Abstract
Low-dose oral interferon could exert immune-modulating effects in human. We conducted a clinical trial to investigate the efficacy of oral interferon-alpha in preventing hepatitis C relapse. Totally 169 genotype 1b chronic hepatitis C patients having achieved end-of-therapy virological clearance were randomized to receive interferon-alpha lozenge 500 IU/day (n=59), 1,500 IU/day (n=53), or placebo (n=57) for 24 weeks. Overall, no significant differences were found for the relapse rates in the 3 groups (P>0.05). However, in patients with fibroindex 1.4–1.7, relapse occurred in 1/12 (8.3%) 500 IU-group patients versus 9/21 (42.9%) patients of the other groups (P=0.05). In 158 patients receiving at least 4 weeks of oral interferon, significantly higher platelet count was found at the end of trial in the 500 IU group (P=0.003). In thrombocytopenic patients, a significantly expedited recovery of platelet count was found in the 500 IU group (P=0.002). No drug-related severe adverse events were reported. In conclusion, at 500 IU/day, oral interferon exerted a borderline suppression effect of virological relapse in chronic hepatitis C patients with mild liver fibrosis. Additionally, it significantly expedited platelet count recovery after the end of peginterferon therapy.
Introduction
There are ∼170 million people currently infected with hepatitis C virus (HCV) in the world. Chronic HCV infection leads to severe diseases such as decompensated cirrhosis and hepatocellular carcinoma (Morgan and others 2013). Previously, the standard treatment for chronic HCV infection was a combination of pegylated interferon and ribavirin (Fried and others 2002). This combination is quite effective against genotypes 2 and 3 HCV infection, but in patients infected with genotype 1, the sustained virological response rate (SVR) is less satisfactory. Recently, several direct-acting antiviral agents (DAAs) were developed (Casey and Lee 2013). The first U.S. food and drug administration–approved DAAs are boceprevir and telaprevir (Petta and Craxi 2012). These are NS3/4A protease inhibitors. Combination of these agents with standard peginterferon plus ribavirin therapy increases about 20% in the SVR in genotype 1 chronic hepatitis C. Other DAAs targeted against other nonstructural proteins are also under development. For example, the polymerase inhibitor sofosbuvir has achieved excellent end-of-therapy response when combined with peginterferon and ribavirin (Lawitz and others 2013). However, relapses still occurred after cessation of treatment. Other new strategies are now under clinical trials, including pangenotypic DAAs and interferon-free oral combination regiments. Preliminary data showed promising results but large-scale phase III data are still pending (Stedman 2013).
A natural human interferon-alpha administered through oral mucosal route has been manufactured by Hayashibara Biochemical Laboratories (HBL) in Okayama, Japan (Diez and others 1987). The same HBL interferon-alpha, formulated for parenteral use by other pharmaceutical companies, has been approved by the Japanese government for the treatment of animal diseases. HBL interferon-alpha has a molecular weight ranging from 16,000 to 25,000 Da. It comprises a mixture of 2 subspecies of interferon-alpha derived from human lymphoblastoid cells (BALL-1 cells) induced by Hemagglutinating Virus of Japan (Diez and others 1987). HBL interferon-alpha lozenge is generated by mixing the bulk powder with anhydrous crystalline maltose and 0.5% magnesium stearate. The product is stable at 4°C–30°C for an extended period of time (Diez and others 1987).
Accumulated data suggest that administration of low-dose interferon-alpha by oral mucosal route can exert protective systemic effects in animals (Schafer and others 1972; Tompkins and Cummins 1982; Steed 1987; Lecce and others 1990; Young and others 1990; Weiss and others 1991; Cummins and others 1993; Beilharz and others 1997; Fleischmann and others 1998; Gilger and others 1999). Various degrees of beneficial effects have also been reported in patients with acquired immune deficiency, chronic aphthous stomatitis, chronic hepatitis B, chronic hepatitis C, Sjogren's syndrome, and measles (Hutchinson and Cummins 1987; Hutchinson and others 1990; Babiuch and others 1993; Balcerska and others 1993; Caban and others 1993; Ratajczak 1993; Zielińska and others 1993a & 1993b; Jordan 1994; Lecciones and others 1998; Shiozawa and others 1998; Russell and others 1999; Ship and others 1999). Interestingly, all observed effects on animal and human were coming from low-dose but not from high-dose interferon. The mechanisms of these effects are not clear. It has been postulated that low-dose interferon-alpha may exert biological effects on the oral mucosa cells, which subsequently induce systemic host defense mechanisms without interferon-alpha actually entering the circulation (Nagura and Sumi 1988; Fleischmann and others 1992; Cummins and others 2005; Mestecky and others 2007; Psetti and others 2011). In this view, the oral-mucosa-mediated interferon effect is totally different from the antiviral effect against HCV by the high-dose injected form of pegylated interferon.
Regardless of the advance of chronic hepatitis C treatments, relapses after the end of treatment remain a problem. With the upcoming strategy of interferon-free oral DAA regiments, it can be expected that post-treatment relapse will be a continuous challenge. In this phase II randomized controlled study, we examined the safety profile as well as the efficacy of low-dose HBL interferon-alpha lozenge therapy in preventing the relapse during a 6-month treatment following peginterferon plus ribavirin treatment for genotype 1b HCV–infected patients. A high-dose treatment group and a placebo group were included for comparison.
