Abstract
Interleukin-33 (IL-33) appears to play a crucial role in the expression of allergic diseases, but its cellular source and regulatory mechanisms remain to be fully elucidated. Mast cells, one of the major effecter cell populations in mediating allergy, express high levels of IL-33 receptor, ST2, and have been shown to express IL-33 transcripts. In this study, we aimed to examine the secretion of IL-33 in mast cells and their response to IL-33. We have successfully detected secreted IL-33 from cell supernatants through a modified enzyme-linked immunosorbent assay (ELISA) technique—cell-based ELISA. Activation of bone marrow-derived cultured mast cells (BMMCs) by crosslinkage of an antigen [ovalbumin (OVA)] and OVA-specific IgE mAbs significantly induced the expression of IL-33 transcripts, cytosolic and secreted proteins. In addition, the Toll-like receptor (TLR) 2 and TLR-9 ligands could trigger IL-33 mRNA expression. Exposure of BMMCs to IL-33 significantly increased the levels of IL-13 and IL-6 expression, concomitant with enhanced activation of mitogen-activated protein kinase (MAPKs) (ERK, p38, and JNK) and nuclear factor-kappa B. These results suggest that mouse BMMCs are capable of producing and serving as endogenous sources of IL-33, and that IL-33 plays an important role in regulating mast cell functions.
Introduction
Interleukin-33 (IL-33) is a cytokine that has recently been classified as a member of the IL-1 cytokine superfamily and the ligand for the orphan receptor—ST2 (Schmitz and others 2005; Liew and others 2010; Smith 2011). IL-33 was first named nuclear factor from high endothelial venules (NF-HEV), as it was known to interact with nuclear chromatin, although its exact intracellular functions are still to be clarified (Baekkevold and others 2003; Carriere and others 2007). As an extracellular cytokine, IL-33 binds to the ST2 receptor and is involved in promoting systemic Th2 responses (Mjosberg and others 2011; Palmer and Gabay 2011; Seidelin and others 2011). The ST2 gene encodes 3 isoforms of a protein: ST2L, soluble ST2 (sST2), and ST2V (Tago and others 2001). ST2L is preferentially found on the surface of type 2 helper T cells (Th2) and mast cells, and is coexpressed with IL-4, IL-5, and/or IL-10, the classic Th2 cytokines, but not interferon-γ (Schmitz and others 2005). Functionally, secreted IL-33 plays a crucial role in inflammatory, infectious, and autoimmune diseases (Gadina and Jefferies 2007; Kakkar and Lee 2008; Liew and others 2010; Smith 2010; Palmer and Gabay 2011). However, the mechanisms of secretion of IL-33 have not been fully elucidated (Zhao and Hu 2010).
A recent report by Hsu and others (2010) has shown that mast cells, the major effecter cell type in mediating allergen-induced Th2 type immune responses, express IL-33 transcript and regulate IgE-dependent inflammation. Although IL-33 transcripts were detected by RT-PCR in a variety of cell types, such as mast cells, dendritic cells, and macrophages (Ohno and others 2009; Hsu and others 2010; Yanagawa and others 2011), attempts to detect the secreted form of IL-33 were not successful. Further, some have concluded that since IL-33 has only been detected during the induction of necrosis, and that similar to the classical alarmin, HMGB1, IL-33 is predominantly observed in the nuclear compartment of endothelial cells, it functions primarily as an alarmin, instead of an extracellular inflammatory cytokine, as do other members in the IL-1 cytokine family (Haraldsen and others 2009; Lamkanfi and Dixit 2009). Others have made assumptions that IL-33, like IL-1 and IL-18, requires caspase 1 cleavage to release an active 18 kDa IL-33 (Dinarello 2005; Cassel and others 2009), while recently, several reports have indicated that the full-length 31 kDa form is biologically active and does not require caspase cleavage (Cayrol and Girard 2009; Lamkanfi and Dixit 2009; Ohno and others 2009; Talabot-Ayer and others 2009). Considering these recent findings regarding IL-33 secretory mechanisms, we speculate that perhaps the failure to detect IL-33 release in vitro lies in the insensitivity or limitations of the current cytokine detection method—enzyme-linked immunosorbent assay (ELISA), and propose a modified ELISA technique to allow detection of IL-33 from the cell supernatant.
Although the extracellular functions of ST2 as a marker for Th2 cells are well defined, the complexities of IL-33 regulation and secretion are only just being realized (Zhao and Hu 2010). The failure to detect the IL-33 release in vitro hinders the understanding of IL-33 secretory mechanisms, which further hampers the study of the biological roles of IL-33 in inflammatory, autoimmune, and infectious diseases. In this study, it was shown that mast cells are able to secrete and respond to IL-33, providing a basis for gaining further insight into the enigmatic secretory and regulatory mechanisms of IL-33.
