Abstract
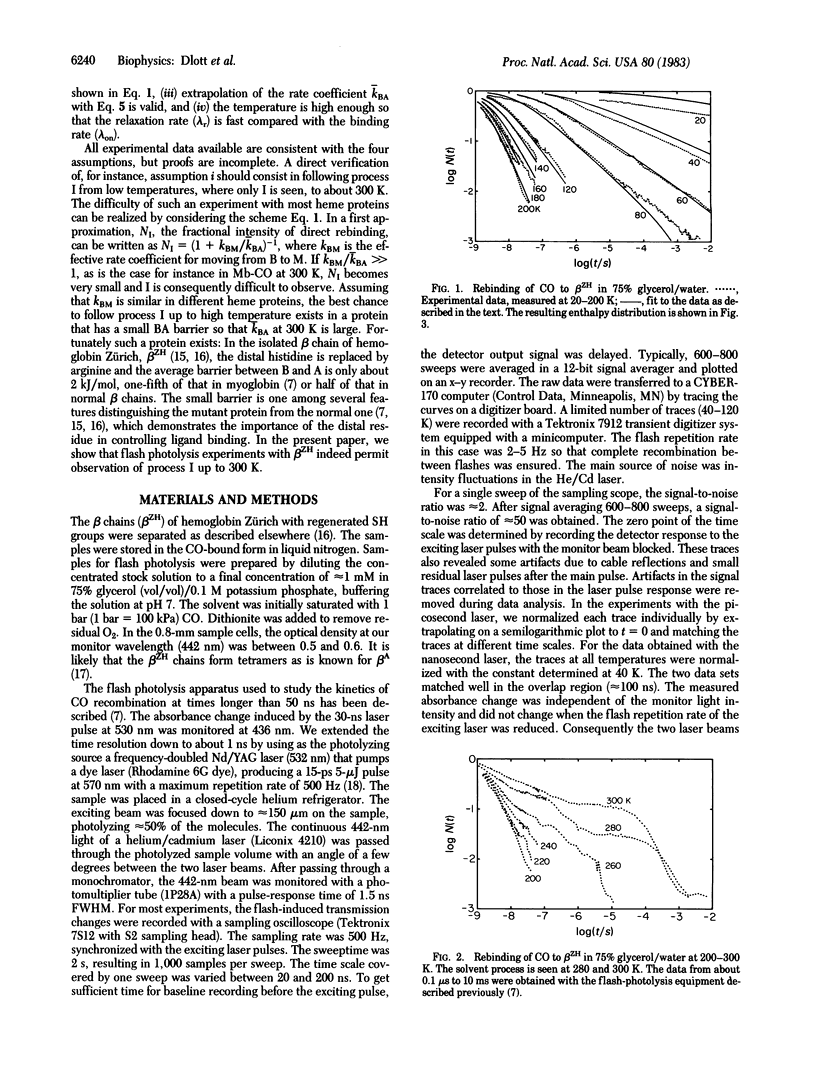
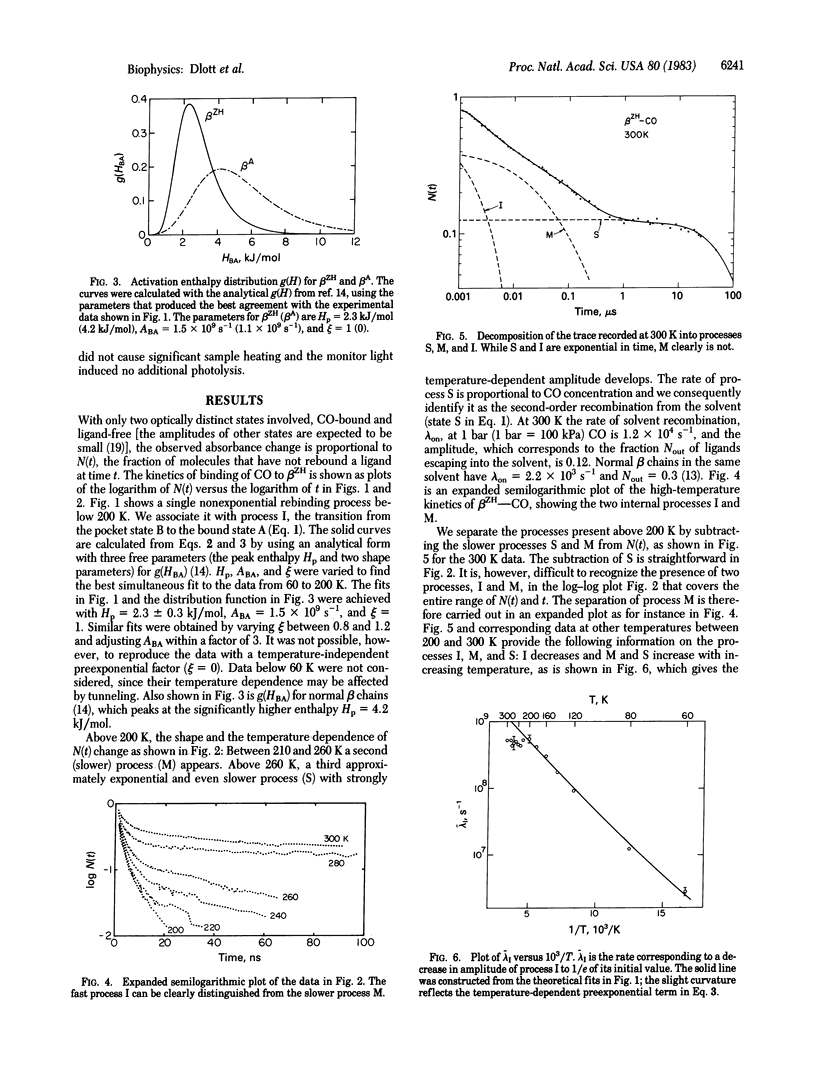
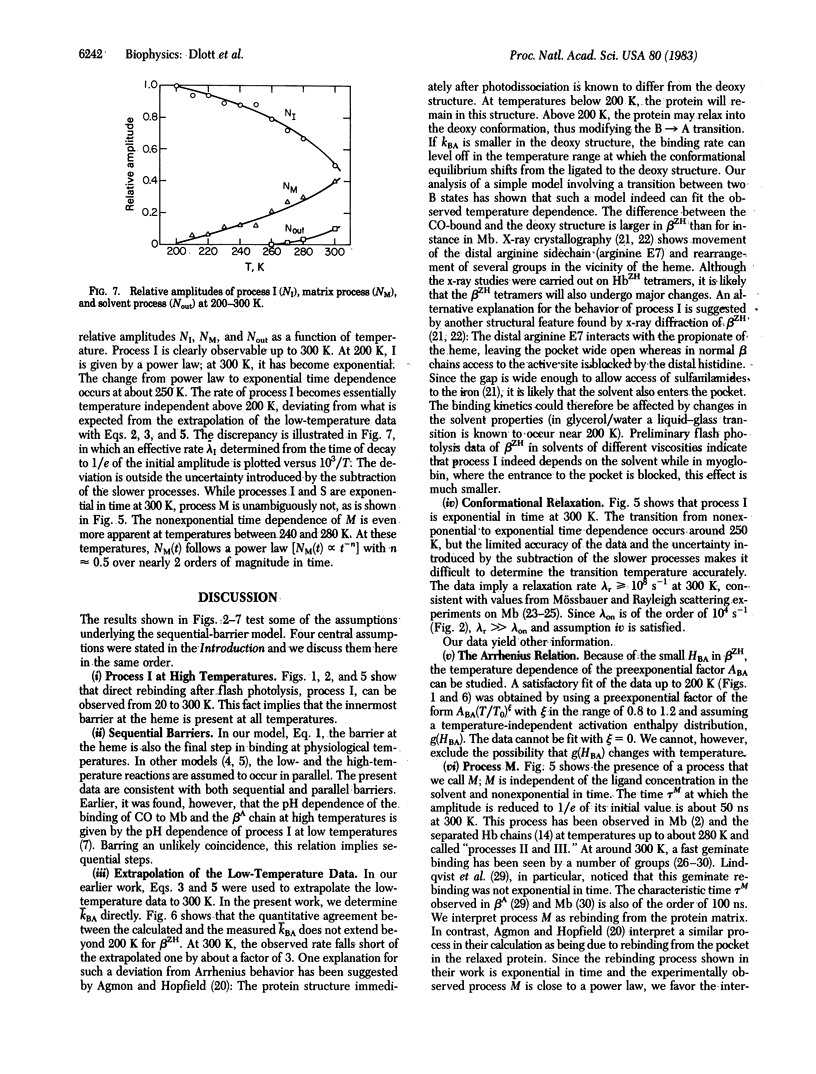
Binding of carbon monoxide to beta chains of hemoglobin Zürich has been studied by flash photolysis over the time range of nanoseconds to seconds at temperatures from 20 to 300 K. From 20 to 200 K a single rebinding process (process I) is seen, characterized by a distribution of barrier heights with a peak enthalpy of 2.3 kJ/mol. Above 200 K some ligands escape from the pocket into the matrix, and above 260 K recombination from the solvent sets in. Process I is visible up to 300 K, but above 200 K its rate remains essentially constant at about 4 X 10(8)s -1. Above about 250 K, process I is exponential in time, indicating rapid conformational relaxation. The results are discussed within the framework of a sequential model for ligand binding.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberding N., Austin R. H., Beeson K. W., Chan S. S., Eisenstein L., Frauenfelder H., Nordlund T. M. Tunneling in ligand binding to heme proteins. Science. 1976 Jun 4;192(4243):1002–1004. doi: 10.1126/science.1273579. [DOI] [PubMed] [Google Scholar]
- Alberding N., Chan S. S., Eisenstein L., Frauenfelder H., Good D., Gunsalus I. C., Nordlund T. M., Perutz M. F., Reynolds A. H., Sorensen L. B. Binding of carbon monoxide to isolated hemoglobin chains. Biochemistry. 1978 Jan 10;17(1):43–51. doi: 10.1021/bi00594a007. [DOI] [PubMed] [Google Scholar]
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Beece D., Eisenstein L., Frauenfelder H., Good D., Marden M. C., Reinisch L., Reynolds A. H., Sorensen L. B., Yue K. T. Solvent viscosity and protein dynamics. Biochemistry. 1980 Nov 11;19(23):5147–5157. doi: 10.1021/bi00564a001. [DOI] [PubMed] [Google Scholar]
