Abstract
Microbodies associated with fossil feathers, originally attributed to microbial biofilm, have been reinterpreted as melanosomes: pigment-containing, eukaryotic organelles. This interpretation generated hypotheses regarding coloration in non-avian and avian dinosaurs. Because melanosomes and microbes overlap in size, distribution and morphology, we re-evaluate both hypotheses. We compare melanosomes within feathers of extant chickens with patterns induced by microbial overgrowth on the same feathers, using scanning (SEM), field emission (FESEM) and transmission (TEM) electron microscopy. Melanosomes are always internal, embedded in a morphologically distinct keratinous matrix. Conversely, microbes grow across the surface of feathers in continuous layers, more consistent with published images from fossil feathers. We compare our results to both published literature and new data from a fossil feather ascribed to Gansus yumenensis (ANSP 23403). ‘Mouldic impressions’ were observed in association with both the feather and sediment grains, supporting a microbial origin. We propose criteria for distinguishing between these two microbodies.
In 1995, approximately 1 μm long elongate microbodies were observed on the surface of fossilized feathers from the Eocene of Messel (Germany), Oligocene of Cereste (France) and Jurassic Solnhofen Limestone (Germany)1 using scanning electron microscopy (SEM). These microstructures were proposed to represent mineralized microorganisms and the glycocalyx they secrete, and, based upon this interpretation, a new mode of fossilization was proposed1. This was further supported by the observation of similar microbodies associated with fish, mammals and other material in the same deposit, and a microbial origin for these has not been disputed2,3. However, a decade after their discovery, the microbodies associated with fossil feathers were reinterpreted as eukaryotic melanosomes4; intracellular, membrane-bound organelles where melanin pigment is synthesized and stored5. Because melanosomes vary in morphology in extant feathers6, it was additionally proposed that color could be inferred in fossil feathers solely on the shape of these structures; round indicating red and brown hues and oblate indicating black and/or grey4,7,8,9,10,11,12,13. Finally, aspects of behavior, physiology and ecology were posited for avian and non-avian dinosaurs4,7,8,9,10,11,12 based solely upon these morphological data.
Bacteria and the extracellular polymeric substances (EPS) they secrete are known to fossilize14,15. Because bacterial cells contain a cell wall composed of resistant, cross-linked peptidoglycan polymers16, they are hypothesized to have greater preservation potential than eukaryotic intracellular organelles17, protected only by a lipid bilayer. The fossil record contains many examples of fossilized bacteria and biofilms2,15,18,19, and some processes leading to their preservation have been elucidated in the lab20. Fossilization of intracellular organelles is extremely rare, even when cell-like microstructures retaining transparency and flexibility persist21,22,23, but morphologies consistent with organelles have been noted24. Whether these represent molecule for molecule replacement in mineral or original components cannot be determined without chemical data. Because melanosomes and microbes overlap in shape and size, differentiating between the two is critical for supporting hypotheses of behavior7, evolutionary significance9,10, and/or ecology11 in extinct organisms.
Because intracellular organelles are definitive evidence for a eukaryotic source, it is imperative to differentiate between melanin and melanosomes before proposing far-reaching interpretations of color, habitat, niche and lifestyle. Melanin is an organic pigment derived from tyrosine residues6 that are highly cross-linked and resistant to degradation. Both eukaryotes and prokaryotes produce melanin pigments, the basic structural unit of which is unknown25. Melanosomes are membrane-bound intracellular organelles within specialized cells called melanocytes where melanin pigments are polymerized by enzymes and stored5. Melanosomes have also been referred to as melanin ‘granules’26, a term more appropriate to describe irregular clusters of the melanin pigment regardless of source. During maturation of keratinous pigmented tissues, including skin, hair, and feathers, melanosomes are transferred from melanocytes to keratin-producing cells, where they become embedded within the keratinous matrix6. While melanin chemistry confers high preservation potential to the pigment27, this resistance to degradation has not been shown to extend to the membrane-bound organelles containing pigment grains. In fact, melanosomes are related to lysosomes, organelles producing enzymes involved in autolytic degradation5, therefore indicating a predisposition for rapid degradation. Finally, bacteria are prokaryotic organisms that may or may not secrete an enzyme-rich exopolymeric substance that facilitates bacterial adherence to a substrate and that may assist in degradative processes28. Melanosomes, as membrane-bound organelles, are not produced by bacteria, although many do produce and utilize the pigment melanin29.
Data presented in previously published works describing fossil material have not eliminated either a eukaryotic melanosome or prokaryotic biofilm source for the microbodies associated with fossil feathers; thus, both hypotheses remain valid. We conducted actualistic experiments on extant feathers to test the hypothesis that microbodies observed in fossil feathers are more consistent with melanosomes than degrading bacteria colonizing the surface. We incubated extant feathers with either a naturally occurring microbial population (see Methods) or a pure culture of biofilm-forming Bacillus cereus, leaving some feathers untreated as controls. We used both pigmented (black and brown) and non-pigmented (white, lacking melanosomes) feathers and compared differences in distribution and morphology between microbes and melanosomes with published data for fossil feathers. We also examined a Chinese fossil feather using SEM-EDX to visualize microbodies or ’mouldic impressions‚ similar to those observed in previous studies of fossil feathers.
Results
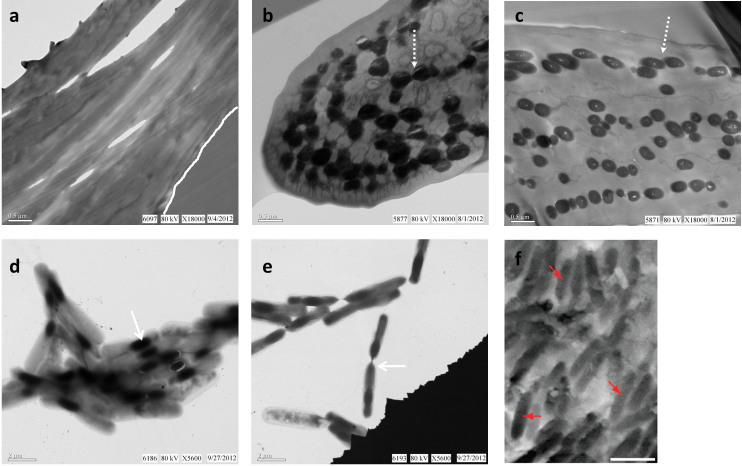
Figure 1 shows that electron-dense melanosomes are visible using transmission electron microscopy (TEM) and are embedded within the keratinous matrix of both pigmented feathers (Fig. 1b, c), but are lacking in white feathers (Fig. 1a). These distinct bodies appear either elongate or round, depending on cutting aspect, but rarely overlap, are often separated, and are always completely surrounded by the keratinous matrix of the feather. Conversely, cells from cultured, endospore-forming B. cereus are external to the feather (Fig. 1d) and are more similar in size, shape, distribution and location to previously published work on fossil melanosomes in side by side comparisons (Fig. 1f7, reprinted with permission).
