Abstract
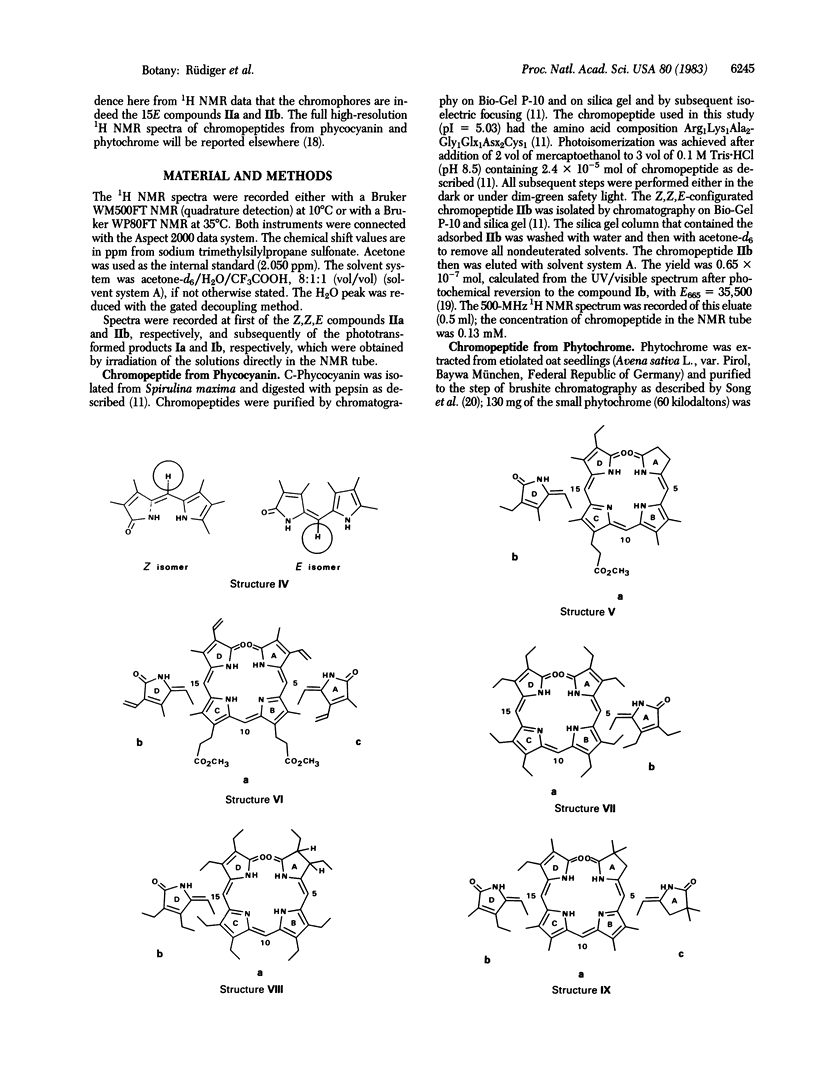
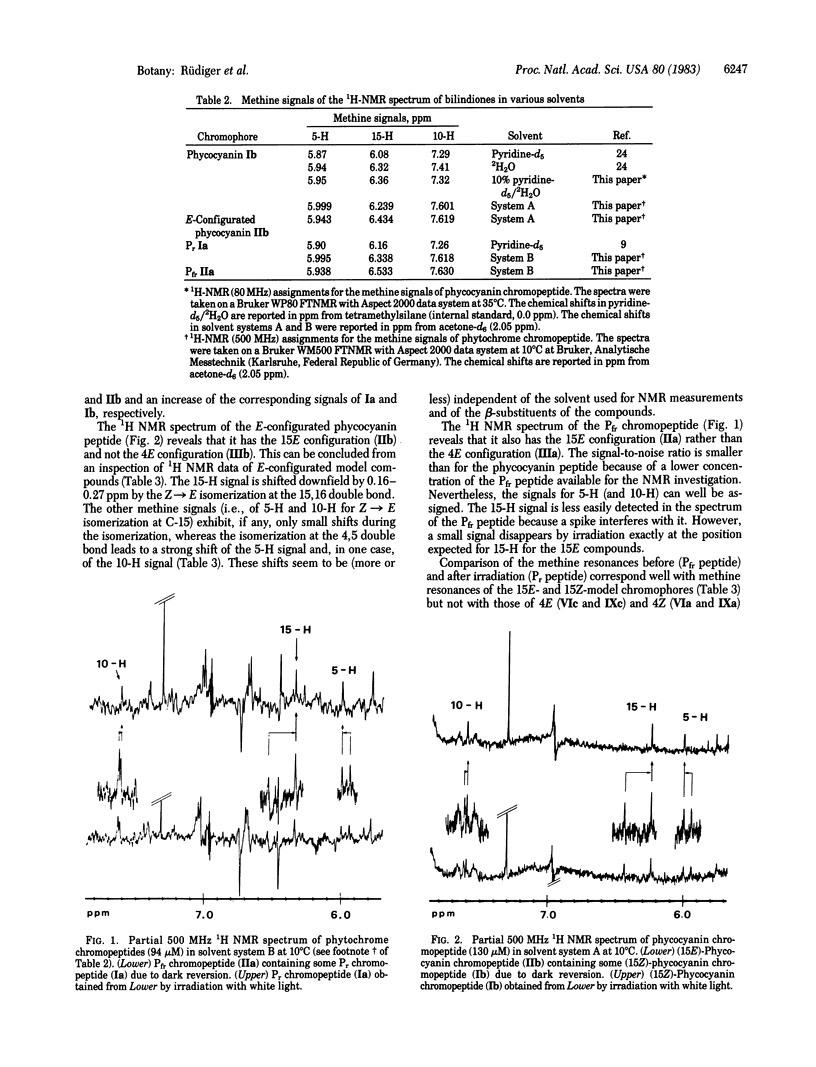
Chromopeptides were prepared by proteolytic digestion of phytochrome (far-red absorbing form, Pfr) and of phycocyanin. The phycocyanobilin peptide, the chromophore of which is Z,Z,Z-configurated, was modified to the Z,Z,E isomeric chromophore. It has been demonstrated earlier that the Pfr chromopeptide and the Z,Z,E-configurated phycocyanin chromopeptide behave similarly with regard to spectral and chromatographic properties and reactivity. We present evidence here, obtained by high-resolution 1H NMR spectroscopy, that both the modified phycocyanobilin chromophore and the phytochrome chromophore obtained directly from Pfr are 15E-configurated.
Keywords: bilipeptides, NMR spectroscopy, cis-trans isomerization, tetrapyrroles
Full text
PDF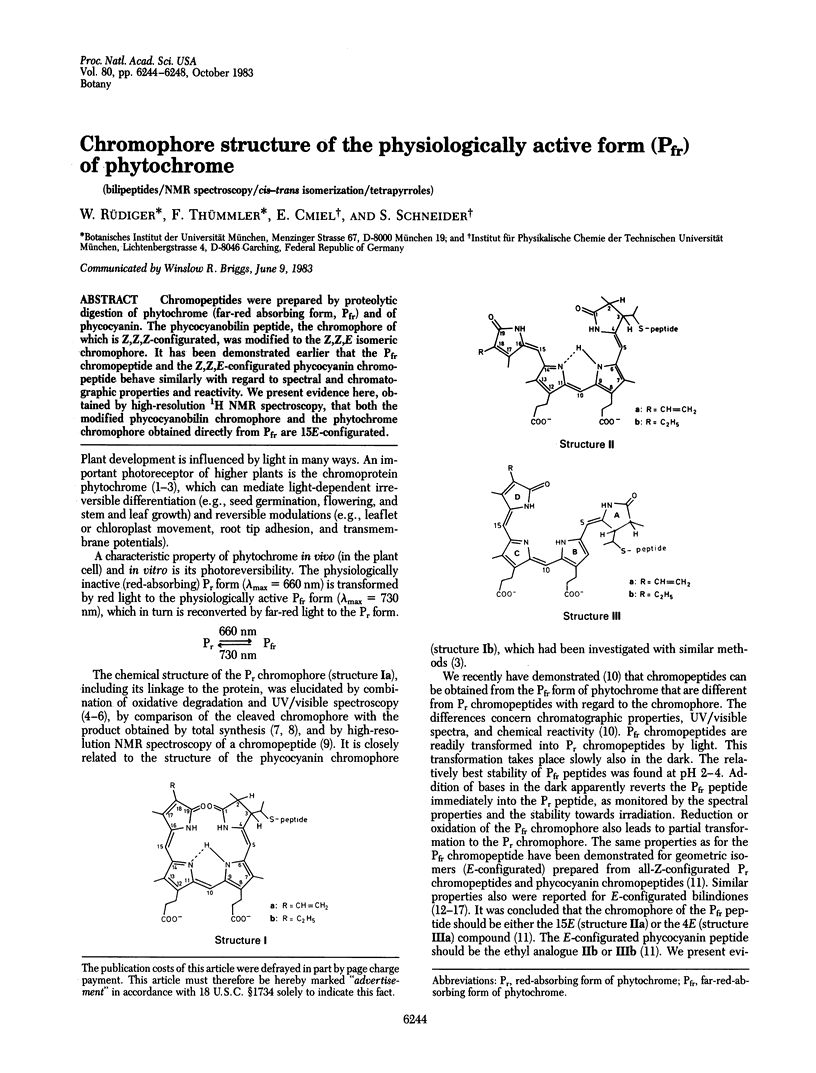


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke M. J., Pratt D. C., Moscowitz A. Low-temperature absorption and circular dichroism studies of phytochrome. Biochemistry. 1972 Oct 24;11(22):4025–4031. doi: 10.1021/bi00772a003. [DOI] [PubMed] [Google Scholar]
