Abstract
Fat distribution is closely linked to metabolic disease risk. Distribution varies with sex, genetic background, disease state, certain drugs and hormones, development, and aging. Preadipocyte replication and differentiation, developmental gene expression, susceptibility to apoptosis and cellular senescence, vascularity, inflammatory cell infiltration, and adipokine secretion vary among depots, as do fatty-acid handling and mechanisms of enlargement with positive-energy and loss with negative-energy balance. How interdepot differences in these molecular, cellular, and pathophysiological properties are related is incompletely understood. Whether fat redistribution causes metabolic disease or whether it is a marker of underlying processes that are primarily responsible is an open question.
Introduction
The prevalence of obesity has increased dramatically in recent years. The clinical description of obesity has largely been based on measurements, such as body mass index (BMI), that gauge total body fat. Over the past 50 years, scientists have recognized that not all adipose tissue is alike (reviewed in Arner, 2005) and that health risk is associated with the location as well as the amount of body fat. Different depots are sufficiently distinct with respect to fatty-acid storage and release as to probably play unique roles in human physiology. The major anatomical fat depots include intra-abdominal (omental and mesenteric depots, also termed visceral fat), lower-body (gluteal fat, subcutaneous leg fat, and intramuscular fat), and upper-body subcutaneous fat (Figure 1). Within the trunk, Scarpa’s fascia separates superficial and deep abdominal subcutaneous fat. Deep subcutaneous fat accumulation is correlated with visceral fat accumulation (Kelley et al., 2000). Fat distribution varies remarkably between sexes, among individuals and families, with aging and disease states, and in response to drugs and hormones. Central obesity is associated with increased risk for diabetes, hypertension, atherosclerosis, dyslipidemia, cancers, and mortality compared to peripheral obesity (reviewed in Shuster et al., 2012). Even humans of a normal weight with a high ratio of central-to-peripheral fat have an increased likelihood of being insulin resistant (Kahn et al., 2001).
Figure 1. Anatomy of Major Fat Depots in Rodents and Humans.
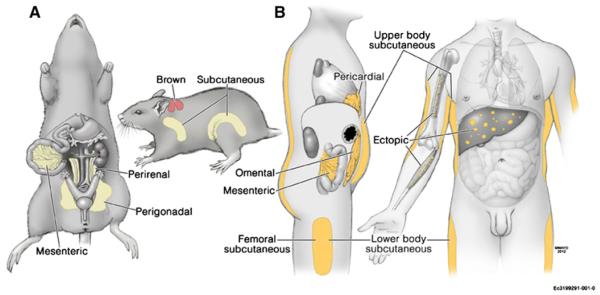
Several different names for particular fat depots in rodents (A) and humans (B) are used, as are different groupings of fat depots for physiological and clinical studies. The names and anatomies of the fat depots reviewed here are indicated.
We begin by reviewing fat-tissue function and its cellular basis. We examine the role of variations in cellular composition and function, adipokine secretion, and fatty-acid storage and release among depots. We conclude by discussing controversies about whether functional characteristics of different fat depots contribute to distinct effects on human physiology and whether intra-abdominal fat is a cause or consequence of systemic metabolic dysfunction.
Fat-Tissue Function
The principal function of adipose tissue is to store and release fat in response to energy-balance needs. Adipose tissue also has immune, endocrine, regenerative, mechanical, and thermal functions (reviewed in Thomou et al., 2010). Both the fuel and nonfuel functions of adipose tissue vary among depots, with depot size, and with body-fat distribution. Potentially, when dysregulation of fatty-acid storage and release occurs in upper-body obesity, fatty-acid overflow into “ectopic” sites leads to lipotoxicity (Tchkonia et al., 2006a). In addition to their role as major sources of cellular fuel, fatty acids can serve as signaling molecules in the form of diacylglycerols, ceramides, and long-chain acyl-coenzymes A. These molecules can exert adverse effects on cell function, including interference with insulin signaling, when present in excess.
Fat is situated under the epidermis and around vital organs, where it plays immunologically defensive and mechanically protective roles (Cousin et al., 1999; Duffaut et al., 2009). Once inflamed, adipose tissue shifts from storing to releasing fatty acids, potentially driven in part through local proinflammatory cytokine release (Faty et al., 2012; Zhang et al., 1996). The proinflammatory response of fat tissue to bacterial antigens such as lipopolysaccharide may combine with high local concentrations of fatty acids during ensuing cytokine-induced lipolysis to mitigate infection (Chung et al., 2006; Desbois and Smith, 2010; Tchkonia et al., 2006a; Thomou et al., 2010). Obesity, aging, and lipodystrophies are associated with sustained fat-tissue immune-response activation, proinflammatory cytokine release, impaired insulin responsiveness, reduced incorporation of fatty acid as triglycerides, and increased lipolysis (Tchkonia et al., 2010; Thomou et al., 2010). This contributes to low-grade “sterile” systemic inflammation, metabolic dysregulation, and lipotoxicity, with different fat depots potentially contributing in distinct ways.
