Abstract
Significance: Ionizing radiation is a vital component in the oncologist's arsenal for the treatment of cancer. Approximately 50% of all cancer patients will receive some form of radiation therapy as part of their treatment regimen. DNA is considered the major cellular target of ionizing radiation and can be damaged directly by radiation or indirectly through reactive oxygen species (ROS) formed from the radiolysis of water, enzyme-mediated ROS production, and ROS resulting from altered aerobic metabolism. Recent Advances: ROS are produced as a byproduct of oxygen metabolism, and superoxide dismutases (SODs) are the chief scavengers. ROS contribute to the radioresponsiveness of normal and tumor tissues, and SODs modulate the radioresponsiveness of tissues, thus affecting the efficacy of radiotherapy. Critical Issues: Despite its prevalent use, radiation therapy suffers from certain limitations that diminish its effectiveness, including tumor hypoxia and normal tissue damage. Oxygen is important for the stabilization of radiation-induced DNA damage, and tumor hypoxia dramatically decreases radiation efficacy. Therefore, auxiliary therapies are needed to increase the effectiveness of radiation therapy against tumor tissues while minimizing normal tissue injury. Future Directions: Because of the importance of ROS in the response of normal and cancer tissues to ionizing radiation, methods that differentially modulate the ROS scavenging ability of cells may prove to be an important method to increase the radiation response in cancer tissues and simultaneously mitigate the damaging effects of ionizing radiation on normal tissues. Altering the expression or activity of SODs may prove valuable in maximizing the overall effectiveness of ionizing radiation. Antioxid. Redox Signal. 20, 1567–1589.
Introduction
The International Agency for Research on Cancer has estimated an annual diagnosis of 12.7 million new cases of cancer and 7.6 million cancer-related deaths worldwide (105). Radiation therapy is used alone, or in conjunction with, chemotherapy, immunotherapy, surgery, and hormone therapy for the treatment of cancer (10). In fact, ∼50% of all cancer patients will receive some form of radiation as an important element in their treatment regimen (43).
The medical application of ionizing radiation was realized early after the discovery of X-rays by Röntgen in 1895, when Emil Grubbé used X-rays to treat an ulcerated breast cancer 60 days after the discovery of X-rays (15). Since that time, efforts have been made to improve the efficacy of radiation therapy, increasing the killing effect on cancer cells while minimizing the detrimental effects on normal tissues. Various drugs have been developed to modulate the DNA damage response in tumor cells, alter the activation of signal transduction pathways activated after irradiation, and control the influence of the tumor microenvironment [reviewed in ref. (12)]. Despite these advances, there is a need for further improvements.
Reactive oxygen species (ROS) are produced as a byproduct of oxygen metabolism (70). ROS, while harmful to cells when produced in excess through oxidative modification of lipids, proteins, and DNA, are also vital mediators of multiple cellular processes, including cell growth and differentiation (18), the immune response, cell adhesion, and apoptosis (47). ROS are also second messengers in cell signaling (69, 81, 181, 210). The rate of ROS production and destruction is carefully maintained in the cell, and interruption of this process contributes to the development of different diseases, including cancer (75, 210, 215).
ROS play a major role in the damaging effects of low linear energy transfer (LET) ionizing radiation on cancer cells. ROS are formed by the radiolysis of water, and these ROS (137), particularly the hydroxyl radical (214), participate in damaging DNA. Roughly two-thirds of radiation-mediated DNA damage is caused by indirect effects from ROS (146). Although radiation is an important treatment for cancer, it can also be harmful to normal tissues (1). Therefore, methods that can simultaneously increase the radiosentivity of cancer cells and radioresistance of normal tissues are needed to improve the treatment outcome in patients.
Mitochondria are the major sites of metabolic ROS production in the cell, with the superoxide radical as the primary ROS generated by the organelle as a byproduct of oxidative phosphorylation (2, 97). Cells are equipped with many systems to scavenge ROS, with the superoxide dismutases (SODs) as the chief ROS scavenging enzymes in the cell (228). Because of the importance of ROS in cancer development, and the role of ROS in the radiation-induced damage, methods to alter the redox environment of cancer cells may enhance the response of cancer cells to ionizing radiation.
In this review, we will discuss the effects of ionizing radiation on the cell. We will also discuss two factors that affect the efficacy of radiation therapy: the bystander effect and the tumor microenvironment. We will also discuss SODs, their role in cancer development, and their importance in the radiation response of both normal tissues and cancer cells. Finally, we will detail different methods to increase SOD expression and/or activity and its effects on simultaneously protecting normal tissues and sensitizing tumor tissues to ionizing radiation.
Effects of Radiation on Biological Tissue
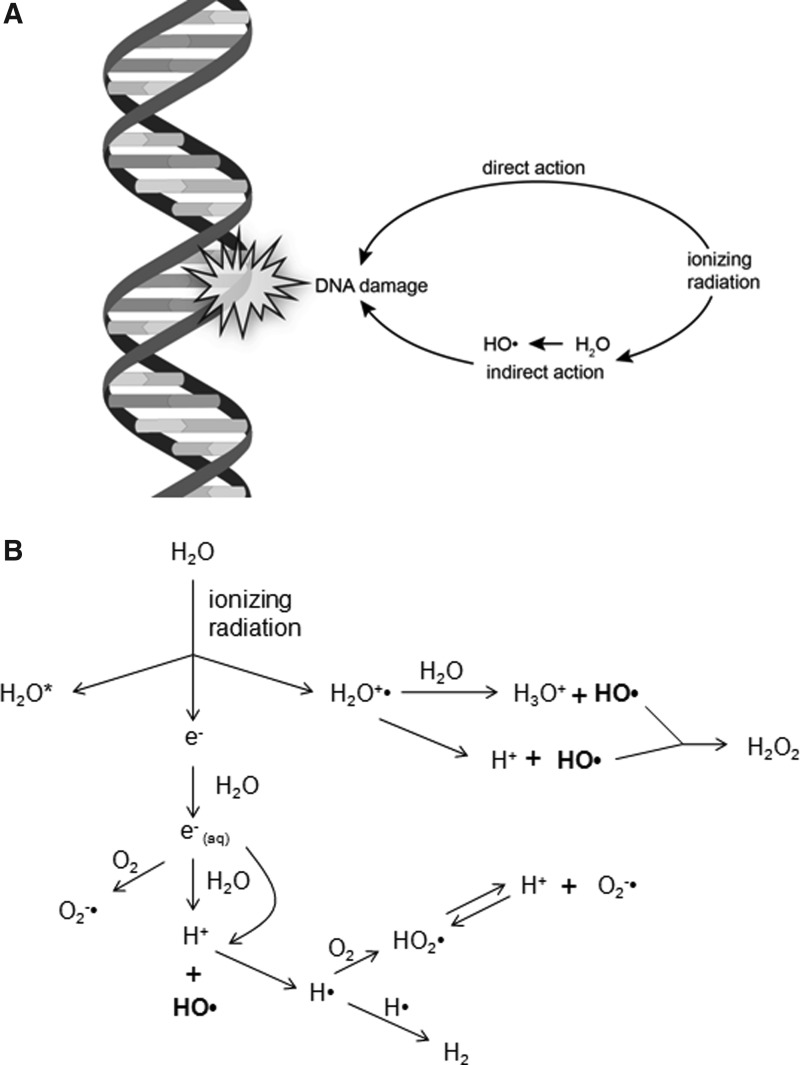
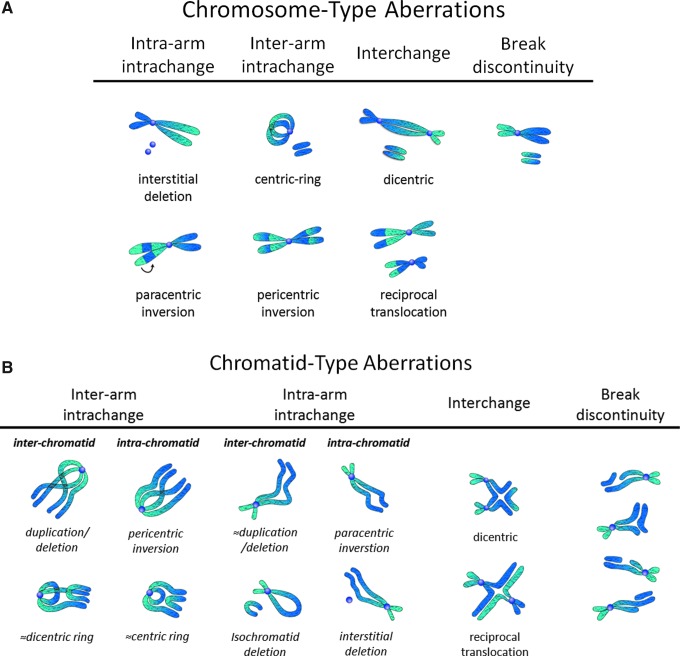
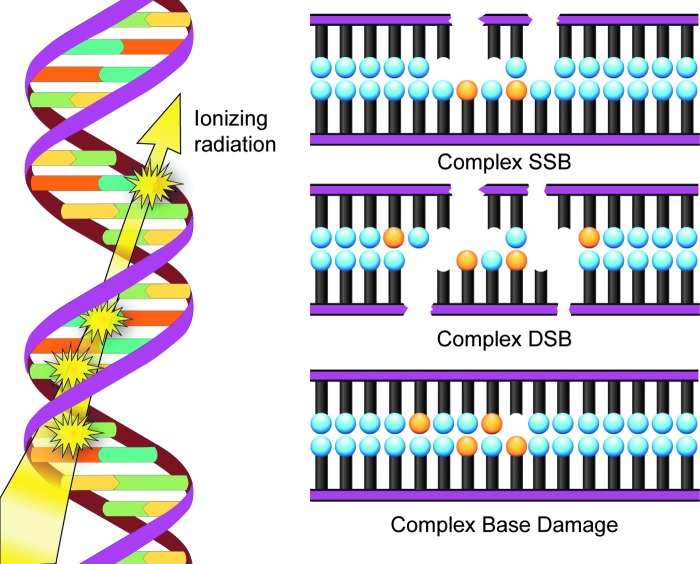
The primary target of ionizing radiation in the cell is chromosomal DNA. Ionizing radiation damages intracellular molecules by direct ionization and through indirect ionizations mediated by water radiolysis products (Fig. 1A), with an estimated two-thirds of radiation-induced DNA damage due to indirect effects (146). This process elicits myriad types of DNA damage, including base and sugar damage, as well as single- and double-strand breaks. Radiolysis of water leads to the formation of a variety of free radicals. For example, water is ionized to form a radical and a free electron. The water radical can either undergo decomposition or interact with a water molecule to form hydroxyl radicals. The free electron can be hydrated by surrounding water molecules, and this stabilized electron can interact with another water molecule to form a hydroxyl radical or a proton to form a hydrogen radical (137) (Fig. 1B). Direct effects of ionizing radiation on DNA lead to a variety of damage products, such as oxidized bases and cleavage of the sugar–phosphate backbone (Fig. 2) (189). Water radiolysis products can damage nucleic acids (Fig. 3) and other cellular molecules, including proteins and lipids (180, 214). Free radical damage of DNA, whether direct or indirect, results in the formation of myriad types of DNA lesions (137, 167, 182). Base lesions may be benign, with no apparent effect on the cell after irradiation, or the base lesions may lead to miscoding of the DNA, resulting in mutation formation. Single-strand breaks can also form, which may result in mutation formation if this damage is not properly repaired. Double-strand breaks also occur, in particular, following the replication of DNA that sustained oxidative damage, resulting in chromosomal abnormalities (137). These double-strand breaks, if not re-annealed at the site of the original break, may re-anneal with other breaks on the same chromosome or a different chromosome to form chromosomal aberrations (88). Chromosomal aberrations are divided into two broad types: chromosome type and chromatid type. Chromosome-type aberrations occur when breaks and rejoining change both sister chromatids at one locus (Fig. 4A). Chromatid-type aberrations occur when breaks and rejoinings alter only one sister chromatid at any one locus (Fig. 4B). The type of damage that occurs after irradiation depends on the stage of the cell cycle at which a cell is irradiated. Chromosome-type aberrations typically occur during G1 of the cell cycle, whereas chromatid-type aberrations typically occur during G2 and S phases of the cell cycle (3).
FIG. 1.
Effects of ionizing radiation on DNA. (A) DNA can either be damaged directly by ionizing radiation to form a base radical, or DNA can be damaged indirectly through radiation-induced ionization of water to form hydroxyl radicals, which cause base radical formation. (B) Interaction of ionizing radiation with water either leads to an excited form of water or ionization of water to form a water radical and a free electron. Hydroxyl radical formation occurs by various mechanisms through the interaction of the free electron with water or decomposition of the water radical.
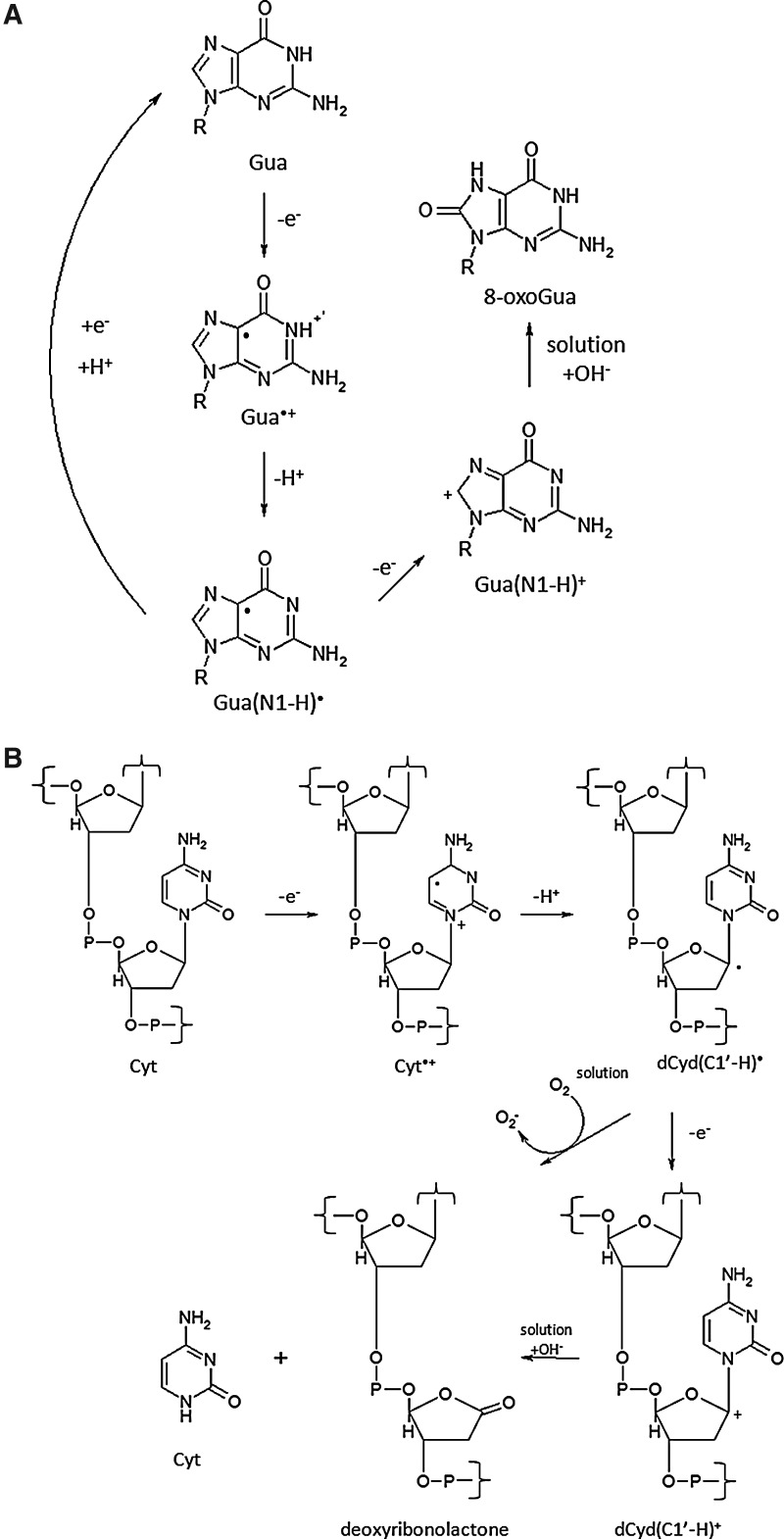
FIG. 2.
Direct damage to DNA by ionizing radiation. (A) Oxidation of guanine (Gua) to 8-oxoGua. (B) Radiation induced cleavage of cytosine (Cyt) from the sugar–phosphate backbone. Modified from Shibata et al. (190).