Patients and Methods
Study design
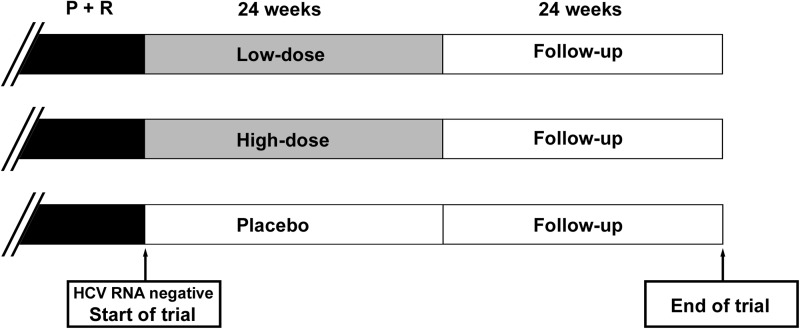
The objectives of this study were to evaluate the efficacy and safety of low-dose human interferon-alpha by the oral mucosal route in reducing the relapse rate in chronic-HCV-infected patients who had achieved end-of-treatment virological responses to the combination therapy of pegylated interferon-alpha and ribavirin. This was a double-blinded, randomized, placebo-controlled, parallel study. Eligible subjects were randomized to placebo or one of the two treatment groups. The low-dose group received oral HBL interferon-alpha lozenge 500 IU once per day. The high-dose group received oral HBL interferon-alpha lozenge 500 IU three times per day. The treatment was either given until virologic relapse occurred or, continued for 24 weeks if no virologic relapse occurred (Fig. 1). Clinical trial registration number was NCT00695019.
FIG. 1.
Clinical trial design. Hepatitis C patients infected with genotype 1b and received pegylated interferon plus ribavirin (P+R) treatment were included, provided that virological clearance was achieved at the end of therapy. Oral interferon was given for 24 weeks (low dose 500 IU/day, high dose 1,500 IU/day, or placebo) followed by 24 weeks of follow-up.
Inclusion and exclusion criteria
A subject was included in the study if he/she met all of the following criteria: (1) was at least 21 years of age; (2) was scheduled to complete hepatitis C treatment, consisting of pegylated interferon-alpha plus ribavirin, within 4 weeks; (3) had a negative serum HCV RNA within 4 weeks before the end of antiviral treatment; (4) had HCV genotype 1b; and (5) was willing and able to comply with study procedures and sign informed consent. The pegylated interferon plus ribavirin given prior to this study was administrated as real-life practice with no specific restriction for dose reduction in intolerant patients. However, patients with rapid virological response could be treated for only 6 months. All patients included had to receive at least 6 months of treatments.
A subject was excluded from the study for any of the following reasons: (1) Child-Pugh stage B or C; (2) history of malignancy within the last 5 years; (3) participating in any other clinical trial or using any other investigational drug within 30 days of entry to this protocol; (4) had any other liver diseases; (5) had any history of decompensated liver function; (6) had uncontrolled diabetes or hypertension; (7) had significant renal function impairment (creatinine>1.5 mg/dL), severe cardiac disease, or suspicion of a disease or condition that might render the subject at high risk from this treatment; (8) had known hypersensitivity to any component of the study medication; (9) had a known substance abuse problem; (10) used any of the listed prohibited medications in protocol; (11) had a medical condition requiring anticoagulant or antiplatelet drugs; (12) a female of childbearing potential; or (13) had human immunodeficiency virus infection.
Efficacy analysis
Relapse was defined as undetectable serum HCV RNA at the end of combination treatment followed by a positive HCV RNA test during follow-ups. In this study, SVR was defined as undetectable serum HCV RNA 24 weeks after the end of oral HBL interferon-alpha lozenge (or placebo) treatment. The overall relapse rate as well as the relapse rates in patients stratified by fibroindex was evaluated (Koda and others 2007). HCV RNA was detected using Roche Cobas TaqMan HCV test (Roche Molecular Systems, Inc., Branchburg, NJ).
Fibroindex
The fibroindex was calculated according to the published formula (Koda and others 2007):
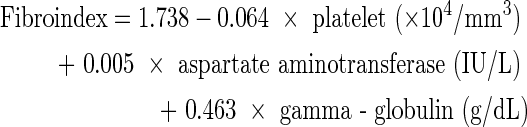 |
Statistical methods
Continuous variables were analyzed by analysis of variance for comparing the difference among 3 groups, and by 2-sample t-test or Wilcoxon Rank Sum test for comparing the difference between 2 groups. Chi-square test or Fisher's exact test was used to compare the difference for categorical variables. All statistical assessments were evaluated at the significance level of alpha=0.05.
Results
Basic clinical data of patients included
A total of 169 patients were included. Of them, 59, 53, and 57 patients were randomly assigned to low-dose (500 IU), high-dose (1,500 IU), and placebo groups, respectively. The basic clinical data were listed in Table 1. No statistical difference was found in terms of age, gender, biochemistry data, and hemogram data. However, a slightly but significantly higher body mass index (BMI) was observed in the placebo group (P=0.038). Previous peginterferon plus ribavirin treatment period in real-life practice was 7.2±2.6, 7.7±2.9, and 7.7±3.0 months, respectively, for the 3 groups of patients (P=0.805). All patients included had received at least 6 months of peginterferon plus ribavirin treatment and achieved end-of-therapy virological response. In real-life practice, liver biopsy was not performed for every patient. Thus, fibroindex was evaluated at the end of peginterferon treatment (before the start of oral HBL interferon-alpha treatment).