Cellular Mechanisms of Fat Growth and Function
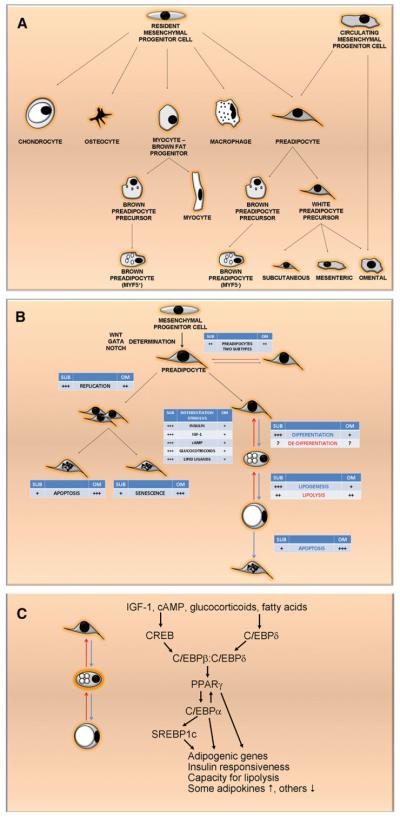
New fat cells appear throughout life (Spalding et al., 2008;Tchoukalova et al., 2010). There is controversy and some confusion about nomenclature for the progenitor cells in stromalvascular digests of fat tissue that give rise to new fat cells (see Supplemental Information available online; Cawthorn et al., 2012). In this review, these cells are collectively termed “preadipocytes.” They account for 15 to 50 percent of cells in fat tissue, perhaps the largest pool of progenitors in humans, facilitating regenerative responses to nutrient excess and injury. Preadipocytes tend to be associated with blood vessels and may be derived from fat-tissue endothelial cells or pericytes (Gupta et al., 2012; Tang et al., 2008; Tran et al., 2012). They are multipotent, appearing to be capable of differentiating into macrophages, muscle or bone progenitors, brown fat, and other cell types (Figure 2A; Charrière et al., 2003; Rodriguez et al., 2004;Schulz et al., 2011; Zuk et al., 2002). Preadipocytes generate adipokines, paracrine factors, hormones, and metabolic signals in a manner distinct from fat cells. Several adipokines are secreted in a fat-depot-dependent fashion (Table 1). Like macrophages, preadipocytes exhibit robust innate immune responses to bacterial antigens, recruit macrophages and other immune effectors, and participate in initiating and regulating fat-tissue immune activation (Gustafson et al., 2009; Lacasa et al., 2007; Thomou et al., 2010). Preadipocyte gene-expression profiles are closer to those of macrophages, into which they may be able to convert, than to those of fat cells (Charrière et al., 2003; Prunet-Marcassus et al., 2006).
Figure 2. Regional Variation in Fat-Tissue Cell Dynamics.
(A) Regional variation in fat-tissue cell dynamics. Preadipocytes arise from resident multipotent mesenchymal progenitor cells, which are generally associated with blood vessels and may be related to endothelial cells or pericytes, and can possibly become committed to brown fat, myocyte, osteocyte, chondrocyte, or macrophage lineages (see Supplemental Information for a note regarding nomenclature). Circulating progenitors may contribute, especially to visceral fat development (Crossno et al., 2006).
(B) Committed preadipocytes can replicate, differentiate into adipocytes, or possibly revert into multipotent progenitors again. At least two interconvertible preadipocyte subtypes exist; one is more capable of replication, differentiation, and resistance of apoptosis than the other. Preadipocytes can be induced to differentiate by IGF-1 and other stimuli. Differentiated fat cells vary lipid content through esterifying exogenous fatty acids to glycerol, de novo lipogenesis, or fatty-acid release through lipolysis.
(C) Key transcription factors involved in adipogenesis. IGF-1 and other signals combine to induce adipogenesis, with early increases in cyclic AMP (cAMP)-and protein kinase A-mediated phosphorylation and activation of CREB, which along with GSK3β contributes to activation of C/EBPβ. Activated C/EBPβ forms homodimers or heterodimers with glucocorticoid-induced C/EBPδ that enhance PPARγ expression and activity. Upon binding to a lipid ligand, as well as to RXRα and its retinoid ligands, PPARγ transactivates C/EBPα, the other major adipogenic transcription factor. PPARγ and C/EBPα cooperate to maintain their own expression, activate SREBP1c (ADD1 in mice), and, together with SREBP1c, transactivate around 2,500 downstream differentiation-dependent genes. This leads to the acquisition of capacities for adipogenesis, lipid storage, insulin responsiveness, and lipolysis; increased secretion of some adipokines (e.g., leptin) and decreased secretion of others (e.g., PAI-1); and changes in extracellular matrix component production, micro-RNAs, histones, and chromatin structure (reviewed in Cristancho and Lazar, 2011; Tang and Lane, 2012).
Table 1.
Adipokines Reported to Exhibit Fat-Depot-Specific Expression
| Adipokine or Secreted Factor |
Source Cells | Depot | References |
|---|---|---|---|
| Leptin* | fat cells | subcutaneous > omental | (Wiest et al., 2010) |
| Adiponectin* HMW |
fat cells | omental > subcutaneous | (Kovacova et al., 2012) |
| PAI-1 | preadipocytes | possibly, omental > subcutaneous |
(Xu et al., 2012) |
| IL-6* | preadipocytes, macrophages, activated endothelial cells, large fat cells |
visceral > subcutaneous | (Fontana et al., 2007) |
| TNF-α* | preadipocytes, macrophages, adipocytes |
mesenteric > omental = subcutaneous |
(Cartier et al., 2008; Xu et al., 2012) |
| MCP-1* | preadipocytes, macrophages | visceral > subcutaneous | (Madani et al., 2009; Miller et al., 2011) |
| Angiotensinogen | fat tissue | omental > subcutaneous | (Dusserre et al., 2000; van Harmelen et al., 2000) |
| RANTES* | stromal vascular fraction, fat cells |
gastric fat pad > omental = subcutaneous |
(Madani et al., 2009) |
| CSF-1 | endothelial cells, fibroblasts | visceral > subcutaneous | (Harman-Boehm et al., 2007) |
| Omentin | stromal vascular cells | omental > subcutaneous | (Yang et al., 2006) |
| RBP4 | preadipocytes, adipocytes | visceral < subcutaneous | (Kos et al., 2011) |
| Chimerin | unknown | visceral < subcutaneous | (Alfadda et al., 2012) |
| Vaspin | fat cells | visceral > subcutaneous | (Hida et al., 2005; Klöting et al., 2006) |
Many adipokines are secreted by adipose tissue. Those that reportedly exhibit regional variation in expression or secretion are included. The cell types in which they are expressed are indicated. Secretion of several of these adipokines (marked by *) has been verified in studies of arteriovenous concentration gradients across adipose depots. Expression or secretion of several of these adipokines is affected by body mass index, fat cell size, or gender in addition to the adipose depot. The following abbreviations were used: HMW, high molecular weight isoform; PAI-1, plasminogen-activated inhibitor 1; IL-6, interleukin-6; TNF-α, tumor necrosis factor α; MCP-1, monocyte chemoattractant protein 1; RANTES, regulated on activation, normal T cell expressed and secreted; CSF-1, colony-stimulating factor 1; and RBP4, retinol binding protein 4.
