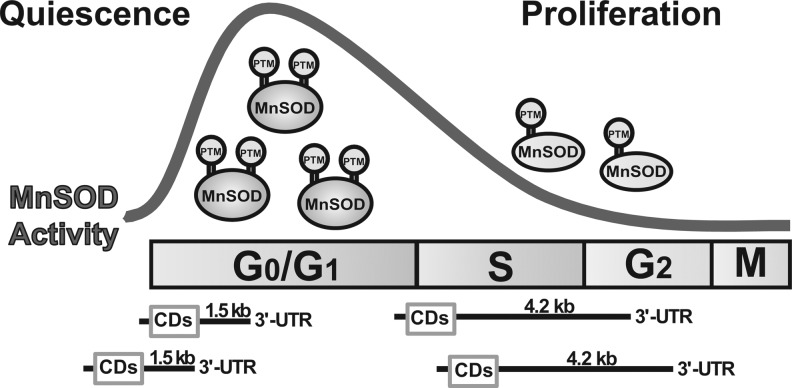
The mechanisms regulating the cyclic pattern of MnSOD activity during the cell cycle appear to be highly complex (Fig. 4). Forkhead transcription factors (FOXO1 and FOXO3a) have been shown to increase MnSOD transcription during quiescence (1, 38). NFκB, a well-studied transcription factor, positively regulates MnSOD transcription. During entry into the proliferative cycle, E2F (transcription factor required for transcription of multiple genes before the initiation of DNA synthesis) competes with the p65 subunit of NFκB and inhibits the binding of p65 to its heterodimer subunit (p50), leading to an inactivation of NFκB transcriptional activity. E2F-induced inactivation of NFκB suppressed MnSOD transcription, which correlated with a lower MnSOD activity during transition from quiescence to the proliferating cycle (84).
FIG. 4.
Regulation of MnSOD expression during quiescence and proliferation. Human MnSOD has two transcripts that carry the same CD but which differ in their 3′-untranslated region. The 1.5 kb transcript is more abundant during quiescence, while the 4.2 kb transcript is enriched during proliferation. MnSOD activity is also regulated by methylation-demethylation PTM. Ser-89 methylation is associated with a higher MnSOD activity during quiescence. CD, coding region; PTM, post-translational modifications.
We recently demonstrated a preferential abundance of MnSOD transcripts during quiescent and proliferative growth states (12). Human MnSOD has two transcripts (1.5 and 4.2 kb) with the same open reading frame but differing in the length of their 3'-untranslated regions. MnSOD 1.5 kb transcript is more abundant in quiescent cells, which correlated with a higher MnSOD activity (Fig. 4). A higher abundance of the 4.2 kb MnSOD transcript in proliferating cells was associated with a lower level of MnSOD activity (Fig. 4).
The complexity of MnSOD expression during the cell cycle is affirmed from our recent finding of a post-translational modification of MnSOD methylation pattern during quiescence and proliferation (74). Results from tandem mass spectrometry (MS2) analysis showed that MnSOD is methylated at both lysine (68, 89, 122, and 202) and arginine (197 and 216) residues. Lysine-89 is mono-methylated during quiescence and un-methylated in proliferating cells. Additional results from site-directed mutagenesis demonstrate that lysine-89 methylation significantly enhanced MnSOD activity. These results are supported by computational-based molecular modeling simulations. It is anticipated that lysine and arginine methylation of MnSOD during quiescence will increase the accessibility of the active site and the surface area of positive electrostatic potential around and within the active site. These post-translational modifications could then increase the accessibility of superoxide (negatively charged substrate) to the enzyme active site more during quiescence compared with the proliferative state. This is consistent with our observation of a higher MnSOD activity during quiescence and a decrease in MnSOD activity in proliferating cells (74).
MnSOD Regulates a ROS Switch During the Cell Cycle
MnSOD and ROS (superoxide and H2O2) levels during the cell cycle
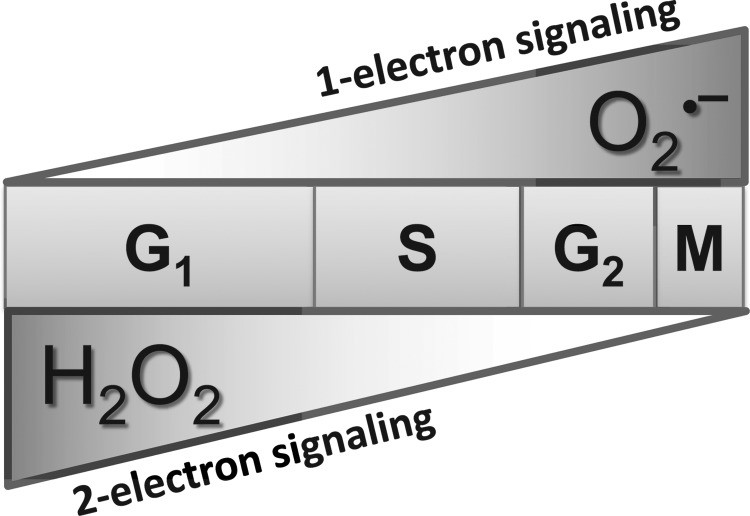
The cyclic pattern of MnSOD activity during the cell cycle correlates with changes in cellular ROS levels. Flow cytometry measurements of dihydroethidium (DHE) oxidation showed increases in probe-oxidation that is consistent with a significant increase in the steady-state levels of superoxide during proliferation compared with quiescent normal human fibroblasts (74, 77). MitoSOX Red oxidation showed an approximately 4-fold increase in proliferating cells, suggesting that the proliferation-associated increase in superoxide could be of mitochondrial origin. Indeed, a direct correlation was observed between DHE oxidation and the percentage of S-phase cells. The rate of extracellular H2O2 production during proliferation and quiescence showed an opposite effect: 3 fmole cell−1 h−1 in proliferating cells and 8 fmole cell−1 h−1 in quiescent cells (77). Accumulation of H2O2 exhibited an inverse correlation with the percentage of S-phase cells. These results, combined with the cyclical pattern of MnSOD activity during the cell cycle, suggest that MnSOD activity regulates a mitochondrial “ROS Switch” favoring superoxide-signaling (one-electron reduction reactions) regulating proliferation and H2O2-signaling (two-electron reduction reactions) supporting quiescence (Fig. 5).