- Doster W., Beece D., Bowne S. F., DiIorio E. E., Eisenstein L., Frauenfelder H., Reinisch L., Shyamsunder E., Winterhalter K. H., Yue K. T. Control and pH dependence of ligand binding to heme proteins. Biochemistry. 1982 Sep 28;21(20):4831–4839. doi: 10.1021/bi00263a001. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Petsko G. A., Tsernoglou D. Temperature-dependent X-ray diffraction as a probe of protein structural dynamics. Nature. 1979 Aug 16;280(5723):558–563. doi: 10.1038/280558a0. [DOI] [PubMed] [Google Scholar]
- Giacometti G. M., Brunori M., Antonini E., Di Iorio E. E., Winterhalter K. H. The reaction of hemoglobin Zürich with oxygen and carbon monoxide. J Biol Chem. 1980 Jul 10;255(13):6160–6165. [PubMed] [Google Scholar]
- Hasinoff B. B., Chishti S. B. Viscosity dependence of the kinetics of the diffusion-controlled reaction of carbon monoxide with the separated alpha and beta chains of hemoglobin. Biochemistry. 1983 Jan 4;22(1):58–61. doi: 10.1021/bi00270a008. [DOI] [PubMed] [Google Scholar]
- Henry E. R., Sommer J. H., Hofrichter J., Eaton W. A. Geminate recombination of carbon monoxide to myoglobin. J Mol Biol. 1983 May 25;166(3):443–451. doi: 10.1016/s0022-2836(83)80094-1. [DOI] [PubMed] [Google Scholar]
- Hofrichter J., Sommer J. H., Henry E. R., Eaton W. A. Nanosecond absorption spectroscopy of hemoglobin: elementary processes in kinetic cooperativity. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2235–2239. doi: 10.1073/pnas.80.8.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupyanskii YuF, Parak F., Goldanskii V. I., Mössbauer R. L., Gaubman E. E., Engelmann H., Suzdalev I. P. Investigation of large intramolecular movement within metmyoglobin by Rayleigh scattering of Mössbauer radiation (RSMR). Z Naturforsch C. 1982 Jan-Feb;37(1-2):57–62. doi: 10.1515/znc-1982-1-211. [DOI] [PubMed] [Google Scholar]
