Abstract
Graft versus host disease (GVHD) is the main complication following allogeneic hematopoietic stem cell transplantation (allo-HSCT). Recent data indicated that regulatory T (Treg) cells might relate to GVHD, and such functions might be mediated by certain T-cell receptor (TCR) subfamily of Treg cells. Thus, we analyzed the distribution and clonality of the TCR Vα and Vβ repertoire of Treg cells from leukemia patients with and without GVHD after allo-HSCT. Numerous TCR Vα subfamilies, including Vα1, Vα9, Vα13, Vα16–19, and Vα24–29, were absent in Treg cells after allo-HSCT. The usage numbers for the TCR Vα and Vβ subfamilies in Treg cells from patients without GVHD appeared more widely. The expression frequencies of Vα10 or Vα20 between both groups were significantly different. Moreover, the expression frequency of TCR Vβ2 subfamily in patients without GVHD was significantly higher than that in patients with GVHD. Oligoclonally expanded TCR Vα and Vβ Treg cells were identified in a few samples in both groups. Restricted utilization of the Vα and Vβ subfamilies and the absence of some important TCR rearrangements in Treg cells may be related to GVHD due to a lower regulating function of Treg subfamilies.
Introduction
Although several salvage regimens have been successfully used to induce remission in leukemia, the duration of remission for refractory leukemia patients is often brief, and there are few long-term survivors (Thomas et al., 2003; Mato et al., 2008; Liu et al., 2013). Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is an important therapeutic option for a number of malignant and refractory hematological diseases (Wu et al., 2011; Lv and Huang, 2012), and it is commonly perceived as the only curative option for refractory leukemia (Oyekunle et al., 2006). However, early or late allo-HSCT complications are responsible for significant morbidity and mortality. Graft versus host disease (GVHD) is the main complication following allo-HSCT (Lv and Huang, 2012). Recent data indicated that CD4+CD25+ regulatory T (Treg) cells might participate in mediating GVHD and graft-versus-leukemia effects after allo-HSCT (Hoffmann et al., 2005; Zhai et al., 2007; Brown and Boussiotis, 2008; Semple et al., 2011). GVHD has been associated with abnormal Treg cell number and function (Giorgini and Noble, 2007). Treg cell numbers are lower in patients with acute and chronic GVHD than in normal patients or those without GVHD (Rieger et al., 2006; Kapur et al., 2008). Chronic GVHD can be resolved by adoptive immunotherapy with Treg cells (Dvorak and Cowan, 2008).
T cells recognize specific ligands by specific T-cell receptors (TCR), which are heterodimers comprising α/β chains. TCR signal stimulation is important for the activation and immune function of Treg cells. Tuovinen et al. (2006) reported that the TCR on Treg cells in most normal thymi and peripheral blood express two different chains (Vα 2 and Vα 12), and two α chains could match a β chain to form αβ TCRs that could transmit signals to make Treg cells have dual specificity, which plays a positive role in guiding T-cell differentiation into the Treg cell lineage. Föhse L et al. (Fohse et al., 2011) suggested that efficient immunoregulation by Treg cells requires high TCR diversity, and high TCR diversity ensures the optimal function and homeostasis of Treg cells. Previous studies showed that some clonally expanded TCR Vβ subfamily T cells are related to GVHD pathogenesis (Friedman et al., 2001; Beck et al., 2005, Du et al., 2007, Fu et al., 2007); nevertheless, little is known about which TCR subfamily members may be related to reducing GVHD onset. High TCR diversity was required for the optimal suppressive function of Treg cells in experimental acute GVHD in mice (Fohse et al., 2011). However, the distribution and clonality of the TCR repertoire for specific Treg cells in human GVHD remains unclear. Based on the importance of TCR signaling stimulation for the activation of Treg cells, in this study, we first analyzed the molecular characteristics of the TCRs (including the TCR spectrum and T-cell clones) on Treg cells from leukemia patients after allo-HSCT.