Figure 1. Electron micrographs of modern Gallus gallus feathers and Bacillus cereus pure culture, compared with an SEM image of a fossil feather from published work.
Chicken feathers were sectioned, stained, and viewed in transmission EM as described (see Methods). Melanosomes are observed (dashed arrow) in brown (b) and black (c) feathers but are absent in similarly prepared white feathers (a) (contact between feather and embedding medium delineated by white line in (a)). (d) Aggregation of B. cereus cells containing electron opaque internal endospores (arrow). (e) Two endospore-containing B. cereus cells aligned and connected (arrow), prepared and stained as described (see Methods). (f) SEM image of isolated feather of Jurassic bird Archaeopteryx with “[…] melanosomes (arrows) preserved […] as moulded imprints” (scale bar: 1 μm). Reprinted by permission from Macmillan Publishers Ltd: [NATURE COMMUNICATIONS]7, copyright (2012).
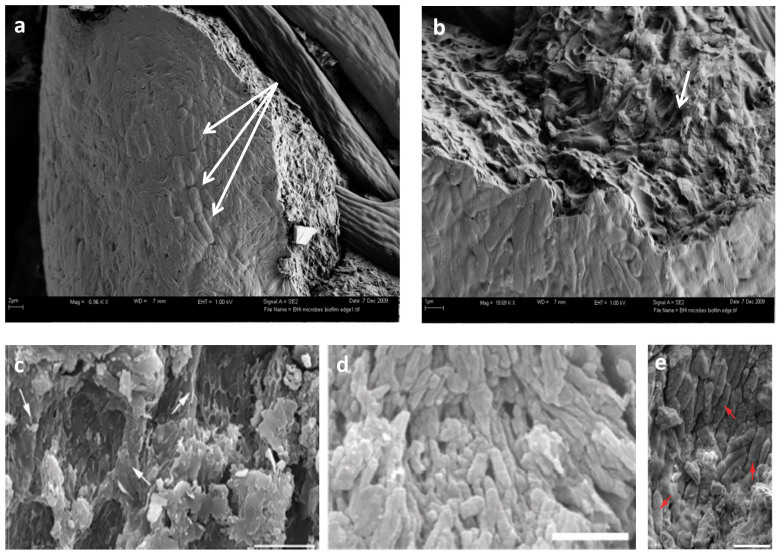
Field emission-scanning electron microscopy (FESEM) images of guineafowl feathers incubated with pond inoculum (Fig. 2a and b) show microbial growth on the surface of the feather. Microbial bodies are dense and overlapping, form a virtually continuous mat across the feather surface and follow feather contours (Supplementary Fig. S2), in contrast to the relatively less abundant and non-overlapping distribution of melanosomes depicted in Fig. 1. In some regions, microbial bodies demonstrate a high degree of alignment (Fig. 2a, arrows), similar to patterns ascribed to fossil melanosomes (Figure 2d13 and e7, republished with permission). Also see Fig. 1b in Vinther et al. (2008) and Fig. 1c and e in Vinther et al. (2010).
Figure 2. FE-SEM micrographs of biofilm overgrowth of extant feathers compared with published images of fossil feathers.
(a) Guineafowl feathers exposed to naturally occurring culture show strong alignment of microbial cells (arrows). (b) Higher magnification of biofilm edge in (a) showing where bacteria cells have been eliminated from the surrounding matrix (arrow), leaving voids similar to those figured in (c), which were identified as “[…] eumelanosomes preserved as moulds inside small areas that are separated from each other by anastomosing ridges of degraded feather (at arrows in c)” (scale bar: 5 μm). Reprinted by permission from Macmillan Publishers Ltd: [NATURE]13, copyright (2010). (d) “Strongly aligned, closely spaced, eumelanosomes preserved as solid bodies,” in Confuciusornis feathers (scale bar: 2 μm). Reprinted by permission from Macmillan Publishers Ltd: [NATURE]13, copyright (2010). (e) “melanosomes (arrows)” figured in Carney et al. 2012 (scale bar: 1 μm). Reprinted by permission from Macmillan Publishers Ltd: [NATURE COMMUNICATIONS]7, copyright (2012).
It was previously noted that purported melanosomes are easier to observe in degraded fossil feathers4,13, but we show that microbial cells can also be removed from their EPS, leaving ‘mouldic impressions’ (Fig. 2b) similar to those noted in fossil feathers (Fig. 2c13, reprinted with permission)7,10,13. We propose that because microbes and their EPS are participants in degradation, it is more parsimonious to attribute these bodies to microbial overgrowth than to melanosome exposure, and that these cannot be differentiated from melanosomes embedded in a keratinous matrix without chemical data.
We have examined many modern pigmented feathers (at least 20) prepared by fresh fracture, cryofracture, and/or sectioning in multiple planes, but definitively identifying melanosomes under SEM is not trivial. Consistent with other observations10,30,31, we found that, in most cases, modern feathers must be treated to reduce or remove keratin before melanosomes embedded within the matrix are visible; alternatively, feathers embedded in resin and longitudinally sectioned10 allow visualization under SEM. Without treatment, melanosomes were seen in only one feather after fresh fracturing (Supplementary Fig. S3). Melanosomes in extant chicken feathers are more sparse and have a non-overlapping distribution (Fig. 1b and c) compared to bacteria (Fig. 1d and e) when both are viewed in TEM. Furthermore, under our experimental conditions, microbes grew across the surfaces of the feathers in densely packed layers, more similar to what have been presented as fossil melanosomes than to in situ melanosomes in TEM sections of modern feathers. Never were melanosomes observed in whole mounts of extant feathers, as has been reported for visualization of fossil feather melanosomes, nor were they observed without extensive manipulation.