Preadipocytes differentiate into fat cells in response to insulin-like growth factor-1 (IGF-1), lipids, glucocorticoids, and other signals (Figure 2B; Cristancho and Lazar, 2011). IGF-1, rather than insulin, is probably the main driver of adipogenesis, given that insulin receptors are not highly expressed until preadipocytes are partially differentiated (Cawthorn et al., 2012). A cascade involving CCAAT/enhancer binding proteins (C/EBPs), peroxisome proliferator-activated receptor γ (PPARγ), and other transcription factors orchestrates changes in expression of around 2,500 genes during adipogenesis (Figure 2C; reviewed in Tang and Lane, 2012).
At least two preadipocyte subtypes are present in rodent and human fat (Prunet-Marcassus et al., 2006; Tchkonia et al., 2005). The first is capable of more extensive replication, differentiation, and adipogenic transcription-factor expression and less apoptosis in response to tumor necrosis factor α (TNF-α) than the second. Both subtypes develop in colonies cultured from single preadipocytes, confirming that both are within the adipocyte lineage and that they can interconvert. The adipogenesis-resistant subtype may sustain the progenitor pool, because its presence ensures that not all preadipocytes become fat cells under conditions favoring adipogenesis. The subtypes might facilitate tissue plasticity, for example, through differentiation into fat cells with distinct properties or through selection for the apoptosis-resistant subtype by increased local inflammatory cytokines (Tchkonia et al., 2005). More investigation is needed for determining the functional relevance of preadipocyte subtypes and whether they account for the two fat cell populations that differ in size observed in rodents and humans (Blüher et al., 2004; Julien et al., 1989).
Preadipocytes can undergo cellular senescence, particularly with aging and obesity (Minamino et al., 2009; Tchkonia et al., 2010). Senescent cells release proinflammatory cytokines, chemokines, and extracellular matrix proteases, termed the senescence-associated secretory phenotype, or SASP (reviewed in Freund et al., 2010). Preadipocytes from old rats are proinflammatory and express SASP components (Cartwright et al., 2010; Tchkonia et al., 2010). Interleukin-6 (IL-6), plasminogen-activator inhibitor 1 (PAI-1), and other SASP components are much more highly expressed in senescent cells isolated by fluorescence-activated cell sorting from the stromal-vascular fraction of mouse adipose tissue than in neighboring nonsenescent cells (Baker et al., 2011). When senescent cells are eliminated from older mice, age-related fat-tissue loss is delayed or prevented, confirming the relevance of senescent cells to age-related fat-tissue dysfunction (Baker et al., 2011).
Regional Differences in Preadipocyte Characteristics
Replication
During weight gain, different fat depots enlarge via hyperplasia, hypertrophy, or both (Tchoukalova et al., 2010). New adipocytes can be generated more rapidly in some depots than others (Figure 3). Modest femoral subcutaneous fat tissue enlargement in response to overfeeding in young adults is due to increases in cell number, not size. The opposite is true for moderate amounts of abdominal subcutaneous fat gain. At least in rats, visceral fat mass changes principally through adjustments in adipocyte size, rather than number (DiGirolamo et al., 1998). Regional differences in preadipocyte replication, differentiation, subtype abundance, susceptibility to apoptosis or senescence, and gene expression may contribute to regional variation in fat-tissue function (Figure 2B; Tchkonia et al., 2010), a speculation that requires more research to test. It is noteworthy that genome-wide association studies suggest that body-fat distribution, as measured by waist-hip ratio, is associated with variations in genes involved in pattern formation during embryonic development, angiogenesis, preadipocyte signaling, and adipocyte development, all pointing toward heritable differences in adipocyte characteristics (Heid et al., 2010). Other gene variations associated with waist-hip ratio include those in the insulin signaling, lipase-activity, lipid-biosynthesis, and intracellular-calcium signaling pathways. These relationships are independent of BMI and differ between women and men.
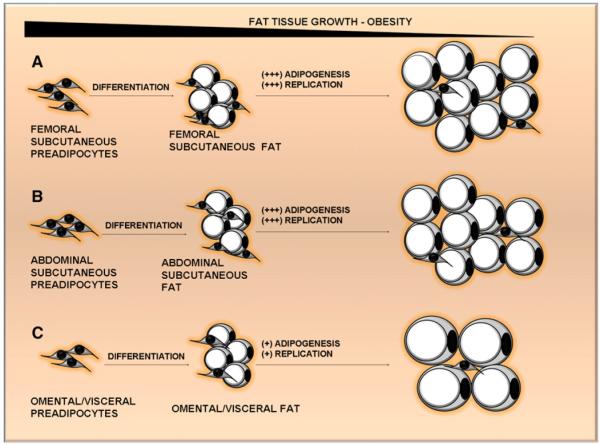
Figure 3. Mechanisms of Fat-Tissue Growth during the Progression of Obesity Vary among Depots.