FIG. 5.
Superoxide (one-electron) and H2O2 (two-electron) signaling during the cell cycle. MnSOD activity is inversely correlated with ROS levels during the cell cycle: A lower level of MnSOD activity is associated with an increase in the steady-state levels of superoxide during S phase, while a higher level of MnSOD activity is correlated with a higher level of H2O2 during quiescence. H2O2, hydrogen peroxide.
MnSOD and cell cycle regulatory proteins
Although the details of the one- and two-electron signaling pathways regulating cell cycle progression (Fig. 5) are not clearly delineated, manipulation of the cellular redox environment is known to affect cell cycle regulatory protein levels. Metal cofactors in cell cycle regulatory kinases and phosphatases could be the site for one-electron reduction reactions, while thiol-disulfide exchange reactions in specific cysteine residues in proteins could be the site for two-electron reduction reactions (7, 8). A decrease in MnSOD activity along with an increase in superoxide levels during proliferation correlates with an increase in cyclin D1 and cyclin B1 protein levels (77). Cyclin D1 and cyclin B1 protein levels decreased when MnSOD activity and H2O2 levels increased during quiescence. The failure to exit from the cell cycle in MnSOD heterozygous MEFs was associated with a persistent increase in cyclin D1 and cyclin B1 protein levels (77). An inverse correlation between MnSOD and cyclin D1 was also observed in NAC-treated mouse fibroblasts (47–49).
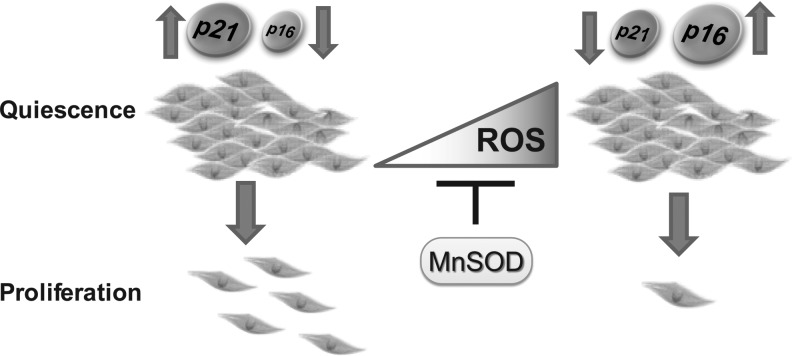
Overexpression of MnSOD suppressed age-associated accumulation of ROS and p16 as well as stabilized p21 protein levels in quiescent human normal fibroblasts (72, 73, 77). Quiescent fibroblasts kept in culture for a long duration lost their capacity to re-enter the proliferative cycle. This loss in the proliferative capacity of quiescent fibroblasts was associated with increased accumulation of ROS and the CKI, p16. However, overexpression of MnSOD inhibits age-associated accumulation of ROS and p16, and it extends the proliferative capacity of quiescent fibroblasts (Fig. 6). Genetic and pharmacological-mediated overexpression of MnSOD has also been shown to increase p27 CK inhibitor protein levels (1, 48). These results led us to the hypothesis that MnSOD activity regulates cell cycle regulatory proteins: cyclins during the proliferative cycle and CKIs during quiescence.
FIG. 6.
MnSOD protects cellular proliferative potential. Overexpression of MnSOD suppresses age-associated increase in intracellular ROS levels and p16 accumulation, which was associated with an extension of the proliferative capacity of quiescent fibroblasts.