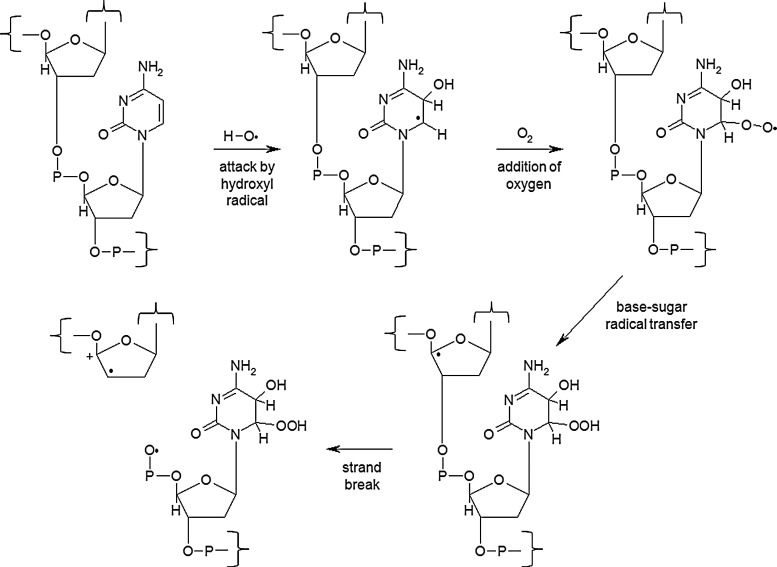
FIG. 3.
Hydroxyl radical-induced DNA damage. A DNA base is attacked by a hydroxyl radical to form a base radical. In the presence of oxygen, this base radical is stabilized by the formation of a peroxide. A base-sugar radical transfer occurs, placing the free radical on the ribose. Hydrolytic cleavage then occurs, resulting in a strand break. Modified from Rhee et al. (181) and Waris and Ahsan (215).
FIG. 4.
Chromosomal aberrations after ionizing radiation. (A) Chromosome-type aberrations occur when breaks and rejoinings change both sister chromatids at one locus. (B) Chromatid-type aberrations occur when breaks and rejoinings alter only one sister chromatid at any one locus. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Another important type of radiation-induced DNA damage identified is referred to as clustered damage, where two or more closely spaced types of damage occur, such as abasic sites, oxidized bases, and tandem lesions, contributing to the formation of double-strand breaks (77, 78, 198, 213) (Fig. 5). This clustered damage is thought to have significant biological consequences because it is much more difficult to repair. Clustered damage is longer-lived, increasing the likelihood of incorporation of an inappropriate base, resulting in mutation formation [reviewed in ref. (152)]. Unrepaired clustered damage also leads to a multitude of chromosomal aberrations (5), and attempts to repair clustered damage can result in further double-strand break formation (191). Not only does clustered damage form from the direct interaction of ionizing radiation with DNA, it can also be induced by ROS, including hydroxyl radicals (180).
FIG. 5.
Model of clustered damage of DNA after ionizing radiation. Clustered DNA damage can occur after direct ionization by radiation or by radiation-induced ROS formation, leading to the formation of base damage (orange circles), abasic sites, and tandem lesions, resulting in single- and double-strand breaks. ROS, reactive oxygen species. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Although radiation therapy has proven valuable in the treatment of cancer, it can also have detrimental side effects. Tissues most susceptible to ionizing radiation are those with a high replication rate and are least differentiated (1). This characteristic makes cancer cells, with their high proliferation rate, more prone to radiation-induced damage than normal tissues (12), but can also affect normal tissues (1). One example of the detrimental effects of ionizing radiation is the formation of secondary cancers. Diallo et al. studied the frequency of secondary cancer formation in relation to irradiated volume in patients with childhood cancer that received radiation therapy. The researchers found that the majority of secondary tumors (66%) formed in the region bordering planning target volume, and a small percentage of tumors formed in a region >5 cm from the irradiated volume (22%) or within the irradiated volume (12%) (46). Therefore, methods that increase the radiosensitivity of cancer tissues and simultaneously increase the radioresistance of normal tissue are needed.
Radiation-Induced Bystander Effect
Description
The bystander effect is defined as an induction of some biological effect in cells that have not been directly traversed by radiation but are in close proximity to a cell that has received radiation (87). Radiation-mediated changes in unirradiated cells can be divided into three classifications: bystander effects, abscopal effects, and cohort effects. Bystander effects occur in cells in an irradiated tissue that are not directly bombarded by radiation. Abscopal effects occur to unirradiated cells outside the irradiated volume. The cohort effect is an indirect effect within an irradiated cell due to some signal received by another irradiated cell within the same irradiated tissue (17) (Fig. 6). Several biological endpoints have been used to prove the presence of radiation-induced bystander effect, including micronuclei formation, clonogenic survival, mutation formation, apoptosis, and neoplastic transformation, among others [reviewed in refs. (34) and (89)]. Another important consequence of the bystander effect is increased radioresistance in nonirradiated tissue. For example, the exposure of normal human lung fibroblasts (NHLFs) to medium from HLFs irradiated with 1 cGy γ-rays increased clonogenic survival after exposure to 10 and 19 cGy radiation. The increased radioresistance in bystander cells correlated with an increase in the expression of AP-endonuclease (102).
FIG. 6.
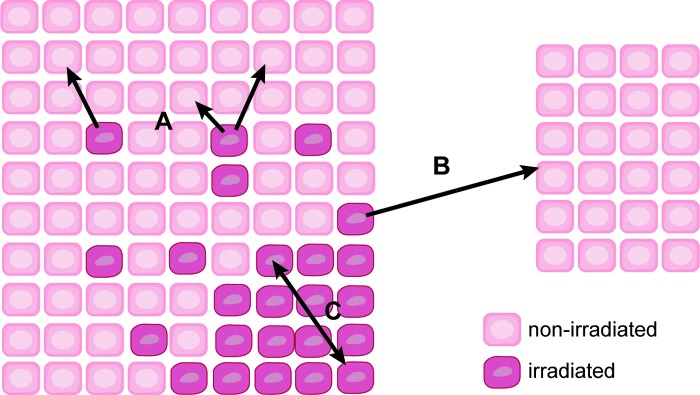
The bystander effect. Three major types of bystander effects occur in irradiated tissues. Bystander effects (A) occur in unirradiated cells within a low-dose-irradiated tissue. Abscopal effects (B) occur to unirradiated cells outside the irradiated volume. The cohort effect (C) occurs between irradiated cells within an irradiated tissue volume. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
One of the first reports of a radiation-induced bystander effect was a study by Nagasawa and Little (145), in which the researchers reported the induction of sister chromatid exchanges in Chinese hamster ovary (CHO) cell cultures exposed to very low mean doses of α-particles (0.31 mGy). While less than 1% of the cell nuclei were struck by an α-particle, 30% of the cells had increased sister chromatid exchange. Irradiation with X-rays (65, 112, 226) and low LET β-particles (163) also produced stressful bystander effects.
Irradiated cells can induce a bystander effect long after the irradiation event. Lyng et al. (129) found that medium collected from progeny cells of γ-irradiated HPV-G human keratinocytes up to passage 7 after irradiation induced rapid calcium fluxes, loss of mitochondrial membrane potential, and increased ROS production in bystander HPV-G cells. No differences were observed in the bystander effect between medium collected from irradiated cells or from the cells passaged from the irradiated cells (129). A better understanding of the mechanisms of the bystander effect can lead to improvements in cancer therapy that mitigate harmful effects of radiation on nontarget tissue.
Mechanisms of the bystander effect
Direct intercellular communication by junctional channels.
Microbeam technology has proven invaluable for study of the bystander effect, allowing for the delivery of a specific dose of radiation to an individual cell or groups of cells, leaving surrounding cells untouched [reviewed in ref. (170)]. Alpha particle irradiation of 20% of AL human hamster hybrid cells in a microbeam dish resulted in a threefold higher mutation fraction than predicted due to the formation of mutations in nonirradiated cells. When cells were pretreated with the gap junction intercellular communication (GJIC) inhibitor lindane, there was a significant reduction in mutation formation, indicating the importance of cell–cell communication in the induction of the bystander effect (231). Further studies revealed that GJIC is vital for propagating signals involved in the bystander effect. Inhibition of gap junction communication by chemical inhibitors or by expression of a dominant-negative form of connexin-43 (Cx43) resulted in diminution of bystander effect-induced mutation frequency (162, 233). Using various fibroblast and epithelial cell lines, Azzam et al. (8) demonstrated the importance of GJIC in the bystander effect. The researchers found that treatment of the cells with α-particles resulted in the induction of p21Waf1, and pretreatment with the GJIC inhibitor lindane, use of cells that are GJIC incompetent or use of Cx43 knockout cells, resulted in diminished radiation-induced p21Waf1 levels (8).
GJIC is also important in potentiating the cell-killing effects of ionizing radiation due to the propagation of stress signals throughout the irradiated volume. Little effect is seen with low LET radiation, but a significant enhancement is observed for high LET radiation. Using confluent cultures of AG1522 human diploid fibroblasts, Autsavapromporn et al. (6) discovered that incubation of the cells for 3 h after irradiation with α-particles resulted in decreased survival and increased DNA damage compared to cells irradiated with γ-rays. This effect was due to GJIC and was attenuated by pretreatment with the GJIC inhibitor AGA or knockdown of Cx43 by siRNA (7). A similar enhancement of cell killing and DNA damage was observed for high-charge high-energy iron ions compared to protons (6).
Intercellular communication via release of soluble factors
Numerous studies have found that conditioned medium obtained from irradiated cells induces a bystander effect in unirradiated cells, suggesting soluble factors released by irradiated cells are involved in the bystander effect. For example, Mothersill and Seymour (141) found that treatment of various epithelial cell lines with the medium from γ-ray-treated cells led to a decrease in clonogenic survival in both a radiation dose- and irradiated cell number-dependent manner. This effect was only seen in the medium from irradiated cells, and not irradiated medium in the absence of cells (141). Similar results have been reported in various cell types using different methods, including medium transfer (123, 159), double mylar dishes (which allow exchange of medium between irradiated and nonirradiated cells) (232), and microbeam treatment (14, 169), leading to increased apoptosis (14, 123, 159, 169), ROS formation (159), as well as micronuclei formation (14, 169).
Extracellular DNA (ecDNA) has recently been identified as an important soluble factor that stimulates the bystander effect. Ermakov et al. (60) discovered that release of ecDNA from X-ray-treated primary human lymphocytes induced a bystander effect in nonirradiated lymphocytes. Both the release of ecDNA and induction of the bystander effect were blocked by the administration of the antioxidant α-tocopherol. Based on these results, the researchers suggested that ROS are important for both the response to radiation, leading to apoptosis and release of ecDNA, and induction of DNA damage in bystander cells (60). Similar results have been observed in X-ray-treated human umbilical vein endothelial cells (HUVEC) (61, 118). The authors proposed that ecDNA is released due to ROS-mediated apoptosis induced by ionizing radiation. DNA-binding receptors (TLR9) on bystander cells detect these DNA fragments, stimulating ROS production and DNA damage. Inhibition of TLR9 by treatment with an oligonucleotide inhibitor or chloroquine blocked the bystander effect, whereas treatment of unirradiated cells with ecDNA isolated from irradiated cell medium stimulated the bystander effect (61, 118). These results suggest that scavenging of ROS may be a valuable strategy for assuaging the bystander effect.
Altered gene expression and signal transduction
Induction of the bystander effect leads to the activation of various signal transduction pathways and different transcription factors, resulting in changes in gene expression, culminating in the biological endpoints that characterize the bystander effect. Two important genes identified in the bystander effect are cyclooxygenase-2 (COX-2) and insulin growth factor binding protein 3 (IGFBP-3). Zhou et al. (229) using NHLFs found that α-particle irradiation resulted in a bystander effect by a reduction in the surviving fraction and an increased mutagenesis rate. Using a signal transduction gene array, the researchers identified a significant increase in the expression of COX-2 gene and a decrease in IGFBP-3 gene expression. Co-treatment of NHLFs with NS-398 (a COX-2 inhibitor) reduced the mutagenesis rate in bystander cells, and addition of IGFBP-3 increased survival and decreased the mutagenesis rate in bystander cells. Treatment of the cells with either the MAP kinase inhibitor PD98059 or an anti-tumor necrosis factor-alpha (TNF-α) antibody significantly blocked the bystander effect (229).
Bystander effect in vivo
While a multitude of studies demonstrate an in vitro bystander effect, the bystander effect has also been confirmed in vivo in various model systems, such as Caenorhabditis elegans, zebra fish, and Arabidpopsis thaliana [reviewed in ref. (30)]. Studies of partial lung γ-ray treatment in rats revealed that the lower lung is much more sensitive to the in-field effects of radiation (as determined by micronuclei formation) than the upper lung, whereas the upper lung demonstrated greater response to bystander effects after lower lung irradiation. Copper-zinc superoxide dismutase (CuZnSOD), manganese superoxide dismutase (MnSOD), or the nitric oxide synthase inhibitor nitro-l-arginine methyl ester dissolved in saline and administered by intraperitoneal injection, significantly reduced both in-field and bystander effects, suggesting a role for ROS in bystander effects in vivo (114, 115). In-field and bystander effect induction of inflammatory cytokines is also involved in the response to partial lung γ-ray treatment (28), as well as X-ray treatment of the abdomen (211). Koturbash et al. found that X-irradiation of the cranium of mice increased double-strand DNA breaks, p53 expression, cell proliferation, and apoptosis in the lead-shielded spleen of the animals (120), and these bystander effects on the spleen were sex specific, with male bystander spleens demonstrating significantly greater DNA damage and loss of DNA methylation compared to bystander female spleens. These sex-specific differences in bystander effect were diminished in animals that had undergone surgical removal of their gonads (119). These results indicate that the bystander effect is a complex phenomenon in vivo and that strategies designed to assuage the bystander effect may need to take into account sex-specific differences.
Bystander effect in neoplastic transformation
Not only can the bystander effect result in cellular damage and death in nonirradiated cells, it can also induce neoplastic transformation in nonirradiated cells. Exposure of CGL1 HeLa skin-fibroblast hybrid cells to medium collected from X-ray-treated HeLa-skin fibroblast hybrid (CGL1) cells significantly increased the neoplastic transformation frequency (123). A similar increase in neoplastic transformation has been observed in JB6 mouse epithelial cells co-cultured with JB6 cells treated with γ-rays (216). Bystander effect-mediated neoplastic transformation has also been observed in vivo. Mancuso et al., using Patched-1 (Ptch1) heterozygous knockout mice (Ptch1+/−), discovered that medulloblastoma formation was significantly greater in Ptch1+/− mice with their heads shielded (but the remainder of their body receiving radiation treatment) compared to untreated mice but was not as great as mice receiving whole-body radiation (134). A later study investigated the role of GJIC on propagation of bystander effect signaling in vivo and its role in radiation-associated bystander effect cancer formation in a Cx43 heterozygous knockout mouse (Cx43+/−) model. Radiation-induced medulloblastoma formation was significantly lower in shielded Cx43+/−/Ptch+/− mice compared to shielded Cx43+/+/Ptch+/− mice, confirming the role for GJIC in bystander effect-mediated neoplastic transformation (133).
The bystander effect may also contribute to neoplastic transformation in humans. In a study by Diallo et al. (46) investigating the formation of secondary cancer formation in childhood cancer patients who received radiation therapy, there was a substantially greater number of secondary cancers that developed at distant sites from the irradiated volume (25 neoplasms) than what was predicted (nine neoplasms). In this study, the authors defined distant as any area farther than 5 cm outward from the edge of the irradiated volume (46). Given the effects of the bystander effect on neoplastic transformation discussed above, these results suggest that an important bystander effect may also be occurring in humans that may contribute to the secondary cancer formation after radiation treatment. Therefore, adjuvant therapies that can mitigate these unwanted effects of radiation therapy would prove valuable in the clinic.
Although irradiated cells can communicate to unirradiated cells through the bystander effect, bystander cells can communicate back to the irradiated cells. Chen et al. found that incubation of α-particle-irradiated NHLFs with bystander NHLFs significantly reduced 53BP1 foci formation (a marker of double-strand breaks), as well as diminished micronuclei formation and apoptosis, in the irradiated cells compared to irradiated cells not incubated with bystander cells (36). Similar reductions in micronuclei formation have been observed in α-particle-irradiated HeLa incubated with NHLFs (36) and incubation of X-irradiated Me45 human melanoma cells with normal human dermal fibroblasts (219), and the reduction in micronuclei formation corresponded with reduced ROS formation in the irradiated cells (219). These reports suggest that the bystander effect and reciprocal communication from bystander cells back to irradiated cells is an important component in the complex response of an organism to ionizing radiation. This type of communication may have important implications in radiation resistance in cancer cells and may provide an important target to increase radiosensitivity in cancer, especially within the context of therapies that exploit oxidative stress.
ROS in the bystander effect
ROS are important mediators of the bystander effect by myriad mechanisms. Incubation of bystander cells with the conditioned medium from γ-ray-treated human keratinocytes in the presence of the antioxidant N-acetylcysteine (NAC) or the caspase-9 inhibitor Z-LEHD-FMK abrogated the effects of the conditioned medium on bystander keratinocytes (130). Increased lipid oxidation after irradiation with γ-rays (as measured by malondialdehyde formation) has also been observed in both irradiated and bystander Me45 cells and correlated with a significant decrease in the MnSOD activity (171). ROS formation was associated with micronuclei formation and γ-H2AX foci formation in bystander AG01522 normal human fibroblasts, which was inhibited by the addition of either CuZnSOD or catalase (final concentration 500 U/ml and 8×103 U/ml, respectively) directly into the culture medium immediately after irradiation (226). Similar results have been reported in human peripheral blood lymphocytes (13). In HaCaT human keratinocytes, viability of bystander cells was rescued by incubation of either the bystander cells or the γ-ray-treated cells with SOD or catalase (added directly to the cell culture medium), suggesting ROS are both a source of bystander signals and carry out the bystander effect in recipient cells (127). ROS also initiate the bystander effect by stimulating ecDNA release from irradiated cells and response of bystander cells (see discussion above). Therefore, methods that increase ROS scavenging ability in cells may simultaneously abrogate both the generation of bystander signals and the response to bystander signals.