Materials and Methods
Patients
Thirty leukemia patients (15 males and 15 females, median age: 30.6 years, range: 20–45 years) were prospectively monitored after allo-HSCT to determine the expression pattern and clonality of the TCR repertoire. The diagnosis of all of the patients was based on the criteria reported in the guidelines of the American Society of Hematology. According to WHO classification (Sabattini et al., 2010), the 30 patients with leukemia included 16 patients with acute myeloid leukemia (AML), 10 patients with chronic myeloid leukemia, and 4 patients with acute lymphoid leukemia. Twenty-eight patients were in first CR (CR1), and two patients had no complete remission (NR) before transplantation. The patient median age at the time of transplantation was 26 years (range: 20–45 years). All of the cases were related donors including 28 patients who were HLA locus matched and 2 patients who were HLA locus mismatched transplants. The standard conditioning regimens included total body irradiation (TBI)+cyclophosphamide (CY) (TBI: 4.5 Gy/d on days -5 and -4; CY: 60 mg/kg·day, i.v., on days -3 and -2), and BuCY (busulfan: 4.0 mg/kg·day, p.o., or 3.2 mg/kg·day, i.v., on days -7 to -4; CY: 60 mg/kg·day, i.v., on days -3 and -2). As described previously (Xuan et al., 2012a), cyclosporine A (CsA) alone or CsA plus methotrexate (MTX) were administered to patients undergoing HLA-matched sibling donor transplantation for GVHD prophylaxis. CsA+MTX+human anti-thymocyte globulin and/or mycophenolate were used in patients undergoing HLA-mismatched related donor transplants. Acute and chronic GVHD were diagnosed and graded as previously described (Wu et al., 2011). All of the procedures were conducted according to the guidelines of the Medical Ethics committee of the health bureau of the Guangdong Province of China.
Flow cytometry
Peripheral blood mononuclear cells (PBMCs) were separated from freshly drawn anticoagulated blood by Ficoll-Hypaque (Pharmacia) density gradient centrifugation. Freshly isolated human PBMCs were suspended in PBS containing 1% BSA. For the staining, cells were incubated with PerCP/Cy5.5-, FITC-, and PE-conjugated mAbs (including mouse anti–human CD3, CD4, and CD25; BD Biosciences) or their isotype control Abs for 30 min at 4°C. The APC-conjugated FoxP3 (BD Biosciences) staining was performed according to the manufacturer's manual. All samples were assayed by BD FACS Canto™ II (BD Biosciences) and the acquired data were further analyzed using BD-FACS Diva Software.
Treg cells isolation
The CD4+CD25+ Treg cells were sorted from PBMCs using a human CD4+CD25+ Treg cell isolation kit and the MACS® magnetic cell sorting technique (Miltenyi Biotec).
RNA isolation and cDNA synthesis
RNA was extracted from the Treg cells according to the manufacturer's recommendations (TRIzol; Invitrogen). Two micrograms of RNA was reverse transcribed into cDNA with random hexamer primers and reverse transcriptase using the Superscript II Kit (Gibco). The quality of cDNA was confirmed by reverse transcriptase PCR (RT-PCR) for β2 microglobulin (β2M) gene amplification.
GeneScan analysis for TCR repertoire clonality
The complementarity-determining region 3 (CDR3) sizes of the TCR repertoire (TCR Vα and Vβ subfamilies) were analyzed on the Treg cells of recipients at GVHD onset using RT-PCR and the GeneScan technique (Li et al., 2007; Zhang et al., 2009; Xuan et al., 2012b). The TCR V sense primers and the TCR C reverse primers were used in an unlabeled PCR to amplify the TCR subfamilies, and the sequences of the primers are described in previous studies (Li et al., 2007; Zhang et al., 2009). Aliquots of the cDNA (1 μL) were amplified in 25 μL reactions with one of the TCR V primers and a TCR C primer. The final reaction mixture contained 0.5 μM forward and reverse primers, 0.1 mM dNTP, 1.5 mM MgCl2, 1× PCR buffer, and 1.25 U Taq polymerase (Promega). The amplification was performed in a DNA thermal cycler (BioMetra). After a 3 min denaturation at 94°C, 40 PCR cycles were performed with each cycle consisting of 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min, and a final 7 min elongation at 72°C. The products were then stored at 4°C.