Some regions of human subcutaneous fat, which is specialized to provide long-term nutrient storage, grow through increases in fat cell number, such as femoral fat (A), and others grow through increases in fat cell size, such as abdominal subcutaneous fat (B). Intraperitoneal fat—the omental depot, for example (C)—generally enlarges through increases in fat cell size rather than number, consistent with its role in storing and releasing nutrients rapidly and its limited space for growth. These interdepot differences are related to higher potentials of subcutaneous than visceral preadipocytes for replication and adipogenesis.
Differences in cell-dynamic properties of preadipocytes are partly cell autonomous. Strains derived from single human or rodent preadipocytes from different depots and cultured under identical conditions retain distinct capacities for adipogenesis and replication, susceptibilities to apoptosis, and developmental gene-expression patterns after many generations, despite originating from the same individuals (Adams et al., 1997; Gesta et al., 2006; Kirkland et al., 1990, 1996; Tchkonia et al., 2002, 2006b, 2007; Wang et al., 1989). For example, rat perirenal preadipocytes are capable of more extensive replication than epididymal preadipocytes (Kirkland et al., 1990; Wang et al., 1989). These differences remain evident for at least 3 weeks in colonies arising from single perirenal or epididymal preadipocytes (Kirkland et al., 1990). The greater replicative potential of rat perirenal than epididymal preadipocytes is reflected in bigger increases in perirenal fat cell numbers during development in vivo (Wang et al., 1989). In humans, colonies derived from individual abdominal subcutaneous preadipocytes are capable of more extensive replication on average than omental clones are, with mesenteric preadipocytes being intermediate (Tchkonia et al., 2006b, 2005; Van Harmelen et al., 2004). Abundance of the more rapidly replicating preadipocyte subtype is greater in subcutaneous than omental preadipocyte populations (Tchkonia et al., 2005). These regional differences in subtype abundance persisted even after subcloning. Consistent with the greater replicative potential of subcutaneous than omental preadipocytes, telomeres are shorter in subcutaneous than in omental cells (Tchkonia et al., 2006b). Stable expression of human telomere reverse transcriptase (hTERT) in single abdominal subcutaneous and omental human preadipocytes (in order to increase survival in culture) showed that, after 35 population doublings, the rate of subcutaneous clonal replication remained 2.5-fold higher than that of omental replication (Tchkonia et al., 2006b). Thus, regional differences in preadipocyte replicative potential are heritable over many cell generations, do not depend on the presence of other cell types, and are independent of telomerase activity. It should be noted that individual preadipocytes isolated from, for example, subcutaneous fat sometimes behave more like cells characteristic of omental fat, just as some individual cells from old subjects behave like typical cells from the young (Kirkland et al., 1990; Tchkonia et al., 2002, 2006b). Characteristics at the cell-population level are determined by the aggregate behavior of individual clones.
Adipogenesis
Expansion in times of sustained nutrient excess is most effectively achieved through increases in fat cell number, a mechanism especially prominent during fat-tissue expansion in human femoral subcutaneous depots (Tchoukalova et al., 2010). Human preadipocyte capacity for adipogenesis appears to vary among depots in most studies (Adams et al., 1997; Digby et al., 2000; Hauner et al., 1988; Macotela et al., 2012; Montague et al., 1998; Niesler et al., 1998; Tchkonia et al., 2002, 2006b), but not all (Shahparaki et al., 2002; Van Harmelen et al., 2004). Discrepancies might be due to variability among subjects, the point at which differentiation was assessed, or culture conditions. Where differences were found, abdominal subcutaneous preadipocytes had a greater capacity for adipogenesis than omental cells. As with replication, regional differences in adipogenesis are evident in colonies derived from individual preadipocytes (Tchkonia et al., 2002), as well as strains made from single subcutaneous, mesenteric, and omental hTERT-expressing preadipocytes after 40 population doublings (Tchkonia et al., 2006b). If left long enough in differentiation-promoting medium, omental preadipocytes eventually catch up to subcutaneous cells, obscuring regional differences evident earlier during differentiation (Tchkonia et al., 2002). Abdominal subcutaneous human preadipocytes tend to differentiate to a greater extent in response to thiazolidinediones (TZDs) than omental preadipocytes do (Adams et al., 1997; Digby et al., 2000; Hauner et al., 1988; Montague et al., 1998; Tchkonia et al., 2002). Consistent with this, administering TZDs promotes more subcutaneous than visceral fat accumulation (Mori et al., 1999).
PPARg and C/EBPα expression is higher in human abdominal subcutaneous than in omental differentiating preadipocytes (Tchkonia et al., 2002, 2006b, 2007). Within clones, C/EBPα is increased in those cells that acquire lipid inclusions, irrespective of depot origin, suggesting that regional variation in adipogenesis depends on adipogenic transcription-factor expression and upstream mechanisms (Tchkonia et al., 2002). In rats, interdepot differences in cultured preadipocyte-differentiation-dependent gene expression are reflected in patterns of fat-tissue expression of the same genes (Kirkland et al., 1996). Despite exposure to hormonal manipulations in vivo, such as estrogen treatment, hypophysectomy, or castration, preadipocytes cultured from various depots of rats retain differences in adipogenesis, consistent with these differences being cell autonomous (Kirkland et al., 1992; Lacasa et al., 1997).
Apoptosis and Senescence
Apoptotic fat cells are more abundant in human omental than abdominal subcutaneous fat (Niesler et al., 1998). Omental preadipocytes are more susceptible to apoptosis induced by serum deprivation or TNF-α (Niesler et al., 1998; Tchkonia et al., 2002; Tchkonia et al., 2006b). Differential susceptibility to TNF-α-induced apoptosis is retained in hTERT-expressing omental and subcutaneous strains. Like replication and adipogenesis, mechanisms underlying regional variation in apoptosis appear to be partly cell autonomous.