Mitochondria and the bystander effect
Mitochondria are the primary source of ROS in the cell due to oxygen metabolism through oxidative phosphorylation (22, 122, 144), with complexes I (83, 200) and III (205) as major contributors of superoxide produced by mitochondria. Because mitochondria produce ROS, and the role of ROS in propagation of the bystander effect (discussed above), logic dictates that mitochondria may be important in the bystander effect. Rajendran et al. (178) used various human lymphoblastoid cell lines treated with X-rays to study the effects of mitochondrial DNA (mtDNA) mutations on the bystander effect. The researchers found that medium from irradiated cell lines with normal mtDNA stimulated a bystander effect (micronucleus formation) in the same cell line or other cell lines with normal mtDNA, but not in cell lines harboring mtDNA mutations. Medium from irradiated cell lines with mtDNA mutations was unable to stimulate a bystander effect in either normal cell lines or cell lines with mtDNA mutations. These results suggest that functioning mitochondria are vital for both generation and reception of bystander signals (178).
Mitochondria as sources of the bystander effect
Studies using AL human-hamster hybrid cells with either normal mtDNA (ρ+) or depleted of mtDNA (ρ0) have established the contribution of mitochondria in generation of bystander signals from irradiated cells. Either depletion of mtDNA or inhibition of the electron transport chain, as well as inhibition of NO production or calcium uptake, significantly reduced γ-H2AX foci, a marker of DNA damage, in bystander cells (37). A reduction in the bystander effect has also been observed in AG1522 cells incubated with the medium from α-particle-irradiated ρ0 AL cells (38). Depletion of mtDNA had similar results in α-particle-irradiated HeLa cells (202) and human skin fibroblasts (230).
Mitochondria as targets of the bystander effect
Not only are mitochondria important initiators of the bystander effect, they are also recipients of bystander signals. For example, treatment of human keratinocytes with the medium from keratinocytes treated with γ-rays resulted in rapid calcium flux, increased ROS production, and decreased mitochondrial membrane potential, as well as increased apoptosis and decreased clonogenic survival (128). Direct irradiation of Chinese hamster ovary K1 cells or HPV-G cells, or exposure of these cells to irradiated cell-conditioned medium, significantly altered mitochondrial oxygen consumption and increased mitochondrial mass (151), as well as decreased expression of mtDNA-associated genes (150).
mtDNA is an important target of the bystander effect. Several reports indicate that mtDNA mutations (79) and deletions (79, 143, 185) occur upon exposure to the conditioned medium from irradiated cells, with these mutations correlating with a decrease in mitochondrial membrane potential (79). The bystander effect can also affect mtDNA gene expression (35, 150). For instance, Chaudhry and Omaruddin (35) found that in both X-irradiated and bystander TK6 human lymphoblast cells, there was a statistically significant change in the expression of various mtDNA encoded genes for different components of the electron transport chain. MT-ND1, MT-ND5, and MT-ND6 (components of NADH dehydrogenase) were upregulated in directly-irradiated cells but were downregulated in bystander cells, whereas MT-ATP6 and MT-ATP8 were upregulated in both cell types (35).
Although cytochrome c (cytc) does not affect the direct response of cells to ionizing radiation or the generation of bystander signals, it has a significant impact on response to bystander signals from irradiated cells. Co-culture of mouse embryonic fibroblasts (MEFs) that are cytc-null (cytc−/−) with α-particle-irradiated cytc−/− or cytc+/+ cells resulted in no significant induction of micronuclei formation in cytc−/− bystander cells. The conditioned medium from irradiated cytc+/+ or cytc−/− cells stimulated ROS production in cytc+/+ bystander cells, but not cytc−/− bystander cells (225).
Genomic DNA damage occurs in bystander cells as a result of signaling events modulated by mitochondria. Telomere shortening and anaphase bridge formation caused by incubation of bystander SW480 cells with the medium from explanted tumor tissue exposed to γ-rays correlated with a decrease in mitochondrial membrane potential and an increase in ROS formation. Overexpression of MnSOD in the SW480 cells not only diminished the effects of the conditioned medium on mitochondrial membrane potential and ROS formation, but also significantly reduced the effects of the conditioned medium on telomere shortening and anaphase bridge formation. These results suggest that MnSOD may be important for inhibition of the bystander effect in nontarget cells (80).
Tumor Architecture and Its Effects on Radiation Medicine
Tumors as organs
Long thought to be only a clonal expansion of mutated cells, tumors are actually complex mixtures of various cell types organized as abnormal organs. A variety of cells have been identified in tumors, many of which contribute to tumor progression, including adipocytes, fibroblasts, endothelial cells, and myriad immune cells [reviewed in ref. (49)]. During tumor progression, changes in the interactions between the cancer cells and supporting cells, including cancer stem cells [recently reviewed in ref. (26)], as well as the differences in the interaction between cancer cells with extracellular matrix (and the composition of the extracellular matrix) occur, which can contribute to radiation resistance in tumors. For example, Josson et al. (107) found that coculture of ARCaPE (with an epithelial morphology and phenotype) human prostate cancer cells with either bone fibroblasts or stromal fibroblasts isolated from normal or cancer-bearing prostate tissue significantly enhanced radioresistance of ARCaPE cells. This radioprotective effect was mediated by both E-cadherin and integrin signaling. Interestingly, coculture of ARCaPM (with a mesenchymal morphology and phenotype) human prostate cancer cells with bone or prostate fibroblasts did not affect radioresistance (107), indicating an important role for phenotype on cancer cell responsiveness. Coculture of Suit-2 human pancreatic cancer cells with γ-ray-treated MRC5 human fibroblast cells or primary pancreatic fibroblasts significantly enhanced invasiveness both in vitro and in vivo. Enhancement of Suit-2 invasiveness and cell scattering was induced by incubation with the conditioned medium from irradiated MRC5, coinciding with an increase in p44/p42 MAPK and c-Met activation, and was blocked by pretreatment with the hepatocyte growth factor inhibitor NK4 (156). In a study by Tsai et al., (206) coculture of MCF-7 or MDA-MB-231 human breast cancer cells with senescent primary human mammary fibroblasts (induced by X-ray treatment) significantly enhanced cell growth due to the stimulation of the expression of mitotic genes. Senescent fibroblasts, as well as senescent fibroblast-conditioned medium, also conferred radioresistance to the breast cancer cells, which was partially attenuated by expression of dominant-negative AKT in the breast cancer cells (206). These results demonstrate the importance of supporting cells for the response of cancer cells to ionizing radiation. Because of the role of soluble factors secreted by the supporting cells in conferring radioprotection and stimulation of aggressiveness of cancer cells, the bystander effect may be vital contributor of this process. Methods that target the bystander effect between cancer cells and the supporting cells within the tumor may prove attractive for enhancing the effectiveness of radiation therapy.
Tumor hypoxia and radiation treatment
Given the importance of oxygen in the response of cells to radiation (23, 82, 106), hypoxia becomes a major problem in the treatment of tumors by radiation. Two types of hypoxia exist in tumors: chronic and acute (Fig. 7). Chronic hypoxia occurs due to the diffusion limits of oxygen through tissues, whereas acute hypoxia occurs due to temporary blockage of a tumor blood vessel (25). Chronic hypoxia was first identified by Deschner and Gray, who found that oxygen supply to cells within a tumor is limited to a region ∼150 μm from the vasculature (44). Acute hypoxia was first identified by Brown (24) and can have important implications in the response of tumors to radiation (32). Several reports show that radiosensitivity of cancer cells is inversely proportional to the distance from the blood supply (142, 201). Chaplin et al. discovered that tumor cells closest to the blood vessels of the tumor were most sensitive to ionizing radiation (as measured by clonogenic survival), whereas the tumor cells farthest from the blood vessel were most resistant to ionizing radiation. Ionizing radiation resulted in acute hypoxia in the tumor due to transient changes in blood flow, and this acute hypoxia increased radioresistance in the tumor (33). Hsieh et al. compared the influence of either cycling hypoxia or uninterrupted hypoxia on radiation resistance of U87 glioma cells and found that cycling hypoxia greatly increased U87 glioma cell radiation resistance compared to uninterrupted hypoxia due to increased ROS production, leading to greater stabilization, expression, and transcriptional activity of hypoxia-inducible factor-1α (HIF-1α). Suppression of HIF-1α induction by siRNA resulted in radiosensitization of U87 cells both in vitro and in vivo (98).
FIG. 7.
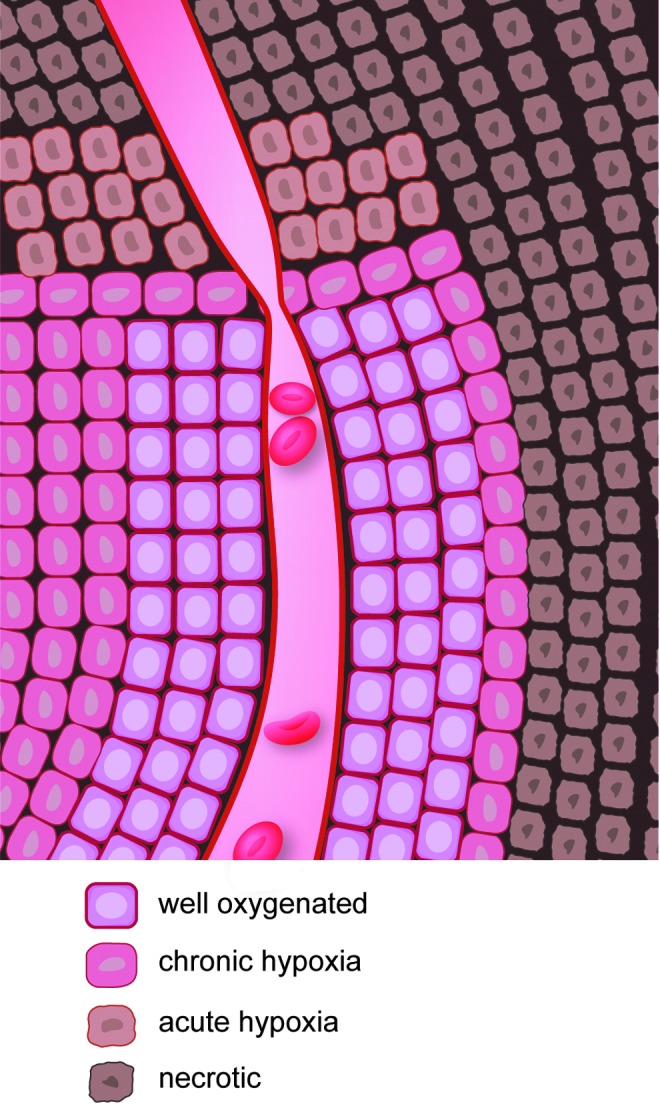
Hypoxia within a tumor. Two types of hypoxia occur within a tumor: chronic hypoxia and acute hypoxia. Chronic hypoxia occurs due to the diffusion limits of oxygen from blood vessels. Acute hypoxia may be due to blockage of blood flow in a region of the tumor. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Because hypoxia diminishes the effectiveness of radiation therapy, several methods have been devised to increase oxygen concentrations at the tumor. These methods include increasing blood oxygen saturation, diminishing tumor oxygen consumption (106), and normalization of tumor vasculature (76). Secomb et al. (186) studied the effects of blood flow, arterial pO2, and oxygen consumption on tumor cell hypoxia using a simulation based on observations from a transplanted mammary adenocarcinoma. While hypoxia was reduced with increasing blood flow and arterial pO2 and decreased oxygen consumption, tumors were much more sensitive to changes in oxygen consumption, with a 30% reduction in oxygen consumption, compared to controls, completely eliminating tumor hypoxia (186). McGee et al. (136) studied the effects of interferon-β (IFN-β) or the monoclonal human vascular endothelial growth factor (VEGF) antibody bevacizumab, on the radiation response of orthotopic U87 glioma xenografts. IFN-β or bevacizumab decreased tumor hypoxia by normalization of tumor vasculature. Combination of radiation with either IFN-β or bevacizumab synergistically reduced tumor size compared to any individual therapy alone, demonstrating the importance of tumor oxygenation by normalization of vasculature in radiation therapy (136).
Another important method to treat tumors that can affect tumor oxygenation is fractionated radiotherapy. By applying radiation in a fractionated regimen, normal tissues can recover through repair of sublethal damage and repopulation of normal cells (10). Fractionated therapy takes advantage of the increased proliferation rate in cancer cells compared to normal tissues (15). Fractionation, especially hyperfractionation, permits a higher total radiation dose to be delivered. Fractionated therapy also allows for the reoxygenation of tumors and increases their radiosensitivity (Fig. 8) (137). The combination of radiation with chemotherapy postradiotherapy can enhance drug delivery and improve patient outcome.
FIG. 8.
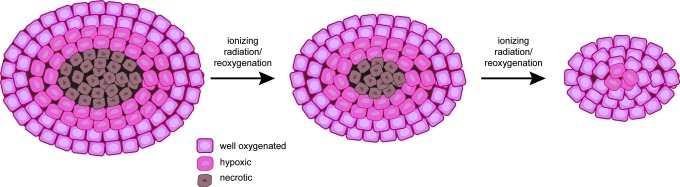
The effects of fractionated radiotherapy on tumors. Fractionated therapy allows the recovery and repair of normal tissues and the reoxygenation of tumor tissues. Reoxygenation of the tumor increases the efficacy of radiation against the tumor. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
SODs in Normal and Tumor Tissues
General description of SODs
Because SODs are discussed in much greater detail in other review articles in this Forum, we will only briefly describe the SODs. SODs are the chief superoxide-scavenging enzymes in a cell (71), and three types exist in cells, with each form encoded by a separate gene [reviewed in ref. (228)]: CuZnSOD (SOD1), MnSOD (SOD2), and extracellular superoxide dismutase (SOD3, ECSOD). CuZnSOD is a cytoplasmic homodimer (113, 135), although small amounts have been identified in the intermembrane space of mitochondria (157, 217). ECSOD shares 40–60% amino acid homology with CuZnSOD, and like CuZnSOD, contains both copper and zinc in its active site. However, ECSOD is a homotetramer found in the extracellular region of the cell (68, 92). MnSOD is a homotetramer found exclusively in the mitochondrial matrix (19, 157, 179, 217, 220). All three enzymes catalyze the dismutation of superoxide radicals to hydrogen peroxide and molecular oxygen. Hydrogen peroxide, itself a ROS, is decomposed to water by myriad enzyme systems, including peroxiredoxins, glutathione peroxidase (GPx), and catalase (4). All three enzymes have forms localized to mitochondria (31, 62, 131, 155, 160, 177, 183, 188, 190) (Fig. 9).
FIG. 9.
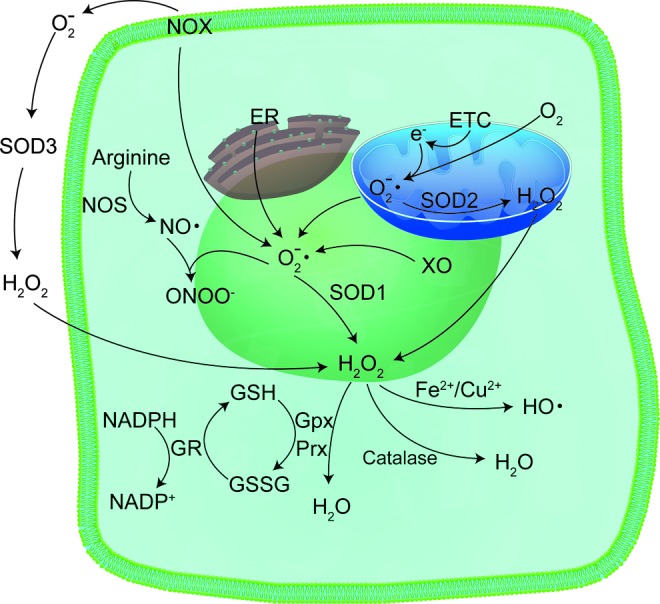
Sources and means of detoxification of ROS in the cell. Superoxide radicals are generated at different sites in the cell, such as the ER, ETC in mitochondria, as well as various enzymatic sources, like NOX and XO, all of which contribute to the generation of ROS after ionizing radiation. Superoxide radicals are detoxified by SODs to form hydrogen peroxide, which is further detoxified by GPx and Prx. GR is used to regenerate GSH. ER, endoplasmic reticulum; ETC, electron transport chain; GPx, glutathione peroxidase; GR, glutathione reductase; GSH, reduced glutathione; NOS, NADPH oxidase; Prx, peroxiredoxin; SOD, superoxide dismutase; XO, xanthine oxidase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
SOD in cancer development
Given the role of ionizing radiation in the development of cancer (46) and the significance of ROS in the development and progression of cancer (75, 209, 215), the importance of MnSOD in cancer development becomes quite evident due to its ability to scavenge ROS. However, a careful examination of the literature reveals a dual function for MnSOD in cancer [recently reviewed in ref. (94)]. Many studies show a reduction in MnSOD expression in various types of cancer compared to normal tissues (39, 42, 100, 153, 192), suggesting MnSOD acts a tumor suppressor. Conversely, other studies report an elevation in MnSOD expression in cancer (93, 99, 103, 104, 132, 203, 207) and its association with cancer aggressiveness, growth, survival (140, 158), and metastatic potential (95, 132, 149, 184), implying that MnSOD supports progression of tumors to a more aggressive stage.