MnSOD is a central molecular player for the Warburg effect
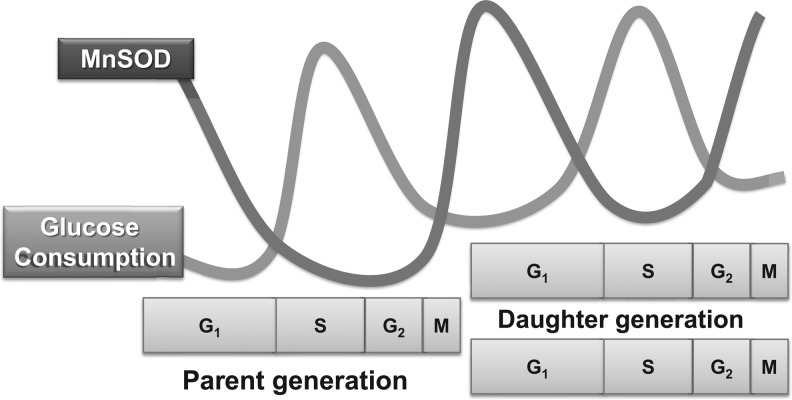
Our recent observations (74, 77) of a cyclical pattern of MnSOD activity during the cell cycle combined with the concept of an “ROS Switch” regulating the cell cycle and an increase in glucose consumption during the cell cycle provide substantial experimental evidence in support of the hypothesis of a “unified theory” of cellular proliferation as proposed by Oberley et al. in 1981 (58). Glucose consumption rate (GCR) increased in proliferating cells to support the high demand of bioenergetics and biosynthetic processes of a growing cell population. A direct correlation between GCR and percent S phase was observed in MnSOD wild-type MEFs (74). The GCR of MEFs with 10% S phase was approximately 40 pg cell−1 h−1, which increased to 120 pg cell−1 h−1 in cultures with 25% S-phase cells. GCR decreased as MEFs exit the proliferative cycle and entered quiescence. It is worth noting that a correlation between the percentage of S-phase cells and GCR was absent in MnSOD homozygous knockout MEFs. A lack of correlation was also associated with these cells' inability to exit the proliferative cycle. The inability to exit the proliferative cycle was associated with a relatively constant GCR. Furthermore, we have shown that an increase in MnSOD activity and subsequently a decrease in GCR were accompanied with a decrease in proliferation (74). These results further support our hypothesis that MnSOD regulates a “metabolic switch,” coordinating GCR with cell cycle progression (Fig. 7). We propose MnSOD as a central molecular player that contributes to both the “Warburg effect” and “unified theory” of cellular proliferation.
FIG. 7.
MnSOD activity regulates a “metabolic cycle” during the cell cycle. An illustration showing the cyclic pattern of an inverse correlation between MnSOD activity and glucose consumption during the cell cycle.
CuZnSOD and the Cell Cycle
Although the majority of research reports MnSOD-dependent regulation of the cell cycle, adenovirus-mediated overexpression of CuZnSOD suppresses low-density lipoprotein-induced stimulation of human aortic smooth muscle cell proliferation (42). This suppression in proliferation was associated with an accumulation of cells in G0/G1 phase, which correlated with a decrease in cyclins (D1 and E) and CDKs (CDK4 and CDK2), and an increase in CKIs (p21 and p27) protein levels. Fibroblasts incubated with antibodies to CuZnSOD exhibited accelerated proliferation (50). Likewise, fibroblasts isolated from CuZnSOD knockout mice exhibited an approximately 25% slower rate of proliferation compared with wild-type fibroblasts (31). These results suggest that CuZnSOD activity may regulate cell cycle progression via ROS signaling of non-mitochondrial origin.
Summary
The mechanisms regulating the cell cycle are complex. A sequential activation of phase-specific cyclin/CDK activity facilitates progression from G0/G1 to S to G2 and M phases. Although the transcriptional, post-transcriptional, translational, and post-translational regulation of cyclin/CDK complexes is well known, interesting questions remain about the cyclic nature of their expression during the cell cycle. The literature discussed here supports the hypothesis that a periodic change in the cellular redox status (a redox cycle) integrates cellular metabolism to the cell cycle machinery during progression from G0 to G1 to S to G2 and M phases.
Since MnSOD is a redox enzyme that regulates mitochondria-generated ROS, changes in MnSOD activity can significantly influence cellular redox status during the cell cycle. We have shown that the cellular redox state shifts toward a more oxidizing environment during S, G2, and M phases (24). Cellular oxidation state increases three- to fourfold in mitotic cells compared with cells in the G1 phase. Interestingly, after cell division, the cellular redox state is reset to the redox state of cells in the G1 phase. Inhibition of this oxidation state before S phase negatively impacts DNA synthesis (49).
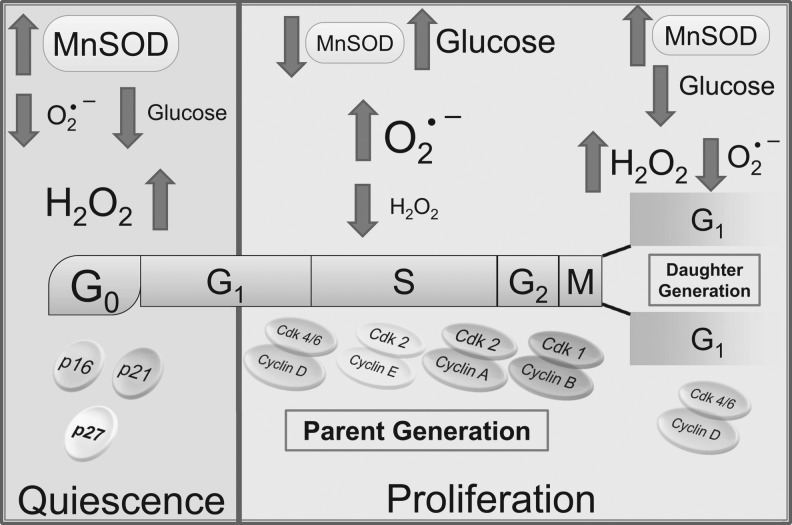
Recent evidence (74, 77) suggests that a periodic change in MnSOD activity during the cell cycle may regulate the redox cycle within the cell cycle (Fig. 8). The changes in MnSOD activity during the cell cycle could result from a preferential selection of its two transcripts (12), thereby influencing its translation and protein levels as well as a post-translational modification of methylation-demethylation at specific serine and arginine residues (74). Such an intricate regulation is needed to maintain an appropriate level of MnSOD activity in each cell cycle phase, which, in turn, will maintain a specific threshold of ROS in each phase of the cell cycle, permitting a sequential transition from one cell cycle phase to the next. For example, in order to maintain a higher oxidation state during S, G2, and M phases, one would expect that MnSOD activity should be lower in these cell cycle phases compared with the G1 phase. Likewise, it is anticipated that MnSOD activity would be higher in the G1 phase to facilitate a lower oxidation state. Indeed, this observation led to the notion that MnSOD activity regulates an “ROS switch,” facilitating superoxide signaling that promotes proliferation and H2O2 signaling which supports quiescence.