- Martin J. L., Migus A., Poyart C., Lecarpentier Y., Astier R., Antonetti A. Femtosecond photolysis of CO-ligated protoheme and hemoproteins: appearance of deoxy species with a 350-fsec time constant. Proc Natl Acad Sci U S A. 1983 Jan;80(1):173–177. doi: 10.1073/pnas.80.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondrias M. R., Rousseau D. L., Simon S. R. Resonance Raman spectra of photodissociated carbonmonoxy hemoglobin and deoxy hemoglobin at 10 K. J Biol Chem. 1983 May 10;258(9):5638–5642. [PubMed] [Google Scholar]
- Parak F., Frolov E. N., Mössbauer R. L., Goldanskii V. I. Dynamics of metmyoglobin crystals investigated by nuclear gamma resonance absorption. J Mol Biol. 1981 Feb 5;145(4):825–833. doi: 10.1016/0022-2836(81)90317-x. [DOI] [PubMed] [Google Scholar]
- Phillips S. E., Hall D., Perutz M. F. Structure of deoxyhaemoglobin Zürich (HisE7(63 beta) - greater than Arg). J Mol Biol. 1981 Jul 25;150(1):137–141. doi: 10.1016/0022-2836(81)90329-6. [DOI] [PubMed] [Google Scholar]
- Tucker P. W., Phillips S. E., Perutz M. F., Houtchens R., Caughey W. S. Structure of hemoglobins Zürich [His E7(63)beta replaced by Arg] and Sydney [Val E11(67)beta replaced by Ala] and role of the distal residues in ligand binding. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1076–1080. doi: 10.1073/pnas.75.3.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes R., Jr, Ackers G. K. Thermodynamic studies on subunit assembly in human hemoglobin. Self-association of oxygenated chains (alphaSH and betaSH): determination of stoichiometries and equilibrium constants as a function of temperature. J Biol Chem. 1977 Jan 10;252(1):74–81. [PubMed] [Google Scholar]
- Winterhalter K. H., Anderson N. M., Amiconi G., Antonini E., Brunori M. Functional properties of hemoglobin Zürich. Eur J Biochem. 1969 Dec;11(3):435–440. doi: 10.1111/j.1432-1033.1969.tb00793.x. [DOI] [PubMed] [Google Scholar]