Recent work by this laboratory has begun to shed light on the dual role of MnSOD in cancer development. Using the 7,12-dimethylbenz(a)-anthracene (DMBA)/12-O-tetradecanoylphorbol-13-acetate (TPA) two-stage model of skin cancer development in a unique mouse model expressing a MnSOD promoter-linked luciferase reporter gene (45), this laboratory discovered that treatment with the tumor initiator DMBA, followed by repeated exposure to TPA over 25 weeks, resulted in a significant reduction in MnSOD luciferase reporter gene activity, MnSOD mRNA, protein, and enzyme activity in both DMBA/TPA-treated skin and papillomas compared to vehicle controls. When the observation period was extended to allow squamous cell carcinoma (SCC) formation, MnSOD expression significantly increased at the reporter gene activity, mRNA, protein, and enzyme activity levels during the transition from the relatively nonaggressive papilloma to the more aggressive SCC. These differences in MnSOD expression between papillomas and SCCs are due, in part, to changes in Sp1 and p53 transcription factor binding activity on the Sod2 promoter (45).
One potential mechanism by which MnSOD is involved in tumor suppression and tumor aggressiveness is altered hydrogen peroxide flux with changes in MnSOD levels. Buettner et al. (27) showed that steady-state levels of hydrogen peroxide are affected by MnSOD where the equilibrium constant for superoxide production (K) is less than 1. Under these conditions, the rate constant for the back reaction (conversion of superoxide back to molecular oxygen) is greater than the rate constant for the forward reaction (conversion of molecular oxygen to superoxide). This condition is seen for superoxide production by the electron transport chain. When MnSOD is present, there is a proportional increase in hydrogen peroxide production with increasing MnSOD levels. The greatest effect occurs when MnSOD levels are low, but at sufficiently high levels of MnSOD, there is only a modest further increase in hydrogen peroxide production. Increased hydrogen peroxide production occurs because MnSOD is driving the equilibrium of the system to the right, resulting in increased superoxide production. A small amount of superoxide is consumed for the production of hydrogen peroxide and does not participate in the reverse reaction to regenerate molecular oxygen, in agreement with Le Chatelier's principle (27).
Changes in hydrogen peroxide flux with modifications in MnSOD levels can have broad repercussions in cancer progression. During early stages of cancer, when MnSOD levels are low (153), MnSOD overexpression may suppress cancer growth through various mechanisms because of greater hydrogen peroxide flux (126). At later stages of cancer progression, when cancer cells experience persistent oxidative stress (16, 161, 168), increased MnSOD expression may benefit cancer cells by the stimulation of metastasis (40, 90, 95, 147).
SOD in radiation-induced neoplastic transformation
Two important papers established the importance of SODs in radiation-induced neoplastic transformation of normal tissues. This laboratory was the first to demonstrate the protective effects of MnSOD overexpression in the protection of normal tissue from ionizing radiation-induced neoplastic transformation. In C3H 10T1/2 MEFs expressing either an empty vector or a construct containing the Sod2 gene, St. Clair et al. discovered that overexpression of MnSOD did not protect the cells from transformation by the DNA intercalating agent 3-methylcholanthrene but did protect the cells from transformation by ionizing radiation (194). Du et al., (48) using MEFs derived from MnSOD+/+, MnSOD+/−, and MnSOD−/− mice, found a fivefold increase in transformation frequency in MnSOD−/− MEFs compared to MnSOD+/+ MEFs. The lack of MnSOD in MnSOD−/− MEFs enhanced the formation of late ROS, micronuclei (a marker of DNA damage), and binucleated bearing micronuclei cells at 72 h post-irradiation compared to MnSOD+/− and MnSOD+/+ MEFs. MnSOD+/− and MnSOD−/− MEFs also demonstrated decreased exit from the G2 cell cycle checkpoint compared to MnSOD+/+ MEFs, which was reversed in MnSOD−/− cells by overexpression of MnSOD (48). These results suggest that mitochondrial ROS generation is an important component of ionizing radiation-mediated transformation and the vital role for MnSOD for the prevention of cancer formation.
SOD-mediated radioprotection
Many studies have revealed the importance of SOD in protection of normal tissue from the harmful effects of ionizing radiation. Lee et al. (121) used a yeast model with knockdown of MnSOD, CuZnSOD, or both MnSOD and CuZnSOD, to study radiation response. The researchers found that in wild-type cells, there was a dose-dependent increase in the activity of different antioxidant enzymes (catalase, glutathione reductase, and glucose 6-phosphate dehydrogenase), whereas knockdown of either SOD alone or the combination of MnSOD and CuZnSOD resulted in a much smaller increase in the activities of these enzymes. The researchers also found a radiation dose-dependent increase in the levels of ROS and ROS-mediated protein and lipid damage (protein carbonyl formation and thiobarbituric acid-reactive substances, respectively) (121).
Overexpression of MnSOD alone is enough to bestow radioprotection in normal cells. In CHO cells, overexpression of MnSOD, but not CuZnSOD, protected the cells from radiation-induced cell death. Overexpression of GPx was only partially protective against radiation compared to MnSOD (195). Overexpression of MnSOD by retroviral transduction in either K562 human erythroleukemic cells or primary mouse bone marrow-derived myeloid progenitor cells resulted in a significant increase in radioresistance (193). MnSOD is also important for radiation-induced adaptive response in normal cells. In JB6 mouse epidermal cells, exposure to low-dose ionizing radiation significantly increased the clonogenic survival of cells subsequently challenged to 2 Gy radiation due to activation of several NF-κB target genes, including MnSOD (63). An important mechanism by which MnSOD confers radioprotection of normal tissues is to prevent apoptosis (50, 56), in part, by maintaining mitochondrial integrity (56). These results suggest that mitochondria are major targets of ionizing radiation and mitochondria-specific scavenging of ROS by MnSOD may be an important mechanism to safeguard cells against radiation-induced damage.
CuZnSOD also has a radioprotective effect in normal tissue. Petkau et al. found that the administration of mice with bovine SOD by intravenous injection before and after ionizing radiation treatment significantly increased the LD50 dose (164, 165), and exogenous SOD protected the proliferative capacity of bone marrow stem cells from ionizing radiation, indicating a protective effect of SOD (164). The addition of CuZnSOD either before irradiation or after irradiation also had a protective effect in HUVEC, although pretreatment with CuZnSOD gave the greatest level of radioprotection. CuZnSOD pretreatment also partially attenuated the antiangiogenic effects of radiation, as determined by tube formation of HUVEC cells in vitro. The radioprotective effect of CuZnSOD leading to increased angiogenesis required the activation of the mitogen-activated protein/extracellular signal-regulated kinase1/2 pathway, and the pretreatment of HUVECs with the MEK inhibitor PD98059 suppressed the effects of CuZnSOD (204).
ECSOD, like MnSOD and CuZnSOD, confers protection from the harmful effects of ionizing radiation. Lung-specific overexpression of ECSOD in B6C3 mice resulted in a significant reduction in radiation-induced lung damage compared to wild-type mice. For example, ECSOD overexpression delayed the onset of increased breathing frequency and significantly reduced breathing rate compared to irradiated wild-type mice. ECSOD also reduced radiation-induced increases in macrophage and lymphocyte lung infiltration compared to irradiated wild-type mice, as well as decreased TGFβ activation, Smad3 expression, and Smad2/3 activation and reduced lipid peroxidation (111, 174).
While a multitude of studies show a role for SOD in radioprotection of normal tissue, other studies suggest an apparent radiosensitization effect. Fishman et al. (67) discovered that knockdown of either MnSOD or CuZnSOD in C57BL/6J mice attenuated radiation-induced reductions in neuronal cells and cells differentiated into either neurons, astrocytes, or microglia in the dentate subgranular zone of the hippocampus compared to wild-type mice. The authors suggest that one potential mechanism of the protective effect of MnSOD or CuZnSOD knockdown may be increased superoxide levels. This hypothesis was supported by experiments using xanthine or xanthine plus xanthine oxidase. Incubation of neural precursor cells with either xanthine alone or xanthine/xanthine oxidase had a protective effect against radiation-induced diminution of cell numbers. Although the authors did not address the specific mechanisms by which decreased SOD, whether MnSOD or CuZnSOD, imparted a radioprotective effect on neuronal cells, they suggested that the effect may be similar to an adaptive response for radiation, whereby low doses of ionizing radiation are protective against later, high doses of ionizing radiation (67). It would be interesting to determine whether the observed effect of SOD knockdown can occur in other normal cells and tumor cells.
SOD and Radiation Response in Neoplastic Cells
Radiosensitization
Using the MnSOD-overexpressing Fsa-II cells implanted in mice, this laboratory found that MnSOD expression resulted in a significant reduction in the radiation dose required to control one-half of the irradiated tumors compared to control mice (208). Overexpression of MnSOD by transfection with a MnSOD cDNA-expressing plasmid/liposome complex (MnSOD-PL) proved effective in increasing radiosensitivity of SCC-VII mouse SCC cells (D0=1.244 Gy compared to 3.246 Gy for control cells), and the combination of MnSOD-PL with the EGFR inhibitor gefitinib further increased radiosensitization (D0=0.785 Gy) (54). The administration of recombinant MnSOD (rMnSOD) was also effective in enhancing radiosensitivity of cancer cells (20).
Radioresistance
Although some studies suggest a radiosensitizing role for MnSOD, other studies show that MnSOD is important in radioresistance of cancer cells. Qu et al. (172, 173) found that the CNE1 human nasopharyngeal cell line was more radioresistant than the CNE2 cell line, which correlated with increased expression and activity of MnSOD in the CNE1 cell line. Knockdown of MnSOD in CNE1 cells decreased radioresistance (172, 173). Feng et al. generated a radioresistant CNE2 cell line (CNE2-IR) by treating parental CNE2 cells with five rounds of sublethal IR. Gene expression between CNE2 and CNE2-IR cells was compared, and there was an increase in MnSOD expression, among other genes, in the radioresistant cell line (64). MnSOD expression correlated well with radioresistance in nasopharyngeal tumors (64, 172), suggesting that MnSOD may be predictive in determining radioresistance of tumors. An important mechanism of radioresistance is cell cycle arrest at the G2 phase of the cell cycle after exposure (66, 109). Scavenging of hydrogen peroxide by catalase expression or treatment with NAC abolished MnSOD-induced radioresistance (199). Using a systems biology approach, Niciforovic et al. discovered that radioresistance was associated with a positive feed-forward cycle with hydrogen peroxide-induced elevation of MnSOD expression (148).
MnSOD is a major target gene for the NF-κB pathway (116, 117, 223). The NF-κB pathway is vital for conferring radiation resistance in cancer cells (124), and a major mechanism of NF-κB-dependent radioresistance is induction of MnSOD [reviewed in ref. (96)]. For example, Guo et al. (84) discovered that MnSOD expression was significantly increased as part of a radioadaptive response in MCF-7 human breast cancer cells. This radioresistance was replicated by stable overexpression of MnSOD, and several NF-κB target genes important for survival were expressed in both radioadapted cells and cells overexpressing MnSOD (84). Work by this laboratory has focused on the mechanisms by which the alternative NF-κB pathway increases radioresistance in prostate cancer cells. In a study by Josson et al., (108) nuclear localization of RelB (a component of the alternative NF-κB pathway) (187) was significantly higher in aggressive PC-3 human prostate cells compared to LNCaP cells and correlated with increased MnSOD expression and radioresistance (108). Inhibition of the alternative NF-κB pathway by expression of RelB-specific siRNA, overexpression of a dominant-negative p100 (108), treatment with the peptide inhibitor SN52 (to prevent nuclear translocation of RelB) (222), or administration of 1α,25-dihyroxyvitamin D3 (to inhibit RelB expression) (221), all resulted in a decrease in radiation-induced MnSOD expression and an increase in radiosensitivity in PC-3 cells.
CuZnSOD can also confer radioresistance in cancer cells. Gao et al. (72) reported that the overexpression of CuZnSOD in U118-9 human glioma cells increased radioresistance compared to vector control and parental cells. ROS accumulation occurred in parental and vector control cells starting at 2 days postirradiation and remained elevated up to 8 days after radiation. This late accumulation of ROS was suppressed in CuZnSOD-overexpressing cells. CuZnSOD overexpression also increased the accumulation of the cells at the G2 phase of the cell cycle, as well as decreased cyclin B1 expression (72).
SODs are a double-edged sword in the radiation response of cancer cells
The studies highlighted above suggest a more complicated role for SODs in the response of cancer cells to ionizing radiation. This dual role for SODs may be due to differences in the expression and/or activity of other antioxidant enzymes in various types of cancer cells. For example, well-differentiated hepatocellular carcinoma cell lines have higher activity or expression levels of catalase, glutathione reductase, CuZnSOD, and MnSOD compared to poorly differentiated cell lines (227). In patients with renal cell carcinoma, there was a significant reduction in the activity of GPx and catalase in cancer tissues compared to normal tissues (166). On the other hand, other studies have demonstrated an elevation in the expression and activity of some antioxidant enzymes in cancer cells. Increased expression of MnSOD and catalase were observed in gastric carcinoma cells compared to noncancerous cells (101), and increased MnSOD expression was associated with lymph node metastasis in gastric cancer patients (132). Overexpression of SODs in cancer cells expressing low levels of other antioxidant enzymes that remove hydrogen peroxide may have a radiosensitization effect, perhaps because of increased hydrogen peroxide levels after irradiation. Conversely, overexpression of SODs in cancer cells with high levels of hydrogen peroxide-scavenging enzymes may have a radioresistant effect because of increased dismutation of superoxide radicals generated after irradiation and the ability to handle the resultant increase in hydrogen peroxide produced.
Given the dual effect of SODs on the response of cancer cells to ionizing radiation, careful screening of the antioxidant status of tumor tissues should be considered before embarking on the use of SOD therapy in parallel with radiation therapy. This screening of tumor tissues may include expression of different antioxidant enzymes alone, or even ratios of different enzymes, as well as a comparison of antioxidant enzyme expression between tumor and normal tissues. This approach would better tailor the therapy to each patient and maximize the effectiveness of the therapy to destroy cancer tissue and mitigate normal tissue injury.
Methods Exploiting SOD for Radiation Response
Because of the detrimental effects of ionizing radiation on normal tissues, the resistance of tumor tissues to radiation therapy, and the role of ROS in these processes, methods that can increase SOD expression and/or activity may prove to be important adjuvants to radiotherapy to increase radiosensitivity in tumor tissues and protect normal tissues. An important mechanism for the effects of SOD overexpression on the simultaneous radioprotection of normal tissues and radiosensitization of tumor tissues may be diminished expression of other antioxidant enzymes, especially catalase, in most cancer tissues compared to normal tissues (9, 20, 21, 41, 154, 196, 197). These enzymes catalyze the decomposition of hydrogen peroxide to molecular oxygen and water. Cancer cells are under higher levels of oxidative stress than nontumorigenic cells (91, 153). Therefore, overexpression of SODs in cancer cells may set up a condition whereby exposure to ionizing radiation results in a greater ROS insult than in normal tissues, resulting in greater cell death and cell cycle arrest. In contrast, overexpression of SODs in normal tissues is radioprotective because of the increased antioxidant capacity in normal tissues to handle the ROS burst resulting from radiation exposure. Discussed below are several strategies that have been devised to increase SOD activity and/or expression in vivo for modulation of the radiation response in normal and tumor tissues.
Overexpression of SOD
Plasmid/liposome therapy
MnSOD-PL are useful for increasing the expression of MnSOD in tissues. This particular approach has proven effective in protecting lung tissue (29, 53, 57), the oral cavity (52, 85), and the esophagus (55, 58) from ionizing radiation-induced damage. Interestingly, this therapy also simultaneously sensitizes cancer cells to ionizing radiation. For example, Epperly et al. (50) discovered that the overexpression of MnSOD using MnSOD-PL inhibited radiation-induced GPx expression in SCC-VII murine and OC-19 human SCC cells and 3LL murine lung carcinoma cells and increased radiosensitization of SCC-VII and OC-19 cells, but did not affect the radiosensitivity of 3LL cells, in vitro. In MEFs, radiation had no effect on GPx activity, but significantly reduced reduced glutathione (GSH) levels, and the administration of MnSOD-PL significantly increased GSH levels after irradiation, suggesting a radioprotective effect on normal cells in vitro. In vivo, the administration of MnSOD-PL had no effect on radiation-induced reduction in GSH in oral cavity SCC-VII orthotopic tumor tissue, but significantly reduced GPx activity in conjunction with radiation. In adjacent normal oral mucosal tissue, MnSOD-PL protected from radiation-induced changes in GSH levels and GPx activity (59). Intraoral administration of the MnSOD-PL therapy significantly decreased the number of ulcerations 5 days after irradiation compared to irradiated control mice. Interestingly, the administration of the MnSOD-PL therapy did not protect SCC-VII tumors from the effects of radiation (85). These results suggest a potential for radioprotection of normal tissue and radiosensitization of tumor tissue by MnSOD-PL.
