Abstract
Vitellogenic oocytes and eggs of the frog Xenopus laevis contain intermediate-size filaments that are resistant to extractions in high-salt buffers and Triton X-100 and are specifically stained with antibodies to cytokeratins. Gel electrophoresis of cytoskeletal proteins from Xenopus oocytes shows a specific enrichment of three polypeptides designated components 1 [Mr, 56,000; IEP (pI obtained by two-dimensional gel electrophoresis in the presence of 9.5 M urea), ca. 5.9], 2 (Mr, 46,000; IEP, 5.38), and 3 (Mr, 42,000; IEP, ca. 5.3). The same three cytoskeletal polypeptides are found in eggs and early embryos, in intestinal mucosa of adult frogs, and in cultured kidney epithelial cells. They are different from amphibian vimentin and desmin and from the keratins present in the epidermis of adult frogs. Peptide mapping and immunoblotting experiments indicate that Xenopus cytokeratin component 1 is related to cytokeratin A of higher vertebrates but is different from the two smaller cytoskeletal polypeptides 2 and 3. Incorporation of [35 S]methionine shows that all three polypeptides are synthesized in both oocytes and embryos. Our observations show that maternal storage is not only restricted to proteins serving basic cellular functions but also can extend to proteins related to a specific form of cell differentiation (i.e., epithelial formation) in the early embryo. The data suggest that mechanisms of epithelial differentiation in Xenopus embryogenesis are different from those of early mammalian embryos in which no such intermediate-size-filament storage pool has been detected.
Full text
PDF
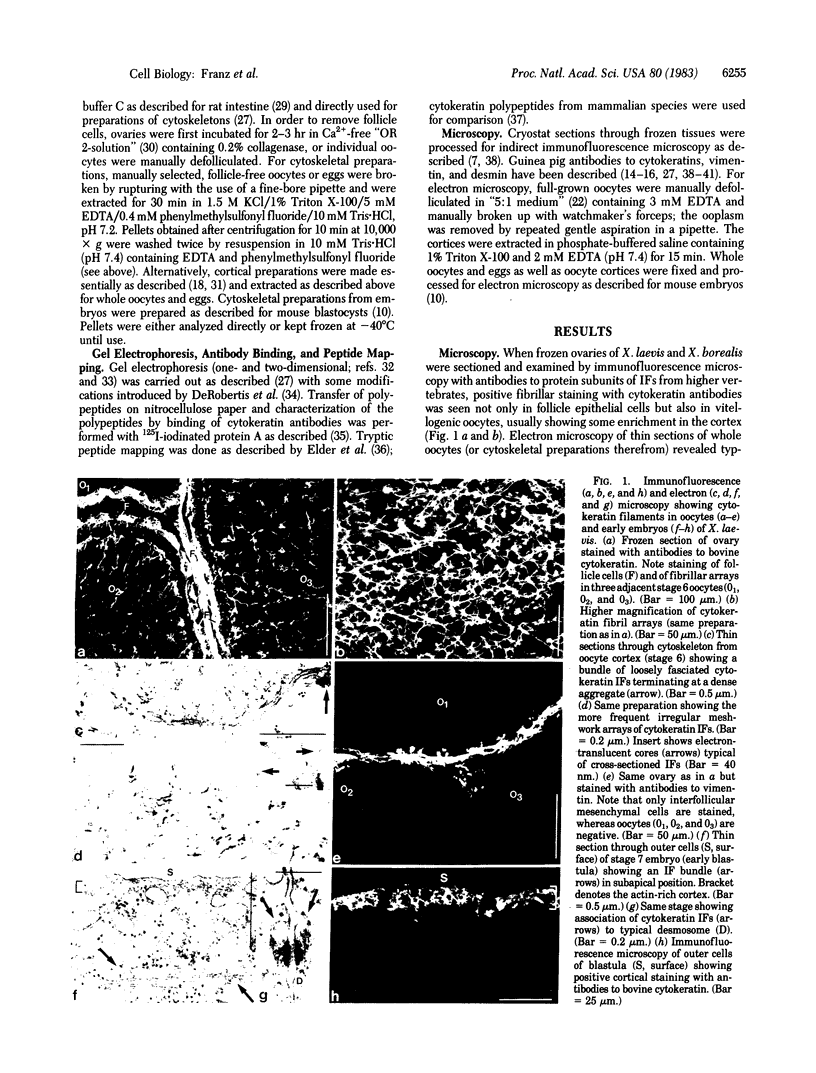


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderton B. H. Intermediate filaments: a family of homologous structures. J Muscle Res Cell Motil. 1981 Jun;2(2):141–166. doi: 10.1007/BF00711866. [DOI] [PubMed] [Google Scholar]
- Ballantine J. E., Woodland H. R., Sturgess E. A. Changes in protein synthesis during the development of Xenopus laevis. J Embryol Exp Morphol. 1979 Jun;51:137–153. [PubMed] [Google Scholar]
- Bluemink J. G. Cytokinesis and cytochalasin-induced furrow regression in the first-cleavage zygote of Xenopus laevis. Z Zellforsch Mikrosk Anat. 1971;121(1):102–126. doi: 10.1007/BF00330921. [DOI] [PubMed] [Google Scholar]
- Brûlet P., Babinet C., Kemler R., Jacob F. Monoclonal antibodies against trophectoderm-specific markers during mouse blastocyst formation. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4113–4117. doi: 10.1073/pnas.77.7.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capco D. G., Jeffrey W. R. Transient localizations of messenger RNA in Xenopus laevis oocytes. Dev Biol. 1982 Jan;89(1):1–12. doi: 10.1016/0012-1606(82)90288-3. [DOI] [PubMed] [Google Scholar]
- Carpenter C. D., Klein W. H. A gradient of poly(A)+ RNA sequences in Xenopus laevis eggs and embryos. Dev Biol. 1982 May;91(1):43–49. doi: 10.1016/0012-1606(82)90006-9. [DOI] [PubMed] [Google Scholar]
- De Robertis E. M., Partington G. A., Longthorne R. F., Gurdon J. B. Somatic nuclei in amphibian oocytes: evidence for selective gene expression. J Embryol Exp Morphol. 1977 Aug;40:199–214. [PubMed] [Google Scholar]
- Dixon L. K., Ford P. J. Regulation of protein synthesis and accumulation during oogenesis in Xenopus laevis. Dev Biol. 1982 Oct;93(2):478–497. doi: 10.1016/0012-1606(82)90136-1. [DOI] [PubMed] [Google Scholar]