Both senescent endothelial cells and preadipocytes accumulate in adipose tissue with obesity in a depot-dependent manner (Minamino et al., 2009; Tchkonia et al., 2009, 2010; Villaret et al., 2010). Although visceral preadipocytes are more proinflammatory than intraperitoneal cells, the trajectory of age-related increases in SASP components is greater in extra- than intraperitoneal preadipocytes, consistent with the earlier age-related loss of subcutaneous fat compared to intra-abdominal fat (Cart-wright et al., 2010). Additionally, although macrophage infiltration is more extensive in visceral than in subcutaneous fat, the trajectory of the increase in subcutaneous macrophages with aging is greater in subcutaneous than in visceral fat (Einstein et al., 2010), paralleling depot-dependent changes in SASP macrophage chemokines with aging (Cartwright et al., 2010). Inflammatory cytokines, including TNF-α, that result from the SASP and macrophage infiltration are profoundly antiadipogenic (Zhang et al., 1996). Thus, cellular senescence may contribute to regional variation in age- and obesity-related increases in fat-tissue inflammation, glucose intolerance, loss of capacity to store fat in subcutaneous depots, and lipotoxicity (Tchkonia et al., 2010).
Inherent Differences
IGF-1 is an important driver of adipogenesis (Cawthorn et al., 2012; Cristancho and Lazar, 2011). Consistent with the greater capacity for differentiation of subcutaneous than omental preadipocytes, AKT phosphorylation in response to IGF-1 is higher in subcutaneous cells, despite similar tyrosine phosphorylation of IGF-1 receptors (Cleveland-Donovan et al., 2010). Serine phosphorylation of IRS-1, which impedes the capacity of IRS-1 to transduce insulin and IGF-1 signaling, is more extensive in omental than subcutaneous preadipocytes, potentially contributing to the insulin resistance and reduced adipogenic potential of omental fat.
Genome-wide expression profiles of primary preadipocytes cultured in parallel from different depots of mice and humans are highly distinct (Gesta et al., 2006; Tchkonia et al., 2007). Expression of >500 genes varies significantly among human abdominal subcutaneous, omental, and mesenteric preadipocytes and fat tissue. Around 25% of transcripts whose expression varies among depots in both humans and rodents are developmental regulators, the gene category that differs most (Gesta et al., 2011, 2006; Karastergiou et al., 2013; Tchkonia et al., 2007). Prominent among these is the homeobox (HOX) superfamily, which consists of master regulators of developmental processes controlling the diversification of segments along the anteroposterior axis of animals. Particular HOX gene combinations, the HOX code, are expressed in adult cells based on their embryonic origin. Other genes that regulate important developmental pathways also vary, including secreted frizzled-related protein 2 (SFRP2), pregnancy-associated plasma protein A-1 (PAPP-A1), transcription factor T-box 15 (TBX15), and engrailed 1 (En-1) (Gesta et al., 2006; Tchkonia et al., 2007). Depot-specific developmental gene-expression signatures persist for 40 population doublings in hTERT clones and remain evident in fat cells. The mesodermal developmental regulator, Tbx15, is expressed at a >250-fold higher level in rodent subcutaneous cells than in epididymal cells and affects adipogenesis when overexpressed (Gesta et al., 2011). There is emerging evidence that genes important for adipogenesis, including C/EBPα, are regulated by HOX epigenetic networks (Huang et al., 2012). Whether developmental regulators directly cause regional differences in preadipocyte and fat-depot characteristics is being actively investigated.
Most developmental genes are subject to epigenetic regulation via DNA cytosine methylation and histone lysine methylation. Cytosine methylation is associated with transcriptional repression through many cell divisions, whereas histone methylation can result in either inherent activation or repression of transcription (reviewed in Bernstein et al., 2007). The HOX code is regulated by cellular memory mechanisms involving histone H3 lysine di- and trimethylation patterns that span large areas. Other developmental genes that differ among depots, such as EN-1 and SHOX2, are controlled by DNA cytosine methylation (Schmidt et al., 2010), whereas SFRP2 is subject to H3 lysine-4 trimethylation for transcriptional activation (Sharma et al., 2010). Epigenetic mechanisms, including DNA methylation or histone modifications, appear to be subject to metabolic control (for example, maternal undernutrition in rodents) (Raychaudhuri et al., 2008). HOXA5, TBX15, and EN1 change in response to systemic metabolic challenges, including obesity, in humans (Gesta et al., 2006). A set of HOX genes (HOXA5, HOXA9, and EMX2) are induced in response to extreme weight loss after bariatric surgery (Dankel et al., 2010), in tandem with downregulation of inflammatory genes. Whether regional differences in developmental gene expression are caused by metabolically regulated epigenetic mechanisms needs to be determined.
Regional differences in preadipocyte cell-dynamic properties and gene expression could contribute to variation in function among depots. Consistent with this, interdepot differences in adipogenesis predict which depots enlarge during development in rats (Wang et al., 1989). Differences in PPARγ and C/EBPα expression appear to predict interdepot variation in the extent of new-adipocyte accumulation that is a result of increased caloric intake in humans (Tchoukalova et al., 2010). Furthermore, fat tissue transplanted into different depots exerts an impact on systemic metabolism characteristic of its anatomic origin (Tran et al., 2008). Additional work is needed for establishing how inherent properties of fat progenitors affect systemic metabolic function and, particularly, whether this represents an opportunity for developing new therapeutic interventions.