Virus-mediated overexpression
The use of viruses is another efficient method for overexpression of MnSOD to alter radiation response. Adenovirus-mediated overexpression of MnSOD, both in vitro (234) and in vivo (51), confers protection to lung tissue and lung epithelial cells against radiation-induced damage. For example, intratracheal injection of MnSOD adenovirus significantly decreased alveolitis, in part, by suppressing radiation-induced expression of different inflammatory cytokines (transforming growth factor-beta [TGF-β], TNF-α, and interleukin-1) (51). Retroviral transduction of a MnSOD construct in hematopoietic cells protected the cells from ionizing radiation-induced DNA damage both in vitro and in vivo (193). Intraluminal administration of a herpes simplex virus-MnSOD virus had a radioprotective effect on the small intestine (86). Transfection of urothelia (bladder) with MnSOD transgene did not protect bladders from the acute effects of radiation early after exposure (as measured by transepithelial resistance and permeabilities to water and urea) but did enhance recovery at 4 weeks after irradiation compared to controls (110). These results suggest a persistent effect of ionizing radiation on oxidative metabolism lasting for weeks after radiotherapy and the ability of SOD overexpression to ameliorate this effect.
Recombinant protein
Administration of a recombinant form of MnSOD can also be radioprotective. Borrelli et al. (20) found that treatment with rMnSOD sensitized cancer cells to ionizing radiation and protected normal cells from ionizing radiation in vitro. In vivo, intraperitoneal administration of rMnSOD after irradiation, with subsequent rMnSOD treatments for an additional 6 days, significantly diminished radiation-induced organ damage and increased survival compared to animals receiving phosphate-buffered saline only (20).
SOD mimetics
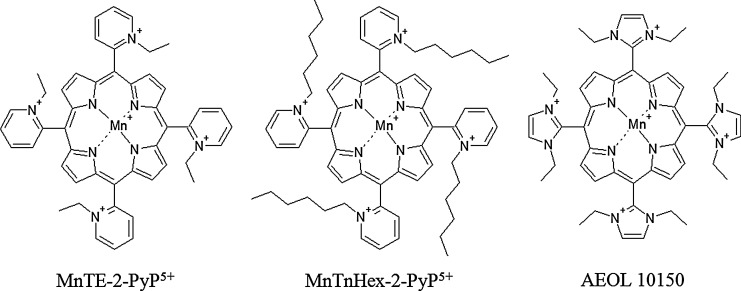
Another attractive approach to increase the SOD activity in normal tissue and cancer is the use of SOD mimetics. Several manganese porphyrin compounds, as well as non-metal-based compounds, have been developed with variable superoxide-scavenging properties, pharmacokinetics and tissue and subcellular localization (11, 138), and several of these porphyrin-based mimetics (Fig. 10) are effective in decreasing radiation-induced damage in normal tissue and radiosensitizing tumor tissue.
FIG. 10.
Various SOD mimetics used in studies on radiotherapy in vivo. All three mimetics discussed in this article have the same porphyrin core and differ only in the substituents on porphyrin. AEOL 10150 contain N,N′-diethylimidazolium, whereas MnTE-2-PyP5+ and MnTnHex-2-PyP5+ contain an ethyl and hexyl hydrocarbon chain, respectively, on the nitrogen of the pyridine ring. AEOL 10150, Mn(III) tetrakis(N,N′-diethylimidazolium-2-yl)porphyrin; MnTE-2-PyP5+, Mn(III) 5,10,15,20-tetrakis(N-ethylpyridinium-2-yl)porphyrin; MnTnHex-2-PyP5+, Mn(III) 5,10,15,20-tetrakis(N-hexylpyridinium-2-yl)porphyrin.
Mn(III) 5,10,15,20-tetrakis(N-ethylpyridinium-2-yl)porphyrin (MnTE-2-PyP5+) protects from bone marrow suppression induced by total body irradiation by inhibiting oxidative damage induced by ionizing radiation in hematopoietic stem cells and progenitor cells (125). MnTE-2-PyP5+ has also proven effective in delaying the onset of ionizing radiation-induced lung injury in rats, as indicated by diminished breathing frequency, reduced lung fibrosis, and less hydroxyproline content (indicating collagen deposition) compared to irradiated rats receiving no mimetic (212). MnTE-2-PyP5+ decreased oxidative stress, blocked the activation of HIF-1α and TGF-β, and suppressed the upregulation of carbonic anhydrase-IX and VEGF expression. The protective effect of MnTE-2-PyP5+ can also occur long after the irradiation event. The administration of MnTE-2-PyP5+ at 3 days and 8 weeks postirradiation, while not affecting radiation-induced oxidative DNA damage, significantly reduced histopathological damage in the lungs compared to animals not receiving mimetic (74).
While MnTE-2-PyP5+ is effective in radioprotection of normal tissues, Mn(III) 5,10,15,20-tetrakis(N-hexylpyridinium-2-yl)porphyrin (MnTnHex-2-PyP5+) has been proven to be even more efficacious than MnTE-2-PyP5+ due to its greater lipophilicity and higher biodistribution (218). At high concentrations, MnTnHex-2-PyP5+ can cause toxicity, which may be due to its micellar properties (73). Another SOD mimetic useful in suppressing radiation-induced lung damage after both single-dose (175) and fractionated radiotherapy (176) is Mn(III) tetrakis(N,N′-diethylimidazolium-2-yl)porphyrin (AEOL 10150). Potential mechanism of diminution of radiation-induced lung damage include reduction in inflammatory cytokines in both plasma (212) and lung tissue (74), suppression of oxidative DNA and protein damage (176), as well as a decrease in radiation-induced apoptosis (224).
Not only do SOD mimetics have radioprotective effects on normal tissues, but they can also have radiosensitization effects on cancerous tissues. Moeller et al. (139) found that the SOD mimetic MnTE-2-PyP5+, at all doses tested, was not cytotoxic on the different tumor and endothelial cell lines tested and neither sensitized nor protected the cells from radiation treatment in vitro. Interestingly, MnTE-2-PyP5+ inhibited the radioprotective effects of tumor cell-conditioned medium on endothelial cells in vitro and enhanced radiation-induced damage to tumor vasculature and delayed tumor growth in vivo (139), suggesting that SOD mimetics may disrupt communication between tumor and endothelial cells, leading to breakdown of tumor vasculature after radiation and disruption of the tumor microenvironment.
Concluding Remarks
Ionizing radiation is an efficient method for the treatment of cancer. However, the development of normal tissue injury can limit the effectiveness of radiotherapy. ROS are important mediators of radiation therapy and can also participate in the differential effect of radiation in cancer cells and in normal cells due to differences in the antioxidant potential between tumor and normal tissues. Bystander effects play an important role in the response of cancerous and normal tissues to ionizing radiation, potentially leading to radioresistance of cancer cells and radiosensitization of normal cells. ROS are key mediators of radiation-induced bystander effects. Bystander effects are also important for the effects of tumor microenvironment on radiation responsiveness, with communication between tumor and stromal cells conferring radioprotection and increased aggressiveness of tumor cells. SODs, the main ROS scavengers of the cell, play a complex role in the response of cancer cells to ionizing radiation. Increasing the expression and/or activity of SODs, especially MnSOD, through the use of gene-expressing plasmid delivery systems, viral vectors, administration of recombinant protein, or the use of SOD mimetics, can increase the radiosensitivity of cancer cells while simultaneously increasing the resistance of normal tissues to radiation-induced injury in vivo. Development of these techniques for use in humans may provide unique tools for the radiation oncologist to improve treatment efficacy and enhance the quality of life for cancer patients in the future.
Abbreviations Used
- AEOL 10150
Mn(III) tetrakis(N,N′-diethylimidazolium-2-yl)porphyrin
- CHO
Chinese hamster ovary cells
- COX-2
cyclooxygenase-2
- CuZnSOD
copper-zinc superoxide dismutase
- Cx43
connexin-43
- cytc
cytochrome c
- DMBA
7,12-dimethylbenz(a)-anthracene
- ecDNA
extracellular DNA
- ECSOD
extracellular superoxide dismutase
- ER
endoplasmic reticulum
- ETC
electron transport chain
- GJIC
gap junction intercellular communication
- GPx
glutathione peroxidase
- GR
glutathione reductase
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- HIF-1α
hypoxia-inducible factor-1α
- HUVEC
human umbilical vein endothelial cells
- IFN-β
interferon-β
- IGFBP-3
insulin growth factor binding protein 3
- LET
linear energy transfer
- MEF
mouse embryonic fibroblast
- MnSOD
manganese superoxide dismutase
- MnSOD-PL
MnSODcDNA-expressing plasmid/liposome complexes
- MnTE-2-PyP5+
Mn(III) 5,10,15,20-tetrakis(N-ethylpyridinium-2-yl)porphyrin
- MnTnHex-2-PyP5+
Mn(III) 5,10,15,20-tetrakis(N-hexylpyridinium-2-yl)porphyrin
- mtDNA
mitochondrial DNA
- NAC
N-acetylcysteine
- NHLF
normal human lung fibroblasts
- NOX
NADPH oxidase
- PRX
peroxiredoxin
- Ptch1
Patched-1
- rMnSOD
recombinant MnSOD
- ROS
reactive oxygen species
- SCC
squamous cell carcinoma
- SOD
superoxide dismutase
- SOD1
copper-zinc superoxide dismutase
- SOD2
manganese superoxide dismutase
- SOD3
extracellular superoxide dismutase
- TGF-β
transforming growth factor-beta
- TNF-α
tumor necrosis factor-alpha
- TPA
12-O-tetradecanoylphorbol-13-acetate
- VEGF
vascular endothelial growth factor
- XO
xanthine oxidase
Acknowledgments
The authors thank Mr. Tom Dolan and Mr. Matt Hazzard of the Graphics and Multimedia/Academic Technology Group at the University of Kentucky for their assistance in generating the figures used in this article. This work was supported by NIH grants 2T32ES007266 and CA143428.
References
- 1.Grupen C. Biological effects of ionizing radiation. In: Introduction to Radiation Protection, edited by Grupen C. Heidelberg, Germany: Springer-Verlag; 2010, pp. 212–228 [Google Scholar]
- 2.Adam-Vizi V. and Chinopoulos C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol Sci 27: 639–645, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Adriaens I, Smitz J, and Jacquet P. The current knowledge on radiosensitivity of ovarian follicle development stages. Human Reprod Update 15: 359–377, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Andreyev AY, Kushnareva YE, and Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Moscow) 70: 246–264, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Asaithamby A, Hu B, and Chen DJ. Unrepaired clustered DNA lesions induce chromosome breakage in human cells. Proc Natl Acad Sci U S A 108: 8293–8298, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Autsavapromporn N, de Toledo SM, Buonanno M, Jay-Gerin J-P, Harris AL, and Azzam EI. Intercellular communication amplifies stressful effects in high-charge high-energy (HZE) particle-irradiated human cells. J Radiat Res 52: 408–414, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Autsavapromporn N, de Toledo SM, Little JB, Jay-Gerin J-P, Harris AL, and Azzam EI. The role of gap junction communication and oxidative stress in the propagation of toxic effects among high-dose α-particle-irradiated human cells. Radiat Res 175: 347–357, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azzam EI, de Toledo SM, and Little JB. Direct evidence for the participation of gap juction-mediated intecellular communication in the transmission of damage signals from α-particle irradiated to nonirradiated cells. Proc Natl Acad Sci U S A 98: 473–478, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balasubramaniyan N, Subramanian S, and Govindasamy S. Status of antioxidant systems in human carcinoma of uterine cervix. Cancer Lett 87: 187–192, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Baskar R, Lee KA, Yeo R, and Yeoh K-W. Cancer and radiation therapy: current advances and future directions. Int J Med Sci 9: 193–199, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batinic-Haberle I, Reboucas JS, and Spasojevic I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid Redox Signal 13: 877–918, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Begg AC, Steward FA, and Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer 11: 239–253, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Belloni P, Latini P, and Palitti F. Radiation-induced bystander effect in healthy G0 human lymphocytes: biological and clinical significance. Mutat Res 713: 32–38, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Belyakov OV, Malcolmson AM, Folkard M, Prise KM, and Michael BD. Direct evidence for a bystander effect of ionizing radiation in primary human fibroblasts. Br J Cancer 84: 674–679, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernier J, Hall EJ, and Giaccia A. Radiation oncology: a century of achievements. Nat Rev Cancer 4: 737–747, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Biaglow JE. and Miller RA. The thioredoxin reductase/thioredoxin system: novel redox targets for cancer therapy. Cancer Biol Ther 4: 6–13, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Blyth BJ. and Sykes PJ. Radiation-induced bystander effects: what are they, and how relevant are they to human radiation exposures? Radiat Res 176: 139–157, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Boonstra J. and Post JA. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene 337: 1–13, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Borgstahl GEO, Parge HE, Hickey MJ, Beyer WF, Jr., Hallewell RA, and Tainer JA. The structure of human mitochondrial manganese superoxide dismutase reveals a novel tetrameric interface of two 4-helix bundles. Cell 71: 107–118, 1992 [DOI] [PubMed] [Google Scholar]
- 20.Borrelli A, Schiattarella A, Mancini R, Morrica B, Cerciello V, Mormile M, d'Alesio V, Bottalico L, Morelli F, D'Armiento M, D'Armiento FP, and Mancini A. A recombinant MnSOD is radioprotective for normal cells and radiosensitizing for tumor cells. Free Radic Biol Med 46: 110–116, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Bostwick DG, Alexander EE, Singh R, Shan A, Qian J, Santella RM, Oberley LW, Yan T, Zhong W, Jiang X, and Oberley TD. Antioxidant enzyme expression and reactive oxygen species damage in prostatic intraepithelial neoplasia and cancer. Cancer 89: 123–134, 2000 [PubMed] [Google Scholar]
- 22.Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol 45: 466–472, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Broerse JJ, Barendsen GW, and van Kersen GR. Survival of cultured human cells after irradiation with fast neutrons of different energies in hypoxic and oxygenated conditions. Int J Radiat Biol 13: 559–572, 1968 [DOI] [PubMed] [Google Scholar]
- 24.Brown JM. Evidence for acutely hypoxic cells in mouse tumours, and a possible mechanism of reoxygenation. Br J Radiol 52: 650–656, 1979 [DOI] [PubMed] [Google Scholar]
- 25.Brown JM. Tumor hypoxia, drug resistance, and metastases. J Natl Cancer Inst 82: 338–339, 1990 [DOI] [PubMed] [Google Scholar]
- 26.Brunner TB, Kunz-Schughart LA, Grosse-Gehling P, and Baumann M. Cancer stem cells as a predictive factor in radiotherapy. Semin Radiat Oncol 22: 151–174, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Buettner GR, Ng CF, Wang M, Rodgers VGJ, and Schafer FQ. A new paradigm: manganese superoxide dismutase influences the production of H2O2 in cells and thereby their biological state. Free Radic Biol Med 41: 1338–1350, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calveley VL, Khan MA, Yeung IWT, Vandyk J, and Hill RP. Partial volume rat lung irradiation: temporal fluctuations of in-field and out-of-field DNA damage and inflammatory cytokines following irradiation. Int J Radiat Biol 81: 887–899, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Carpenter M, Epperly MW, Agarwal A, Nie S, Hricisak L, Niu Y, and Greenberger JS. Inhalation delivery of manganese superoxide dismutase-plasmid/liposomes protects the murine lung from irradiation damage. Gene Ther 12: 685–693, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Chai Y. and Hei TK. Radiation induced bystander effect in vivo. Acta Med Nagasaki 53: S65–S69, 2008 [PMC free article] [PubMed] [Google Scholar]
- 31.Chang T-S, Cho C-S, Park S, Yu S, and Kang SW. Peroxiredoxin III, a mitochondrion-specific peroxidase, regulate apoptotic signaling by mitochondria. J Biol Chem 279: 41975–41984, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Chaplin DJ, Durand RE, and Olive PL. Acute hypoxia in tumors: implications for modifiers of radiation effects. Int J Radiat Oncol Biol Phys 12: 1279–1282, 1986 [DOI] [PubMed] [Google Scholar]