Aliquots of the unlabeled PCR products (2 μL) were subjected to a cycle of a runoff reaction with a fluorophore-labeled TCR C-fam primer. The labeled runoff PCR products (2 μL) were heat-denatured at 94°C for 4 min with 9.5 μL formamide (Hi-Di Formamide; ABI) and 0.5 μL of size standards (GENESCAN™-500-LIZ™ ABI); the samples were then loaded into a 3100 POP-4™ gel (Performance Optimized Polymer-4; ABI) and resolved by electrophoresis in 3100 DNA sequencer (ABI) for size and fluorescence intensity determination using GeneScan software.
Statistical analysis
Differences between numerical variables were calculated with the independent-sample t test. The p values were two-tailed, and p<0.05 was considered statistically significant. The SPSS software package 13.0 (SPSS) was used for all data analysis.
Results
Patient status after allo-HSCT
All patients achieved hematopoietic reconstitution. The median time for neutrophil (absolute neutrophil count >0.5×109/L) and platelet (platelet count >20×109/L) engraftment was 12 and 15 days, respectively.
With a median follow-up time of 12.5 months, the 30 patients were alive, and the incidence of total GVHD was 73.3% (n=22), while 8 patients were without acute or chronic GVHD. The incidence of I°∼IV° (grade I, skin, n=9; grade II, skin and liver, n=6; grade III, gastrointestinal, n=3; and grade IV, gastrointestinal, n=1) and III°∼IV° acute GVHD was 63.3% (n=19) and 13.3% (n=4), respectively. The incidence of limited (skin, n=8 or liver, n=2) and extensive chronic GVHD (skin and liver, n=2) was 33.3% (n=10) and 6.7% (n=2), respectively. Therefore, in this study, we compared the TCR Vα and Vβ repertoire characteristics of Treg cells from patients without GVHD (8 cases, numbered NG01 to NG08) and patients with acute and/or chronic GVHD (22 cases, numbered G01 to G22) after allo-HSCT.
Treg cell frequencies in PBMCs after HSCT
The frequencies of Treg cells were compared by measuring CD4+ CD25+ cells, FoxP3+ cells, FoxP3+ CD4+ cells, and FoxP3+ CD25+ cells from freshly acquired peripheral blood samples in patients at GVHD onset (n=22) and patients who did not develop GVHD at the same time point (n=8). Frequencies of CD4+ CD25+ cells in patients at GVHD onset (2.05%±1.77%) were lower in patients without GVHD (5.06%±3.24%) (p=0.003). Frequencies of FoxP3+ cells (1.96%±1.11% vs. 9.28%±8.63%), FoxP3+ CD25+ cells (0.21%±0.18% vs. 1.34%±1.27%), and FoxP3+ CD4+ cells (0.21%±0.18% vs. 1.34%±1.27%) in patients at GVHD onset were significantly lower than that in patients without GVHD (p<0.001, <0.001, and <0.001).
The expression frequency of the TCR repertoire of Treg cells from patients with and without GVHD
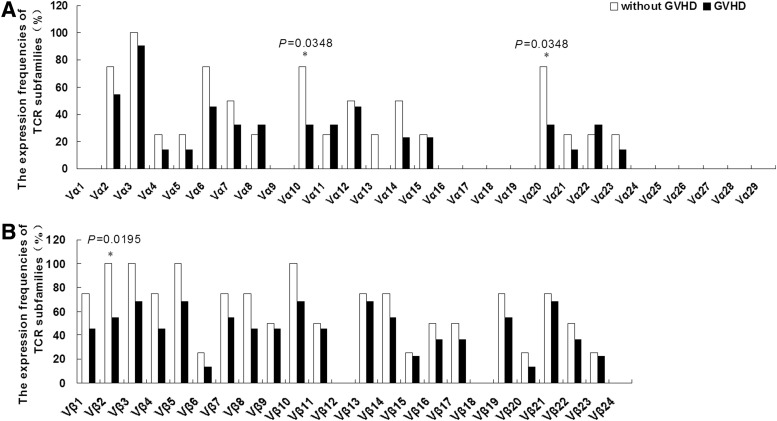