FIG. 8.
MnSOD activity regulates a redox cycle within the cell cycle integrating cellular metabolism to the cell cycle regulatory machinery. An illustration representing changes (upward arrow represents increase, while downward arrow represents decrease) in MnSOD activity, glucose consumption, ROS (superoxide and H2O2), and cell cycle regulatory proteins during quiescence and progression through the cell cycle of the parental and daughter generations. p16, p21, and p27 are CKIs. Cyclin/CDKs are the positive regulators of the cell cycle, and CKIs are negative regulators. Redox, oxidation-reduction.
The literature discussed here points toward the idea that MnSOD activity regulates a “metabolic switch” during the cell cycle (Fig. 8). It is well known that proliferating cells consume more glucose to meet the high demand of bioenergetics and biosynthetic processes that are required for cell division. As anticipated, GCR increases as cells transit through S to G2 to M phases, and after mitosis, GCR is reset to cells in the G1 phase. It is worth noting that this periodic pattern of glucose consumption during the cell cycle is inversely correlated with MnSOD activity (74). Furthermore, genetic manipulations of MnSOD expression inversely affected GCR and cellular proliferation. These exciting observations led us to propose MnSOD as a central molecular player for the “Warburg effect.” These observations also provide experimental evidence for the “unified theory” of cellular proliferation put forward by Oberley et al. in 1981, linking MnSOD to ROS and glucose consumption, and initiation of cellular proliferation (58).
Future Directions
The concept of a redox cycle integrating cellular metabolism to the cell cycle regulatory machinery is gaining acceptance from the scientific community. We believe that this concept will also be successful in bridging cell cycle and free radical biology research, which will further advance our understanding of the redox biology of cell cycle regulation. Future research is needed to (i) decipher specific one- and two-electron signaling processes that regulate the cell cycle; (ii) source, pulse duration (flux), and threshold of ROS that regulate specific events during the cell cycle; and (iii) quantitative redox biology to distinguish between ROS levels which are necessary for physiological processes and ROS levels that lead to pathology. Since increasing evidence suggests that ROS have both beneficiary (normal physiological processes) and deleterious (cell death, pathological conditions, etc.) effects, we propose that “eustress” should be used when discussing ROS processes which regulate normal physiological functions, while “oxidative stress” should be used to discuss the deleterious effects of ROS.
Abbreviations Used
- CAT
catalase
- CDK
cyclin-dependent kinase
- CKI
cyclin-dependent kinase inhibitors
- CuZnSOD
copper zinc superoxide dismutase
- DHE
dihydroethidium
- EcSOD
extracellular superoxide dismutase
- FoxO
forkhead transcription factor
- GCR
glucose consumption rate
- GPx
glutathione peroxidase
- GSH
glutathione
- GSK
glycogen synthase kinase
- GSSG
glutathione disulfide
- H2O2
hydrogen peroxide
- IR
ionizing radiation
- MEFs
mouse embryonic fibroblasts
- MnSOD
manganese superoxide dismutase
- NAC
N-acetyl-L-cysteine
- NADPH
nicotinamide adenine dinucleotide phosphate
- Rb
retinoblastoma
- Redox
oxidation-reduction
- ROS
reactive oxygen species
Acknowledgments and Grant Support
The authors thank all previous and current members of their laboratory for their efforts and contributions in advancing the concept of a redox cycle regulating the cell cycle. They thank Garry R. Buettner for introducing them to the concept of one- and two-electron signaling concepts as well as to his continuous interest and feedback of their research, Sue Goo Rhee (Ewha Womans University, Seoul, Korea) for his interest and invaluable suggestions throughout the development of the concept of a redox cycle regulating the cell cycle, and Gareth Smith for editorial assistance. Funding from NIH CA111365 and NIEHS P42 ES 013661 supported this work. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the U. S. Government.