Following the protocol in Li et al. 2010, we were able to observe melanosomes in longitudinally-sectioned black and brown chicken feather barbs. We observed both morphological types of melanosomes, eumelanosomes (oblate) and phaeomelanosomes (round) in the black feather (Fig. 3b). Only phaeomelanosomes, displaying unsmooth, granular surfaces, were observed in the brown feather (Fig. 3c and d). All melanosomes detected by this method vary in size and appear to be randomly oriented. They are sparse and non-uniform in distribution, never occurring in dense mats or closely spaced, as described for the microbodies in fossil feathers9,11,13,32,33.
Figure 3. SEM images of melanosomes from extant Gallus gallus feathers.
Black (a,b) and brown (c,d) chicken feathers sectioned longitudinally. (b) Although the feather is visually black, both ovate eumelanosomes (arrow) and round phaeomelanosomes (arrowhead) are present. (c) Phaeomelanosomes (arrowhead) are also observed in the brown feather. (d) Melanosomes exhibit unsmooth, granular surfaces. Using this method, like those observed in TEM, melanosomes appear randomly oriented, rather than in dense mats as reported for fossils (see text). Considerable size variation (e.g. ~.5 μm–~2 μm for the eumelanosomes) is observed between all melanosomes.
Microbial bodies are easily distinguished from melanosomes in extant feathers, because they are not electron-dense and because melanosomes are always internal and embedded in the keratinous matrix while microbes are present as surface overgrowth11 (Fig. 2a, b and Supplementary Fig. S1). Additionally, their electron-dense nature makes melanosomes visible in both stained and unstained sections (Fig. 4), while microbes are only clearly visible after heavy metal staining (Fig. 4a and b).
Figure 4. TEM images of Bacillus cereus-treated black chicken feather.
Stained (a,b) and unstained (c,d) TEM images of feather are compared. Feathers were incubated with cultured B. cereus for three days (See SI for details). (a) Superficial B. cereus cells (arrow) extending from the barb surface (white line; feather tissue is to the right). Melanosomes (dashed arrow) are always internal to the feather surface, sparsely distributed and non-overlapping. (b) Higher magnification of boxed area in (a) shows interaction (arrowhead) of bacteria (arrow) with barb surface (white line). White dashed arrow depicts internal, electron-opaque melanosome. Without staining, (c) bacteria are not visible on the external surface, as they are not normally electron-opaque, in contrast with easily visualized, internal, electron-opaque melanosomes (dashed arrow). The melanin pigment is inherently electron dense; no staining is necessary. (d) Enlarged image of unstained section in (c) shows vacuoles associated with internal melanosomes, as has been noted previously4. Keratinous matrix completely surrounds melanosomes, making them difficult to image in SEM without additional treatment.
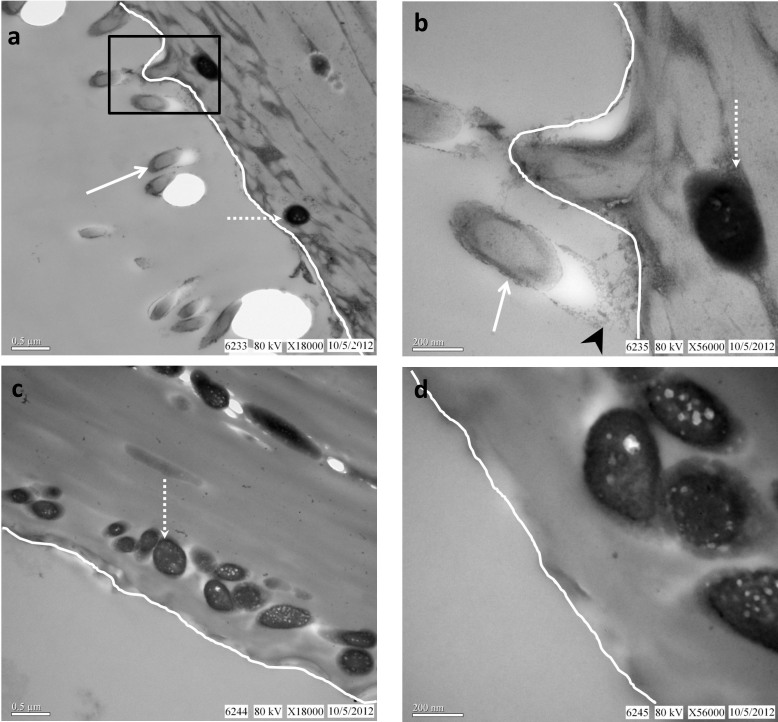
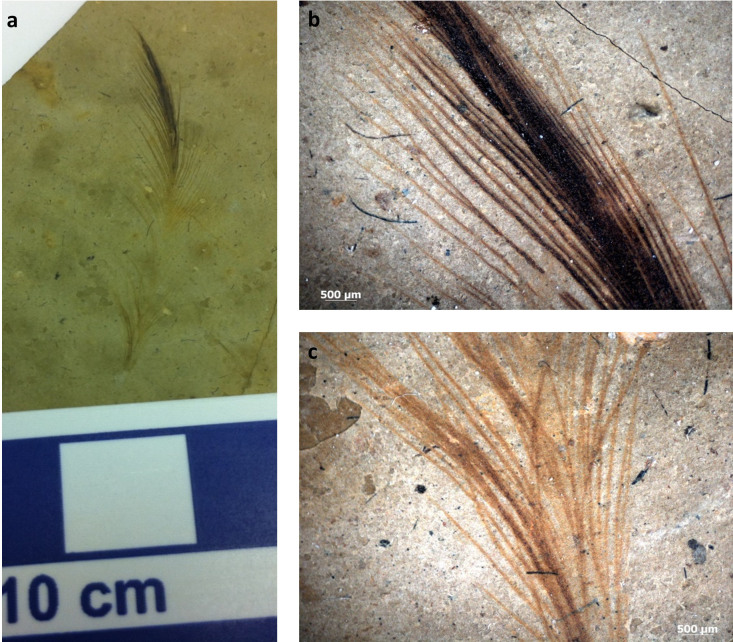
We also examined an isolated fossil feather (Fig. 5) (ANSP 23403) collected from the Xiagou Formation (Lower Cretaceous) in northwestern Gansu Province, China, and ascribed to the bird Gansus yumenensis34,35. As reported in other work7,8,9,10,13,32 ’mouldic impressions‚ (Fig. 6) were observed associated with the fossil feather. Although the feather exhibited regions of different color (black apically (Fig. 5a), brown more basally (Fig. 5b)) the ’mouldic impressions‚ did not differ in type or distribution between the black and brown regions. In addition, these impressions were also observed on sediment grains (the identity of which is confirmed by EDS (Fig. 7)) superficially associated with the feather. Because the sediment grains are clearly not part of the feather structure, yet retain ’mouldic impressions‚, a microbial origin for these impression structures is favored.