To compare the expression frequency of the TCR Vα and Vβ repertoire of Treg cells from patients with GVHD onset and those without GVHD, the expression of 29 TCR Vα and 24 TCR Vβ subfamilies was detected. The most frequently expressing Treg cell subfamily, TCR Vα 3, was similar for both groups that is, 8/8 (100%) for patients without GVHD vs. 20/22 (90.9%) for patients with GVHD after allo-HSCT, while the Vα1, Vα9, Vα13, Vα16–19, and Vα24–29 subfamilies could not be detected in the samples from both groups. The usage numbers for the TCR Vα subfamilies in Treg cells from patients without GVHD appeared more widely, the mean value of detectable TCR Vα subfamilies in Treg cells was 5.3±3.4 for patients with GVHD and 7.5±4.2 for those without GVHD, but the utilization of TCR Vα subfamilies in Treg cells was not significantly different between the groups (p=0.1518). However, the expression frequency for Vα10 (6/8 vs. 7/22) and Vα20 (6/8 vs. 7/22) between both groups was significantly different (p=0.0348, p=0.0348). Skewed usage of TCR Vβ subfamilies was found in Treg cells from both groups; the mean value of detectable TCR Vβ subfamily in Treg cells from patients without GVHD was 13.5±4.2, while it was only 9.7±4.7 in patients with GVHD, and the difference was significant (p=0.0451). Moreover, the expression frequency of the TCR Vβ2 subfamily in patients without GVHD (6/8, 75%) was significantly higher than that in patients with GVHD (12/22, 54.5%) (p=0.0195) (Fig. 1).
FIG. 1.
The expression frequencies of T-cell receptor (TCR) Vα (A) and Vβ (B) subfamilies in Treg cells from patients after allogeneic hematopoietic stem cell transplantation (allo-HSCT) with and without graft versus host disease (GVHD). *Compared the expression frequency of TCR Vα or Vβ subfamilies of Treg cells in patients with GVHD onset and without GVHD.
The clonality of TCR subfamily Treg cells in patients with or without GVHD
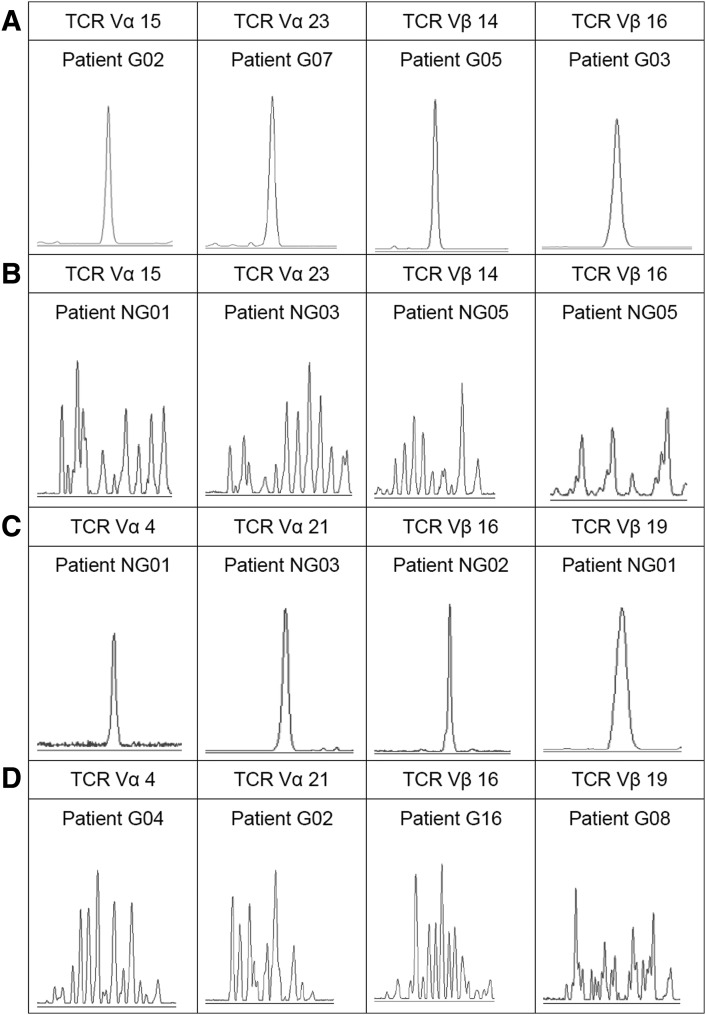
We further analyzed the clonality of the expressed TCR Vα and Vβ subfamilies on Treg cells, and the most TCR Vα and Vβ subfamily expression from most samples indicated polyclonal Treg cells. The distribution of clonally expanded TCR Vα and Vβ subfamilies of Treg cells were listed in Figure 2. The oligoclonal or monoclonal expanded T cells were identified in TCR Vα15, Vα23 and TCR Vβ14, Vβ16 subfamilies in GVHD patients, and TCR Vα4, Vα21 and TCR Vβ16, Vβ19 subfamilies in patients without GVHD. Three out of 8 patients without GVHD and 6 out of 22 patients with GVHD contained TCR Vα subfamily oligoclonally expanded Treg cells; 2 out of 8 patients without GVHD and 2 out of 22 patients with GVHD contained TCR Vβ subfamily oligoclonally expanded Treg cells. The clonality differences of the TCR Vα and Vβ subfamilies in Treg cells between patients with and without GVHD were shown in Figure 3.