Table 1.
Basic Data for All Patients Enrolled
| Variable | Low-dose group | High-dose group | Placebo | PA | PHP | PLP |
|---|---|---|---|---|---|---|
| No. of patients | 59 | 53 | 57 | |||
| Age (years) | 54.9±11.9 | 55.7±10.8 | 56.8±9.5 | 0.606 | 0.542 | 0.342 |
| Gender, male (%) | 32 (54.2%) | 27 (50.9%) | 32 (56.1%) | 0.859 | 0.585 | 0.837 |
| BMI (kg/m2) | 23.2±3.4 | 23.1±3.1 | 24.5±3.1 | 0.038 | 0.019 | 0.034 |
| Biochemistry | ||||||
| Total bilirubin (mg/dL) | 0.8±0.3 | 0.9±0.4 | 0.8±0.3 | 0.233 | 0.130 | 0.808 |
| AST (U/L) | 46.3±36.3 | 43.6±26.6 | 43.5±29.8 | 0.877 | 0.647 | 0.965 |
| ALT (U/L) | 44.4±43.8 | 40.1±28.1 | 38.2±29.9 | 0.923 | 0.693 | 0.888 |
| Blood urine nitrogen (mg/dL) | 12.6±4.3 | 12.4±3.2 | 12.0±3.7 | 0.557 | 0.470 | 0.605 |
| Uric acid (mg/dL) | 6.3±2.1 | 5.9±1.7 | 6.1±1.8 | 0.042 | 0.442 | 0.066 |
| Creatinine (mg/dL) | 0.82±0.20 | 0.80±0.21 | 0.80±0.19 | 0.841 | 0.869 | 0.648 |
| Prothrombin time (s) | 10.7±0.8 | 10.7±0.8 | 10.7±0.7 | 0.932 | 0.898 | 0.808 |
| Alfa-fetoprotein (ng/mL) | 6.1±5.2 | 6.8±12.8 | 7.7±8.5 | 0.845 | 0.744 | 0.950 |
| TSH (mIU/L) | 1.90±1.19 | 2.90±5.02 | 2.01±1.47 | 0.787 | 0.667 | 0.749 |
| Free T4 (μg/dL) | 1.07±0.25 | 1.04±0.26 | 1.06±0.32 | 0.725 | 0.704 | 0.381 |
| Fasting blood sugar (mg/dL) | 97.3±13.6 | 101.5±19.2 | 100.9±19.8 | 0.643 | 0.520 | 0.637 |
| Hemogram | ||||||
| Red blood cells (×106/μL) | 3.53±0.56 | 3.33±0.63 | 3.50±0.65 | 0.183 | 0.171 | 0.753 |
| White blood cells (×103/μL) | 2.98±0.98 | 2.71±1.02 | 2.77±0.90 | 0.198 | 0.412 | 0.351 |
| Platelet (×103/μL) | 147.9±54.7 | 137.7±70.6 | 133.9±51.6 | 0.283 | 0.820 | 0.162 |
| Hemoglobin (g/dL) | 10.9±1.7 | 10.2±1.8 | 10.9±1.8 | 0.084 | 0.061 | 0.956 |
A, among 3 groups; HP, high-dose versus placebo group; LP, low-dose versus placebo group; BMI, body mass index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; TSH, thyroid stimulating hormone.
The fibroindex was 1.82±0.45, 1.79±0.56, and 1.87±0.59, respectively, for the 3 groups of patients (P=0.986).
Efficacy of oral HBL interferon-alpha in preventing HCV relapse
Overall, relapse of HCV infection occurred in 18 (30.5%), 20 (37.7%), and 20 (35.1%) of low-dose, high-dose, and placebo groups, respectively (P=0.716). The patients were then stratified by fibroindex into 4 subgroups (fibroindex <1.40, 1.4–1.7, 1.7–2.0, and ≥2.0) (Table 2). It was found that in patients with fibroindex between 1.4 and 1.7, relapse of HCV infection occurred in 1/12 (8.3%), 4/9 (44.4%), and 5/12 (41.7%) patients for low-dose, high-dose, and placebo groups, respectively. The low-dose group had a borderline significantly lower relapse rate (8.3%) when compared with that of the non-low-dose groups (high-dose plus placebo groups, 9/21 [42.9%], P=0.05).
Table 2.
Relapse of Hepatitis C Virus Infection in Patents Stratified by Fibroindex
| Fibroindex | Low-dose group | High-dose group | Placebo | P1 | P2 |
|---|---|---|---|---|---|
| <1.40 | |||||
| No. of patients | 9 | 10 | 10 | ||
| No. of relaspers | 2 (22.2%) | 3 (30.0%) | 2 (25.0%) | 1.00 | 1.00 |
| ≥1.40, <1.70 | |||||
| No. of patients | 12 | 9 | 12 | ||
| No. of relaspers | 1 (8.3%) | 4 (44.4%) | 5 (41.7%) | 0.12 | 0.05 |
| ≥1.70, <2.0 | |||||
| No. of patients | 20 | 15 | 20 | ||
| No. of relaspers | 8 (40.0%) | 4 (26.7%) | 6 (30.0%) | 0.77 | 0.38 |
| ≥2.0 | |||||
| No. of patients | 18 | 19 | 15 | ||
| No. of relaspers | 7 (38.9%) | 9 (47.4%) | 7 (46.7%) | 0.85 | 0.57 |
P1, overall comparison; P2, low-dose group versus high-dose group plus placebo.