- 33.Chaplin DJ, Olive PL, and Durand RE. Intermittent blood flow in a murine tumor: radiobiological effects. Cancer Res 47: 597–601, 1987 [PubMed] [Google Scholar]
- 34.Chaudhry MA. Bystander effect: biological endpoints and microarray analysis. Mutat Res 597: 98–112, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Chaudhry MA. and Omaruddin RA. Mitochondrial gene expression in directly irradiated and nonirradiated bystander cells. Cancer Biother Radiopharmaceut 26: 657–663, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Chen S, Zhao Y, Chiu SK, Zhu L, Wu L, and Yu KN. Rescue effects in radiobiology: unirradiated bystander cells assist irradiated cells through intercellular signal feedback. Mutat Res 706: 59–64, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Chen S, Zhao Y, Han W, Zhao G, Zhu L, Wang J, Bao L, Jiang E, Xu A, Hei TK, Yu Z, and Wu L. Mitochondria-dependent signalling pathway are involved in the early process of radiation-induced bystander effects. Br J Cancer 98: 1839–1844, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen S, Zhao Y, Zhao G, Han W, Zao L, Yu KN, and Wu L. Up-regulation of ROS by mitochondria-dependent bystander signaling contributes to genotoxicity of bystander effects. Mutat Res 666: 68–73, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Chuang T-C, Liu J-Y, Lin C-T, Tang Y-T, Yeh M-H, Chang S-C, Li J-W, and Kao M-C. Human manganese superoxide dismutase suppresses HER2/neu-mediated breast cancer malignancy. FEBS Lett 581: 4443–4449, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Connor KM, Hempel N, Nelson KK, Dabiri G, Gamarra A, Balarmino J, van de Water L, Mian BM, and Melendez JA. Manganese superoxide dismutase enhances the invasive and migratory activity of tumor cells. Cancer Res 67: 10260–10267, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Cullen JJ, Mitros FA, and Oberley LW. Expression of antioxidant enzymes in diseases of the human pancreas: another link between chronic pancreatitis and pancreatic cancer. Pancreas 26: 23–27, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Cullen JJ, Weydert C, Hinkhouse MM, ritchie J, Domann FE, Spitz D, and Oberley LW. The role of manganese superoxide dismutase in the growth of pancreatic adenocarcinoma. Cancer Res 63: 1297–1303, 2003 [PubMed] [Google Scholar]
- 43.Delaney G, Jacob S, Featherstone C, and Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 104: 1129–1137, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Deschner EE. and Gray LH. Influence of oxygen tension on x-ray-induced chromosomal damage in Ehrlich ascites tumor cells irradiated in vitro and in vivo. Radiat Res 11: 115–146, 1959 [PubMed] [Google Scholar]
- 45.Dhar SK, Tangpong J, Chaiswing L, Oberley TD, and St. Clair DK. Manganese superoxide dismutase is a p53-regulated gene that switches cancers between early and advanced stages. Cancer Res 71: 6684–6695, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diallo I, Haddy N, Adjadj E, Samand A, Quiniou E, Chavaudra j, Alziar I, Perret N, Guerin S, Lefkopoulos D, and de Vathaire F. Frequency distribution of second solid cancer locations in relation to the irradiated volume among 115 patients treated for childhood cancer. Int J Radiat Oncol Biol Phys 74: 876–883, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Droge W. Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Du C, Gao Z, Venkatesha VA, Kalen AL, Chaudhuri L, Spitz DR, Cullen JJ, Oberley LW, and Goswami PC. Mitochondrial ROS and radiation induced transformation in mouse embryonic fibroblasts. Cancer Biol Ther 8: 1962–1971, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Egeblad M, Nakasone ES, and Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell 18: 884–901, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Epperly MW, Bray JA, Esocobar P, Bigbee WL, Watkins S, and Greenberger JS. Overexpression of the human manganese superoxide dismutase (MnSOD) transgene in subclones of murine hematopoietic progenitor cell line 32D cl 3 decreases irradiation-induced apoptosis but does not alter G2/M or G1/S phase cell cycle arrest. Radiat Oncol Investig 7: 331–342, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Epperly MW, Bray JA, Krager S, Berry LM, Gooding W, Engelhardt JF, Zwacka R, Travis EL, and Greenberger JS. Intratracheal injection of adenovirus containing the human MnSOD transgene protects athymic nude mice from irradiation-induced organizing alveolitis. Int J Radiat Oncol Biol Phys 43: 169–181, 1999 [DOI] [PubMed] [Google Scholar]
- 52.Epperly MW, Carpenter M, Agarwal A, Mitra P, Nie S, and Greenberger JS. Intraoral manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) radioprotective gene therapy decreases ionizing irradiation-induced murine mucosal cell cycling and apoptosis. In Vivo 18: 401–410, 2004 [PubMed] [Google Scholar]
- 53.Epperly MW, Epstein CJ, Travis EL, and Greenberger JS. Decreased pulmonary radiation resistance of manganese superoxide dismutase (MnSOD)-deficient mice is corrected by human manganese superoxide dismtuase-plasmid/liposome (SOD2-PL) intratracheal gene therapy. Radiat Res 154: 365–374, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Epperly MW, Franicola D, Zhang X, Nie S, and Greenberger JS. Effect of EGFR antagonists gefitinib (Iressa) and C225 (Cetuximab) on MnSOD-plasmid liposome transgene radiosensitization of a murine squamous cell carcinoma cell line. In Vivo 20: 791–796, 2006 [PubMed] [Google Scholar]
- 55.Epperly MW, Gretton JA, DeFilippi SJ, Sikora CA, Liggitt D, Koe G, and Greenberger BA. Modulation of radiation-induced cytokine elevation associated with esophagitis and esophageal stricture by manganese superoxide dismutase-plasmid/liposome (SOD2-PL) gene therapy. Radiat Res 155: 2–14, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Epperly MW, Sikora CA, DeFilippi SJ, Gretton JE, Zhan Q, Kufe DW, and Greenberger JS. Manganese superoxide dismutase (SOD2) inhibits radiation-induced apoptosis by stabilization of the mitochondrial membrane. Radiat Res 157: 568–577, 2002 [DOI] [PubMed] [Google Scholar]
- 57.Epperly MW, Travis EL, Whitsett JA, Raineri I, Epstein CJ, and Greenberger JS. Overexpression of manganese superoxide dismutase (MnSOD) in whole lung or alveolar type II cells of MnSOD transgenic mice does not provide intrinsic lung irradiation protection. Int J Cancer (Radiat Oncol Invest) 96: 11–21, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Epperly MW, Tyurina YY, Nie S, Niu YY, Zhang X, Kagan VE, and Greenberger JS. MnSOD-plasmid liposome gene therapy decreases ionizing irradiation-induced lipid peroxidation of the esophagus. In Vivo 19: 997–1004, 2005 [PubMed] [Google Scholar]
- 59.Epperly MW, Wegner R, Kanai AJ, Kagan VE, Greenberger EE, Nie S, and Greenberger JS. Effects of MnSOD-plasmid liposome gene therapy on antioxidant levels in irradiated murine oral cavity orthotopic tumors. Radiat Res 167: 289–297, 2007 [DOI] [PubMed] [Google Scholar]
- 60.Ermakov AV, Konkova MS, Kostyuk SV, Egolina NA, Efremova LV, and Veiko NN. Oxidative stress as a significant factor for development of an adaptive response in irradiated and nonirradiated human lymphocytes after inducing the bystander effect by low-dose X-radiation. Mutat Res 669: 155–161, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Ermakov AV, Konkova MS, Kostyuk SV, Smirnova TD, Malinovskaya EM, Efremova LV, and Veiko NN. An extracellular DNA mediated bystander effect produced from low dose irradiated endothelial cells. Mutat Res 712: 1–10, 2011 [DOI] [PubMed] [Google Scholar]
- 62.Esworthy RS, Ho Y-S, and Chu F-F. The Gpx1 gene encodes mitochondrial glutathione peroxidase in the mouse liver. Arch Biochem Biophys 340: 59–63, 1997 [DOI] [PubMed] [Google Scholar]
- 63.Fan M, Ahmed KM, Coleman MC, Spitz DR, and Li JJ. Nuclear factor-κB and manganese superoxide dismutase mediate adaptive radioresistance in low-dose irradiated mouse skin epithelial cells. Cancer Res 67: 3220–3228, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Feng X-P, Yi H, Li M-Y, Li X-H, Yi B, Zhang P-F, Li C, Peng F, Tang C-E, Li J-L, Chen Z-C, and Xiao Z-Q. Identification of biomarkers for predicting nasopharyngeal carcinoma response to radiotherapy by proteomics. Cancer Res 70: 3450–3462, 2010 [DOI] [PubMed] [Google Scholar]
- 65.Fernandez-Palomo C, Schultke E, Smith R, Brauer-Krisch E, Laissue J, Schroll C, Fazzari J, Seymour C, and Mothersill C. Bystander effects in tumor-free and tumor-bearing rat brains following irradiation by synchrotron X-rays. Int J Radiat Biol 89: 445–453, 2013 [DOI] [PubMed] [Google Scholar]
- 66.Fisher CJ. and Goswami PC. Mitochondria-targeted antioxidant enzyme activity regulates radioresistance in human pancreatic cancer cells. Cancer Biol Ther 7: 1271–1279, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fishman K, Baure J, Zou Y, Huang T-T, Andres-Mach M, Rola R, Suarez T, Acharya M, Limoli CL, Lamborn KR, and Fike JR. Radiation-induced reductions in neurogenesis are ameliorated in mice deficient in CuZnSOD or MnSOD. Free Radic Biol Med 47: 1459–1467, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Folz RJ. and Crapo JD. Extracellular superoxide dismutase (SOD3): tissue-specific expression, genomic characterization, and computer-assisted sequence analysis of the human EC SOD gene. Genomics 22: 162–171, 1994 [DOI] [PubMed] [Google Scholar]
- 69.Forman HJ, Fukuto JM, and Torres M. Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am J Physiol Cell Physiol 287: C246–C256, 2004 [DOI] [PubMed] [Google Scholar]
- 70.Fridovich I. The biology of oxygen radicals. Science 201: 875–880, 1978 [DOI] [PubMed] [Google Scholar]
- 71.Fridovich I. Superoxide dismutases. An adaptation to a paramagnetic gas. J Biol Chem 264: 7761–7764, 1989 [PubMed] [Google Scholar]
- 72.Gao Z, Sarsour EH, Kalen AL, Li L, Kumar MG, and Goswami PC. Late ROS accumulation and radiosensitivity in SOD1-overexpressing human glioma cells. Free Radic Biol Med 45: 1501–1509, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jiang C, Batinic-Haberle I, and Vujaskovic Z. Comparison of two Mn porphyrin-based mimics of superoxide dismutase in pulmonary radioprotection. Free Radic Biol Med 44: 982–989, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jiang C, Reboucas JS, Batinic-Haberle I, and Vujaskovic Z. Early and late administration of MnTE-2-PyP5+ in mitigation and treatment of radiation-induced lung damage. Free Rad Biol Med 48: 1034–1043, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gius D. and Spitz DR. Redox signaling in cancer biology. Antioxid Redox Signal 8: 1249–1252, 2006 [DOI] [PubMed] [Google Scholar]
- 76.Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, and Jain RK. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev 91: 1071–1121, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goodhead DT. The initial physical damage produced by ionizing radiations. Int J Radiat Biol 56: 623–634, 1989 [DOI] [PubMed] [Google Scholar]
- 78.Goodhead DT. Initial events in the cellular effects of ionizing radiations: clustered damage in DNA. Int J Radiat Biol 65: 7–17, 1994 [DOI] [PubMed] [Google Scholar]
- 79.Gorman S, Fox E, O'Donoghue D, Sheahan K, Hyland J, Mulcahy H, Loeb LA, and O'Sullivan J. Mitochondrial mutagenesis induced by tumor-specific radiation bystander effects. J Mol Med 88: 701–708, 2010 [DOI] [PubMed] [Google Scholar]
- 80.Gorman S, Tosetto M, Lyng F, Howe O, Sheahan K, O'Donoghue D, Hyland J, Mulcahy H, and O'Sullivan J. Radiation and chemotherapy bystanders effects induce early genomic instability events: telomere shortening and bridge formation coupled with mitochondrial dysfunction. Mutat Res 669: 131–138, 2009 [DOI] [PubMed] [Google Scholar]
- 81.Gough DR. and Cotter TG. Hydrogen peroxide: a Jekyll and Hyde signalling molecule. Cell Death Disease 2: e213, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gray LH, Conger AD, Ebert M, Hornsey S, and Scott OCA. The concentration of oxygen dissolved in tissues at the time or irradiation as a factor in radiotherapy. Br J Radiol 26: 638–648, 1953 [DOI] [PubMed] [Google Scholar]
- 83.Grivennikova VG. and Vinogradov AD. Generation of superoxide by the mitochondrial complex I. Biochim Biophys Acta 1757: 553–561, 2006 [DOI] [PubMed] [Google Scholar]
- 84.Guo G, Yan-Sanders Y, Lyn-Cook BD, Wang T, Tamae D, Ogi J, Khaletskiy A, Li Z, Weydert C, Longmate JA, Huang T-T, Spitz DR, Oberley LW, and Li JJ. Manganese superoxide dismutase-mediated gene expression in radiation-induced adaptive responses. Mol Cell Biol 23: 2362–2378, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo H, Seixas-Silva JA, Jr, Epperly MW, Gretton JE, Shin DM, Bar-Sagi D, Archer H, and Greenberger JS. Prevention of radiation-induced oral cavity mucositis by plasmid/liposome delivery of the human manganese superoxide dismutase (SOD2) transgene. Radiat Res 159: 361–370, 2003 [DOI] [PubMed] [Google Scholar]
- 86.Guo HL, Wolfe D, Epperly MW, Huang S, Liu K, Glorioso JC, Greenberger J, and Blumberg D. Gene transfer of human manganese superoxide dismutase protects small intestinal villi from radiation injury. J Gastrointest Surg 7: 229–236, 2003 [DOI] [PubMed] [Google Scholar]
- 87.Hall EJ. The bystander effect. Health Phys 85: 31–35, 2003 [DOI] [PubMed] [Google Scholar]
- 88.Hall EJ. and Giaccia AJ. Radiobiology for the Radiologist. New York: Lippincott, Williams, and Wilkins, 2006 [Google Scholar]
- 89.Hei TK, Ballas LK, Brenner DJ, and Geard CR. Advances in radiobiological studies using a microbeam. J Radiat Res 50: A7–A12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hempel N, Carrico PM, and Melendez JA. Manganese superoxide dismutase (Sod2) and redox-control of signaling events that drive metastasis. Anti Cancer Agents Med Chem 11: 191–201, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hileman EO, Liu J, Albitar M, Keating MJ, and Huang P. Intrinsic oxidative stress in cancer cells: a biochemical basis for therapeutic selectivity. Cancer Chemother Pharmacol 53: 209–219, 2004 [DOI] [PubMed] [Google Scholar]
- 92.Hjalmarsson K, Marklund SL, Engstrom A, and Edlund T. Isolation and sequence of complimentary DNA encoding human extracellular superoxide dismutase. Proc Natl Acad Sci U S A 84: 6340–6344, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ho JC-m, Zheng S, Comhair SAA, Farver C, and Erzurum SC. Differential expression of manganese superoxide dismutase and catalase in lung cancer. Cancer Res 61: 8578–8585, 2001 [PubMed] [Google Scholar]
- 94.Holley AK, Dhar SK, and St. Clair DK. Curbing cancer's sweet tooth: is there a role for MnSOD in regulation of the Warburg effect? Mitochondrion 13: 170–188, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Holley AK, Kiningham KK, Spitz DR, Edwards DP, Jenkins JT, and Moore MR. Progestin stimulation of manganese superoxide dismutase and invasive properties in T47D human breast cancer cells. J Steroid Biochem Mol Biol 117: 23–30, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Holley AK, Xu Y, St. Clair DK, and St. Clair WH. RelB regulates manganese superoxide dismutase gene and resistance to ionizing radiation of prostate cancer cells. Ann N Y Acad Sci 1201: 129–136, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hoye AT, Davoren JE, Wipf P, Fink MP, and Kagan VE. Targeting mitochondria. Acc Chem Res 41: 87–97, 2008 [DOI] [PubMed] [Google Scholar]
- 98.Hsieh C-H, Lee C-H, Liang J-A, Yu C-Y, and Shyu W-C. Cycling hypoxia increases U87 glioma cell radioresistance via ROS induced higher and long-term HIF-1 signal transduction activity. Oncol Rep 24: 1629–1636, 2010 [DOI] [PubMed] [Google Scholar]
- 99.Hu H, Luo M-L, Du X-L, Feng Y-B, Zhang Y, Shen X-M, Xu X, Cai Y, Han Y-L, and Wang M-R. Up-regulated manganese superoxide dismutase expression increases apoptosis resistance in human esophageal squamous cell carcinomas. Chin Med J 120: 2092–2098, 2007 [PubMed] [Google Scholar]