FIG. 2.
The distribution and clonality of TCR Vα (A) and Vβ (B) subfamilies in Treg cells from patients after allo-HSCT. Patients with GVHD: numbered G01 to G22; Patients without GVHD: numbered NG01 to NG08.
FIG. 3.
Clonality differences of the TCR Vα and Vβ subfamilies in Treg cells between patients with and without GVHD. (A) Oligoclonal expansion of the TCR Vα and Vβ subfamilies in Treg cells from patients with GVHD. (B) Polyclonal expansion of the TCR Vα and Vβ subfamilies in Treg cells from patients without GVHD. (C) Oligoclonal expansion of the TCR Vα and Vβ subfamilies in Treg cells from patients without GVHD. (D) Polyclonal expansion of the TCR Vα and Vβ subfamilies in Treg cells from patients with GVHD.
Discussion
The occurrence of GVHD is due to a reaction in which donor-derived T-cell clones recognize a host allogeneic antigen, demonstrating the expansion cloning of a specific T cell and even the predominant expansion of some Vβ subfamilies (Du et al., 2007; Fu et al., 2007). Different degrees of chronic GVHD are related to allo-HSCT recipient T-cell immune recovery delay (Fu et al., 2007). Thus, specific immune therapy targeting such specific T-cell clones would help to improve curative effects. The effector T cells that mediate GVHD are an abnormal distribution of donor CD8+ T cells with specific TCR subfamilies, while CD4+ T cells play a role in reducing GVHD in patients after MHC-matching allo-HSCT (Friedman et al., 2001). Therefore, T cell subfamily analysis is expected to be an effective means for monitoring the progress of the disease after transplantation, formulating individualized treatment options according to TCR repertoire analysis, and determining the T-cell clones closely related to GVHD pathogenesis (O'Keefe et al., 2004). Moreover, it is interesting to determine which T-cell clones may regulate GVHD onset.
Previous studies tried to investigate the potential role of Treg cells in the development of GVHD. The initial phase of GVHD might be associated with a decrease of CD4+ CD25+ Treg cells in the peripheral blood of patients after allo-HSCT (Schneider et al., 2006). The Treg frequencies measured within 24 h of GVHD diagnosis were significantly less than patient without GVHD, and correlated inversely with GVHD severity (Magenau et al., 2010). In this study, we also observed that frequencies of CD4+ CD25+ cells, FoxP3+ cells, FoxP3+ CD25+ cells, and FoxP3+ CD4+ cells in patients at GVHD onset were all significantly lower than that in patients without GVHD. However, the relationship between Treg cells and GVHD in clinical research are inconsistent and may be due to the different functions of different Treg cell subsets, and it is difficult to distinguish Treg cell subsets simply based on the CD4+CD25high and FoxP3 markers. Thus, it is necessary to study the complex biological characteristics of Treg cells to establish a better classification of the different subfamilies. In this study, we focused on the distribution and clonality of the TCR subfamilies in Treg cells and attempted to determine the specific characteristic TCR repertoires of Treg cells in patients with and without GVHD after allo-HSCT.