Figure 5. Images of the fossil feather ascribed to Gansus yumenensis (ANSP 23403).
(a) Photograph of the isolated feather from the Xiagou Formation showing varying color pattern; black at the top, and red-brown toward the base. It is beyond the scope of this paper to determine whether this apparent color variation reflects the actual biological color of the feather or taphonomic alteration. (b,c) Higher magnification images of the dense black distal region (b) and the finer, brown proximal region.
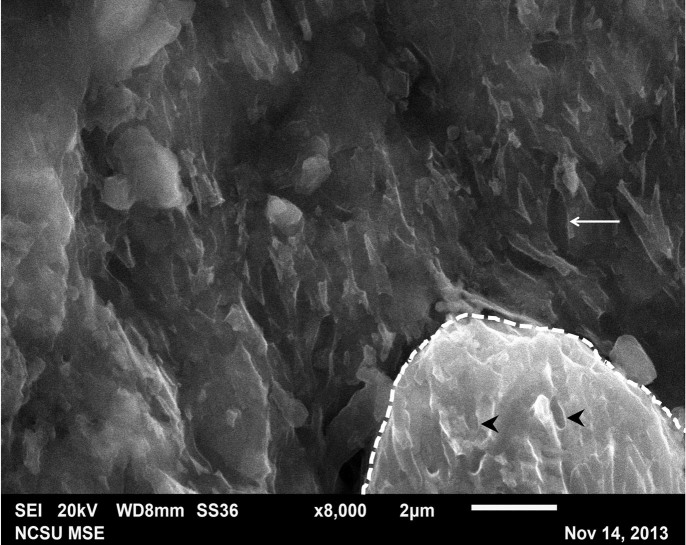
Figure 6. High magnification image of the black region of the fossil feather.
’Mouldic impressions‚ (~1–1.5 μm) co-localize to both the fossil feather (arrow) and the sediment grains (arrowheads) intimately associated with but superficial to the fossil. The sediment grain is encircled by the dashed line.
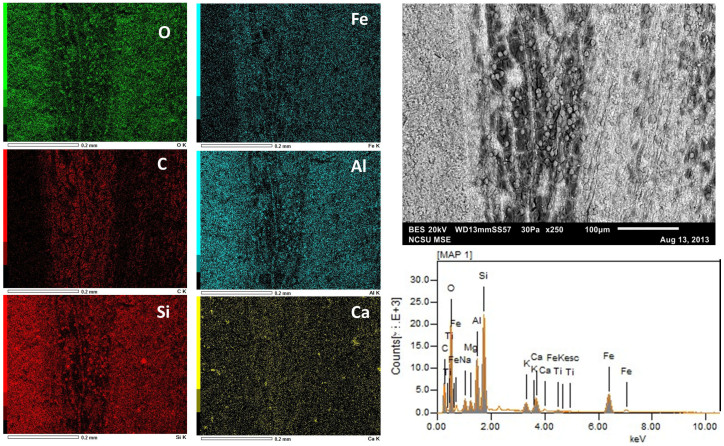
Figure 7. SEM-EDS data derived from the black fossil feather region.
The six dominant elements (~95% of detected electrons) are presented in order of decreasing abundance by weight percent. Elemental map and quantitative data (Table 1) suggest the fossil feather is composed primarily of C although Fe is also abundant. In contrast, Al is localized to the sediments and not in the feather. The surrounding sediment is composed primarily of O and Si which is consistent with previous analyses32. The data demonstrate that the ~8 μm-sized spheres shown in Fig. S4 are composed of O and Si, consistent with sediment grains and not part of the original feather structure.
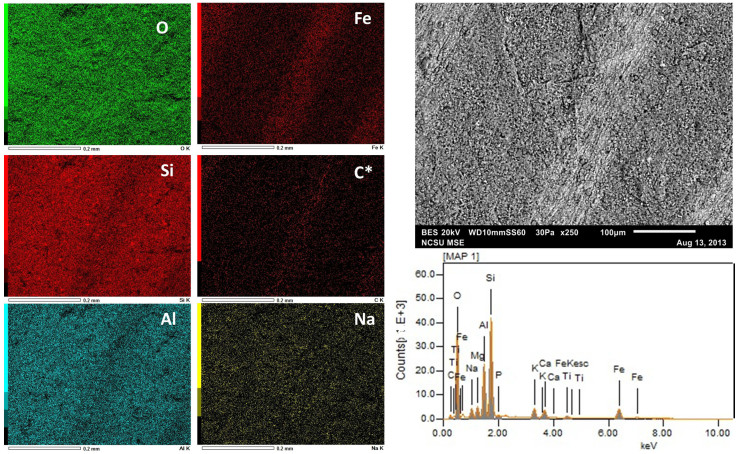
SEM-EDS analysis revealed that the fossil feather (Fig. 7 and 8) is composed primarily of C and Fe, although quantitative data from the brown portion (Fig. 8) indicates a reduced amount of C compared to the black region (Fig. 7) (see Table 1 for quantitative elemental data). The sedimentary matrix surrounding the feather, as well as the sediment grains observed associated with the fossil material, are composed primarily of O, Si and Al.
Figure 8. SEM-EDS data derived from the brown, basal part of the fossil feather.