- 100.Hu Y, Rosen DG, Zhou Y, Feng L, Yang G, Liu J, and Huang P. Mitochondrial manganese-superoxide dismutase expression in ovarian cancer: role in cell proliferation and response to oxidative stress. J Biol Chem 280: 39485–39492, 2005 [DOI] [PubMed] [Google Scholar]
- 101.Hwang TS, Choi HK, and Han HS. Differential expression of manganese superoxide dismutase, copper/zinc superoxide dismutase, and catalase in gastric adenocarcinoma and normal gastric mucosa. EJSO 33: 474–479, 2007 [DOI] [PubMed] [Google Scholar]
- 102.Iyer R. and Lehnert BE. Alpha-particle induced increases in the radioresistance of normal human bystander effects. Radiat Res 157: 3–7, 2002 [DOI] [PubMed] [Google Scholar]
- 103.Izutani R, Asano S, Imano M, Kuroda D, Kato M, and Ohyanagi H. Expression of manganese superoxide dismutase in esophageal and gastric cancers. J Gastroenterol 33: 816–822, 1998 [DOI] [PubMed] [Google Scholar]
- 104.Janssen AML, Bosman CB, van Duijn W, Oostendorp-van de Ruit MM, Kubben FJGM, Griffioen G, Lamers BBHW, van Krieken JHJM, van de Velde CJH, and Verspaget HW. Superoxide dismutases in gastric and esophageal cancer and the prognostic impact in gastric cancer. Clin Cancer Res 6: 3183–3192, 2000 [PubMed] [Google Scholar]
- 105.Jemal A, Bray F, Center MM, Ferlay J, Ward E, and Forman D. Global cancer statistics. CA Cancer J Clin 61: 69–90, 2011 [DOI] [PubMed] [Google Scholar]
- 106.Jordan BF. and Sonveaux P. Targeting tumor perfusion and oxygenation to improve the outcome of anticancer therapy. Front Pharmacol 3: 1–15, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Josson S, Sharp S, Sung S-Y, Johnstone PAS, Aneja R, Wang R, Gururajan M, Turner T, Chung LWK, and Yates C. Tumor-stromal interactions influence radiation sensitivity in epithelial-versus mesenchymal-like prostate cancer cells. J Oncol pii: 232831, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Josson S, Xu Y, Fang F, Dhar SK, St. Clair DK, and St. Clair WH. RelB regulates manganese superoxide dismutase gene and resistance to ionizing radiation of prostate cancer cells. Oncogene 25: 1554–1559, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kalen AL, Sarsour EH, Venkataraman S, and Goswami PC. Mn-superoxide dismutase overexpression enhances G2 accumulation and radioresistance in human oral squamous carcinoma cells. Antioxid Redox Signal 8: 1273–1281, 2006 [DOI] [PubMed] [Google Scholar]
- 110.Kanai AJ, Zeidel ML, Lavelle JP, Greenberger JS, Birder LA, De Groat WC, Apodaca GL, Meyers SA, Ramage R, and Epperly MW. Manganese superoxide dismutase gene therapy protects against irradiation-induced cystitis. Am J Physiol Renal Physiol 283: F1304–F1312, 2002 [DOI] [PubMed] [Google Scholar]
- 111.Kang SK, Rabbani ZN, Folz RJ, Golson ML, Huang H, Yu D, Samulski TS, Dewhirst MW, Anscher MS, and Vujaskovic Z. Overexpression of extracellular superoxide dismutase protects mice from radiation-induced lung injury. Int J Radiat Oncol Biol Phys 57: 1056–1066, 2003 [DOI] [PubMed] [Google Scholar]
- 112.Kashino G, Prise KM, Schettino G, Folkard M, Vojnovic B, Michael BD, Suzuki K, Kodama S, and Watanabe M. Evidence for induction of DNA double strand breaks in the bystander response to targeted soft X-rays in CHO cells. Mutat Res 556: 209–215, 2004 [DOI] [PubMed] [Google Scholar]
- 113.Keele BB, Jr., McCord JM, and Fridovich I. Further characterization of bovine superoxide dismutase and its isolation from bovine heart. J Biol Chem 246: 2875–2880, 1971 [PubMed] [Google Scholar]
- 114.Khan MA, Hill RP, and Van Dyk J. Partial volume rat lung irradiation: an evaluation of early DNA damage. Int J Radiat Oncol Biol Phys 40: 467–476, 1998 [DOI] [PubMed] [Google Scholar]
- 115.Khan MA, Van Dyk J, Yeung IWT, and Hill RP. Partial volume rat lung irradiation; assessment of early DNA damage in different lung regions and effect of radical scavengers. Radiother Oncol 66: 95–102, 2003 [DOI] [PubMed] [Google Scholar]
- 116.Kiningham KK, Daosukho C, and St. Clair DK. IκBα (inhibitory κBα) identified as labile repressor of MnSOD (manganese superoxide dismutase) expression. Biochem J 384: 543–549, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kiningham KK, Xu Y, Daosukho C, Popova B, and St. Clair DK. Nuclear factor κB-dependent mechanisms coordinate the synergistic effect of PMA and cytokines on the induction of superoxide dismutase 2. Biochem J 353: 147–156, 2001 [PMC free article] [PubMed] [Google Scholar]
- 118.Kostyuk SV, Ermakov AV, Alekseeva AY, Smirnova TD, Glebova KV, Efremova LV, Baranova A, and Veiko NN. Role of extracellular DNA oxidative modification in radiation induced bystander effects in human endotheliocytes. Mutat Res 729: 52–60, 2012 [DOI] [PubMed] [Google Scholar]
- 119.Koturbash I, Kutanzi K, Hendrickson K, Rodriguez-Juarez R, Kogosov D, and Kovalchuk O. Radiation-induced bystander effects in vivo are sex specific. Mutat Res 642: 28–36, 2008 [DOI] [PubMed] [Google Scholar]
- 120.Koturbash I, Loree J, Kutanzi K, Koganow C, Pogribny I, and Kovalchuk O. In vivo bystander effect: cranial x-irradiation leads to elevated DNA damage, altered cellular proliferation and apoptosis, and increased p53 levels in shielded spleen. Int J Radiat Oncol Biol Phys 70: 554–562, 2008 [DOI] [PubMed] [Google Scholar]
- 121.Lee JH, Choi IY, Kil IS, Kim SY, Yang ES, and Park J-W. Protective role of superoxide dismutases against ionizing radiation in yeast. Biochim Biophys Acta 1526: 191–198, 2001 [DOI] [PubMed] [Google Scholar]
- 122.Lenaz G. The mitochondrial production of reactive oxygen species: mechanisms and implications in human pathology. IUBMB Life 52: 159–164, 2001 [DOI] [PubMed] [Google Scholar]
- 123.Lewis DA, Mayhugh BM, Qin Y, Trott K, and Mendonca MS. Production of delayed death and neoplastic transformation in CGL1 cells by radiation-induced bystander effects. Radiat Res 156: 251–258, 2001 [DOI] [PubMed] [Google Scholar]
- 124.Li F. and Sethi G. Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochim Biophys Acta 1805: 167–180, 2010 [DOI] [PubMed] [Google Scholar]
- 125.Li H, Wang Y, Pazhanisamy SK, Shao L, Batinic-Haberle I, Meng A, and Zhou D. Mn(III) meso-tetrakis-(N-ethylpyridinium-2-yl) porphyrin mitigates total body irradiation-induced long-term bone marrow suppression. Free Radic Biol Med 51: 30–37, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li S, Yan T, Yang J-Q, Oberley TD, and Oberley LW. The role of cellular glutathione peroxidase redox regulation in the suppression of tumor cell growth by manganese superoxide dismutase. Cancer Res 60: 3927–3939, 2000 [PubMed] [Google Scholar]
- 127.Lyng FM, Howe OL, and McClean B. Reactive oxygen species-induced release of signalling factors in irradiated cells triggers membrane signalling and calcium influx in bystander cells. Int J Radiat Biol 87: 683–695, 2011 [DOI] [PubMed] [Google Scholar]
- 128.Lyng FM, Seymour CB, and Mothersill C. Production of a signal by irradiated cells which leads to a response in unirradiated cells characteristic of initiation of apoptosis. Br J Cancer 83: 1223–1230, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lyng FM, Seymour CB, and Mothersill C. Initiation of apoptosis in cells exposed to medium from the progeny of irradiated cells: a possible mechanism for bystander-induced genomic instability? Radiat Res 157: 365–370, 2002 [DOI] [PubMed] [Google Scholar]
- 130.Maguire P, Mothersill C, Seymour C, and Lyng F. Medium from irradiated cells induces dose-dependent mitochodnrial changes and BCL2 responses in unirradiated human keratinocytes. Radiat Res 163: 384–390, 2005 [DOI] [PubMed] [Google Scholar]
- 131.Maiorino M, Scapin M, Ursini F, Biasolo M, Bosello V, and Flohe L. Distinct promoters determine alternative transcription of gpx-4 into phospholipid-hydroperoxide glutathione peroxidase variants. J Biol Chem 278: 34286–34290, 2003 [DOI] [PubMed] [Google Scholar]
- 132.Malafa M, Margenthaler J, Webb B, Neitzel L, and Christophersen M. MnSOD expression is increased in metastatic gastric cancer. J Surg Res 88: 130–134, 2000 [DOI] [PubMed] [Google Scholar]
- 133.Mancuso M, Pasquali E, Leonardi S, Rebessi S, Tanori M, Giardullo P, Borra F, Pazzaglia S, Naus CC, Di Majo V, and Saran A. Role of connexin43 and ATP in long-range bystander radiation damage and oncogenesis in vivo. Oncogene 30: 4601–4608, 2011 [DOI] [PubMed] [Google Scholar]
- 134.Mancuso M, Pasquali E, Leonardi S, Tanori M, Rebessi S, Di Majo V, Pazzaglia S, Toni MP, Pimpinella M, Covelli V, and Saran A. Oncogenic bystander radiation effects in patched heterozygous mouse cerebellum. Proc Natl Acad Sci U S A 105: 12445–12450, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McCord JM. and Fridovich I. Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244: 6049–6055, 1969 [PubMed] [Google Scholar]
- 136.McGee MC, Hamner JB, Williams RF, Rosati SF, Sims TL, Ng CY, Gaber MW, Calabrese C, Wu J, Nathwani AC, Duntsch C, Merchant TE, and Davidoff AM. Improved intratumoral oxygenation through vascular normalization increases glioma sensitivity to ionizing radiation. Int J Radiat Oncol Biol Phys 76: 1537–1545, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McParland BJ. Nuclear Medicine Radiation Dosimetry. London: Springer-Verlag, 2010 [Google Scholar]
- 138.Miriyala S, Spasojevic I, Tovmasyan A, Salvemini D, Vujaskovic Z, St. Clair D, and Batinic-Haberle I. Manganese superoxide dismutase, MnSOD and its mimics. Biochim Biophys Acta 1822: 794–814, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moeller BJ, Batinic-Haberle I, Spasojevic I, Rabbani ZN, Anscher MS, Vujaskovic Z, and Dewhirst MW. A manganese porphyrin superoxide dismutase mimetic enhances tumor radioresponsiveness. Int J Radiat Oncol Biol Phys 63: 545–552, 2005 [DOI] [PubMed] [Google Scholar]
- 140.Mohr A, Buneker C, Gough RP, and Zwacka RM. MnSOD protects colorectal cancer cells from TRAIL-induced apoptosis by inhibition of Smac/DIABLO release. Oncogene 27: 763–774, 2008 [DOI] [PubMed] [Google Scholar]
- 141.Mothersill C. and Seymour C. Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of unirradiated cells. Int J Radiat Biol 71: 421–427, 1997 [DOI] [PubMed] [Google Scholar]
- 142.Mottram JC. A factor of importance in the radio sensitivity of tumors. Br J Radiol 9: 606–614, 1936 [Google Scholar]
- 143.Murphy JEJ, Nugent S, Seymour C, and Mothersill C. Mitochondrial DNA point mutations and a novel deletion induced by direct low-LET radiation and by medium from irradiated cells. Mutat Res 585: 127–136, 2005 [DOI] [PubMed] [Google Scholar]
- 144.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 417: 1–13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nagasawa H. and Little JB. Induction of sister chromatid exchanges by extremely low doses of α-particles. Cancer Res 52: 6394–6396, 1992 [PubMed] [Google Scholar]
- 146.Nias AHW. An Introduction to Radiobiology. Chichester, United Kingdom: John Wiley and Sons Ltd, 1998 [Google Scholar]
- 147.Nelson KK, Ranganathan AC, Mansouri J, Rodriguez AM, Providence KM, Rutter JL, Pumiglia K, Bennett JA, and Melendez JA. Elevated Sod2 activity augments matrix metalloproteinase expression: evidence for the involvement of endogenous hydrogen peroxide in regulating metastasis. Clin Cancer Res 9: 424–432, 2003 [PubMed] [Google Scholar]
- 148.Niciforovic A, Djordjevic J, Adzic M, Vucic V, Mitrasinovic PM, and Radojcic MB. Experimental and systems biology studies of the molecular basis for the radioresistance of prostate carcinoma cells. Ann Biomed Eng 36: 831–838, 2008 [DOI] [PubMed] [Google Scholar]
- 149.Nozoe T, Honda M, Inutsuka S, Yasuda M, and Korenaga D. Significance of immunohistochemical expression of manganese superoxide dismutase as a marker of malignant potential in colorectal carcinoma. Oncol Rep 10: 39–43, 2003 [PubMed] [Google Scholar]
- 150.Nugent S, Mothersill CE, Seymour C, McClean B, Lyng FM, and Murphy JEJ. Altered mitochondrial function and genome frequency post exposure to γ-radiation and bystander factors. Int J Radiat Biol 86: 829–841, 2010 [DOI] [PubMed] [Google Scholar]
- 151.Nugent SME, Mothersill CE, Seymour C, McClean B, Lyng FM, and Murphy JEJ. Increased mitochondrial mass in cells with functionally compromised mitochondria after exposure to both direct γ radiation and bystander factors. Radiat Res 168: 134–142, 2007 [DOI] [PubMed] [Google Scholar]
- 152.O'Neill P. and Wardman P. Radiation chemistry comes before radiation biology. Int J Radiat Biol 85: 9–25, 2009 [DOI] [PubMed] [Google Scholar]
- 153.Oberley LW. and Buettner GR. Role of superoxide dismutase in cancer: a review. Cancer Res 39: 1141–1149, 1979 [PubMed] [Google Scholar]
- 154.Oberley TD. and Oberley LW. Antioxidant enzyme levels in cancer. Histol Histopathol 12: 525–535, 1997 [PubMed] [Google Scholar]
- 155.Oberley TD, Verwiebe E, Zhong W, Kang SW, and Rhee SG. Localization of the thioredoxin system in normal rat kidney. Free Radic Biol Med 30: 412–424, 2001 [DOI] [PubMed] [Google Scholar]
- 156.Ohuchida K, Mizumoto K, Murakami M, Qian L-W, Sato N, Nagai E, Matsumoto K, Nakamura T, and Tanaka M. Radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal interactions. Cancer Res 64: 3215–3222, 2004 [DOI] [PubMed] [Google Scholar]
- 157.Okado-Matsumoto A. and Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver. Cu, Zn-SOD in mitochondria. J Biol Chem 276: 38388–38393, 2001 [DOI] [PubMed] [Google Scholar]
- 158.Palazzotti B, Pani G, Colavitti R, de Leo ME, Bedogni B, Borrello S, and Galeotti T. Increased growth capacity of cervical-carcinoma cells over-expressing manganous superoxide dismutase. Int J Cancer 82: 145–150, 1999 [DOI] [PubMed] [Google Scholar]
- 159.Pandey BN, Kumar A, Ali M, and Mishra KP. Bystander effect of conditioned medium from low and high doses of γ-irradiated human leukemic cells on normal lymphocytes and cancer cells. J Environ Pathol Toxicol Oncol 30: 333–340, 2011 [DOI] [PubMed] [Google Scholar]
- 160.Panfili E, Sandri G, and Ernster L. Distribution of glutathione peroxidases and glutathione reductase in rat brain mitochondria. FEBS Lett 290: 35–37, 1991 [DOI] [PubMed] [Google Scholar]
- 161.Pani G, Galeotti T, and Chiarugi P. Metastasis: cancer cell's escape from oxidative stress. Cancer Metastasis Rev 29: 351–378, 2010 [DOI] [PubMed] [Google Scholar]
- 162.Persaud R, Zhou H, Baker SE, Hei TK, and Hall EJ. Assessment of low linear energy transfer radiation-induced bystander mutagenesis in a three-dimensional culture model. Cancer Res 65: 9876–9882, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Persaud R, Zhou H, Hei TK, and Hall EJ. Demonstration of a radiation-induced bystander effect for low dose low LET β-particles. Radiat Environ Biophys 46: 395–400, 2007 [DOI] [PubMed] [Google Scholar]
- 164.Petkau A. Radiation protection by superoxide dismutase. Photochem Photobiol 28: 765–774, 1978 [DOI] [PubMed] [Google Scholar]
- 165.Petkau A, Chelack WS, Pleskach SD, Meeker BE, and Brady CM. Radioprotection of mice by superoxide dismutase. Biochem Biophys Res Commun 65: 886–893, 1975 [DOI] [PubMed] [Google Scholar]
- 166.Pljesa-Ercegova M, Mimic-Oka J, Dragicevic D, Savic-Radojevic A, Opacic M, Pljesa S, Radosavljevic R, and Simic T. Altered antioxidant capacity in human renal cell carcinoma: role of glutathione associated enzymes. Urol Oncol 26: 175–181, 2008 [DOI] [PubMed] [Google Scholar]
- 167.Pouget J-P, Frelon S, Ravanat J-L, Testard I, Odin F, and Cadet J. Formation of modified DNA bases in cells exposed either to gamma radiation or to high-LET particles. Radiat Res 157: 589–595, 2002 [DOI] [PubMed] [Google Scholar]
- 168.Powis G. and Kirkpatrick DL. Thioredoxin signaling as a target for cancer therapy. Curr Opin Pharmacol 7: 392–397, 2007 [DOI] [PubMed] [Google Scholar]
- 169.Prise KM, Belyakov OV, Folkard M, and Michael BD. Studies of bystander effects in human fibroblasts using a charged particle microbeam. Int J Radiat Biol 74: 793–798, 1998 [DOI] [PubMed] [Google Scholar]