Association between other clinical factors and HCV relapse was evaluated. It was found that none of the age, gender, BMI, and the biochemical and hematological factors in Table 1 were associated with HCV relapse.
Effect of oral HBL interferon-alpha on biochemistry and hemogram data changes
Biochemistry data and hemogram data were reviewed at 24 (end-of-oral interferon treatment) and 48 weeks (end-of-follow up) after the start of oral HBL interferon-alpha. A small but significant decrease of bilirubin levels between the high-dose and placebo groups was found at week 24 and week 28 (0.09±0.30 and 0.08±0.24 mg/dL of decrease, respectively; P=0.027 and 0.004). No other significant changes were observed for other biochemistry data. Interestingly, a significant difference of the absolute platelet count was found between the low-dose and placebo groups, when reviewing the hemogram data. While the initial platelet counts were not significantly different between the 3 groups (Table 1, P=0.283), the subsequent platelet counts were 186.9±50.4 versus 164.6±58.4×103/μL (low-dose versus placebo; P=0.030) at week 24 and 190.7±54.9 versus 169.6±58.7×103/μL (low-dose versus placebo; P=0.047) at week 48, respectively.
Effect of oral HBL interferon-alpha on platelet count recovery
Because in some of our patients, relapse of HCV occurred shortly after the start of oral interferon, a very short duration of oral interferon treatment was given to them. To understand better the effects of oral interferon on platelet count recovery, we thus reviewed the data in only the patients receiving at least 4 weeks of oral interferon. The data were summarized in Table 3. Totally, 55, 48, and 55 patients in the 3 groups received oral interferon for more than 4 weeks. The results were similar to those of total patient analysis and the difference was even more significant. In week 48, the platelet counts were 203.4±52.7 versus 167.6±59.0×103/μL in low-dose versus placebo groups (P=0.003). Interestingly, no difference was found between the high-dose and placebo groups (P=0.96).
Table 3.
Platelet Analysis for Patients Treated for More Than 4 Weeks
| Variable | Low-dose group | High-dose group | Placebo | PA | PHP | PLP |
|---|---|---|---|---|---|---|
| No. of patients | 55 | 48 | 55 | |||
| Age (years) | 54.7±12.1 | 55.4±11.0 | 56.7±9.6 | 0.62 | 0.52 | 0.34 |
| Gender, male (%) | 29 (%) | 24 (%) | 31 (%) | 0.81 | 0.78 | 0.70 |
| BMI (kg/m2) | 23.3±3.5 | 23.0±3.1 | 24.5±3.2 | 0.04 | 0.02 | 0.05 |
| Platelet counts (×103/μL) | ||||||
| Week 0 | 150.4±54.1 | 138.7±71.1 | 133.9±50.9 | 0.32 | 0.69 | 0.10 |
| Week 4 | 185.4±56.7 | 165.1±69.5 | 158.8±53.7 | 0.06 | 0.61 | 0.01 |
| Week 12 | 177.1±49.1 | 160.9±67.4 | 151.9±51.5 | 0.07 | 0.47 | 0.01 |
| Week 24 – treatment stopped | 195.7±48.7 | 165.2±59.6 | 162.8±58.3 | 0.007 | 0.85 | 0.004 |
| Week 36 | 194.5±52.0 | 171.8±60.3 | 163.8±56.3 | 0.03 | 0.53 | 0.007 |
| Week 48 | 203.4±52.7 | 167.0±53.1 | 167.6±59.0 | 0.002 | 0.96 | 0.003 |
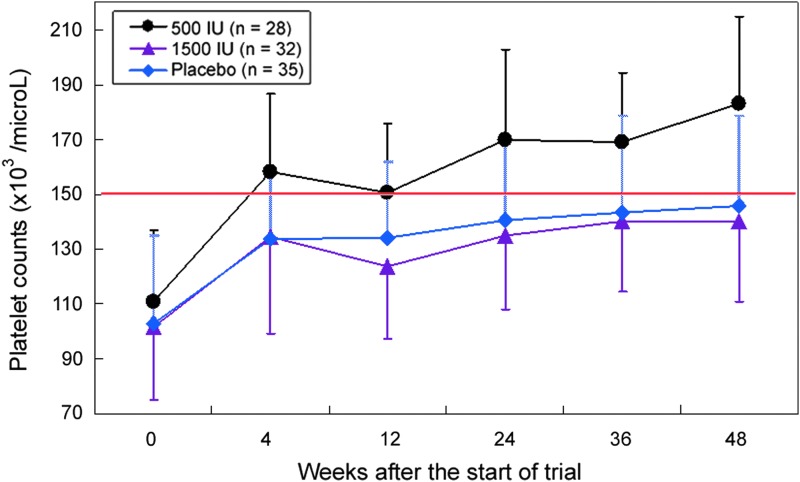
In thrombocytopenic patients with initial platelet counts <150×103/μL, 28, 32, and 35 patients were in the low-dose, high-dose, and placebo groups, respectively. The initial platelet counts were 110.9±26.0, 101.8±26.7, and 103.0±31.8×103/μL, respectively. It was found that the expedited platelet count recovery in the low-dose group was still observed (low-dose versus placebo group, P=0.002 at week 48; Fig. 2). Notably, in the low-dose group, the mean platelet count recovered to 183.2×103/μL, whereas in the other 2 groups, the mean platelet counts only recovered to 140.2 and 146.0×103/μL, respectively, which were still <150×103/μL (Fig. 2).