- Ducibella T., Albertini D. F., Anderson E., Biggers J. D. The preimplantation mammalian embryo: characterization of intercellular junctions and their appearance during development. Dev Biol. 1975 Aug;45(2):231–250. doi: 10.1016/0012-1606(75)90063-9. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Franke W. W., Appelhans B., Schmid E., Freudenstein C., Osborn M., Weber K. Identification and characterization of epithelial cells in mammalian tissues by immunofluorescence microscopy using antibodies to prekeratin. Differentiation. 1979;15(1):7–25. doi: 10.1111/j.1432-0436.1979.tb01030.x. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Mayer D., Schmid E., Denk H., Borenfreund E. Differences of expression of cytoskeletal proteins in cultured rat hepatocytes and hepatoma cells. Exp Cell Res. 1981 Aug;134(2):345–365. doi: 10.1016/0014-4827(81)90435-3. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Rathke P. C., Seib E., Trendelenburg M. F., Osborn M., Weber K. Distribution and mode of arrangement of microfilamentous structures and actin in the cortex of the amphibian oocyte. Cytobiologie. 1976 Dec;14(1):111–130. [PubMed] [Google Scholar]
- Franke W. W., Schiller D. L., Moll R., Winter S., Schmid E., Engelbrecht I., Denk H., Krepler R., Platzer B. Diversity of cytokeratins. Differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. J Mol Biol. 1981 Dec 25;153(4):933–959. doi: 10.1016/0022-2836(81)90460-5. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Freudenstein C., Appelhans B., Osborn M., Weber K., Keenan T. W. Intermediate-sized filaments of the prekeratin type in myoepithelial cells. J Cell Biol. 1980 Mar;84(3):633–654. doi: 10.1083/jcb.84.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Schiller D. L., Winter S., Jarasch E. D., Moll R., Denk H., Jackson B. W., Illmensee K. Differentiation-related patterns of expression of proteins of intermediate-size filaments in tissues and cultured cells. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):431–453. doi: 10.1101/sqb.1982.046.01.041. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Winter S., Osborn M., Weber K. Widespread occurrence of intermediate-sized filaments of the vimentin-type in cultured cells from diverse vertebrates. Exp Cell Res. 1979 Oct 1;123(1):25–46. doi: 10.1016/0014-4827(79)90418-x. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Weber K., Osborn M., Schmid E., Freudenstein C. Antibody to prekeratin. Decoration of tonofilament like arrays in various cells of epithelial character. Exp Cell Res. 1978 Oct 15;116(2):429–445. doi: 10.1016/0014-4827(78)90466-4. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Winter S., Grund C., Schmid E., Schiller D. L., Jarasch E. D. Isolation and characterization of desmosome-associated tonofilaments from rat intestinal brush border. J Cell Biol. 1981 Jul;90(1):116–127. doi: 10.1083/jcb.90.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenstein C., Franke W. W., Osborn M., Weber K. Reaction of tonofilament-like intermediate-sized filaments with antibodies raised against isolated defined polypeptides of bovine hoof prekeratin. Cell Biol Int Rep. 1978 Nov;2(6):591–600. doi: 10.1016/0309-1651(78)90068-1. [DOI] [PubMed] [Google Scholar]
- Gigi O., Geiger B., Eshhar Z., Moll R., Schmid E., Winter S., Schiller D. L., Franke W. W. Detection of a cytokeratin determinant common to diverse epithelial cells by a broadly cross-reacting monoclonal antibody. EMBO J. 1982;1(11):1429–1437. doi: 10.1002/j.1460-2075.1982.tb01334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer H., Bennett G. S., Tapscott S. J., Croop J. M., Toyama Y. Intermediate-size filaments: changes in synthesis and distribution in cells of the myogenic and neurogenic lineages. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):317–329. doi: 10.1101/sqb.1982.046.01.033. [DOI] [PubMed] [Google Scholar]
- Jackson B. W., Grund C., Schmid E., Bürki K., Franke W. W., Illmensee K. Formation of cytoskeletal elements during mouse embryogenesis. Intermediate filaments of the cytokeratin type and desmosomes in preimplantation embryos. Differentiation. 1980;17(3):161–179. doi: 10.1111/j.1432-0436.1980.tb01093.x. [DOI] [PubMed] [Google Scholar]
