Abstract
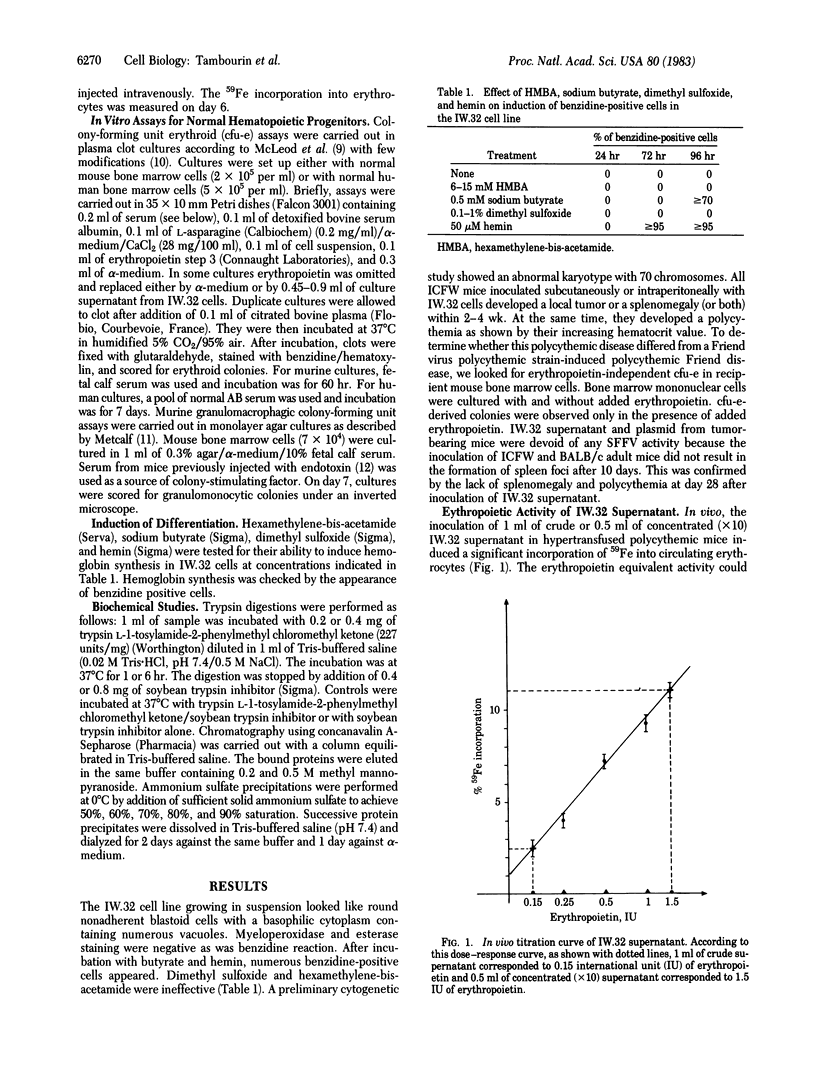
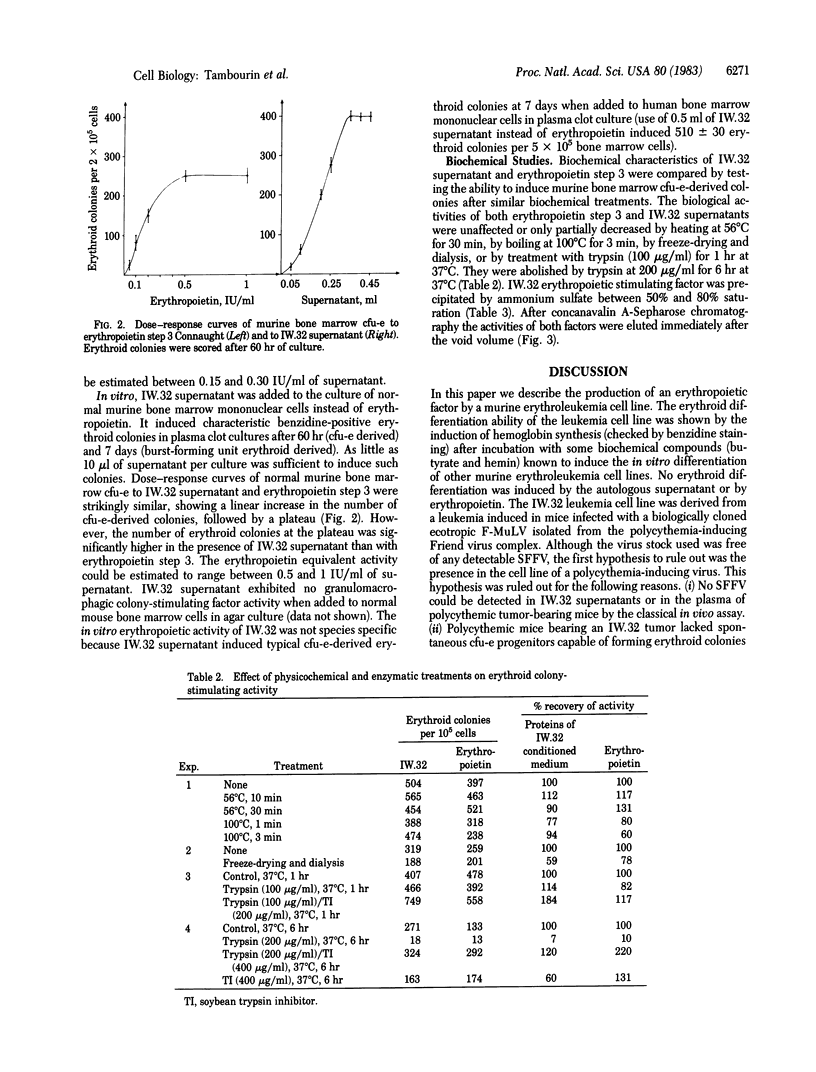
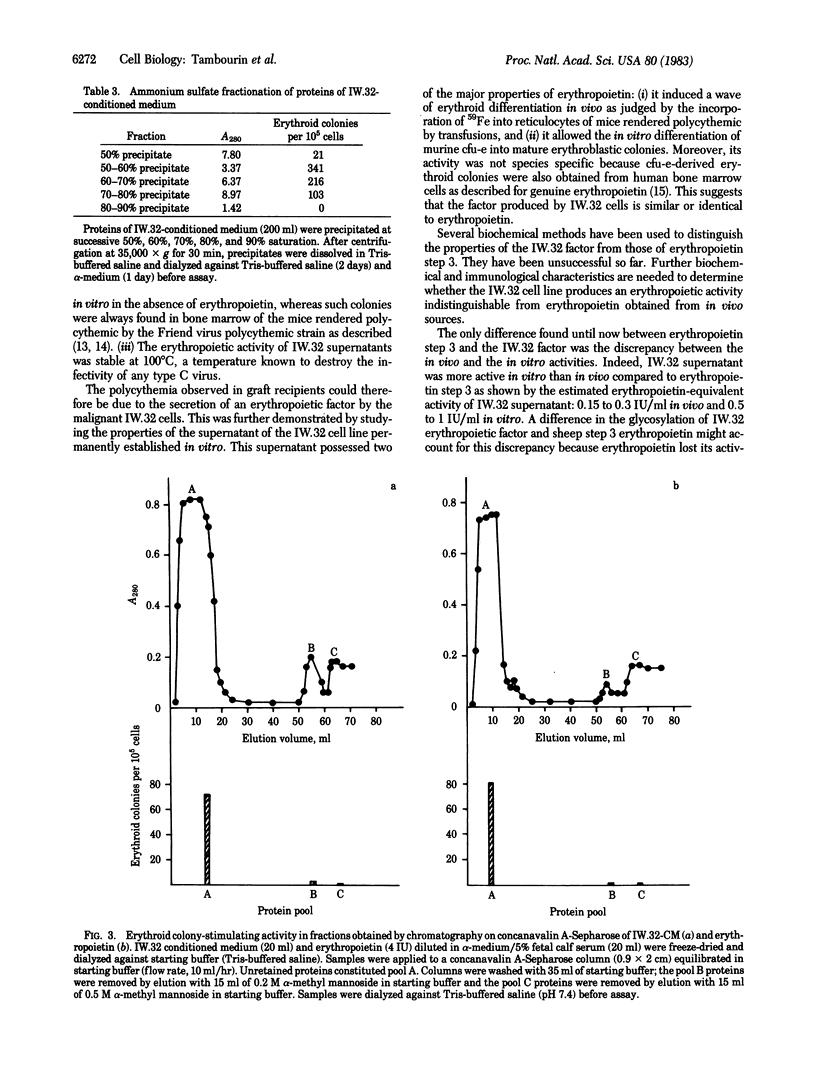
A transplantable murine leukemia, primarily induced by a biologically cloned Friend helper virus, was shown to induce polycythemia in recipient ICFW mice. A leukemia cell line (IW.32) was established in vitro from this transplantable leukemia. Sodium butyrate and hemin induced erythroid differentiation in these leukemia cells as has already been shown with other erythroleukemia cells. The supernatant of this cell line was devoid of spleen focus-forming virus activity. However, it induced the incorporation of 59Fe in polycythemic mice and the in vitro differentiation of murine and human cfu-e into erythroid colonies. Therefore, these erythroleukemia cells produced a factor with all the biological properties of erythropoietin. The erythropoietic activity of IW.32 supernatant was higher in vitro [equivalent to 0.5-1 international unit (IU) of erythropoietin per ml] than in vivo (0.15-0.3 IU/ml). This erythropoietin-like activity was stable at 100 degrees C for 3 min, which ruled out the possibility that a virus was responsible for these effects. Preliminary studies demonstrated that the biochemical properties of the IW.32 factor are strongly similar to those of Connaught step 3 erythropoietin, thus supporting the hypothesis that the IW.32 factor is indeed an erythropoietin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELRAD A. A., STEEVES R. A. ASSAY FOR FRIEND LEUKEMIA VIRUS: RAPID QUANTITATIVE METHOD BASED ON ENUMERATION OF MACROSCOPIC SPLEEN FOCI IN MICE. Virology. 1964 Nov;24:513–518. doi: 10.1016/0042-6822(64)90199-0. [DOI] [PubMed] [Google Scholar]