FIG. 2.
Progressive increase of platelet count in patients receiving at least 4 weeks of oral interferon. Black circle, low dose 500 IU/day; purple triangle, high dose 1,500 IU/day; blue diamond, placebo. Vertical lines, standard deviation.
Despite that there was no statistical difference in the initial platelet counts between the low-dose and placebo groups (150.4±54.1 versus 133.9±50.9×103/μL; P=0.10), the numerically higher mean platelet count might cast some doubt as to whether the expedited platelet count recovery in the low-dose group was due to a higher initial platelet count. To resolve this issue, the changes of platelet count were compared between patients with initial platelet count <150×103/μL (thrombocytopenia patients, n=95) and patients with initial platelet count ≥150×103/μL (n=63). Interestingly, patients with thrombocytopenia at the start of trial had a significantly more rapid recovery of platelet count, compared with those having a high platelet count (P≤0.002 for all comparisons at 4, 12, 24, 36, and 48 weeks after the start of oral interferon). This result suggested that an initial high platelet count was not a favorable factor for platelet recovery, but rather, it was an unfavorable one. Therefore, a numerically higher initial platelet count in the low-dose group actually implied that the low-dose interferon was indeed effective.
Safety
In comparing with the placebo group, no significant increase in the adverse events was found. In fact, 11 (18.6%), 7 (13.2%), and 13 (22.8%) adverse events were reported for the low-dose, high-dose, and placebo groups, respectively (Table 4). Adverse events that appeared only in the treatment groups but not in the placebo group were tonsillitis (1 case), upper respiratory tract infection (1 case), arthralgia (1 case), peripheral neuropathy (1 case), anxiety (1 case), altered mood (1 case), alopecia (1 case), and rash (1 case). All mentioned adverse events were self-limited and mild. These adverse events could also be residual side effects derived from prior peginterferon treatment. No drug-related severe adverse events were reported.
Table 4.
Adverse Events
| Low-dose (n=59) | High-dose (n=53) | Placebo (n=57) | ||||||
|---|---|---|---|---|---|---|---|---|
| Adverse events | Event | Subject | Event | Subject | Event | Subject | PHP | PLP |
| Overall | 13 | 11 (18.6%) | 10 | 7 (13.2%) | 26 | 13 (22.8%) | 0.192 | 0.580 |
| Ear and labyrinth disorders | ||||||||
| Tinnitus | 0 | 0 | 0 | 0 | 1 | 1 (1.8%) | 1 | 0.491 |
| Gastrointestinal disorders | ||||||||
| Abdominal pain upper | 0 | 0 | 0 | 0 | 1 | 1 (1.8%) | 1 | 0.491 |
| Constipation | 0 | 0 | 1 | 1 (1.9%) | 1 | 1 (1.8%) | 1 | 0.491 |
| Dry mouth | 0 | 0 | 1 | 1 (1.9%) | 0 | 0 | 0.482 | NA |
| Irritable bowel syndrome | 0 | 0 | 0 | 0 | 1 | 1 (1.8%) | 1 | 0.491 |
| Mouth ulceration | 1 | 1 (1.7%) | 1 | 1 (1.9%) | 1 | 1 (1.8%) | 1 | 1 |
| Tongue ulceration | 0 | 0 | 0 | 0 | 1 | 1 (1.8%) | 1 | 0.491 |
| General disorder | ||||||||
| Malaise | 0 | 0 | 0 | 0 | 1 | 1 (1.8%) | 1 | 0.491 |
| Infections | ||||||||
| Tonsillitis | 0 | 0 | 1 | 1 (1.9%) | 0 | 0 | 0.482 | NA |
| Upper respiratory tract infection | 0 | 0 | 1 | 1 (1.9%) | 0 | 0 | 0.482 | NA |
| Biochemistry | ||||||||
| ALT increased | 0 | 0 | 0 | 0 | 1 | 1 (1.8%) | 1 | 0.491 |
| AST increased | 0 | 0 | 0 | 0 | 1 | 1 (1.8%) | 1 | 0.491 |
| Immunoglobulins increased | 0 | 0 | 0 | 0 | 1 | 1 (1.8%) | 1 | 0.491 |
| Musculoskeletal | ||||||||
| Arthralgia | 1 | 1 (1.7%) | 0 | 0 | 0 | 0 | NA | 1 |
| Myalgia | 0 | 0 | 0 | 0 | 2 | 2 (3.5%) | 0.496 | 0.239 |
| Nervous system disorders | ||||||||
| Dizziness | 0 | 0 | 0 | 0 | 1 | 1 (1.8%) | 1 | 0.491 |
| Headache | 2 | 2 (3.4%) | 0 | 0 | 3 | 3 (5.3%) | 0.244 | 0.677 |
| Neuropathy peripheral | 1 | 1 (1.7%) | 0 | 0 | 0 | 0 | NA | 1 |
| Somnolence | 0 | 0 | 0 | 0 | 1 | 1 (1.8%) | 1 | 0.491 |
| Psychiatric disorders | ||||||||
| Anxiety | 1 | 1 (1.7%) | 0 | 0 | 0 | 0 | NA | 1 |
| Depression | 0 | 0 | 1 | 1 (1.9%) | 1 | 1 (1.8%) | 1 | 0.491 |
| Insomnia | 2 | 2 (3.4%) | 1 | 1 (1.9%) | 2 | 2 (3.5%) | 1 | 1 |
| Mood altered | 1 | 1 (1.7%) | 0 | 0 | 0 | 0 | NA | 1 |
| Sleep disorder | 0 | 0 | 0 | 0 | 1 | 1 (1.8%) | 1 | 0.491 |
| Respiratory disorders | ||||||||
| Cough | 0 | 0 | 1 | 1 (1.9%) | 2 | 1 (1.8%) | 1 | 0.491 |
| Skin and subcutaneous tissue disorders | ||||||||
| Alopecia | 1 | 1 (1.7%) | 0 | 0 | 0 | 0 | NA | 1 |
| Pruritus | 2 | 2 (3.4%) | 2 | 2 (3.8%) | 3 | 3 (5.3%) | 1 | 0.677 |
| Rash | 1 | 1 (1.7%) | 0 | 0 | 0 | 0 | NA | 1 |
Discussion