The six dominant elements (~93% of detected electrons) are presented in order of decreasing abundance by weight percent. See Table 1 for the quantitative data. Carbon is denoted with an asterisk because it was not auto-detected by the instrument and was manually inserted. Although this basal portion of the fossil is also dominated by Fe and C, as above, the quantitative data indicate the C is greatly decreased in abundance relative to the black portion.
Table 1. SEM-EDS quantitative data for the fossil feather. Both black (Fig. 7) and brown (Fig. 8) regions of the feather are presented as weight (ms%) and molar (mol%) percentages in decreasing abundance. * indicates element not auto-detected by instrument.
| Black | ms% | mol% | Brown | ms% | mol% |
|---|---|---|---|---|---|
| O | 37.92 | 41.36 | O | 45.29 | 57.03 |
| C | 28.06 | 40.76 | Si | 19.12 | 13.72 |
| Si | 10.99 | 6.83 | Al | 9.53 | 7.11 |
| Fe | 9.90 | 3.09 | Fe | 8.39 | 3.03 |
| Al | 5.75 | 3.72 | C* | 7.30 | 12.24 |
| Ca | 2.20 | 0.96 | Na | 2.59 | 2.27 |
Discussion
We evaluate statements used by others to support a melanosome origin for microbodies in fossil feathers, and put forth alternative interpretations equally supported by the same data. (1.) Localization of microbodies to ‘dark’ and absence from ‘light’ regions of one fossil feather support a melanosome origin4. Three hypotheses exist to explain the striped patterning of the fossil feather reported in Vinther et al. 2008. First, the original feather, in life, was also striped, with melanosomes distributed in the colored regions of the feather and no melanosomes in the uncolored parts, and the fossils preserve this original pattern. Second, microbial overgrowth on the surface of the feather is distributed according to the relatively more nutrient-rich pigmented feather regions over unpigmented areas36. Third, microbes preferentially colonized and completely degraded those feather regions most easily broken down (e.g. unpigmented)37,38; thus were no longer present in these regions during fossilization, but continued to act on the more resistant, melanin-containing regions. (2.) Densely packed and aligned/organized layers support a melanosome origin (Fig. 2c–e)7,13. Recently published research indicates original melanosome geometry and distribution are altered with heat and pressure31, diagenetic processes affecting fossils, and that have not been taken into account in previous research claiming melanosome morphology in fossil feathers reflects their original color.
Data presented here show microbial overgrowth is dense and can be aligned, whereas internal melanosomes are more sparsely distributed and relatively random in orientation as observed in both SEM and TEM. Our data show this description is more consistent with biofilm overgrowth (Fig. 2a, b) than melanosomes (Fig. 1b and c). In addition, bacteria can align (Fig. 1e, 2a, and Supplementary Fig. S1c2) and follow the contours of a feather (Supplementary Fig. S2) in layers. Unlike most bacteria, which usually exhibit more uniform and smooth cell surfaces39,40, melanosomes, as observed in a previous study26, are not smooth, but rather have bumpy and non-uniform surfaces (Fig. 3b and d). Because bacteria have a tough cell wall external to the plasma membrane41, this granular topography26 may be more difficult to observe in melanin-containing bacteria than in eukaryotic melanosomes. This remains to be tested. (3.) Elements consistent with melanin, including Ca2+, Cu2+ and Zn2,43,44, are associated with feathers and thus support a melanosome origin. However, these biomarkers are also used and/or sequestered by bacteria45,46, including common soil bacteria and other microorganisms, and are also part of the sedimentary environment. These microorganisms are also capable of synthesizing melanin29, thus elemental data alone cannot be used to discriminate microbes from melanosomes.
Our examination of the fossil feather ascribed to Gansus yumenensis showed no microbodies of any type, but did reveal ‘mouldic impressions’ (Fig. 6) similar to those described in previous studies. However, these impressions did not vary between black and brown regions of the feather, and were also observed on sediment grains (Fig. 6) (confirmed with EDS data) associated with the fossil. Therefore, because sediment grains do not contain melanosomes, it more parsimonious to propose these ‘mouldic impressions’ represent a microbial origin (remnant EPS) than intracellular structures derived from the original feather. A very similar image was presented in Barden et al. 2010 (Fig. 1H) but elemental data were not mapped, so identification as a sediment grain cannot be confirmed in their paper. Visual, textural (Fig. 5 and Fig. S4), and elemental data (Fig. 7 and 8 and Table 1) from the fossil feather suggest differential diagenetic processes acting on the different regions of the feather. The reason for these differences is beyond the scope of this paper and requires additional studies.
Pending the identification of definitive molecular or chemical signals unique to either melanosomes or microbes in extant feathers that are likely to persist across geological time, distinguishing microbes from melanosomes in fossils may be difficult. Until new data are presented, we propose the following criteria to support a melanosome origin for microbodies associated with fossil feathers: 1) a taphonomic mechanism must be demonstrated for removing resistant keratin while leaving the intracellular organelles intact; 2) electron-dense material should be localized to the microbodies using TEM-EELS (TEM coupled with energy loss spectroscopy) or TEM-EDX; 3) melanosome-specific (e.g. cargo proteins5) or bacteria-specific (e.g., peptidoglycans16) biomolecules should be localized to the structures to eliminate the alternative, using in situ surface techniques (e.g., time of flight secondary ion mass spectrometry (TOF-SIMS42)), or other softer mass spectrometry imaging methods.
The ‘mouldic impressions’ described in fossil feathers imply that the microbodies were once present, and subsequently degraded from an amorphous material that retained the impression through fossilization. Therefore, if the structures are melanosomes, this material should resemble β-keratin, because the β-keratin matrix of feathers is highly resistant to degradation47. β-keratin is a rigid structural protein comprising ~80% of the organic matrix of mature feathers48. Its multiple cross-links, twisted-pleated-sheet tertiary structure and hydrophobic amino acid composition49 confer high preservation potential to structures comprised of this protein50. Mammals do not produce β-keratin51, thus common contamination by human keratins can be easily recognized. If microbial, the material should retain microbial-specific biomarkers in the environment immediately surrounding these microbodies.