- Fry K. T., Mumford F. E. Isolation and partial characterization of a chromophore-peptide fragment from pepsin digests of phytochrome. Biochem Biophys Res Commun. 1971 Dec 17;45(6):1466–1473. doi: 10.1016/0006-291x(71)90185-9. [DOI] [PubMed] [Google Scholar]
- Glazer A. N., Fang S. Chromophore content of blue-green algal phycobiliproteins. J Biol Chem. 1973 Jan 25;248(2):659–662. [PubMed] [Google Scholar]
- Grombein S., Rüdiger W., Zimmermann H. The structures of the phytochrome chromophore in both photoreversible forms. Hoppe Seylers Z Physiol Chem. 1975 Nov;356(11):1709–1714. doi: 10.1515/bchm2.1975.356.2.1709. [DOI] [PubMed] [Google Scholar]
- Klein G., Grombein S., Rüdiger W. On the linkages between chromophore and protein in biliproteins, VI[1]. Structure and protein linkage of the phytochrome chromophore. Hoppe Seylers Z Physiol Chem. 1977 Aug;358(8):1077–1079. [PubMed] [Google Scholar]
- Linschitz H., Kasche V. The kinetics of phytochrome conversion. J Biol Chem. 1966 Jul 25;241(14):3395–3403. [PubMed] [Google Scholar]
- Sarkar H. K., Song P. S. Phototransformation and dark reversion of phytochrome in deuterium oxide. Biochemistry. 1981 Jul 21;20(15):4315–4320. doi: 10.1021/bi00518a012. [DOI] [PubMed] [Google Scholar]