References
- 1.Adachi M, Osawa Y, Uchinami H, Kitamura T, Accili D, and Brenner DA. The forkhead transcription factor FoxO1 regulates proliferation and transdifferentiation of hepatic stellate cells. Gastroenterology 132: 1434–1446, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, and Rhee SG. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem 272: 217–221, 1997 [PubMed] [Google Scholar]
- 3.Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M, Mc Henry KT, Pinchback RM, Ligon AH, Cho YJ, Haery L, Greulich H, Reich M, Winckler W, Lawrence MS, Weir BA, Tanaka KE, Chiang DY, Bass AJ, Loo A, Hoffman C, Prensner J, Liefeld T, Gao Q, Yecies D, Signoretti S, Maher E, Kaye FJ, Sasaki H, Tepper JE, Fletcher JA, Tabernero J, Baselga J, Tsao MS, Demichelis F, Rubin MA, Janne PA, Daly MJ, Nucera C, Levine RL, Ebert BL, Gabriel S, Rustgi AK, Antonescu CR, Ladanyi M, Letai A, Garraway LA, Loda M, Beer DG, True LD, Okamoto A, Pomeroy SL, Singer S, Golub TR, Lander ES, Getz G, Sellers WR, and Meyerson M. The landscape of somatic copy-number alteration across human cancers. Nature 463: 899–905, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blakely E, Chang P, Lommel L, Bjornstad K, Dixon M, Tobias C, Kumar K, and Blakely WF. Cell-cycle radiation response: role of intracellular factors. Adv Space Res 9: 177–186, 1989 [DOI] [PubMed] [Google Scholar]
- 5.Bokoch GM. and Knaus UG. NADPH oxidases: not just for leukocytes anymore! Trends Biochem Sci 28: 502–508, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Borgstahl GE, Parge HE, Hickey MJ, Beyer WF, Jr., Hallewell RA, and Tainer JA. The structure of human mitochondrial manganese superoxide dismutase reveals a novel tetrameric interface of two 4-helix bundles. Cell 71: 107–118, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Buettner GR. Superoxide dismutase in redox biology: the roles of superoxide and hydrogen peroxide. Anticancer Agents Med Chem 11: 341–346, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buettner GR, Ng CF, Wang M, Rodgers VG, and Schafer FQ. A new paradigm: manganese superoxide dismutase influences the production of H2O2 in cells and thereby their biological state. Free Radic Biol Med 41: 1338–1350, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burdon RH. and Gill V. Cellularly generated active oxygen species and HeLa cell proliferation. Free Radic Res Commun 19: 203–213, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Buttrick GJ. and Wakefield JG. PI3-K and GSK-3: Akt-ing together with microtubules. Cell Cycle 7: 2621–2625, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee A. and Jacob-Raman M. Effect of superoxide dismutase on X-ray induced chromosome aberrations and cell cycle delay in muntjac lymphocytes. Pharmacol Ther 39: 323–325, 1988 [DOI] [PubMed] [Google Scholar]
- 12.Chaudhuri L, Nicholson AM, Kalen AL, and Goswami PC. Preferential selection of MnSOD transcripts in proliferating normal and cancer cells. Oncogene 31: 1207–1216, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conour JE, Graham WV, and Gaskins HR. A combined in vitro/bioinformatic investigation of redox regulatory mechanisms governing cell cycle progression. Physiol Genomics 18: 196–205, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Cullen JJ, Weydert C, Hinkhouse MM, Ritchie J, Domann FE, Spitz D, and Oberley LW. The role of manganese superoxide dismutase in the growth of pancreatic adenocarcinoma. Cancer Res 63: 1297–1303, 2003 [PubMed] [Google Scholar]
- 15.Di Mascio P, Murphy ME, and Sies H. Antioxidant defense systems: the role of carotenoids, tocopherols, and thiols. Am J Clin Nutr 53: 194S–200S, 1991 [PubMed] [Google Scholar]
- 16.Diehl JA, Cheng M, Roussel MF, and Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev 12: 3499–3511, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epperly MW, Epstein CJ, Travis EL, and Greenberger JS. Decreased pulmonary radiation resistance of manganese superoxide dismutase (MnSOD)-deficient mice is corrected by human manganese superoxide dismutase-Plasmid/Liposome (SOD2-PL) intratracheal gene therapy. Radiat Res 154: 365–374, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Evans T, Rosenthal ET, Youngblom J, Distel D, and Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell 33: 389–396, 1983 [DOI] [PubMed] [Google Scholar]