Some papers state the keratin matrix is completely degraded in fossil feathers and no feather structure remains4,9,11,13, but fail to state what material, then, retains ‘mouldic impressions’. If feather structure is not preserved, how is the object identifiable as a fossil feather, and how has a biofilm source been eliminated? The presence or absence of keratin has not been tested in any fossil feather purporting to contain melanosomes. Yet, the melanosome hypothesis posits that melanosomes are preserved ‘in life position’. Modern feather melanosomes (‘in life’) are always embedded in a keratinous matrix, thus the ‘mouldic impressions’ cited by many in support of the melanosome hypothesis are assumed to be made in the original keratinous matrix of the feather, an assumption that has never been tested.
It should be noted that handling history and full depositional description are often not included in studies purporting to recover fossil melanosomes. Excavation of a fossil feather as part and counterpart could be interpreted differently than a feather collected as a whole specimen. This information is critical for determining where these microbodies are localized (ie. inside versus on the surface of the feather).
More importantly, even if irrefutable data support a melanosome origin for microbodies in a given fossil, imparting color to the entire organism, or even the entire feather, based upon their presence cannot be inferred. All melanized feathers in extant birds contain both eumelanin and phaeomelanin6; it is the relative abundances of these two melanins that determine the expressed color of a pigmented feather6. Claiming a ‘red-orange’ or ‘black-grey’ color for entire fossil organisms based upon identification of round or elongate morphologies is overly simplistic, because in living birds, pigment molecules of multiple types are employed to confer hues of brown, red, orange, etc.6. Coloration of feathers is complex, the result of expression of more than one type of pigment (e.g. porphyrins, carotenoids)6, which may be more labile, with lower preservation potential, than the relatively resistant melanins. Without preservation of all pigments originally employed, original organismal color cannot be interpreted with accuracy.
The initially proposed hypothesis of a microbial origin1 for these microstructures observed in multiple fossilized feathers, as well as other fossil material from the Messel deposits2,3 has not been refuted, or indeed addressed, with data presented in previous studies, but is supported by the data we present herein. The present data do not support the melanosome hypothesis for these fossilized microstructures. Morphology alone is insufficient to distinguish between a melanosome and/or microbial origin, but data that capitalize on distinct chemical differences between melanosomes and microbes are needed to support one hypothesis over the other. With the exception of one fossil feather study where the chemical data are not of high resolution32, the only in-depth chemical data presented for microbodies in the fossil record that seem to support a melanosome origin are derived from marine42,52,53,54 rather than terrestrial environments, where preservational conditions are very different than the lacustrine environments from which most feathers have been recovered1. Additionally, geochemical data from fossil feather ‘melanosomes’ are compared only with that derived from extant melanosomes; microbes are not included in the comparative data50.
Furthermore, because the shape of melanosomes has been used to interpret color4,7,8,9,10,11 and behavior7,11 in extinct animals, distinguishing melanosomes from microbes is critical to acceptance or rejection of these hypotheses. As McGraw warned “[…] it is wise to withhold classification of a color as partially or wholly melanin-based before the appropriate biochemical tests are conducted”6. How much more should this caution be applied to extinct organisms?
Methods
Feather and microbial preparations
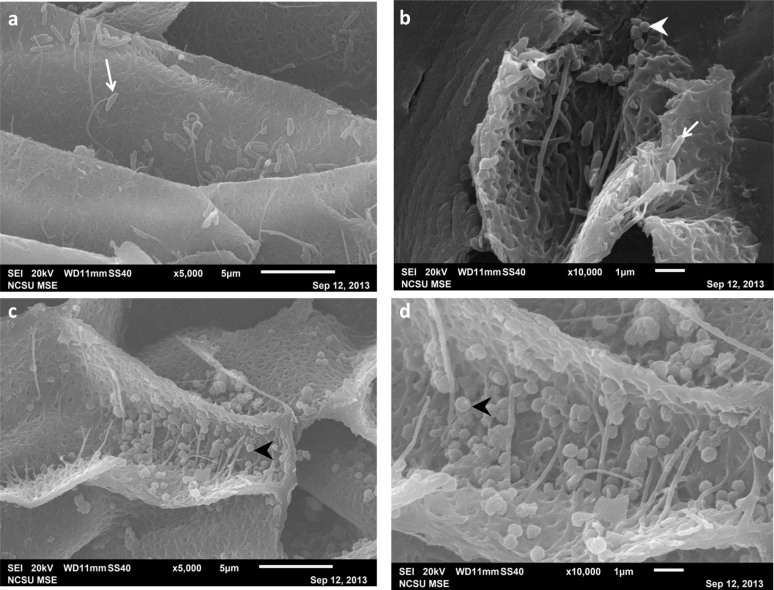
A culture from an environmental sample of pond water (Bozeman, Montana) was grown in brain heart infusion broth (BHI) on pigmented feathers taken from Numida meleagris prepared using aseptic techniques. Biofilms were air dried, coated with ~5 Å of iridium, and visualized using FESEM (Zeiss Supra 55VP).
Pigmented and non-pigmented chicken feathers (Gallus gallus domesticus) were collected from the Poultry Teaching Unit at North Carolina State University. (All experiments involving live vertebrates were performed in accordance with relevant guidelines and regulations of North Carolina State University.) Primary and secondary flight feathers were plucked and placed in clean Ziploc® bags. Feathers were incubated in 5% tryptic soy broth inoculated with Bacillus cereus (ATTC 14579) for three days at room temperature with agitation, then fixed in 10% formalin. Feathers of each type were reserved in 10% formalin without inoculation and used as negative controls.
Fossil feather specimen
For more information on the geologic context of the fossil feather specimen (ANSP 23403) ascribed to Gansus yumenensis see the Supplementary Methods section online.
Scanning electron microscopy (SEM) and SEM with energy dispersive x-ray spectroscopy (SEM-EDX)
For whole mount/surface analysis, G. gallus feathers were gently washed in E-pure water and air dried.
To view melanosomes, approximately ~1 mm sections were taken from G. gallus feathers, and washed in phosphate buffered saline, dehydrated in two changes of 70% ethanol for 30 minutes with rocking followed by a one hour incubation of (2:1) LR white: 70% ethanol. Samples were then incubated for one hour each in two changes of 100% LR white, followed by a third incubation overnight. Feathers were placed in gelatin capsules with the long axis parallel to the length of the capsule, filled with LR white and covered to exclude oxygen. Resin was polymerized for 24 hours at 60°C. A Leica EMUC6 ultra-microtome with a Diatome 45° knife was used to cut 5 and 10 μm longitudinal (parallel to long axis of the barb) sections.