Materials and Methods
Mice
C57BL/6 mice from the Jackson Laboratory were maintained under specific pathogen-free conditions, and all experiments were approved by the Animal Care and Use Committee at the Johns Hopkins University School of Medicine.
Bone marrow-derived cultured mast cells
As described (Zhou and others 2013), mouse bone marrow mast cells were cultured at a starting density of 5×105 cells/mL at 37°C in a humidified atmosphere containing 5% CO2 in the RPMI-1640 medium, supplemented with 2 mM GlutaMAX™, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 100 U/mL penicillin, 100 μg/mL streptomycin, 10% heat-inactivated fetal bovine serum (FBS; Invitrogen), 50 μM β-2-mercaptoethanol, 10 mM HEPES, and 30% WEHI-3-conditioned medium, as a source of IL-3 for 4 to 6 weeks. Mast cell phenotypes were confirmed by flow cytometry analysis with Abs specific for c-kit (2B8; eBioscience), FcɛRI (MAR-1; eBioscience), and CD49b (DX5; eBioscience) and by histochemical staining with acid toluidine blue.
Real-time RT-PCR analysis
RNA was extracted with RNeasy mini kits (Qiagen) and was analyzed by real-time RT-PCR with GeneAmp 7300 Sequence Detection System (Applied Biosystems). Real-time RT-PCR was performed with the SYBR Green (Applied Biosciences) method as previously described (Zhou and others 2010), and the gene expression levels were calculated by the change in the cycling threshold (ΔCT) method as 2−ΔCT, where ΔCT is [CT (IL-33)–CT (actin)], then further normalized to the medium control group. IL-33, primer sequence: sense: 5′-CTACTGCATGAGACTCCGTTCTG-3′; antisense: 5′-AGAATCCCGTGGATAGGCAGAG-3′. β-actin, forward: 5′- CATTGCTGACAGGATGCAGAAGG-3′; reverse: 5′- TGCTGGAAGGTGGACAGTGAGG-3′.
Measurement of intracellular IL-33 by flow cytometry
Bone marrow-derived cultured mast cells (BMMCs) were sensitized with 1 μg/mL of anti-ovalbumin (OVA) IgE antibody (E-C1, Chondrex INC) overnight, and then challenged with 10 μg/mL OVA (Sigma) for 8 h, with or without the addition of 2 μM monensin for the last 5 h after OVA challenge. Cells were then fixed with the BD Cytofix/Cytoperm™ buffer and stained with PE-labeled anti-mouse IL-33 (R&D Systems) for 1 h before flow cytometry analysis using a FACSCalibur flow cytometer (BD Biosciences).
Western blotting
For detection of intracellular IL-33 protein levels, unprimed or IgE primed mast cells were stimulated for 8 or 24 h with 10 μg/mL OVA in mast cell growth media. Mast cells were then lysed in the RIPA buffer (Sigma) supplemented with protease inhibitor cocktails (Roche), followed by sonication. Cell lysates were separated by NuPAGE Bis-Tris gels (Invitrogen Life Technologies) and transferred to nitrocellulose membranes. Membranes were probed with polyclonal rabbit anti-mouse IL-33 (Abcam) and were stripped and reprobed for β-actin for normalization during analysis. For IL-33-induced mast cell IL-6 and IL-13 production signaling studies, mast cells were starved of the WEHI-3-conditioned medium overnight followed by treatment with 10 ng/mL of mouse recombinant IL-33 (R&D Systems) for 0, 2, 5, 15, and 30 min before lysis with the RIPA buffer supplemented with protease inhibitor cocktails and 1 mM Na3VO4 (Sigma-Aldrich). Proteins reactive with primary Abs were visualized with an HRP-conjugated secondary Ab and ECL reagents (Amersham). Anti-ERK, anti-p38, anti-JNK, anti-NFκB-p65, anti-phospho-p44/42 mitogen-activated protein kinase (MAPK) (Thr202/Tyr204), anti-phospho-p38 MAPK (Thr180/Tyr182), anti-phospho-JNK (Thr183/Tyr185), and anti-phospho-NFκB-p65 (Ser536) were purchased from Cell Signaling Technology.
IL-33 secretion measurement by cell-based ELISA
Cell-based ELISA was adapted from previous publications, where it was used to detect low levels of specific antigen-induced cytokines (Beech and others 1997; Fillatreau and others 2002). BMMCs were sensitized with anti-OVA IgE (E-C1) overnight, then washed, and resuspended in the RPMI-1640 medium. The mouse IL-33 precoated plate (Biolegend) was washed 4 times with a sterile washing buffer (PBS plus 0.05% Tween 20), then twice with sterile PBS. BMMCs were seeded to the precoated plate at different densities, along with standards diluted in a medium. Cells were then challenged with 10 μg/mL OVA and incubated at 37°C in a humidified atmosphere containing 5% CO2 for 24 h. Unstimulated cells in the RPMI medium were used as control for comparison. All of the above procedures were performed under sterile conditions. After this period, plates were washed 5 times in PBS/Tween and incubated with 100 μl per well of biotinylated anti-cytokine detecting antibody for 1 hr at room temperature. The rest of the procedures were performed following standard ELISA methods according to the manufacturer's instructions (Biolegend).