- 19.Fernandes AP. and Holmgren A. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid Redox Signal 6: 63–74, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Field LS, Furukawa Y, O'Halloran TV, and Culotta VC. Factors controlling the uptake of yeast copper/zinc superoxide dismutase into mitochondria. J Biol Chem 278: 28052–28059, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Finkel T. and Hwang PM. The Krebs cycle meets the cell cycle: mitochondria and the G1-S transition. Proc Natl Acad Sci U S A 106: 11825–11826, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher CJ. and Goswami PC. Mitochondria-targeted antioxidant enzyme activity regulates radioresistance in human pancreatic cancer cells. Cancer Biol Ther 7: 1271–1279, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folz RJ. and Crapo JD. Extracellular superoxide dismutase (SOD3): tissue-specific expression, genomic characterization, and computer-assisted sequence analysis of the human EC SOD gene. Genomics 22: 162–171, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Goswami PC, Sheren J, Albee LD, Parsian A, Sim JE, Ridnour LA, Higashikubo R, Gius D, Hunt CR, and Spitz DR. Cell cycle-coupled variation in topoisomerase IIalpha mRNA is regulated by the 3'-untranslated region. Possible role of redox-sensitive protein binding in mRNA accumulation. J Biol Chem 275: 38384–38392, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Grana X. and Reddy EP. Cell cycle control in mammalian cells: role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs). Oncogene 11: 211–219, 1995 [PubMed] [Google Scholar]
- 26.Guo G, Yan-Sanders Y, Lyn-Cook BD, Wang T, Tamae D, Ogi J, Khaletskiy A, Li Z, Weydert C, Longmate JA, Huang TT, Spitz DR, Oberley LW, and Li JJ. Manganese superoxide dismutase-mediated gene expression in radiation-induced adaptive responses. Mol Cell Biol 23: 2362–2378, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halliwell B. and Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford, New York: Clarendon Press; Oxford University Press; 1999, xxxi, p. 936 [Google Scholar]
- 28.Harbour JW. and Dean DC. The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev 14: 2393–2409, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Hartwell LH, Culotti J, and Reid B. Genetic control of the cell-division cycle in yeast. I. Detection of mutants. Proc Natl Acad Sci U S A 66: 352–359, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu Y, Rosen DG, Zhou Y, Feng L, Yang G, Liu J, and Huang P. Mitochondrial manganese-superoxide dismutase expression in ovarian cancer: role in cell proliferation and response to oxidative stress. J Biol Chem 280: 39485–39492, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Huang TT, Yasunami M, Carlson EJ, Gillespie AM, Reaume AG, Hoffman EK, Chan PH, Scott RW, and Epstein CJ. Superoxide-mediated cytotoxicity in superoxide dismutase-deficient fetal fibroblasts. Arch Biochem Biophys 344: 424–432, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Jirawatnotai S, Hu Y, Livingston DM, and Sicinski P. Proteomic identification of a direct role for cyclin d1 in DNA damage repair. Cancer Res 72: 4289–4293, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalen AL, Sarsour EH, Venkataraman S, and Goswami PC. Mn-superoxide dismutase overexpression enhances G2 accumulation and radioresistance in human oral squamous carcinoma cells. Antioxid Redox Signal 8: 1273–1281, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Kawamura N. and Dan K. A cytochemical study of the sulfhydryl groups of sea urchin eggs during the first cleavage. J Biophys Biochem Cytol 4: 615–619, 1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keele BB, Jr, McCord JM, and Fridovich I. Superoxide dismutase from escherichia coli B. A new manganese-containing enzyme. J Biol Chem 245: 6176–6181, 1970 [PubMed] [Google Scholar]
- 36.Kim A, Zhong W, and Oberley TD. Reversible modulation of cell cycle kinetics in NIH/3T3 mouse fibroblasts by inducible overexpression of mitochondrial manganese superoxide dismutase. Antioxid Redox Signal 6: 489–500, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol 77: 598–625, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, and Burgering BM. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419: 316–321, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Laurent A, Nicco C, Chereau C, Goulvestre C, Alexandre J, Alves A, Levy E, Goldwasser F, Panis Y, Soubrane O, Weill B, and Batteux F. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res 65: 948–956, 2005 [PubMed] [Google Scholar]
- 40.Li N. and Oberley TD. Modulation of antioxidant enzymes, reactive oxygen species, and glutathione levels in manganese superoxide dismutase-overexpressing NIH/3T3 fibroblasts during the cell cycle. J Cell Physiol 177: 148–160, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Li Z, Xia L, Lee LM, Khaletskiy A, Wang J, Wong JY, and Li JJ. Effector genes altered in MCF-7 human breast cancer cells after exposure to fractionated ionizing radiation. Radiat Res 155: 543–553, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Lin SJ, Shyue SK, Shih MC, Chu TH, Chen YH, Ku HH, Chen JW, Tam KB, and Chen YL. Superoxide dismutase and catalase inhibit oxidized low-density lipoprotein-induced human aortic smooth muscle cell proliferation: role of cell-cycle regulation, mitogen-activated protein kinases, and transcription factors. Atherosclerosis 190: 124–134, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Massey V, Strickland S, Mayhew SG, Howell LG, Engel PC, Matthews RG, Schuman M, and Sullivan PA. The production of superoxide anion radicals in the reaction of reduced flavins and flavoproteins with molecular oxygen. Biochem Biophys Res Commun 36: 891–897, 1969 [DOI] [PubMed] [Google Scholar]