- Siegelman H. W., Hendricks S. B. Purification and properties of phytochrome: a chromoprotein regulating plant growth. Fed Proc. 1965 Jul-Aug;24(4):863–867. [PubMed] [Google Scholar]
- Song P. S., Chae Q., Gardner J. D. Spectroscopic properties and chromophore conformations of the photomorphogenic receptor: phytochrome. Biochim Biophys Acta. 1979 Feb 26;576(2):479–495. doi: 10.1016/0005-2795(79)90423-9. [DOI] [PubMed] [Google Scholar]
- Song P. S., Kim I. S., Hahn T. R. Purification of phytochrome by Affi-Gel Blue chromatography; an effect of lumichrome on purified phytochrome. Anal Biochem. 1981 Oct;117(1):32–39. doi: 10.1016/0003-2697(81)90687-4. [DOI] [PubMed] [Google Scholar]
- Song P. S., Sarkar H. K., Kim I. S., Poff K. L. Primary photoprocesses of undegraded phytochrome excited with red and blue light at 77 K. Biochim Biophys Acta. 1981 Apr 13;635(2):369–382. doi: 10.1016/0005-2728(81)90035-9. [DOI] [PubMed] [Google Scholar]
- Wald G. The molecular basis of visual excitation. Nature. 1968 Aug 24;219(5156):800–807. doi: 10.1038/219800a0. [DOI] [PubMed] [Google Scholar]