IL-6 and IL-13 measurements
Cytokines in BMMC supernatants were analyzed with ELISA. Coating and detection Abs specific for IL-6 and IL-13 were from eBioscience. For IL-33-induced mast cell activation study, specific inhibitors for JNK (SP600125; Calbiochem), ERK (PD98059; Cayman), p38 (SB203580; Cayman), and nuclear factor-kappa B (NFκB) (Bay11-7082; Cayman) were added to BMMCs 30 min before 24 h of 10 ng/mL rmIL-33 treatment. Cytokines in supernatants were then analyzed with ELISA.
Statistical analysis
Data are expressed as the mean±s.e.m. GraphPad Prism 5.0 was used for statistical analysis. P values of less than 0.05 were considered statistically significant.
Results
BMMCs produce and secrete IL-33 through IgE-mediated activation
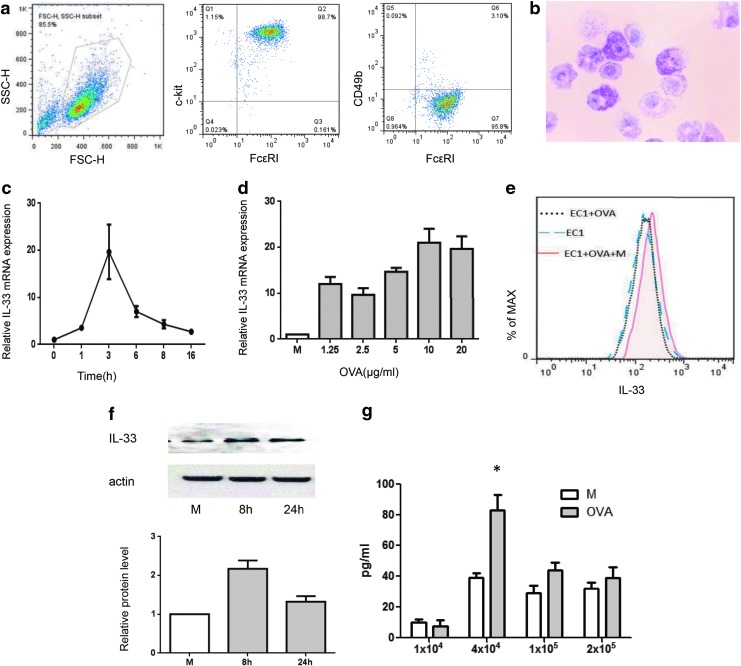
We initially wanted to confirm that mast cells do express and produce IL-33 through IgE-mediated activation as claimed by Hsu and others (2010). To explore this, mouse BMMCs (c-kit+FcɛRI+CD49b-) were used. The phenotype and purity were determined by flow cytometry (Fig. 1a) and toluidine blue staining (Fig. 1b). The purity of BMMCs was more than 95%. BMMCs were sensitized for 16 hr with 1 μg/mL of OVA-specific IgE mAbs (E-C1), followed by crosslinkage of surface-bound IgE with the antigen, OVA. The results showed that IL-33 mRNA was upregulated within an hour of stimulation and peaked at 3 h after antigen-driven activation with OVA-IgE (Fig. 1c, d). Further, we determined whether intracellular IL-33 could be detected within mast cells by flow cytometry for comparison of the following groups: with or without antigen challenge and with or without antigen plus monensin, a protein transport inhibitor commonly used to enhance intracellular cytokine staining signals by blocking transport processes during cell activation (Mollenhauer and others 1990). We found that even though there was no difference in intracellular IL-33 levels between the unstimulated and antigen stimulated mast cells without monensin, there was an enhanced level observed in BMMCs treated with antigen plus monensin (Fig. 1e), suggesting that intracellular IL-33 may primarily be secreted extracellularly.
FIG. 1.