- 44.Masui Y. and Markert CL. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool 177: 129–145, 1971 [DOI] [PubMed] [Google Scholar]
- 45.Mauro F, Grasso A, and Tolmach LJ. Variations in sulfhydryl, disulfide, and protein content during synchronous and asynchronous growth of HeLa cells. Biophys J 9: 1377–1397, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCord JM. and Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244: 6049–6055, 1969 [PubMed] [Google Scholar]
- 47.Menon SG. and Goswami PC. A redox cycle within the cell cycle: ring in the old with the new. Oncogene 26: 1101–1109, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Menon SG, Sarsour EH, Kalen AL, Venkataraman S, Hitchler MJ, Domann FE, Oberley LW, and Goswami PC. Superoxide signaling mediates N-acetyl-L-cysteine-induced G1 arrest: regulatory role of cyclin D1 and manganese superoxide dismutase. Cancer Res 67: 6392–6399, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Menon SG, Sarsour EH, Spitz DR, Higashikubo R, Sturm M, Zhang H, and Goswami PC. Redox regulation of the G1 to S phase transition in the mouse embryo fibroblast cell cycle. Cancer Res 63: 2109–2117, 2003 [PubMed] [Google Scholar]
- 50.Michiels C, Raes M, Zachary MD, Delaive E, and Remacle J. Microinjection of antibodies against superoxide dismutase and glutathione peroxidase. Exp Cell Res 179: 581–589, 1988 [DOI] [PubMed] [Google Scholar]
- 51.Mitra K, Wunder C, Roysam B, Lin G, and Lippincott-Schwartz J. A hyperfused mitochondrial state achieved at G1-S regulates cyclin E buildup and entry into S phase. Proc Natl Acad Sci U S A 106: 11960–11965, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nevins JR. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science 258: 424–429, 1992 [DOI] [PubMed] [Google Scholar]
- 53.Nordenson I. Effects of superoxide dismutase and catalase on radiation-induced chromosome aberrations: dose and cell cycle dependence. Hereditas 89: 163–167, 1978 [DOI] [PubMed] [Google Scholar]
- 54.Oberley LW. Anticancer therapy by overexpression of superoxide dismutase. Antioxid Redox Signal 3: 461–472, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Oberley LW. and Buettner GR. Role of superoxide dismutase in cancer: a review. Cancer Res 39: 1141–1149, 1979 [PubMed] [Google Scholar]
- 56.Oberley LW, McCormick ML, Sierra-Rivera E, and Kasemset-St Clair D. Manganese superoxide dismutase in normal and transformed human embryonic lung fibroblasts. Free Radic Biol Med 6: 379–384, 1989 [DOI] [PubMed] [Google Scholar]
- 57.Oberley LW, Oberley TD, and Buettner GR. Cell differentiation, aging and cancer: the possible roles of superoxide and superoxide dismutases. Med Hypotheses 6: 249–268, 1980 [DOI] [PubMed] [Google Scholar]
- 58.Oberley LW, Oberley TD, and Buettner GR. Cell division in normal and transformed cells: the possible role of superoxide and hydrogen peroxide. Med Hypotheses 7: 21–42, 1981 [DOI] [PubMed] [Google Scholar]
- 59.Oberley TD, Oberley LW, Slattery AF, Lauchner LJ, and Elwell JH. Immunohistochemical localization of antioxidant enzymes in adult Syrian hamster tissues and during kidney development. Am J Pathol 137: 199–214, 1990 [PMC free article] [PubMed] [Google Scholar]
- 60.Oberley TD, Schultz JL, Li N, and Oberley LW. Antioxidant enzyme levels as a function of growth state in cell culture. Free Radic Biol Med 19: 53–65, 1995 [DOI] [PubMed] [Google Scholar]
- 61.Ough M, Lewis A, Zhang Y, Hinkhouse MM, Ritchie JM, Oberley LW, and Cullen JJ. Inhibition of cell growth by overexpression of manganese superoxide dismutase (MnSOD) in human pancreatic carcinoma. Free Radic Res 38: 1223–1233, 2004 [DOI] [PubMed] [Google Scholar]
- 62.Ozeki M, Tamae D, Hou DX, Wang T, Lebon T, Spitz DR, and Li JJ. Response of cyclin B1 to ionizing radiation: regulation by NF-kappaB and mitochondrial antioxidant enzyme MnSOD. Anticancer Res 24: 2657–2663, 2004 [PMC free article] [PubMed] [Google Scholar]
- 63.Pan W, Cox S, Hoess RH, and Grafstrom RH. A cyclin D1/cyclin-dependent kinase 4 binding site within the C domain of the retinoblastoma protein. Cancer Res 61: 2885–2891, 2001 [PubMed] [Google Scholar]
- 64.Paravicini TM. and Touyz RM. Redox signaling in hypertension. Cardiovasc Res 71: 247–258, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Pardee AB. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci U S A 71: 1286–1290, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pines J. and Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell 58: 833–846, 1989 [DOI] [PubMed] [Google Scholar]
- 67.Rapkine L. Su les processus chimiques au cours de la division cellulaire. Ann Physiol Physiochem Biol 7: 382–418, 1931 [Google Scholar]
- 68.Rhee SG, Kang SW, Netto LE, Seo MS, and Stadtman ER. A family of novel peroxidases, peroxiredoxins. Biofactors 10: 207–209, 1999 [DOI] [PubMed] [Google Scholar]
- 69.Rigoulet M, Yoboue ED, and Devin A. Mitochondrial ROS generation and its regulation: mechanisms involved in H(2)O(2) signaling. Antioxid Redox Signal 14: 459–468, 2011 [DOI] [PubMed] [Google Scholar]