Regional Variation in Adipokines
Different fat depots have distinct glycoprotein, adipokine, and paracrine factor secretion profiles and capacities to activate or respond to hormones (Table 1). Changes in preadipocyte inflammatory cytokine and chemokine production due to obesity and aging are, in many cases, fat depot dependent (Cartwright et al., 2010; Tchkonia et al., 2010; Thomou et al., 2010; Xu et al., 2012). Given that preadipocyte adipokine and paracrine factor profiles differ from those of fat cells (Table 1; reviewed in Thomou et al., 2010), regional variation in preadipocyte numbers relative to differentiated fat cell numbers probably also adds to interdepot differences in secretory profiles. Furthermore, macrophage abundance varies up to 7-fold among depots (Zhang et al., 2009), which probably contributes to regional variation in cytokine secretion. Macrophage infiltration is associated with insulin resistance, metabolic syndrome, and morbidity (Cancello et al., 2006; Harman-Boehm et al., 2007). Macrophage infiltration into omental fat, but not subcutaneous fat, is associated with obesity comorbidity and severity of steatosis (Cancello et al., 2006). Because macrophage characteristics vary among organs (Murray and Wynn, 2011), fat-tissue macrophages could differ from other tissue-specific macrophage subtypes and might even vary among depots. T lymphocyte subsets, mast cells, and humoral immune-response elements are involved in the fat-tissue inflammation associated with obesity (Liu et al., 2009; Nishimura et al., 2009). Whether these immune elements or other types of cells vary among fat depots needs further investigation.
Preadipocytes have toll-like receptors for bacterial antigens and proinflammatory cytokines (Vitseva et al., 2008), as well as functional receptors for TNF-α and neuropeptides, such as substance P (Karagiannides et al., 2006) and neurotensin (Koon et al., 2009). Substance P and neurotensin induce expression of proinflammatory cytokines, including IL-6 and I6 8 in preadipocytes, as well as activating NF-κB, at least in mesenteric preadipocytes. Regional differences in abundance of TNF-α receptor 1 have been found (Xu et al., 2012). More information about regional variation in other cytokine receptors and in neuropeptide and proinflammatory signaling pathways is needed.
Brown Fat and Regional Differences in Fat-Tissue Function
Brown adipose tissue generates energy from triglycerides (Seale et al., 2009). Abundance of brown fat, which is concentrated in the neck and upper chest of human adults, is inversely correlated with BMI (Cypess et al., 2009). Brown-fat progenitors may be related to white-fat preadipocytes, muscle cells, and vascular endothelial-like cells (Figure 2A; Gupta et al., 2012; Tran et al., 2012). Preadipocytes in white fat and muscle can become committed to the brown-fat lineage through induction involving bone morphogenetic protein 7 (BMP-7) (Schulz et al., 2011). Human abdominal subcutaneous white-fat preadipocytes have greater brown-adipocyte lineage commitment potential following BMP-7 induction than preadipocytes isolated from mesenteric or omental white depots. Whether brown or “beige” fat cells within different depots have distinct properties needs to be investigated.
Fat Distribution, Adipose-Tissue Blood Flow, and Metabolic Dysfunction
Postabsorptive adipose-tissue blood flow is relatively low compared to other tissues, ranging from 1.5 to 10.0 ml × 100 g−1 × min−1 depending upon the population studied and the method used to make the measurement (Funada et al., 2010; Romanski et al., 2000; Virtanen et al., 2002). Both abdominal and femoral adipose-tissue blood flow increase remarkably following meal ingestion (Romanski et al., 2000) and in response to adrenergic stimulation (Ardilouze et al., 2012). Adipose blood flow is slightly greater in visceral fat than subcutaneous fat (Virtanen et al., 2002). There is some evidence that women may have more increased femoral adipose-tissue blood flow in the postprandial period than men do (Romanski et al., 2000). Obesity, insulin resistance, and endothelial dysfunction are associated with reduced fasting and meal-stimulated adipose-tissue blood flow (Funada et al., 2010), which may not be entirely harmful. Lean adults can increase subcutaneous adipose blood flow by more than 5-fold following meal ingestion; in someone with 15 kg of fat, this would result in an increase in adipose blood flow of over 1 l/min. If a similar proportionate increase in blood flow were to occur in someone with 50 kg of adipose tissue, cardiac output would need to almost double in order to maintain perfusion of other organs. The extent to which adipose-tissue blood flow is rate limiting with regards to nutrient delivery is not clear in most conditions.
Regional Differences in Adipose-Tissue Fatty-Acid Storage
Functional properties differ among the major human fat regions: intra-abdominal, lower-body, and upper-body subcutaneous fat (Figure 1). Storage of dietary fatty acids is generally more efficient in upper-body than in lower-body subcutaneous fat in normal-weight volunteers who consume appropriately calibrated meals (Romanski et al., 2000). Likewise, dietary fat is stored more efficiently in visceral than in upper- or lower-body subcutaneous fat (Jensen et al., 2003). Together, subcutaneous and visceral fat store ~50% of dietary fat in sedentary adults, and the remainder is oxidized within the first 24 hr following meal ingestion (Jensen et al., 2003; Romanski et al., 2000). In obesity, regional differences in dietary fat storage are more profound (Santosa et al., 2008; Votruba et al., 2007). Women with increased leg fat store more dietary fat in this depot. This does not occur in visceral fat. Furthermore, the pattern of dietary fat storage relative to fat mass differs considerably between upper-body subcutaneous, visceral, and leg fat. Obese men store less dietary fat in subcutaneous depots than obese women do, especially lower-body-obese women. The cellular and molecular basis for these differences is an open area for investigation.
Free Fatty Acids
Adipose tissue can take up very low density lipoprotein triglyceride (VLDL-TG) and free fatty acids (FFA) directly (Koutsari et al., 2011, 2012). Direct storage of systemic FFA into adipose tissue in the postabsorptive state is remarkably different between men and women and among depots. Women store a substantially greater portion of systemic FFA in lower-body fat than men do. Surprisingly, patterns of regional FFA release largely mirror meal fatty acid, but VLDL-TG fatty acid and direct FFA storage do not.