Bone marrow-derived cultured mast cells (BMMCs) produce and secrete interleukin (IL)-33 through IgE-mediated activation. (a) Four-week BMMCs were stained with FcɛRI, c-kit, and CD49b, and then detected by flow cytometry. Ninety-five percent of BMMCs were FcɛRI+ c-kit+ CD49b−. (b) Toluidine blue staining of 4-week mast cells. (c) IL-33 mRNA kinetic expression. Ovalbumin (OVA)-specific IgE Ab (E-C1) sensitized mast cells stimulated with 10 μg/mL OVA for different time periods. (d) IL-33 mRNA dose response. OVA-specific IgE Ab (E-C1) sensitized mast cells stimulated with different dose of OVA for 3 h. (e) Intracellular IL-33 expression was measured by flow cytometry for the following comparison: with or without antigen challenge and with or without antigen plus monensin. (f) Western blotting analysis of IL-33 protein expression at 8 h and 24 h following OVA challenge. Below, band intensities normalized to actin and relative to the medium (M) control group were calculated with Quantity One software (Bio-Rad). (g) IL-33 secretions from E-C1 sensitized mast cells with or without OVA stimulation detected by cell-based enzyme-linked immunosorbent assay (ELISA). Data are representative of 4 independent experiments. *P<0.05.
We then tried to detect the protein expression through western blotting. Low levels of cleaved IL-33 protein (approximately 18 kDa) were detected in unstimulated cells with significant upregulated expression detected in antigen/IgE-activated BMMCs at the 8-h time point, which was then downregulated after 24 h of stimulation (Fig. 1f).
Upon confirmation of IL-33 expression at mRNA, intracellular and protein levels in mast cells, measurement of IL-33 secretion from the supernatant was attempted first using the standard sandwich ELISA method, but we failed to detect any IL-33 (data not shown). However, through a modified cell-based ELISA technique, we were subsequently successful in detecting IL-33 from the supernatant. Significantly higher levels of IL-33 were measured from the antigen-stimulated mast cells than from the unstimulated control over 4 independent experiments (Fig. 1g). Interestingly, the levels of IL-33 detection from the supernatants were dependent on the cell seeding density, with the optimal detection at seeding 4×104 BMMCs/well (Fig. 1g). Because the levels of IL-33 detected were all below 100 pg/mL, we speculated that the IL-33 might have been bound to surface ST2 (IL-33 receptor) and performed ST2 blocking experiments. A 20 μg/mL ST2 antibody or equal IgG control was added 30 min before OVA stimulation; however, the levels of IL-33 in the cell supernatant were not enhanced after blocking (data not shown).
Non-IgE-mediated mast cell IL-33 expression
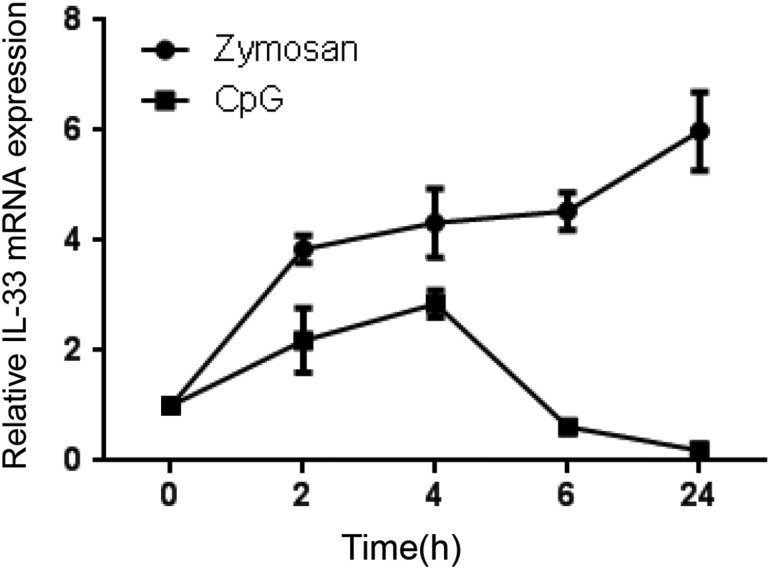
Since mast cells are not only the major effecter cell types in mediating allergen-induced immune responses, but are also important innate immune cells, as suggested by recent studies showing that mast cells expressed functional Toll-like receptors (TLRs) (McCurdy and others 2001; Supajatura and others 2002; Varadaradjalou and others 2003; Matsushima and others 2004), we wanted to investigate whether non-IgE-mediated pathways, such as the TLR pathway, could enhance IL-33 expression and production from BMMCs. We chose to explore TLR-2- and TLR-9-induced IL-33 production by using zymosan and CpG-oligonucleotide as ligands, respectively. Results showed that both zymosan and CpG-oligonucleotide triggered IL-33 mRNA expression. Zymosan induced IL-33 expression with peak expression after 4 h of treatment, which remained elevated up to 24 h of stimulation, whereas CpG induced only transient expression of IL-33, peaking at 2 h after stimulation (Fig. 2).
FIG. 2.