- 70.Roberts JM, Koff A, Polyak K, Firpo E, Collins S, Ohtsubo M, and Massague J. Cyclins, Cdks, and cyclin kinase inhibitors. Cold Spring Harb Symp Quant Biol 59: 31–38, 1994 [DOI] [PubMed] [Google Scholar]
- 71.Sakamaki T, Casimiro MC, Ju X, Quong AA, Katiyar S, Liu M, Jiao X, Li A, Zhang X, Lu Y, Wang C, Byers S, Nicholson R, Link T, Shemluck M, Yang J, Fricke ST, Novikoff PM, Papanikolaou A, Arnold A, Albanese C, and Pestell R. Cyclin D1 determines mitochondrial function in vivo. Mol Cell Biol 26: 5449–5469, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sarsour EH, Agarwal M, Pandita TK, Oberley LW, and Goswami PC. Manganese superoxide dismutase protects the proliferative capacity of confluent normal human fibroblasts. J Biol Chem 280: 18033–18041, 2005 [DOI] [PubMed] [Google Scholar]
- 73.Sarsour EH, Goswami M, Kalen AL, and Goswami PC. MnSOD activity protects mitochondrial morphology of quiescent fibroblasts from age associated abnormalities. Mitochondrion 10: 342–349, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sarsour EH, Kalen AL, Xiao Z, Veenstra TD, Chaudhuri L, Venkataraman S, Reigan P, Buettner GR, and Goswami PC. Manganese superoxide dismutase regulates a metabolic switch during the mammalian cell cycle. Cancer Res 72: 3807–3816, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sarsour EH, Kumar MG, Chaudhuri L, Kalen AL, and Goswami PC. Redox control of the cell cycle in health and disease. Antioxid Redox Signal 11: 2985–3011, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sarsour EH, Kumar MG, Kalen AL, Goswami M, Buettner GR, and Goswami PC. MnSOD activity regulates hydroxytyrosol-induced extension of chronological lifespan. Age (Dordr) 34: 95–109, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sarsour EH, Venkataraman S, Kalen AL, Oberley LW, and Goswami PC. Manganese superoxide dismutase activity regulates transitions between quiescent and proliferative growth. Aging Cell 7: 405–417, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schrader M. and Fahimi HD. Mammalian peroxisomes and reactive oxygen species. Histochem Cell Biol 122: 383–393, 2004 [DOI] [PubMed] [Google Scholar]
- 79.Sebastian B, Kakizuka A, and Hunter T. Cdc25M2 activation of cyclin-dependent kinases by dephosphorylation of threonine-14 and tyrosine-15. Proc Natl Acad Sci U S A 90: 3521–3524, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sherr CJ. Mammalian G1 cyclins and cell cycle progression. Proc Assoc Am Physicians 107: 181–186, 1995 [PubMed] [Google Scholar]
- 81.Sherr CJ. and Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev 18: 2699–2711, 2004 [DOI] [PubMed] [Google Scholar]
- 82.Taguchi N, Ishihara N, Jofuku A, Oka T, and Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem 282: 11521–11529, 2007 [DOI] [PubMed] [Google Scholar]
- 83.Takada Y, Hachiya M, Park SH, Osawa Y, Ozawa T, and Akashi M. Role of reactive oxygen species in cells overexpressing manganese superoxide dismutase: mechanism for induction of radioresistance. Mol Cancer Res 1: 137–146, 2002 [PubMed] [Google Scholar]
- 84.Tanaka H, Matsumura I, Ezoe S, Satoh Y, Sakamaki T, Albanese C, Machii T, Pestell RG, and Kanakura Y. E2F1 and c-Myc potentiate apoptosis through inhibition of NF-kappaB activity that facilitates MnSOD-mediated ROS elimination. Mol Cell 9: 1017–1029, 2002 [DOI] [PubMed] [Google Scholar]
- 85.Tanaka T, Nakamura H, Nishiyama A, Hosoi F, Masutani H, Wada H, and Yodoi J. Redox regulation by thioredoxin superfamily; protection against oxidative stress and aging. Free Radic Res 33: 851–855, 2000 [DOI] [PubMed] [Google Scholar]
- 86.Venkataraman S, Jiang X, Weydert C, Zhang Y, Zhang HJ, Goswami PC, Ritchie JM, Oberley LW, and Buettner GR. Manganese superoxide dismutase overexpression inhibits the growth of androgen-independent prostate cancer cells. Oncogene 24: 77–89, 2005 [DOI] [PubMed] [Google Scholar]
- 87.Wang C, Li Z, Lu Y, Du R, Katiyar S, Yang J, Fu M, Leader JE, Quong A, Novikoff PM, and Pestell RG. Cyclin D1 repression of nuclear respiratory factor 1 integrates nuclear DNA synthesis and mitochondrial function. Proc Natl Acad Sci U S A 103: 11567–11572, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Warburg O, Posener K, and Negelein E. Ueber den Stoffwechsel der Tumoren. Biochemische Zeitschrift 152: 319–344, 1924 [Google Scholar]
- 89.Weydert CJ, Waugh TA, Ritchie JM, Iyer KS, Smith JL, Li L, Spitz DR, and Oberley LW. Overexpression of manganese or copper-zinc superoxide dismutase inhibits breast cancer growth. Free Radic Biol Med 41: 226–237, 2006 [DOI] [PubMed] [Google Scholar]
- 90.Yamamoto K, Volkl A, Hashimoto T, and Fahimi HD. Catalase in guinea pig hepatocytes is localized in cytoplasm, nuclear matrix and peroxisomes. Eur J Cell Biol 46: 129–135, 1988 [PubMed] [Google Scholar]
- 91.Zangar RC, Davydov DR, and Verma S. Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol Appl Pharmacol 199: 316–331, 2004 [DOI] [PubMed] [Google Scholar]
- 92.Zou Y, Ewton DZ, Deng X, Mercer SE, and Friedman E. Mirk/dyrk1B kinase destabilizes cyclin D1 by phosphorylation at threonine 288. J Biol Chem 279: 27790–27798, 2004 [DOI] [PubMed] [Google Scholar]