- Ascensao J. L., Kay N. E., Earenfight-Engler T., Koren H. S., Zanjani E. D. Production of erythroid potentiating factor(s) by a human monocytic cell line. Blood. 1981 Jan;57(1):170–173. [PubMed] [Google Scholar]
- Fagg B. Is erythropoietin the only factor which regulates late erythroid differentiation? Nature. 1981 Jan 15;289(5794):184–186. doi: 10.1038/289184a0. [DOI] [PubMed] [Google Scholar]
- Golde D. W., Bersch N., Quan S. G., Lusis A. J. Production of erythroid-potentiating activity by a human T-lymphoblast cell line. Proc Natl Acad Sci U S A. 1980 Jan;77(1):593–596. doi: 10.1073/pnas.77.1.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootenberg J. E., Ruscetti F. W., Mier J. W., Gazdar A., Gallo R. C. Human cutaneous T cell lymphoma and leukemia cell lines produce and respond to T cell growth factor. J Exp Med. 1981 Nov 1;154(5):1403–1418. doi: 10.1084/jem.154.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard J. M., Fichelson S., Choppin J., Varet B. Autocrine function of murine F-MuLV induced myeloblastic cell lines. Int J Cancer. 1983 Mar 15;31(3):337–344. doi: 10.1002/ijc.2910310314. [DOI] [PubMed] [Google Scholar]
- Horoszewicz J. S., Leong S. S., Carter W. A. Friend leukemia: rapid development of erythropoietin-independent hematopoietic precursors. J Natl Cancer Inst. 1975 Jan;54(1):265–267. doi: 10.1093/jnci/54.1.265. [DOI] [PubMed] [Google Scholar]
- Johnson G. R., Metcalf D. Pure and mixed erythroid colony formation in vitro stimulated by spleen conditioned medium with no detectable erythropoietin. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3879–3882. doi: 10.1073/pnas.74.9.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe C., Casadevall N., Varet B. Polycythaemia vera: in vitro studies of circulating erythroid progenitors. Br J Haematol. 1980 Feb;44(2):189–199. doi: 10.1111/j.1365-2141.1980.tb01201.x. [DOI] [PubMed] [Google Scholar]