Regional Differences in Adipose-Tissue FFA Release
Much of the basic information regarding regional differences in adipocyte lipolysis was gained using sophisticated in vitro models (Arner, 2005). These results prompted a series of whole-body, in vivo studies designed to quantify how these in vitro differences translated into human physiology. We found that overnight postabsorptive FFA release per kg of fat is greater from upper-body than lower-body subcutaneous adipose tissue in both men and women (Jensen, 1995). Upper-body subcutaneous adipose tissue FFA release accounts for the majority (>60%) of systemic FFA under basal (Nielsen et al., 2004) and insulin-suppressed conditions (Guo et al., 1999; Meek et al., 1999). Lipolysis is markedly accelerated by exercise in both upper-and lower-body subcutaneous fat (Burguera et al., 2000). Leg adipose-tissue lipolysis contributes ~15%–20% of basal, systemic FFA release in lean adults (Jensen, 1995; Meek et al., 1999) and ~28% in obese adults (Guo et al., 1999; Nielsen et al., 2004). With hyperinsulinemia (Meek et al., 1999) and meal ingestion (Guo et al., 1999; Jensen, 1995), leg FFA release is more readily suppressed than release from upper-body subcutaneous fat. Hepatic vein FFA, a surrogate measure of visceral adipose lipolysis, appears resistant to insulin’s antilipolytic effects (Jensen, 1995; Meek et al., 1999). Only 6%–17% of systemic FFA comes from the splanchnic bed under overnight postabsorptive conditions, but up to 40% during hyperinsulinemia (Jensen, 1995; Meek et al., 1999). This indicates either that visceral fat is very resistant to insulin’s antilipolytic effects compared to subcutaneous fat or that spillover of fatty acids from hydrolysis of triglyceride-rich lipoproteins is a special issue in the splanchnic bed (Nelson et al., 2007). In either case, the liver is probably exposed to higher FFA concentrations than the periphery during hyperinsulinemia, and this portal-systemic difference may be exaggerated in upper-body/visceral obesity (Ali et al., 2011).
Fat-Tissue Lipolysis
The most consistent finding regarding fat-tissue lipolysis is that much greater FFA release occurs during hyperinsulinemia in upper-body/visceral obesity compared with the nonobese or lower-body-obese state (Guo et al., 1999). Interestingly, individually cultured omental differentiated preadipocytes have greater FFA flux than subcutaneous clones do (Caserta et al., 2001), suggesting that a partially cell-autonomous mechanism could contribute. Excess postprandial FFA release results in elevated FFA concentrations (~3-fold greater in upper- than lower-body obesity [Guo et al., 1999]). Because leg adipose tissue lipolysis is so sensitive to insulin (Meek et al., 1999) and meal suppression (Guo et al., 1999; Jensen, 1995), it does not contribute to the elevated postprandial FFA in obesity. Higher postprandial FFA concentrations in upper- than in lower-body obesity are almost entirely accounted for by excess FFA release from upper-body subcutaneous fat (Guo et al., 1999). This implies that adipocytes in upper-body obesity are resistant to the antilipolytic effects of insulin. The explanation for this resistance is unclear. This may be related to enlarged abdominal or visceral adipocytes in upper-body obesity being inherently resistant to insulin, with large adipocytes having greater rates of lipolysis than small adipocytes, even when they are harvested from the same depot (Laurencikiene et al., 2011). In adults with severe obesity, large omental adipocytes are more predictive of metabolic abnormalities than are large abdominal subcutaneous adipocytes (Hoffstedt et al., 2010). Lower-body obesity, which is much more often associated with greater insulin sensitivity with regards to glucose metabolism, is also associated with greater adipose-tissue insulin sensitivity with regards to suppression of lipolysis (Guo et al., 1999). Given the known adverse effects of elevated FFA on insulin action (Roden et al., 1996), it is possible that the greater adipose-tissue insulin sensitivity in lower-body obesity allows greater muscle and hepatic insulin sensitivity. Certainly weight loss through diet and exercise, which reduces fat cell size, also improves insulin regulation of lipolysis (Shadid and Jensen, 2006), whereas liposuction (no decrease in fat cell size) does not (Klein et al., 2004).
A Special Role for Leg Fat?
The role of leg fat in metabolic function is of growing interest. In addition to the well-known epidemiological association between lower-body fat distribution and improved metabolic health, leg fat appears to serve an important role in disposing of excess dietary fat in women (Votruba and Jensen, 2006). Because adipose tissue lipolysis in leg fat is normally exquisitely sensitive to insulin (Meek et al., 1999), this makes lower-body depots an ideal place to store fat when it is ingested in excess of short-term energy needs. More recently, it has been suggested that leg fat may even contribute to metabolic health via production of the purported lipokine, palmitoleic acid (Pinnick et al., 2012).
In summary, excess visceral fat is associated with but is not the predominant source of excess FFA in humans. Dysregulated release of FFA from upper-body subcutaneous fat is a hallmark of visceral obesity and probably contributes to lipotoxicity. Greater amounts of leg fat signal a lesser metabolic risk and a more normal fatty-acid profile, although whether leg fat plays a protective role or (opposite of visceral fat) signals generally normal function of subcutaneous fat remains to be determined. The relations between these functional differences and regional variations in cellular composition, preadipocyte properties, and cytokine release are poorly understood. Potential local modulators of adipose function with regards to fat storage and release include abnormal perfusion, accumulation of inflammatory cells, or paracrine or autocrine effects of adipocytes themselves.
Mesenteric Fat: An Underappreciated Role?