In general, T cells randomly expressed different TCR repertoires, and all of the TCR Vα and Vβ subfamilies could be detected on T cells from healthy peripheral blood (Li et al., 2007). Little is known about the TCR repertoires characteristic of Treg cells. In this study, we first characterized the expression frequency and clonality of the TCR Vα and Vβ repertoires in Treg cells and compared the difference in their expression pattern in Treg cells from patients with and without GVHD after allo-HSCT. First, we found that a common feature of Treg cells is that some TCR Vα and Vβ subfamilies such as Vα1, Vα9, Vα13, Vα16–19, Vα 24–29, Vβ12, Vβ18, and Vβ24 could not be detected on the Treg cells from all samples, and whether this is a common characteristic that is different from that in healthy peripheral blood needs to be characterized. Second, Treg cells from patients with GVHD demonstrated more restricted TCR Vα and Vβ subfamily utilization, and whether this is the reason that some TCR subfamilies are absent may be related to a lower regulating effect, and its involvement in GVHD needs further investigation. More interestingly, neither Vα2 nor Vα12 were detected in six samples from patients with GVHD, which were thought to have dual specificity that plays a positive role in guiding T cells to differentiate into the Treg cell lineage (Tuovinen et al., 2006), and whether it is also a characteristic related to the lowered regulating function of Treg cells in patients with GVHD remains an open question. Third, unlike CD8+ T cells, which displayed significant clonal expansion in patients with GVHD (Friedman et al., 2001), lower clonally expanded T cells were identified for Treg cells from patients with or without GVHD; therefore, it is thought that the regulatory role of Treg cells for GVHD inhibition may derive from multi-clonal TCR subfamilies rather than clonally expanded Treg cells with specific TCR repertoires. However, the role of the clonally expanded TCR Vα15 (18.2%) and Vα23 (13.6%) subfamilies in Treg cells from patients with GVHD and Vα4 (25%) and Vα21 (25%) in patients without GVHD, which were identified in samples in this study, might need further investigation and follow-up. To expand the analysis of these clonally expanded Treg cells to a larger cohort of patients would help to make definitive conclusions. The characteristic of TCR CDR3 are important to the function of clonally expanded T cells. Recently, Meyer et al. (2013) used TCRβ repertoire sequencing to identify dominant personal T-cell clones in the gastrointestinal tracts of patients with acute GVHD. They found that TCRβ CDR3 repertoire sequencing reveals patterns that could eventually serve as a disease biomarker of T-cell alloreactivity in acute GVHD. In this study, we further characterized the expression frequency and clonality of both TCR Vα and Vβ repertoires in specific T-cell subset—Treg cells, and provided the basic data for further sequencing analysis. Because immune repertoire sequencing-based methods could enable a novel personalized way to guide diagnosis and therapy in diseases where T-cell activity is a major determinant, on the basis of our study, we will focus on TCR repertoire sequencing of clonally expanded Treg cells after HSCT in our further research. And specific TCR genes of Treg repertoires could also provide the benefit of producing T-cell populations of desired specificity and offers new opportunities for antigen-specific T-cell therapy for GVHD (Li et al., 2012).
In conclusion, we characterized the expression profiles of the TCR Vα and Vβ repertoire in Treg cells from patients with and without GVHD and showed more restricted utilization of the Vα and Vβ subfamilies, and the absence of some important TCR rearrangements may be related to GVHD due to a lower regulating function of Treg cell subfamilies.
Acknowledgments
This study was supported by a grant from the Guangdong Natural Science Foundation (No. 10451051501005778), the Science and Technology Planning Project of Guangdong Province of China (No. 2012B031800403), the National Natural Science Foundation of China (No. 81200388), a project of the Zhujiang Science & Technology Star of Guangzhou City (No. 2013027), and the China Postdoctoral Science Foundation (No. 200902332, No. 20080440776).
Authors' Contributions
Y.Q.L. and X.L.W. contributed to the concept development and study design. Z.Y.J., S.H.C., and L.J.Y. performed the GeneScan analysis. Q.F.L. was responsible for collection of the clinical data. Y.Q.L., X.L.W., and Z.Y.J. coordinated the study and helped draft the article. All authors read and approved the final article.
Disclosure Statement
The authors have no potential conflicts of interest.