Non-IgE mediated mast cell IL-33 expression. Toll-like receptor-2 (TLR-2) (100 μg/mL zymosan; Sigma), TLR-9 [1 μM CpG-Oligonucleotide (ODN1668); InvivoGen] mediated IL-33 kinetic mRNA expression using real-time RT-PCR over a time course of 24 h. Data are representative of 3 independent experiments.
IL-3-induced upregulated mast cell cytokine production
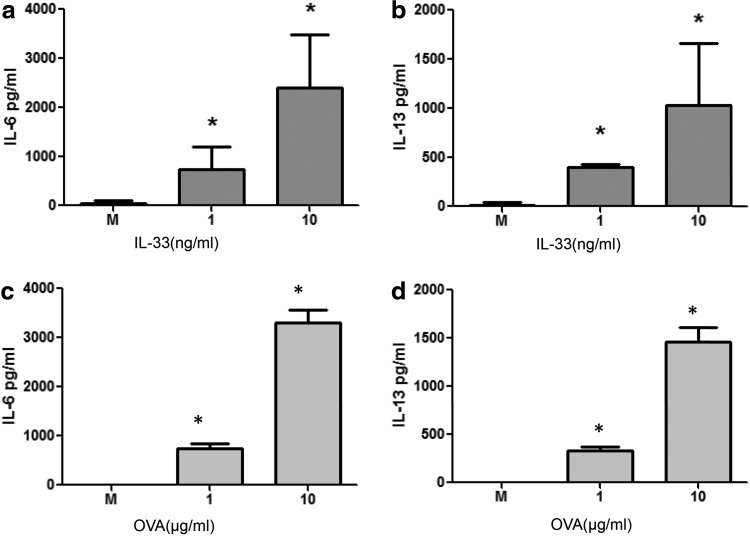
After we had successfully detected IL-33 secretion from cell supernatants to confirm that IL-33 is indeed produced by mast cells, we further explored the functional effects of IL-33 on mast cells, independent of FcɛRI signaling, by examining mast cell IL-6 and IL-13 production after different doses of rmIL-33 stimulation. BMMCs were starved of the WEHI-3-conditioned medium overnight to ensure no interference by the effect of IL-3 to the cells. The results showed that both IL-6 production (Fig. 3a) and IL-13 production (Fig. 3b) from BMMCs were significantly higher (P<0.05) in rmIL-33-treated mast cells than in the vehicle control group in a dose-dependent manner, which is consistent with a previous study (Ho and others 2007). For comparison, crosslinkage of IgE (E-C1) with OVA induced both IL-6 and IL-13. It was noted that IL-33 is also a potent IL-6 and IL-13 inducer, as compared to crosslinkage of IgE with OVA (Fig. 3c, d).
FIG. 3.
IL-33-induced upregulated mast cell cytokine production. Cytokines (a) IL-6 and (b) IL-13 released from untreated mast cells and cells treated with different doses (1 and 10 ng/mL) of recombinant mouse IL-33 for 24 h were measured by ELISA. *P<0.05. Data represents 2 individual experiments. Cytokines (c) IL-6 and (d) IL-13 released after crosslinkage of IgE (E-C1) with Ag (10 μg/mL OVA) for 24 h.
IL-33-induced mast cell signaling events
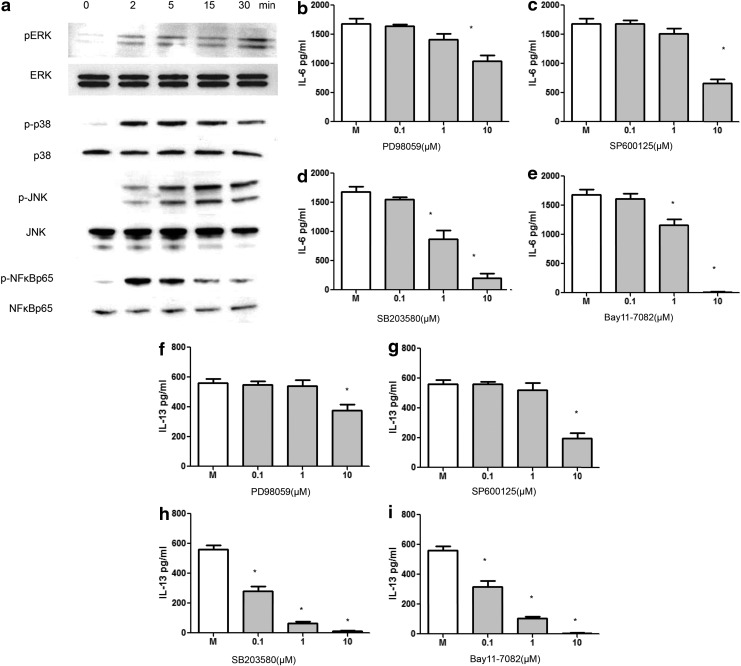
Next, we investigated the signaling events activated by the administration of IL-33 to BMMCs. MAPK and NFκB regulatory pathways were examined. Through western blotting, we found that within 2 min of stimulation, BMMCs showed enhanced levels of phosphorylated ERK, p38, JNK, and NFκB-p65 at Ser536, as compared to their respective total levels (Fig. 4a). To further confirm these signaling events activated by rmIL-33, we treated BMMCs with specific inhibitors for JNK (SP600125), ERK (PD98059), p38 (SB203580), and NFκB (Bay11-7082) 30 min before 24 h of rmIL-33 treatment and collected the supernatants to test for the downstream signaling-induced functional changes by measuring IL-6 and IL-13 in the supernatants. Significant inhibition or complete suppression of both IL-6 (Fig. 4b–e) and IL-13 (Fig. 4f–i) production was observed for cells treated with the inhibitors, as compared to the untreated cells, indicating that IL-33-induced mast cell responses were, in fact, facilitated through the MAPK and NFκB regulatory pathways.