Samples were mounted on double-sided carbon tape and imaged using a JEOL JSM-6010LA analytical SEM controlled by JEOL InTouchScope version 1.05A software. Some images (Fig. 3 and Supplementary Fig. S1b1, b3, c2 and c3) were captured after applying a 3–6 nm gold/palladium coating. All EDS data of the uncoated fossil feather sample were collected at 20 kV accelerating voltage, a working distance of 10 mm and for 100–120 seconds.
Transmission electron microscopy (TEM)
Samples for TEM were prepared as described above for visualizing melanosomes in SEM. One sample of B. cereus-treated black chicken feather was embedded directly in LR white following fixation, eliminating all dehydration and penetration steps (Fig. 4). Cross sections were taken at 90 nm with a diamond knife on a Leica EMUC6 ultra-microtome, mounted on 200 mesh copper grids, and imaged using an Erlangshen ES1000W Model 785 TEM coupled to a CCD 11Megapixel High-speed Digital Camera, and analyzed using Gatan Microscopy Suite (GMS) software. Some sections were stained with 15% methanolic uranyl acetate and Reynolds' lead citrate (Fig. 1a–c and Fig. 4a–b).
Images of B. cereus were obtained from culture growth as indicated above, diluted by 50% and applied directly to a Formvar-coated 200 mesh nickel grid followed by negative staining with 1% phosphotungstic acid (Fig. 1d) or 0.5% uranyl acetate (Fig. 1e).
Author Contributions
A.E.M., E.A.J. and W.Z. performed data collection and research. D.L. and M.C.L. collected and described specimens of Gansus yumenensis. K.J.L. provided the geologic description of the Gansus site and performed initial analyses on the fossil specimen. A.E.M. and M.H.S. wrote the manuscript.
Supplementary Material
Supplementary Information
Acknowledgments
We thank the NCSU Poultry Education Unit, Toby Tung and the NCSU Materials Science and Engineering Department and Jeanette Shipley-Phillips and the NCSU Veterinary School Imaging Center. We also thank Amy Grunden, Lindsay Zanno and Timothy Cleland for their discussion and editorial comments. This research is supported by the National Science Foundation Graduate Research Fellowship (DGE-1252376) to A.E.M. and the David and Lucille Packard Foundation to M.H.S. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
References
- Davis P. G. & Briggs D. E. G. Fossilization of feathers. Geology 23, 783–786 (1995). [Google Scholar]
- Liebig K., Westall F. & Schmitz M. A study of fossil microstructures from the Eocene Messel Formation using transmission electron microscopy. N. Jb. Geol. Paläont. Mh. 4, 218–231 (1996). [Google Scholar]
- Franzen J. L. Exceptional preservation of Eocene vertebrates in the lake deposit of Grube Messel (West Germany). Philos. Trans. R. Soc. B Biol. Sci. 311, 181–186 (1985). [Google Scholar]
- Vinther J., Briggs D. E. G., Prum R. O. & Saranathan V. The colour of fossil feathers. Biol. Lett. 4, 522–525 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks M. S. & Seabra M. C. The melanosome: membrane dynamics in black and white. Nat. Rev. Mol. Cell Biol. 2, 738–748 (2001). [DOI] [PubMed] [Google Scholar]
- McGraw K. J. in Bird Color. Mech. Meas. (Hill, G. E. & McGraw, K. J.) 243–294 (Harvard University Press, 2006). [Google Scholar]
- Carney R. M., Vinther J., Shawkey M. D., D'Alba L. & Ackermann J. New evidence on the colour and nature of the isolated Archaeopteryx feather. Nat. Commun. 3, 637 (2012). [DOI] [PubMed] [Google Scholar]
- Clarke J. A. et al. Fossil evidence for evolution of the shape and color of penguin feathers. Science 330, 954–957 (2010). [DOI] [PubMed] [Google Scholar]
- Li Q. et al. Reconstruction of Microraptor and the evolution of iridescent plumage. Science 335, 1215–1219 (2012). [DOI] [PubMed] [Google Scholar]
- Li Q. et al. Plumage color patterns of an extinct dinosaur. Science 327, 1369–1372 (2010). [DOI] [PubMed] [Google Scholar]
- Vinther J., Briggs D. E. G., Clarke J., Mayr G. & Prum R. O. Structural coloration in a fossil feather. Biol. Lett. 6, 128–131 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q. et al. Melanosome evolution indicates a key physiological shift within feathered dinosaurs. Nature; 10.1038/nature12973 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang F. et al. Fossilized melanosomes and the colour of Cretaceous dinosaurs and birds. Nature 463, 1075–1078 (2010). [DOI] [PubMed] [Google Scholar]
- Pacton M., Fiet N. & Gorin G. E. Bacterial activity and preservation of sedimentary organic matter: the role of exopolymeric substances. Geomicrobiol. J. 24, 571–581 (2007). [Google Scholar]
- Westall F. et al. Early Archean fossil bacteria and biofilms in hydrothermally-influenced sediments from the Barberton greenstone belt, South Africa. Precambrian Res. 106, 93–116 (2001). [Google Scholar]
- Vollmer W., Blanot D. & De Pedro M. A. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 32, 149–167 (2008). [DOI] [PubMed] [Google Scholar]
- Westall F. The nature of fossil bacteria: a guide to the search for extraterrestrial life. J. Geophys. Res. Planets 104, 16437–16451 (1999). [Google Scholar]
- Liebig K. Fossil Microorganisms from the Eocene Messel Oil Shale of Southern Hesse, Germany. (1997). [Google Scholar]
- Toporski J. K. W. et al. Morphologic and spectral investigation of exceptionally well-preserved bacterial biofilms from the Oligocene Enspel Formation, Germany. Geochim. Cosmochim. Acta 66, 1773–1791 (2002). [Google Scholar]
- Westall F., Boni L. & Guerzoni E. The experimental silicification of microorganisms. Palaeontology 48, 495–528 (1995). [Google Scholar]
- Cadena E. & Schweitzer M. Variation in osteocytes morphology vs bone type in turtle shell and their exceptional preservation from the Jurassic to the present. Bone 51, 614–620 (2012). [DOI] [PubMed] [Google Scholar]