It has always been questioned whether low-dose oral interferon can exert any pharmacological effects in human. One major reason for such doubt comes from the demonstration of potent antiviral effect using regular or pegylated interferon (Fried and others 2002; Casey and Lee 2013; Morgan and others 2013). A subcutaneous administration of millions of IU is required to achieve antiviral effect in human. In comparison, 250–500 IU of interferon through mucosa route seems unlikely to have any pharmacological effect. However, accumulated evidence indicated that low-dose interferon by oral route could exhibit some immune modulating function in animal and human diseases (Nagura and Sumi 1988; Fleischmann and others 1992; Mestecky and others 2007; Psetti and others 2011). In this study, we tested whether the low-dose interferon-alpha lozenge exerted any effect in the inhibition of HCV relapse after peginterferon-based therapy. Our data indicated a possible suppressive effect for HCV relapse in patients with fibroindex between 1.4 and 1.7. Such effect was not seen in patients with fibroindex >1.7, suggesting that in patients with more established liver fibrosis or cirrhosis, this effect disappeared. Because of the stratification according to fibroindex, only a small number of patients were included for each subgroup. In the subgroup of patients with fibroindex<1.4, no significant difference was observed between the low-dose and non-low-dose treated patients (Table 2). As a result, even if we combined the 2 lower fibroindex subgroups (fibroindex <1.4 and fibroindex 1.4–1.7), the suppression effect remained insignificant (P=0.136). A larger scale of study that focuses on patients with mild fibrosis is needed to further clarify this issue.
When analyzing the biochemistry and hemogram data, we found that the platelet count recovered more rapidly in the low-dose group. One of the major side effects for pegylated interferon treatment was the induction of thrombocytopenia. The platelet count decreased progressively until the end of treatment, followed by a slow recovery (Mac Nicholas and Norris 2010). This adverse effect was even more severe in patients with advanced fibrosis or cirrhosis. In this study, we discovered that low-dose interferon could expedite the recovery of platelet count and, thus, this treatment potentially reduced the risk of gastrointestinal hemorrhage. Such effect remained prominent in patients with thrombocytopenia (platelet count<150×103/μL) suggesting a possible clinical role.
An interesting observation for both the HCV-relapse-suppressing effect and the platelet-count-recovery-expediting effect was that they only appeared in the low-dose group. Both of the effects were not seen in the high-dose and placebo groups. These results strongly argued for the view that there is an immune modulating pathway mediated by low-dose oral interferon through mucosa route and this pathway is independent of the antiviral effect of the high-dose pegylated interferon. Previous studies have suggested elicitation of systemic immune responses through mucosa-mediated route (Fleischmann and others 1992; Nagura and Sumi 1998; Cummins and others 2005; Psetti and others 2011). In studies that focus on immune responses mediated by intestinal mucosa, it was found that humoral immune responses to antigens in contact with intestinal mucosa are mostly of immunoglobulin A class in secretory form with different degrees of immunoglobulin G class responses. Interestingly, the mucosal immune response could induce systemic T cell hyporesponsiveness to certain immunological reactions. It is believed that the lymphoid cells in the mucosal sites release lymphokines through antigen recognition, thereby regulating regional as well as systemic T cell function. Further, the liver is the major site for removal of IgA immune complexes and thus it plays a role in regulating mucosa-mediated immune process. Other studies on gut mucosal vaccines also showed that induction of immunity in the gastrointestinal mucosa through oral immunization resulted in complex crosstalk between mucosal and systemic immunity (Nagura and Sumi 1988; Psetti and others 2011). Despite our extensive knowledge of mucosa-mediated immune responses in pathogen or vaccine induced immunity, the immunological changes following oral interferon administration are largely unknown. Judging from our present results, there is surely a prominent systemic effect. Further studies should be conducted to illustrate the molecular and cellular mechanisms.