- Jackson B. W., Grund C., Winter S., Franke W. W., Illmensee K. Formation of cytoskeletal elements during mouse embryogenesis. II. Epithelial differentiation and intermediate-sized filaments in early postimplantation embryos. Differentiation. 1981;20(3):203–216. doi: 10.1111/j.1432-0436.1981.tb01177.x. [DOI] [PubMed] [Google Scholar]
- Kemler R., Brûlet P., Schnebelen M. T., Gaillard J., Jacob F. Reactivity of monoclonal antibodies against intermediate filament proteins during embryonic development. J Embryol Exp Morphol. 1981 Aug;64:45–60. [PubMed] [Google Scholar]
- Krohne G., Dabauvalle M. C., Franke W. W. Cell type-specific differences in protein composition of nuclear pore complex-lamina structures in oocytes and erythrocytes of Xenopus laevis. J Mol Biol. 1981 Sep 5;151(1):121–141. doi: 10.1016/0022-2836(81)90224-2. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Nelson W. J., Traub P. Intermediate (10 nm) filament proteins and the Ca2+-activated proteinase specific for vimentin and desmin in the cells from fish to man: an example of evolutionary conservation. J Cell Sci. 1982 Oct;57:25–49. doi: 10.1242/jcs.57.1.25. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Osborn M., Geisler N., Shaw G., Sharp G., Weber K. Intermediate filaments. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):413–429. doi: 10.1101/sqb.1982.046.01.040. [DOI] [PubMed] [Google Scholar]
- Paulin D., Babinet C., Weber K., Osborn M. Antibodies as probes of cellular differentiation and cytoskeletal organization in the mouse blastocyst. Exp Cell Res. 1980 Dec;130(2):297–304. doi: 10.1016/0014-4827(80)90006-3. [DOI] [PubMed] [Google Scholar]
- Renner W., Franke W. W., Schmid E., Geisler N., Weber K., Mandelkow E. Reconstitution of intermediate-sized filaments from denatured monomeric vimentin. J Mol Biol. 1981 Jun 25;149(2):285–306. doi: 10.1016/0022-2836(81)90303-x. [DOI] [PubMed] [Google Scholar]
- Schiller D. L., Franke W. W., Geiger B. A subfamily of relatively large and basic cytokeratin polypeptides as defined by peptide mapping is represented by one or several polypeptides in epithelial cells. EMBO J. 1982;1(6):761–769. doi: 10.1002/j.1460-2075.1982.tb01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid E., Osborn M., Rungger-Brändle E., Gabbiani G., Weber K., Franke W. W. Distribution of vimentin and desmin filaments in smooth muscle tissue of mammalian and avian aorta. Exp Cell Res. 1982 Feb;137(2):329–340. doi: 10.1016/0014-4827(82)90034-9. [DOI] [PubMed] [Google Scholar]
- Steinert P., Zackroff R., Aynardi-Whitman M., Goldman R. D. Isolation and characterization of intermediate filaments. Methods Cell Biol. 1982;24:399–419. doi: 10.1016/s0091-679x(08)60667-6. [DOI] [PubMed] [Google Scholar]
- Sturgess E. A., Ballantine J. E., Woodland H. R., Mohun P. R., Lane C. D., Dimitriadis G. J. Actin synthesis during the early development of Xenopus laevis. J Embryol Exp Morphol. 1980 Aug;58:303–320. [PubMed] [Google Scholar]
- Vandekerckhove J., Franke W. W., Weber K. Diversity of expression of non-muscle actin in amphibia. J Mol Biol. 1981 Oct 25;152(2):413–426. doi: 10.1016/0022-2836(81)90251-5. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W., Dumont J. N., Sega M. W. Protein incorporation by isolated amphibian oocytes. 3. Optimum incubation conditions. J Exp Zool. 1973 Jun;184(3):321–333. doi: 10.1002/jez.1401840305. [DOI] [PubMed] [Google Scholar]
- Wolf D. P., Hedrick J. L. A molecular approach to fertilization. II. Viability and artificial fertilization of Xenopus laevis gemetes. Dev Biol. 1971 Jul;25(3):348–359. doi: 10.1016/0012-1606(71)90036-4. [DOI] [PubMed] [Google Scholar]
- Woodland H. R., Adamson E. D. The synthesis and storage of histones during the oogenesis of Xenopus laevis. Dev Biol. 1977 May;57(1):118–135. doi: 10.1016/0012-1606(77)90359-1. [DOI] [PubMed] [Google Scholar]