References
- Beck R.C., Wlodarski M., Gondek L., Theil K.S., Tuthill R.J., Sobeck R., et al. (2005). Efficient identification of T-cell clones associated with graft-versus-host disease in target tissue allows for subsequent detection in peripheral blood. Br J Haematol 129,411–419 [DOI] [PubMed] [Google Scholar]
- Brown J.A., and Boussiotis V.A. (2008). Umbilical cord blood transplantation: basic biology and clinical challenges to immune reconstitution. Clin Immunol 127,286–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J.W., Gu J.Y., Liu J., Cen X.N., Zhang Y., Ou Y., et al. (2007). TCR spectratyping revealed T lymphocytes associated with graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Leuk Lymphoma 48,1618–1627 [DOI] [PubMed] [Google Scholar]
- Dvorak C.C., and Cowan M.J. (2008). Hematopoietic stem cell transplantation for primary immunodeficiency disease. Bone Marrow Transplant 41,119–126 [DOI] [PubMed] [Google Scholar]
- Fohse L., Suffner J., Suhre K., Wahl B., Lindner C., Lee C.W., et al. (2011). High TCR diversity ensures optimal function and homeostasis of Foxp3+regulatory T cells. Eur J Immunol 41,3101–3113 [DOI] [PubMed] [Google Scholar]
- Friedman T.M., Statton D., Jones S.C., Berger M.A., Murphy G.F., and Korngold R. (2001). Vbeta spectratype analysis reveals heterogeneity of CD4+T-cell responses to minor histocompatibility antigens involved in graft-versus-host disease: correlations with epithelial tissue infiltrate. Biol Blood Marrow Transplant 7,2–13 [DOI] [PubMed] [Google Scholar]
- Fu Y.W., Wu de P., Cen J.N., Feng Y.F., Chang W.R., Zhu Z.L., et al. (2007). Patterns of T-cell reconstitution by assessment of T-cell receptor excision circle and T-cell receptor clonal repertoire after allogeneic hematopoietic stem cell transplantation in leukemia patients—a study in Chinese patients. Eur J Haematol 79,138–145 [DOI] [PubMed] [Google Scholar]
- Giorgini A., and Noble A. (2007). Blockade of chronic graft-versus-host disease by alloantigen-induced CD4+CD25+Foxp3+regulatory T cells in nonlymphopenic hosts. J Leukoc Biol 82,1053–1061 [DOI] [PubMed] [Google Scholar]
- Hoffmann P., Ermann J., and Edinger M. (2005). CD4+CD25+regulatory T cells in hematopoietic stem cell transplantation. Curr Top Microbiol Immunol 293,265–285 [DOI] [PubMed] [Google Scholar]
- Kapur R., Ebeling S., and Hagenbeek A. (2008). B-cell involvement in chronic graft-versus-host disease. Haematologica 93,1702–1711 [DOI] [PubMed] [Google Scholar]
- Li Y., Chen S., Yang L., Yin Q., Geng S., Wu X., et al. (2007). TRAV and TRBV repertoire, clonality and the proliferative history of umbilical cord blood T-cells. Transpl Immunol 18,151–158 [DOI] [PubMed] [Google Scholar]
- Li Y., Lin C., and Schmidt C.A. (2012). New insights into antigen specific immunotherapy for chronic myeloid leukemia. Cancer Cell Int 12,52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Zhai X., Song Z., Sun J., Xiao Y., Nie D., et al. (2013). Busulfan plus fludarabine as a myeloablative conditioning regimen compared with busulfan plus cyclophosphamide for acute myeloid leukemia in first complete remission undergoing allogeneic hematopoietic stem cell transplantation: a prospective and multicenter study. J Hematol Oncol 6,15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv M., and Huang X.J. (2012). Allogeneic hematopoietic stem cell transplantation in China: where we are and where to go. J Hematol Oncol 5,10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magenau J.M., Qin X., Tawara I., Rogers C.E., Kitko C., Schlough M., et al. (2010). Frequency of CD4(+)CD25(hi)FOXP3(+) regulatory T cells has diagnostic and prognostic value as a biomarker for acute graft-versus-host-disease. Biol Blood Marrow Transplant 16,907–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato A.R., Morgans A., and Luger S.M. (2008). Novel strategies for relapsed and refractory acute myeloid leukemia. Curr Opin Hematol 15,108–114 [DOI] [PubMed] [Google Scholar]