- Liao S. K., Axelrad A. A. Erythropoietin-independent erythroid colony formation in vitro by hemopoietic cells of mice infected with friend virus. Int J Cancer. 1975 Mar 15;15(3):467–482. doi: 10.1002/ijc.2910150313. [DOI] [PubMed] [Google Scholar]
- Mathieu-Mahul D., Heard J. M., Fichelson S., Gisselbrecht S., Sola B., Larsen C. J. Viral expression in two myelomonocytic cell lines obtained from long-term bone marrow culture infected with the Friend polycythemia-inducing virus (FV-P). Virology. 1982 May;119(1):59–67. doi: 10.1016/0042-6822(82)90065-4. [DOI] [PubMed] [Google Scholar]
- McLeod D. L., Shreeve M. M., Axelrad A. A. Improved plasma culture system for production of erythrocytic colonies in vitro: quantitative assay method for CFU-E. Blood. 1974 Oct;44(4):517–534. [PubMed] [Google Scholar]
- Metcalf D. Acute antigen-induced elevation of serum colony stimulating factor (CFS) levels. Immunology. 1971 Sep;21(3):427–436. [PMC free article] [PubMed] [Google Scholar]
- Oliff A., Ruscetti S., Douglass E. C., Scolnick E. Isolation of transplantable erythroleukemia cells from mice infected with helper-independent Friend murine leukemia virus. Blood. 1981 Aug;58(2):244–254. [PubMed] [Google Scholar]
- Rich I. N., Heit W., Kubanek B. Extrarenal erythropoietin production by macrophages. Blood. 1982 Oct;60(4):1007–1018. [PubMed] [Google Scholar]
- Schooley J. C., Mahlmann L. J. Inhibition of the biologic activity of erythropoietin by neuraminidase in vivo. J Lab Clin Med. 1971 Nov;78(5):765–770. [PubMed] [Google Scholar]
- Shibuya T., Mak T. W. Host control of susceptibility to erythroleukemia and to the types of leukemia induced by Friend murine leukemia virus: initial and late stages. Cell. 1982 Dec;31(2 Pt 1):483–493. doi: 10.1016/0092-8674(82)90141-6. [DOI] [PubMed] [Google Scholar]