FIG. 4.
IL-33-induced mast cell signaling events. (a) Western blotting analysis of mast cells ERK, p38, JNK, and nuclear factor-kappa B (NFκB)-p65 activation after 10 ng/mL rmIL-33 treatment over a time course of 30 min. To further test the downstream signaling-induced functional effects, specific inhibitors for JNK (SP600125), ERK (PD98059), p38 (SB203580), and NFκB (Bay11–7082) at different dosages were added to BMMCs 30 min before rmIL-33 treatment. Supernatants were collected to test for the inhibitors' effects on IL-33-induced mast cell (b–e) IL-6 and (f–i) IL-13 production.
Discussion
The results of our study demonstrated that mast cells not only express IL-33 at mRNA and protein levels, but also secrete detectable IL-33, indicating that mast cells are, indeed, an endogenous source of IL-33. In addition, IgE antigen crosslinkage stimulation upregulated IL-33 production. Further, we have shown that TLRs, in particular, the TLR-2 pathway with ligand, zymosan, can significantly enhance IL-33 mRNA expression. Surprisingly, while CpG induced transient expression of IL-33, zymosan-induced mast cell IL-33 expression remained elevated after 24 h of zymosan treatment, suggesting the existence of a complex regulatory mechanism operative in the induction of IL-33 in mast cells. This should not be a complete surprise, as it has been reported that zymosan induced both TLR-2 and dectin 1/2 signaling pathways and that mast cells express low levels of dectin-1 receptor on the cell surface (Yang and Marshall 2009). Conceivably, zymosan activated both TLR-2 and dectin-1 to produce the synergetic and prolonged IL-33 expression.
Our results have further provided evidence for the upregulation of mast cell IL-6 and IL-13 production after rmIL-33 treatment by activating the MAPK, including ERK, p38, JNK, and NFκB signaling pathways, which we have confirmed with inhibitor studies. IL-33 binds to the heterodimer receptor complex consisting of ST2 and IL-1 receptor accessory protein (IL-1RAP). The binding leads to the recruitment of myeloid differentiation primary response protein 88 (MYD88) and tumor necrosis factor receptor associated factor 6 (TRAF6) (Palmer and Gabay 2011). This leads to NFκB activation and activation of the MAPK, including p38, ERK, and JNK. However, the signaling pathway is quite complex, depending on the type of ST2-expressing cells, the concentration of IL-33, and other factors. In our inhibitory experiment, it was shown that p38 (SB203580) and NFκB (Bay11-7082) inhibitors have much more potent inhibitory effect on the production of IL-6 and IL-13 in mast cells stimulated with IL-33 than JNK (SP600125) and ERK (PD98059) inhibitors. Thus, the IL-33 effect on mast cells may occur primarily through the P38 and NFκB pathways.
One caveat to our detection of IL-33 from cell supernatants through cell-based ELISA was that the levels of IL-33 detected were in small amount, ranging from 40 to 90 pg/mL from 4 independent experiments, as compared to the amounts of IL-6 and IL-13 detected. Several factors could contribute to the low detection levels of IL-33. As indicated previously, the amount of IL-33 detected was dependent on seeding mast cell density to the 96-well ELISA plate, with the optimal seeding density at 4×104 per well. This number was considerably lower than the usual amount of cells that we seed to the plate for standard ELISA, and could result in less total IL-33 production. It was reported that cell density regulates neutrophil IL-8 synthesis in response to LPS stimulation (Hattar and others 2001). Another factor could be that the medium in which BMMCs were cultured was supplemented with FBS, which contains proteases that could degrade IL-33 and result in less IL-33 remaining to be detected.