In summary, oral HBL interferon-alpha is a safe drug for chronic hepatitis C patients having received peginterferon and ribavirin therapy. Patients who had received low-dose (500 IU) oral HBL interferon-alpha significantly expedited the platelet count recovery, compared with the high-dose and placebo groups. In patients with mild fibrosis subgroup (fibroindex 1.4–1.7), low-dose HBL oral interferon-alpha might be able to reduce the HCV relapse rate. A large-scale trial is needed to confirm this observation.
Acknowledgment
This study is supported by research grants provided by CytoPharm, Inc., Taiwan (XMRPG 380301).
Author Disclosure Statement
No competing financial interests exist.
References
- Babiuch L, Mian M, Kamińska E, Szymańska B, Georgiades JA. 1993. An interim report on the effect of natural human interferon alpha (IFN-alpha) lozenges in patients seropositive for the human immunodeficiency virus type 1 (HIV-1). Arch Immunol Ther Exp (Warsz) 41:213–219 [PubMed] [Google Scholar]
- Balcerska A, Bohdan Z, Drozyńska E, Kozielska E, Szarszewski A, Georgiades JA. 1993. Evaluation of the efficacy of natural human interferon alpha lozenges on the clinical course of childhood neoplasia and in chronic hepatitis B virus infection in patients who were successfully treated for pediatric malignancies. Arch Immunol Ther Exp (Warsz) 41:221–227 [PubMed] [Google Scholar]
- Beilharz MW, McDonald W, Watson MW, Heng J, McGeachie J, Lawson CM. 1997. Low-dose oral type I interferons reduce early virus replication of murine cytomegalovirus in vivo. J Interferon Cytokine Res 17:625–630 [DOI] [PubMed] [Google Scholar]
- Caban J, Mossor-Ostrowska J, Zyrkowska-Bieda T, Zejc M, Janas-Skulina U, Cieśla A, Cummins JM, Georgiades JA. 1993. Treatment of chronic viral hepatitis type B with oral mucosal administration of natural human interferon alpha lozenges. Arch Immunol Ther Exp (Warsz) 41:229–235 [PubMed] [Google Scholar]
- Casey LC, Lee WM. 2013. Hepatitis C virus therapy update 2013. Curr Opin Gastroenterol 29:243–249 [DOI] [PubMed] [Google Scholar]
- Cummins JM, Hutcheson DP, Cummins MJ, Georgiades JA, Richards AB. 1993. Oral therapy with human interferon alpha in calves experimentally injected with infectious bovine rhinotracheitis virus. Arch Immunol Ther Exp 41:193–197 [PubMed] [Google Scholar]
- Cummins JM, Krakowka GS, Thompson CG. 2005. Systemic effects of interferons after oral administration in animals and humans. Am J Vet Res 66:164–176 [DOI] [PubMed] [Google Scholar]
- Diez RA, Perdereau B, Falcoff E. 1987. From old results to new perspectives: a look at interferon's fate in the body. J Interferon Res 7:553–557 [DOI] [PubMed] [Google Scholar]
- Fleischmann WR, Jr, Koren S, Fleischmann CM. 1992. Orally administered interferons exert their white blood cell suppressive effects via a novel mechanism. Proc Soc Exp Biol Med 201:200–207 [DOI] [PubMed] [Google Scholar]
- Fleischmann WR, Jr, Masoor J, Wu TY, Fleischmann CM. 1998. Orally administered IFN-alpha acts alone and in cynergistic combination with introperitoneally administered IFN-gamma to exert an antitumor effect against B16 melanoma in mice. J Interferon Cytokine Res 18:17–20 [DOI] [PubMed] [Google Scholar]
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Jr, Häussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. 2002. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 347:975–982 [DOI] [PubMed] [Google Scholar]
- Gilger BC, Rose PD, Davidson MG, Roberts SM, Miller T. 1999. Low dose oral administration of interferon-alpha for the treatment of immune-mediated keratoconjunctivitis sicca in dogs. J Interferon Cytokine Res 19:901–905 [DOI] [PubMed] [Google Scholar]
- Hutchinson V, Cummins JM. 1987. Low-dose oral interferon in patient with AIDS. Lancet 2:1530–1531 [DOI] [PubMed] [Google Scholar]
- Hutchinson VA, Angenend JL, Mok WL, Cummins JM, Richards AB. 1990. Chronic recurrent aphthous stomatitis: oral treatment with low-dose interferon alpha. Mol Biother 2:160–164 [PubMed] [Google Scholar]
- Jordan WC. 1994. Three open-label studies of oral interferon alpha in the treatment of HIV disease. J Natl Med Assoc 86:257–262 [PMC free article] [PubMed] [Google Scholar]
- Koda M, Matunaga Y, Kawakami M, Kishimoto Y, Suou T, Murawaki Y. 2007. FibroIndex, a practical index for predicting significant fibrosis in patients with chronic hepatitis C. Hepatology 45:297–306 [DOI] [PubMed] [Google Scholar]
- Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR, Jacobson IM, Kowdley KV, Nyberg L, Subramanian GM, Hyland RH, Arterburn S, Jiang D, McNally J, Brainard D, Symonds WT, McHutchison JG, Sheikh AM, Younossi Z, Gane EJ. 2013. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 368:1931–1932 [DOI] [PubMed] [Google Scholar]