- 170.Prise KM, Schettino G, Vojnovic B, Belyakov O, and Shao C. Microbeam studies of the bystander response. J Radiat Res 50: A1–A6, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Przybyszewski WM, Widel M, Szurko A, Lubecka B, Matulewicz L, Maniakowski Z, Polaniak R, Birkner E, and Rzeszowska-Wolny J. Multiple bystander effect of irradiated megacolonies of melanoma cells on non-irradiated neighbors. Cancer Lett 214: 91–102, 2004 [DOI] [PubMed] [Google Scholar]
- 172.Qu Y, Zhang H, Zhao S, Hong J, and Tang C. The effect on radioresistance of manganese superoxide dismutase in nasophryngeal carcinoma. Oncol Rep 23: 1005–1011, 2010 [DOI] [PubMed] [Google Scholar]
- 173.Qu Y, Zhao S, Hong J, and Tang S. Radiosensitive gene therapy through imRNA expression for silencing manganese superoxide dismutase. J Cancer Res Clin Oncol 136: 953–959, 2010 [DOI] [PubMed] [Google Scholar]
- 174.Rabbani ZN, Anscher MS, Folz RJ, Archer E, Huang H, Chen L, Golson ML, Samulski TS, Dewhirst MW, and Vujaskovic Z. Overexpression of extracellular superoxide dismutase reduces acute radiation induced lung toxicity. BMC Cancer 5: 59, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rabbani ZN, Batinic-Haberle I, Anscher MS, Huang J, Day BJ, Alexander E, Dewhirst MW, and Vujaskovic Z. Long-term administration of a small molecular weight catalytic metalloporphyrin antioxidant, AEOL 10150, protects lungs from radiation-induced injury. Int J Radiat Oncol Biol Phys 67: 573–580, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rabbani ZN, Salahuddin FK, Yarmolenko P, Batinic-Haberle I, Thrasher BA, Gauter-Fleckenstein B, Dewhirst MW, Anscher MS, and Vujaskovic Z. Low molecular weight catalytic metalloporphyrin antioxidant AEOL 10150 protects lungs from fractionated radiation. Free Radic Res 41: 1273–1282, 2007 [DOI] [PubMed] [Google Scholar]
- 177.Radi R, Turrens JF, Chang LY, Bush KM, Crapo JD, and Freeman BA. Detection of catalase in rat heart mitochondria. J Biol Chem 266: 22028–22034, 1991 [PubMed] [Google Scholar]
- 178.Rajendran S, Harrison SH, Thomas RA, and Tucker JD. The role of mitochondria in the radiation-induced bystander effect in human lymphoblastoid cells. Radiat Res 175: 159–171, 2011 [DOI] [PubMed] [Google Scholar]
- 179.Ravindranath SD. and Fridovich I. Isolation and characterization of a manganese-containing superoxide dismutase from yeast. J Biol Chem 250: 6107–6112, 1975 [PubMed] [Google Scholar]
- 180.Regulus P, Duroux B, Bayle P-A, Favier A, Cadet J, and Ravanat J-L. Oxidation of the sugar moiety of DNA by ionizing radiation or bleomycin could induce the formation of a cluster DNA lesion. Proc Natl Acad Sci U S A 104: 14032–14037, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rhee SG, Chang T-S, Bae YS, Lee S-R, and Kang SW. Cellular regulation by hydrogen peroxide. J Am Soc Nephrol 14: S211–S215, 2003 [DOI] [PubMed] [Google Scholar]
- 182.Sachs RK, Chen P-L, Hahnfeldt PJ, and Hlatky LR. DNA damage caused by ionizing radiation. Math Biosci 112: 271–303, 1992 [DOI] [PubMed] [Google Scholar]
- 183.Salvi M, Battaglia V, Brunati AM, La Rocca N, Tibaldi E, Pietrangeli P, Marcocci L, Mondovi B, Rossi CA, and Toninello A. Catalase takes part in rat liver mitochondria oxidative stress defense. J Biol Chem 282: 24407–24415, 2007 [DOI] [PubMed] [Google Scholar]
- 184.Salzman R, Kankova K, Pacal L, Tomandl J, Horakova Z, and Kostrica R. Increased activity of superoxide dismutase in advanced stages of head and neck squamous cell carcinoma with locoregional metastases. Neoplasma 54: 321–325, 2007 [PubMed] [Google Scholar]
- 185.Schilling-Toth B, Sandor N, Kis E, Kadhim M, Safrany G, and Hegyesi H. Analysis of the common deletions in the mitochondrial DNA is a sensitive biomarker detecting direct and non-targeted cellular effects of low dose ionizing radiation. Mutat Res 716: 33–39, 2011 [DOI] [PubMed] [Google Scholar]
- 186.Secomb TW, Hsu R, Ong ET, Gross JF, and Dewhirst MW. Analysis of the effects of oxygen supply and demand on hypoxic fraction in tumors. Acta Oncol 34: 313–316, 1995 [DOI] [PubMed] [Google Scholar]
- 187.Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun S-C, and Karin M. Activation by IKKα of a second, evolutionary conserved, NF-κB signaling pathway. Science 293: 1495–1499, 2001 [DOI] [PubMed] [Google Scholar]
- 188.Seo MS, Kang SW, Kim K, Baines IC, Lee TH, and Rhee SG. Identification of a new type of mammalian peroxiredoxin that forms an intramolecular disulfide as a reaction intermediate. J Biol Chem 275: 20346–20354, 2000 [DOI] [PubMed] [Google Scholar]
- 189.Sharma KKK, Swarts SG, and Bernhard WA. Mechanisms of direct radiation damage to DNA: the effect of base sequence on base end products. J Phys Chem B 115: 4843–4855, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shibata E, Nanri H, Ejima K, Araki M, Fukuda J, Yoshimura K, Toki N, Ikeda M, and Kashmura M. Enhancement of mitochondrial oxidative stress and up-regulation of antioxidant protein peroxiredoxin III/SP-22 in the mitochondria of human pre-eclamptic placentae. Placenta 24: 698–705, 2003 [DOI] [PubMed] [Google Scholar]
- 191.Singh SK, Wang M, Staudt C, and Iliakis G. Post-irradiation chemical processing of DNA damage generates double-strand breaks in cells already engaged in repair. Nucleic Acids Res 39: 8416–8429, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Soini Y, Vakkala M, Kahlos K, Paakko P, and Kinnla V. MnSOD expression is less frequent in tumour cells of invasive breast carcinomas than in in situ carcinomas or non-neoplastic breast epithelial cells. J Pathol 195: 156–162, 2001 [DOI] [PubMed] [Google Scholar]
- 193.Southgate TD, Sheard V, Milsom MD, Ward TH, Mairs RJ, Boyd M, and Fairbairn LJ. Radioprotective gene therapy through retroviral expression of manganese superoxide dismutase. J Gene Med 8: 557–565, 2006 [DOI] [PubMed] [Google Scholar]
- 194.St. Clair DK, Wan XS, Oberley TD, Muse KE, and St. Clair WH. Suppression of radiation-induced neoplastic transformation by overexpression of mitochondrial superoxide dismutase. Mol Carcinog 6: 238–242, 1992 [DOI] [PubMed] [Google Scholar]
- 195.Sun J, Chen Y, Li M, and Ge Z. Role of antioxidant enzymes on ionizing radiation resistance. Free Radic Biol Med 24: 586–593, 1998 [DOI] [PubMed] [Google Scholar]
- 196.Sun Y, Colburn NH, and Oberley LW. Depression of catalase gene expression after immortalizationa nd transformation of mouse liver cells. Carcinogenesis 14: 1505–1510, 1993 [DOI] [PubMed] [Google Scholar]
- 197.Sun Y, Oberley LW, Oberley TD, Elwell JH, and Sierra-Rivera E. Lowered antioxidant enzymes in spontaneously transformed embryonic mouse liver cells in culture. Carcinogenesis 14: 1457–1463, 1993 [DOI] [PubMed] [Google Scholar]
- 198.Sutherland BM, Bennett PV, Sidorkina O, and Laval J. Clustered DNA damages induced in isolated DNA and in human cells by low doses of ionizing radiation. Proc Natl Acad Sci U S A 97: 103–108, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Takada Y, Hachiya M, Park S-H, Osawa Y, Ozawa T, and Akashi M. Role of reactive oxygen species in cells overexpressing manganese superoxide dismutase: mechanism for induction of radioresistance. Mol Cancer Res 1: 137–146, 2002 [PubMed] [Google Scholar]
- 200.Takeshige K. and Minakami S. NADH- and NADPH-dependent formation of superoxide anions by bovine heart submitochondrial particles and NADH-ubiquinone reductase preparation. Biochem J 180: 129–135, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tannock IF. Oxygen diffusion and the distribution of cellular radiosensitivity in tumors. Br J Radiol 45: 515–524, 1972 [DOI] [PubMed] [Google Scholar]
- 202.Tartier L, Gilchrist S, Burdak-Rothkamm S, Folkard M, and Prise KM. Cytoplasmic irradiation induces mitochondrial-dependent 53BP1 protein relocalization in irradiated and bystander cells. Cancer Res 67: 5872–5879, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Toh Y, Kuninaka S, Oshiro T, Ikeda Y, Nakashima H, Baba H, Kohnoe S, Okamura T, Mori M, and Sugimachi K. Overexpression of manganese superoxide dismutase mRNA may correlate with aggressiveness in gastric an colorectal adenocarcinomas. Int J Oncol 17: 107–112, 2000 [DOI] [PubMed] [Google Scholar]
- 204.Tominaga T, Hachiya M, Shibata T, Sakamoto Y, Taki K, and Akashi M. Exogenously-added copper/zinc superoxide dismutase rescues damage of endothelial cells from lethal irradiation. J Clin Biochem Nutr 50: 78–83, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Trumpower BL. The protonmotive Q cycle. Energy transduction by coupling of proton translocation to electron transfer by the cytochrome bc1 complex. J Biol Chem 265: 11409–11412, 1990 [PubMed] [Google Scholar]
- 206.Tsai KKC, Stuart J, Chuang Y-YE, Little JB, and Yuan Z-M. Low-dose radiation-induced senescent stromal fibroblasts render nearby breast cancer cells radioresistant. Radiat Res 172: 306–313, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tsanou E, Ioachim E, Briasoulis E, Damala K, Charchanti A, Karavasilis V, Pavlidis N, and Agnantis NJ. Immunohistochemical expression of superoxide dismutase (MnSOD) anti-oxidant enzyme in invasive breast carcinoma. Histol Histopathol 19: 807–813, 2004 [DOI] [PubMed] [Google Scholar]
- 208.Urano M, Kuroda M, Reynolds R, Oberley TD, and St. Clair DK. Expression of manganese superoxide dismutase reduces tumor control radiation dose: gene-radiotherapy. Cancer Res 55: 2490–2493, 1995 [PubMed] [Google Scholar]
- 209.Valko M, Izakovic M, Mazur M, Rhodes CJ, and Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem 266: 37–56, 2004 [DOI] [PubMed] [Google Scholar]
- 210.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, and Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39: 44–84, 2007 [DOI] [PubMed] [Google Scholar]
- 211.Van der Meeren A, Monti P, Vandamme M, Squiban C, Wysocki J, and Griffiths N. Abdominal radiation exposure elicits inflammatory responses and abscopal effects in the lungs of mice. Radiat Res 163: 144–152, 2005 [DOI] [PubMed] [Google Scholar]
- 212.Vujaskovic Z, Batinic-Haberle I, Rabbani ZN, Feng Q-F, Kang SK, Spasojevic I, Samulski TV, Fridovich I, Dewhirst MW, and Anscher MS. A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury. Free Radic Biol Med 33: 857–863, 2002 [DOI] [PubMed] [Google Scholar]
- 213.Ward JF. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog Nucleic Acid Res Mol Biol 35: 95–125, 1988 [DOI] [PubMed] [Google Scholar]
- 214.Wardman P. The importance of radiation chemistry to radiation and free radical biology (The 2008 Silvanus Thompson Memorial Lecture). Br J Radiol 82: 89–104, 2009 [DOI] [PubMed] [Google Scholar]
- 215.Waris G. and Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog 5: 14–21, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Weber TJ, Siegel RW, Markillie LM, Chrisler WB, Lei XC, and Colburn NH. A paracrine signal mediates the cell transformation response to low dose gamma radiation in JB6 cells. Mol Carcinog 43: 31–37, 2005 [DOI] [PubMed] [Google Scholar]
- 217.Weisiger RA. and Fridovich I. Mitochondrial superoxide dismutase. Site of synthesis and intramitochondrial localization. J Biol Chem 248: 4793–4796, 1973 [PubMed] [Google Scholar]
- 218.Weitner T, Kos I, Sheng H, Tovmasyan A, Reboucas JS, Fan P, Waner DS, Vujaskovic Z, Batinic-Haberle I, and Spasojevic I. Comprehensive pharmacokinetic studies and oral bioavailability of two Mn porphyrin-based SOD mimics, MnTE-2-PyP(5+) and MnTnHex-2-PyP(5+). Free Rad Biol and Med 58: 73–80, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Widel M, Przybyszewski WM, Cieslar-Pobuda A, Saenko YV, and Rzeszowska-Wolny J. Bystander normal human fibroblasts reduce damage response in radiation targeted cancer cells through intercellular ROS level modulation. Mutat Res 731: 117–124, 2012 [DOI] [PubMed] [Google Scholar]
- 220.Wispe JR, Clark JC, Burhans MS, Kropp KE, Korfhagen TR, and Whitsett JA. Synthesis and processing of the precursor for human mangano-superoxide dismutase. Biochim Biophys Acta 994: 30–36, 1989 [DOI] [PubMed] [Google Scholar]
- 221.Xu Y, Fang F, St. Clair DK, Josson S, Sompol P, Spasojevic I, and St. Clair WH. Suppression of RelB-mediated manganse superoxide dismutase expression reveals a primary mechanism for radiosensitization effect of 1α-25-dihydroxyvitamin D3 in prostate cancer cells. Mol Cancer Therapeut 6: 2048–2056, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Xu Y, Fang F, St. Clair DK, Sompol P, Josson S, and St. Clair WH. SN52, a novel nuclear factor-κB inhibitor, blocks nuclear import of RelB:p52 dimer and sensitizes prostate cancer cells to ionizing radiation. Mol Cancer Therapeut 7: 2367–2376, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Xu Y, Kiningham KK, Devalaraja MN, Yeh C-C, Majima H, Kasarskis EJ, and St. Clair DK. An intronic NF-κB element is essential for induction of the human manganese superoxide dismutase gene by tumor necrosis factor-α and interleukin-1β. DNA Cell Biol 18: 709–722, 1999 [DOI] [PubMed] [Google Scholar]
- 224.Yakovlev VA, Rabender CS, Sankala H, Gauter-Fleckenstein B, Fleckenstein K, Batinic-Haberle I, Jackson I, Vujaskovic Z, Anscher MS, Mikkelsen RB, and Graves PR. Proteomic analysis of radiation-induced changes in rat lung: modulation by the superoxide dismutase mimetic MnTE-2-PyP5+. Int J Radiat Oncol Biol Phys 78: 547–554, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yang G, Wu L, Chen S, Zhu L, Huang P, Tong L, Zhao Y, Zhao G, Wang J, Mei T, Xu A, and Wang Y. Mitochondrial dysfunction resulting from loss of cytochrome c impairs radiation-induced bystander effect. Br J Cancer 100: 1912–1916, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yang H, Asaad N, and Held KD. Medium-mediated intercellular communication is involved in bystander responses of x-ray-irradiated normal human fibroblasts. Oncogene 24: 2096–2103, 2005 [DOI] [PubMed] [Google Scholar]
- 227.Yang L-Y, Chen W-L, Lin J-W, Lee S-F, Lee C-C, Hung TI, Wei Y-H, and Shih C-M. Differential expression of antioxidant enzymes in various hepatocellular carcinoma cell lines. J Cell Biochem 96: 622–631, 2005 [DOI] [PubMed] [Google Scholar]
- 228.Zelko IN, Mariani TJ, and Felz RJ. Superoxide dismutase multigene family: a comparison of CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structure, evolution, and expression. Free Radic Biol Med 33: 337–349, 2002 [DOI] [PubMed] [Google Scholar]
- 229.Zhou H, Ivanov VN, Gillespie J, Geard CR, Amundson SA, Brenner DJ, Yu Z, Lieberman HB, and Hei TK. Mechanism of radiation-induced bystander effect: role of the cyclooxygenase-2 signaling pathway. Proc Natl Acad Sci U S A 102: 14641–14646, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zhou H, Ivanov VN, Lien Y-C, Davidson M, and Hei TK. Mitochondrial function and nuclear factor-κB-mediated signaling in radiation-induced bystander effects. Cancer Res 68: 2233–2240, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhou H, Randers-Pehrson G, Waldren CA, Vannais D, Hall EJ, and Hei TK. Induction of a bystander mutagenic effect of alpha particles in mammalian cells. Proc Natl Acad Sci U S A 97: 2099–2104, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zhou H, Suzuki M, Geard CR, and Hei TK. Effects of irradiated medium with or without cells on bystander cell responses. Mutat Res 499: 135–141, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhou H, Suzuki M, Randers-Pehrson G, Vannais D, Chen G, Trosko JE, Waldren CA, and Hei TK. Radiation risk to low fluences of α particles may be greater than we thought. Proc Natl Acad Sci U S A 98: 14410–14415, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zwacka RM, Dudus L, Epperly MW, Greenberger JS, and Engelhardt JF. Redox gene therapy protects human IB-3 lung epithelial cells against ionizing radiation-induced apoptosis. Human Gene Ther 9: 1381–1386, 1998 [DOI] [PubMed] [Google Scholar]