- Schweitzer M. H., Wittmeyer J. L., Horner J. R. & Toporski J. K. Soft-tissue vessels and cellular preservation in Tyrannosaurus rex. Science 307, 1952–1955 (2005). [DOI] [PubMed] [Google Scholar]
- Schweitzer M. H. et al. Biomolecular characterization and protein sequences of the Campanian hadrosaur B. canadensis. Science 324, 626–631 (2009). [DOI] [PubMed] [Google Scholar]
- Henwood A. Exceptional preservation of dipteran flight muscle and the taphonomy of insects in amber. Palaios 7, 203–212 (1992). [Google Scholar]
- d'Ischia M. et al. Melanins and melanogenesis: methods, standards, protocols. Pigment Cell Melanoma Res. 26, 616–633 (2013). [DOI] [PubMed] [Google Scholar]
- Simon J. D., Hong L. & Peles D. N. Insights into melanosomes and melanin from some interesting spatial and temporal properties. J. Phys. Chem. B 112, 13201–13217 (2008). [DOI] [PubMed] [Google Scholar]
- Hollingworth N. T. J. & Barker M. J. in Process. Foss. (Donovan, S. K.) 105–119 (Columbia University Press, 1991). [Google Scholar]
- Flemming H.-C. & Wingender J. The biofilm matrix. Nat. Rev. Microbiol. 8, 623–633 (2010). [DOI] [PubMed] [Google Scholar]
- Plonka P. M. & Grabacka M. Melanin synthesis in microorganisms—biotechnological and medical aspects. Acta Biochim Pol 53, 429–443 (2006). [PubMed] [Google Scholar]
- Field D. J. et al. Melanin concentration gradients in modern and fossil feathers. PLoS One 8, e59451 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara M. E., Briggs D. E. G., Orr P. J., Field D. J. & Wang Z. Experimental maturation of feathers: implications for reconstructions of fossil feather colour. Biol. Lett. 9, 1–6 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barden H. E. et al. Morphological and geochemical evidence of eumelanin preservation in the feathers of the Early Cretaceous bird, Gansus yumenensis. PLoS One 6, e25494 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight T. K., Bingham P. S., Lewis R. D. & Savrda C. E. Feathers of the Ingersoll Shale, Eutaw Foramtion (Upper Cretaceous), eastern Alabama: the largest collection of feathers from North American Mesozoic rocks. Palaios 26, 364–376 (2011). [Google Scholar]
- Ma F. Late Mesozoic fossil fishes from the Jiuquan Basin of Gansu Province, China. 118 (Ocean Press, 1993). [Google Scholar]
- You H. et al. A nearly modern amphibious bird from the Early Cretaceous of northwestern China. Science 312, 1640–1643 (2006). [DOI] [PubMed] [Google Scholar]
- Grande J. M., Negro J. J. & Torres M. J. The evolution of bird plumage colouration: a role for feather-degrading bacteria? Ardeola 51, 375–383 (2004). [Google Scholar]
- Goldstein G. et al. Bacterial degradation of black and white feathers. Auk 121, 656–659 (2004). [Google Scholar]
- Gunderson A. R., Frame A. M., Swaddle J. P. & Forsyth M. H. Resistance of melanized feathers to bacterial degradation: is it really so black and white? J. Avian Biol. 39, 539–545 (2008). [Google Scholar]
- Brinkmann V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004). [DOI] [PubMed] [Google Scholar]
- Gaill F., Desbruyères D. & Prieur D. Bacterial communities associated with “Pompei worms” from the East Pacific rise hydrothermal vents: SEM, TEM observations. Microb. Ecol. 13, 129–139 (1987). [DOI] [PubMed] [Google Scholar]
- Schleifer K. H. & Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol. Rev. 36, 407–477 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren J. et al. Molecular preservation of the pigment melanin in fossil melanosomes. Nat. Commun. 3, 1–7 (2012). [DOI] [PubMed] [Google Scholar]
- Wogelius R. A. et al. Trace metals as biomarkers for eumelanin pigment in the fossil record. Science 333, 1622–1626 (2011). [DOI] [PubMed] [Google Scholar]
- McGraw K. J. Melanins, metals, and mate quality. Oikos 102, 402–406 (2003). [Google Scholar]
- Waldron K. J. & Robinson N. J. How do bacterial cells ensure that metalloproteins get the correct metal? Nat. Rev. Microbiol. 7, 25–35 (2009). [DOI] [PubMed] [Google Scholar]
- White C., Wilkinson S. C. & Gadd G. M. The role of microorganisms in biosorption of toxic metals and radionuclides. Int. Biodeterior. Biodegrad. 35, 17–40 (1995). [Google Scholar]
- Brandelli A., Daroit D. & Riffel A. Biochemical features of microbial keratinases and their production and applications. Appl. Microbiol. Biotechnol. 85, 1735–1750 (2010). [DOI] [PubMed] [Google Scholar]
- Fujii T. & Li D. Preparation and properties of protein films and particles from chicken feather citation export. J. Biol. Macromol. 8, 48–55 (2008). [Google Scholar]
- Fraser R. D. B. & Parry D. A. D. Molecular packing in the feather keratin filament. J. Struct. Biol. 162, 1–13 (2008). [DOI] [PubMed] [Google Scholar]
- Manning P. L. et al. Synchrotron-based chemical imaging reveals plumage patterns in a 150 million year old early bird. J. Anal. At. Spectrom. 28, 1024–1030 (2013). [Google Scholar]
- Sawyer R. H. & Knapp L. W. Avian skin development and the evolutionary origin of feathers. J. Exp. Zool. Part B Mol. Dev. Evol. 298B, 57–72 (2003). [DOI] [PubMed] [Google Scholar]
- Lindgren J. et al. Skin pigmentation provides evidence of convergent melanism in extinct marine reptiles. Nature 10.1038/nature12899 (2014). [DOI] [PubMed] [Google Scholar]
- Glass K. et al. Direct chemical evidence for eumelanin pigment from the Jurassic Period. Proc. Natl. Acad. Sci. 109, 10218–10223 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass K. et al. Impact of diagenesis and maturation on the survival of eumelanin in the fossil record. Org. Geochem. 64, 29–37 (2013). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information