The reason for the success in the detection of IL-33 secretion through cell-based ELISA, but not the standard ELISA method, is at present unclear. It could be that once IL-33 is secreted, it quickly binds to surface ST2L, hindering its detection by ELISA. However, in one of our experiments in which we blocked ST2L before IgE-OVA stimulation, the resulting detection of secreted IL-33 was lower than that without the addition of the anti-ST2 blocking antibody (data not shown). It is also possible that IL-33 binds to sST2 (Hayakawa and others 2007; Kakkar and Lee 2008), leading to no or extremely low levels of detectable IL-33 remaining in the cell supernatants. Another possibility could be that cell-to-cell or cell-to-extracellular matrix contact is required for secretion of IL-33 as mast cell–fibroblast interactions have been reported to be required for BMMCs to secrete CCL11 (eotaxin-1), mediated through transmembrane expression of stem cell factor by the fibroblast (Hogaboam and others 1998). These possibilities all could contribute to the failure to detect secreted IL-33 by ELISA compared to the successful detection through cell-based ELISA. Determining the reason(s) could unlock the key to understanding the secretory mechanism of IL-33 not only for mast cells, but perhaps, other cell types as well.
It is also noted from our study that the 18-kDa cleaved IL-33 was detected from western blotting results (data not shown), in contradiction to the full-length form detected by Hsu and others (2010). This might be due to the specificity and binding preference of the antibody used. Nevertheless, this suggests that both forms may be biologically active and may coexist.
Collectively, our results suggest that mouse BMMCs are endogenous sources of IL-33 and are capable of producing IL-33, and that IL-33 plays an important role in regulating IgE and non-IgE-mediated mast cell functions.
Acknowledgments
This work was supported, in part, by NIH grants (AI052468 and AI073610), and grants from the National Health Research Institute, Taiwan (EOPP10-014 and EOSP07-014) and the Ministry of Health, Taiwan (EODOH01).
Authors' Contributions
H.T. and B.P. performed the experiments. H.T. wrote the article. S.-K.H. and Y.Z. planned, designed, supervised, and coordinated the overall research efforts and wrote the article.
Author Disclosure Statement
The authors declare that they have no conflict of financial interests.
References
- Baekkevold ES, Roussigne M, Yamanaka T, Johansen FE, Jahnsen FL, Amalric F, Brandtzaeg P, Erard M, Haraldsen G, Girard JP. 2003. Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am J Pathol 163(1):69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech JT, Bainbridge T, Thompson SJ. 1997. Incorporation of cells into an ELISA system enhances antigen-driven lymphokine detection. J Immunol Methods 205(2):163–168 [DOI] [PubMed] [Google Scholar]
- Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, Bouche G, Girard JP. 2007. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A 104(1):282–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel SL, Joly S, Sutterwala FS. 2009. The NLRP3 inflammasome: a sensor of immune danger signals. Semin Immunol 21(4):194–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayrol C, Girard JP. 2009. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci U S A 106(22):9021–9026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. 2005. An IL-1 family member requires caspase-1 processing and signals through the ST2 receptor. Immunity 23(5):461–462 [DOI] [PubMed] [Google Scholar]
- Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. 2002. B cells regulate autoimmunity by provision of IL-10. Nat Immunol 3(10):944–950 [DOI] [PubMed] [Google Scholar]
- Gadina M, Jefferies CA. 2007. IL-33: a sheep in wolf's clothing? Sci STKE 2007(390):pe31. [DOI] [PubMed] [Google Scholar]
- Haraldsen G, Balogh J, Pollheimer J, Sponheim J, Kuchler AM. 2009. Interleukin-33- cytokine of dual function or novel alarmin? Trends Immunol 30(5):227–233 [DOI] [PubMed] [Google Scholar]
- Hattar K, Fink L, Fietzner K, Himmel B, Grimminger F, Seeger W, Sibelius U. 2001. Cell density regulates neutrophil IL-8 synthesis: role of IL-1 receptor antagonist and soluble TNF receptors. J Immunol 166(10):6287–6293 [DOI] [PubMed] [Google Scholar]
- Hayakawa H, Hayakawa M, Kume A, Tominaga S. 2007. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J Biol Chem 282(36):26369–26380 [DOI] [PubMed] [Google Scholar]
- Ho LH, Ohno T, Oboki K, Kajiwara N, Suto H, Iikura M, Okayama Y, Akira S, Saito H, Galli SJ, Nakae S. 2007. IL-33 induces IL-13 production by mouse mast cells independently of IgE-Fc epsilon RI signals. J Leuk Biol 82(6):1481–1490 [DOI] [PubMed] [Google Scholar]