- Lecce JG, Cummins JM, Richards AB. 1990. Treatment of rotavirus infection in neonate and weanling pigs using natural human interferon alpha. Mol Biother 2:211–216 [PubMed] [Google Scholar]
- Lecciones JA, Abejar NH, Dimaano EE, Bartolome R, Cinco S, Mariano N, Yerro ME, Cobar S, Fuggan B. 1998. A pilot double-blind, randomized, and placebo-controlled study of orally administered IFN-alpha-n1 (Ins) in pediatric patients with measles. J Interferon Cytokine Res 18:647–652 [DOI] [PubMed] [Google Scholar]
- Mac Nicholas R, Norris S. 2010. Review article: optimizing SVR and management of the haematological side effects of peginterferon/ribavirin antiviral therapy for HCV—the role of epoetin, G-CSF and novel agents. Aliment Pharmacol Ther 31:929–937 [DOI] [PubMed] [Google Scholar]
- Mestecky J, Russell MW, Elson CO. 2007. Perspectives on mucosal vaccines: is mucosal tolerance a barrier? J Immunol 179:5633–5638 [DOI] [PubMed] [Google Scholar]
- Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. 2013. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med 158:329–337 [DOI] [PubMed] [Google Scholar]
- Nagura H, Sumi Y. 1988. Immunological functions of the gut-role of the mucosal immune system. Toxicol Pathol 16:154–164 [DOI] [PubMed] [Google Scholar]
- Petta S, Craxi A. 2012. Therapeutic algorithms for chronic hepatitis C in the DAA era during the current economic crisis: whom to treat? how to treat? when to treat? BMC Infect Dis 12 (Suppl 2):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psetti MF, Simon JK, Sztein MB, Levine MM. 2011. Immunology of gut mucosal vaccines. Immunol Rev 239:125–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak B. 1993. Observation of the effect of low oral doses of human leukocytic interferon alpha in children with chronic HBV infection and impaired immune response. Arch Immunol Ther Exp 41:237–240 [PubMed] [Google Scholar]
- Russell IJ, Michalek JE, Kang YK, Richards AB. 1999. Reduction of morning stiffness and improvement in physical function in fibromyalgia syndrome patients treated sublingually with low doses of human interferon-alpha. J Interferon Cytokine Res 19:961–968 [DOI] [PubMed] [Google Scholar]
- Schafer TW, Lieberman M, Cohen M, Came PE. 1972. Interferon administered orally: protection of neonatal mice from lethal virus challenge. Science 176:1326–1327 [DOI] [PubMed] [Google Scholar]
- Shiozawa S, Tanaka Y, Shiozawa K. 1998. Single-blinded controlled trial of low-dose oral IFN-alpha for the treatment of xerostomia in patients with Sjogren's syndrome. J Interferon Cytokine Res 18:255–262 [DOI] [PubMed] [Google Scholar]
- Ship JA, Fox PC, Michalek JE, Cummins MJ, Richards AB. 1999. Treatment of primary Sjögren's syndrome with low-dose natural human interferon-alpha administered by the oral mucosal route: a phase II clinical trial. IFN Protocol Study Group. J Interferon Cytokine Res 19:943–951 [DOI] [PubMed] [Google Scholar]
- Stedman CA. 2013. Current prospects for interferon-free treatment of hepatitis C in 2012. J Gastroenterol Hepatol 28:38–45 [DOI] [PubMed] [Google Scholar]
- Steed VP. 1987. Improved survival of four cats infected with feline leukemia virus after oral administration of interferon. Feline Pract 17:24–30 [Google Scholar]
- Tompkins MB, Cummins JM. 1982. Response of feline leukemia virus-induced nonregenerative anemia to oral administration of an interferon containing preparation. Feline Pract 12:6–15 [Google Scholar]
- Weiss RC, Cummins JM, Richards AB. 1991. Low-dose orally administered alpha interferon treatment for feline leukemia virus infection. J Am Vet Med Assoc 199:1477–1481 [PubMed] [Google Scholar]
- Young AS, Maritim AC, Kariuki DP, Stagg DA, Wafula JM, Mutugi JJ, Cummins JM, Richards AB, Burns C. 1990. Low-dose oral administration of human interferon alpha can control the development of Theileria Parva infection in cattle. Parasitology 101Pt2:201–209 [DOI] [PubMed] [Google Scholar]
- Zielińska W, Paszkiewicz J, Korczak A, Własiuk M, Zółtowska A, Szutowicz A, Cummins JM, Georgiades JA. 1993a. Treatment of fourteen chronic active HBsAg+, HBeAg+ hepatitis patients with low dose natural human interferon alpha administered orally. Arch Immunol Ther Exp (Warsz) 41:241–251 [PubMed] [Google Scholar]
- Zielińska W, Paszkiewicz J, Korczak A, Własiuk M, Zółtowska A, Szutowicz A, Cummins JM, Georgiades JA. 1993b. Treatment of six patients with chronic active HCV hepatitis, with low dose natural human interferon alpha administered orally. Arch Immunol Ther Exp (Warsz) 41:253–257 [PubMed] [Google Scholar]