- Meyer E.H., Hsu A.R., Liliental J., Lohr A., Florek M., Zehnder J.L., et al. (2013). A distinct evolution of the T-cell repertoire categorizes treatment refractory gastrointestinal acute graft-versus-host disease. Blood 121,4955–4962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe C.L., Sobecks R.M., Wlodarski M., Rodriguez A., Bell K., Kuczkowski E., et al. (2004). Molecular TCR diagnostics can be used to identify shared clonotypes after allogeneic hematopoietic stem cell transplantation. Exp Hematol 32,1010–1022 [DOI] [PubMed] [Google Scholar]
- Oyekunle A.A., Kroger N., Zabelina T., Ayuk F., Schieder H., Renges H., et al. (2006). Allogeneic stem-cell transplantation in patients with refractory acute leukemia: a long-term follow-up. Bone Marrow Transplant 37,45–50 [DOI] [PubMed] [Google Scholar]
- Rieger K., Loddenkemper C., Maul J., Fietz T., Wolff D., Terpe H., et al. (2006). Mucosal FOXP3+regulatory T cells are numerically deficient in acute and chronic GvHD. Blood 107,1717–1723 [DOI] [PubMed] [Google Scholar]
- Sabattini E., Bacci F., Sagramoso C., and Pileri S.A. (2010). WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica 102,83–87 [PubMed] [Google Scholar]
- Schneider M., Munder M., Karakhanova S., Ho A.D., and Goerner M. (2006). The initial phase of graft-versus-host disease is associated with a decrease of CD4+CD25+regulatory T cells in the peripheral blood of patients after allogeneic stem cell transplantation. Clin Lab Haematol 28,382–390 [DOI] [PubMed] [Google Scholar]
- Semple K., Yu Y., Wang D., Anasetti C., and Yu X.Z. (2011). Efficient and selective prevention of GVHD by antigen-specific induced Tregs via linked-suppression in mice. Biol Blood Marrow Transplant 17,309–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M.B., Koller C., Yang Y., Shen Y., O'Brien S., Kantarjian H., et al. (2003). Comparison of fludarabine-containing salvage chemotherapy regimens for relapsed/refractory acute myelogenous leukemia. Leukemia 17,990–993 [DOI] [PubMed] [Google Scholar]
- Tuovinen H., Salminen J.T., and Arstila T.P. (2006). Most human thymic and peripheral-blood CD4+CD25+regulatory T cells express 2 T-cell receptors. Blood 108,4063–4070 [DOI] [PubMed] [Google Scholar]
- Wu X., Zhu K., Du X., Chen S., Yang L., Wu J., et al. (2011). Frequency analysis of TRBV subfamily sjTRECs to characterize T-cell reconstitution in acute leukemia patients after allogeneic hematopoietic stem cell transplantation. J Hematol Oncol 4,19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan L., Huang F., Fan Z., Zhou H., Zhang X., Yu G., et al. (2012a). Effects of intensified conditioning on Epstein-Barr virus and cytomegalovirus infections in allogeneic hematopoietic stem cell transplantation for hematological malignancies. J Hematol Oncol 5,46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan L., Wu X., Wu M., Zhang Y., Liu H., Fan Z., et al. (2012b). Effect of granulocyte colony-stimulating factor mobilization on the expression patterns, clonality and signal transduction of TRAV and TRBV repertoire. Immunobiology 217,816–822 [DOI] [PubMed] [Google Scholar]
- Zhai Z., Sun Z., Li Q., Zhang A., Liu H., Xu J., et al. (2007). Correlation of the CD4+CD25high T-regulatory cells in recipients and their corresponding donors to acute GVHD. Transpl Int 20,440–446 [DOI] [PubMed] [Google Scholar]
- Zhang X.L., Li Y.Q., Chen S.H., Yang L.J., Chen S., Wu X.L., et al. (2009). The feature of clonal expansion of TCR Vbeta repertoire, thymic recent output function and TCRzeta chain expression in patients with immune thrombocytopenic purpura. Int J Lab Hematol 31,639–648 [DOI] [PubMed] [Google Scholar]