- Hogaboam C, Kunkel SL, Strieter RM, Taub DD, Lincoln P, Standiford TJ, Lukacs NW. 1998. Novel role of transmembrane SCF for mast cell activation and eotaxin production in mast cell-fibroblast interactions. J Immunol 160(12):6166–6171 [PubMed] [Google Scholar]
- Hsu CL, Neilsen CV, Bryce PJ. 2010. IL-33 is produced by mast cells and regulates IgE-dependent inflammation. PLoS One 5(8):e11944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkar R, Lee RT. 2008. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov 7(10):827–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. 2009. IL-33 raises alarm. Immunity 31(1):5–7 [DOI] [PubMed] [Google Scholar]
- Liew FY, Pitman NI, McInnes IB. 2010. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol 10(2):103–110 [DOI] [PubMed] [Google Scholar]
- Matsushima H, Yamada N, Matsue H, Shimada S. 2004. TLR3-, TLR7-, and TLR9-mediated production of proinflammatory cytokines and chemokines from murine connective tissue type skin-derived mast cells but not from bone marrow-derived mast cells. J Immunol 173(1):531–541 [DOI] [PubMed] [Google Scholar]
- McCurdy JD, Lin TJ, Marshall JS. 2001. Toll-like receptor 4-mediated activation of murine mast cells. J Leukoc Biol 70(6):977–984 [PubMed] [Google Scholar]
- Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. 2011. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol 12(11):1055–1062 [DOI] [PubMed] [Google Scholar]
- Mollenhauer HH, Morre DJ, Rowe LD. 1990. Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity. Biochim Biophys Acta 1031(2):225–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno T, Oboki K, Kajiwara N, Morii E, Aozasa K, Flavell RA, Okumura K, Saito H, Nakae S. 2009. Caspase-1, caspase-8, and calpain are dispensable for IL-33 release by macrophages. J Immunol 183(12):7890–7897 [DOI] [PubMed] [Google Scholar]
- Palmer G, Gabay C. 2011. Interleukin-33 biology with potential insights into human diseases. Nat Rev Rheumatol 7(6):321–329 [DOI] [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. 2005. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23(5):479–490 [DOI] [PubMed] [Google Scholar]
- Seidelin JB, Rogler G, Nielsen OH. 2011. A role for interleukin-33 in T(H)2-polarized intestinal inflammation? Mucosal Immunol 4(5):496–502 [DOI] [PubMed] [Google Scholar]
- Smith DE. 2010. IL-33: a tissue derived cytokine pathway involved in allergic inflammation and asthma. Clin Exp Allergy 40(2):200–208 [DOI] [PubMed] [Google Scholar]
- Smith DE. 2011. The biological paths of IL-1 family members IL-18 and IL-33. J Leukoc Biol 89(3):383–392 [DOI] [PubMed] [Google Scholar]
- Supajatura V, Ushio H, Nakao A, Akira S, Okumura K, Ra C, Ogawa H. 2002. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J Clin Invest 109(10):1351–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tago K, Noda T, Hayakawa M, Iwahana H, Yanagisawa K, Yashiro T, Tominaga S. 2001. Tissue distribution and subcellular localization of a variant form of the human ST2 gene product, ST2V. Biochem Biophys Res Commun 285(5):1377–1383 [DOI] [PubMed] [Google Scholar]
- Talabot-Ayer D, Lamacchia C, Gabay C, Palmer G. 2009. Interleukin-33 is biologically active independently of caspase-1 cleavage. J Biol Chem 284(29):19420–19426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadaradjalou S, Feger F, Thieblemont N, Hamouda NB, Pleau JM, Dy M, Arock M. 2003. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human mast cells. Eur J Immunol 33(4):899–906 [DOI] [PubMed] [Google Scholar]
- Yanagawa Y, Suzuki M, Matsumoto M, Togashi H. 2011. Prostaglandin E(2) enhances IL-33 production by dendritic cells. Immunol Lett 141(1):55–60 [DOI] [PubMed] [Google Scholar]
- Yang Z, Marshall JS. 2009. Zymosan treatment of mouse mast cells enhances dectin-1 expression and induces dectin-1-dependent reactive oxygen species (ROS) generation. Immunobiology 214(4):321–330 [DOI] [PubMed] [Google Scholar]
- Zhao W, Hu Z. 2010. The enigmatic processing and secretion of interleukin-33. Cell Mol Immunol 7(4):260–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Kawasaki H, Hsu SC, Lee RT, Yao X, Plunkett B, Fu J, Yang K, Lee YC, Huang SK. 2010. Oral tolerance to food-induced systemic anaphylaxis mediated by the C-type lectin SIGNR1. Nat Med 16(10):1128–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Tung HY, Tsai YM, Hsu SC, Chang HW, Kawasaki H, Tseng HC, Plunkett B, Gao P, Hung CH, Vonakis BM, Huang SK. 2013. Aryl hydrocarbon receptor controls murine mast cell homeostasis. Blood 121(16):3195–3204 [DOI] [PMC free article] [PubMed] [Google Scholar]