Mesenteric fat may make an important contribution to metabolic dysfunction, acting in a manner distinct from omental fat (Catalano et al., 2010; Liu et al., 2006). Mesenteric fat is the first depot to encounter lipids as they travel within chylomicrons from the gut through lymphatics to join the circulation at the thoracic duct and vena cava, bypassing the liver. These lipids, especially saturated fatty acids, might trigger signaling events and innate immune responses in the various mesenteric fat cell types. In obese women, lipid synthesis and lipolysis differ between omental and mesenteric fat (Edens et al., 1993; Fried et al., 1993). Glucocorticoid deficiency in hydroxysteroid dehydrogenase 1 (HSD-1) knockout (KO) mice protects against visceral obesity by reducing inflammation and preserving β-oxidation in mesenteric fat, but not in other depots (Wamil et al., 2011). The mesentery contains autonomic nervous fibers that connect the spinal ganglia to the gut, potentially linking the nervous system and mesenteric fat. Substance P, a product of sensory neurons, has profound proinflammatory and mitogenic effects on mesenteric preadipocytes, potentially contributing to the development of creeping fat in inflammatory bowel disease (IBD; Gross et al., 2009; Karagiannides et al., 2006). In patients with IBD, mesenteric fat lineage cells themselves, as opposed to macrophages or endothelial cells, are the major source of TNF-α, whereas little TNF-α was observed in either the subcutaneous fat of these patients or the mesenteric or subcutaneous fat of healthy controls (Fink et al., 2012). Mesenteric substance P has recently been implicated in the genesis of insulin resistance (Karagiannides et al., 2011a, 2011b).
The cellular and gene-expression properties of human mesenteric fat are cell-autonomously distinct from omental fat (Tchkonia et al., 2002, 2006b, 2007). However, both depots contribute to intra-abdominal fat as measured radiographically in clinical trials, raising the possibility that distribution of visceral fat between the mesenteric and omental depots may have as-yet-unexplained clinical implications. There is emerging evidence indicating that mesenteric fat is an independent determinant of metabolic syndrome and correlates with thickness of the intima and media of the carotid arteries in humans (Liu et al., 2006). In support of the speculation that mesenteric fat makes a contribution to metabolic function, mice with genetically increased lifespan (Ames, Snell, growth hormone receptor KO, and PAPP-A KO) have substantially reduced mesenteric fat, yet retain perigonadal fat (J.L.K. and T. Tchkonia, unpublished data; rodents have very little omental fat—rodent perigonadal fat is roughly equivalent in function to omental fat in humans). The improved insulin responsiveness that results from calorically restricting obese rats is associated with reductions in mesenteric fat, but not other fat depots (Catalano et al., 2010). A closer link between mesenteric fat and metabolic syndrome than between omental fat and metabolic syndrome could explain why removing the omentum appears to have little effect on glucose tolerance in humans (see the section “Intra-Abdominal Fat: Cause or Indicator?” below). The effects of targeting mesenteric fat on glucose responsiveness remain to be investigated.
Metabolically Protective Role of Subcutaneous Fat
Certain subcutaneous fat regions appear to be metabolically, immunologically, and mechanically protective. Subcutaneous fat, which can expand outward without the anatomic constraints that limit visceral fat growth, is specialized to provide long-term fuel storage, acting as a sink to sequester potentially lipotoxic fatty acids (Kirkland et al., 2003; Tchkonia et al., 2006a; Thomou et al., 2010). Consistent with its role in energy storage, subcutaneous fat is the major source of leptin, which signals the state of lipid stores to the brain (Table 1). Dysfunctional subcutaneous fat is associated with visceral fat enlargement, systemic inflammation, and lipotoxicity (Tchkonia et al., 2006a). Interestingly, subcutaneous fat abundance increases when visceral fat is removed from experimental animals (Mauer et al., 2001). When subcutaneous fat is removed, visceral fat mass, insulin resistance, circulating insulin, and TNF-α increase. This may contribute to the visceral fat enlargement that occurs in tandem with subcutaneous fat loss in lipodystrophies and aging or subcutaneous fat dysfunction in obesity. Reimplantation of healthy subcutaneous fat reverses these effects (Ishikawa et al., 2006). Transplantation of inguinal fat into other subcutaneous regions, or into the abdominal cavity especially, improves the metabolic profile, whereas transplanting epididymal fat does not (Tran et al., 2008). The beneficial effects of subcutaneous transplantation do not depend on adiponectin. These findings are consistent with the hypothesis that subcutaneous fat dysfunction is more important than omental fat in the etiology of metabolic syndrome, a hypothesis that merits further testing.
In obesity, visceral fat generally enlarges through increases in fat cell size, whereas subcutaneous fat can enlarge through increases in fat cell size or number (Figure 3; DiGirolamo et al., 1998; Tchoukalova et al., 2010), which is perhaps related to the greater capacities of subcutaneous preadipocytes for replication and adipogenesis compared to those of visceral cells from the same subjects. Subcutaneous fat is not uniform: femoral subcutaneous fat undergoes increases in fat cell numbers within a few weeks during overfeeding, whereas abdominal subcutaneous fat does not (Tchoukalova et al., 2010). Furthermore, loss of leg fat during negative-energy balance appears to be proportionately lesser than that of other depots, and whereas leg-fat gain results in the accumulation of new fat cells, leg-fat loss does not result in the loss of those new cells, at least over the short term (Singh et al., 2012). In line with this, femoral fat is more metabolically protective than abdominal subcutaneous fat (Koster et al., 2010). Together with the much greater mass of subcutaneous compared to visceral fat, the high capacity for certain subcutaneous regions to generate new fat cells may prevent visceral fat enlargement and systemic lipotoxicity (Tchkonia et al., 2006a).
Intra-abdominal Fat: Cause or Indicator?
Central fat is associated with elevated risk for diabetes, hypertension, atherosclerosis, dyslipidemia, and cancers (reviewed in Shuster et al., 2012) Whether intra-abdominal fat is a cause or consequence of metabolic dysfunction is controversial. Nutrient overload induces both subcutaneous and visceral fat enlargement, but visceral depot size increases rapidly and then plateaus, whereas subcutaneous fat increases steadily as obesity develops (Bergman et al., 2006). In normal-weight or moderately overweight people, visceral obesity is strongly associated with insulin resistance, but in severe obesity, it is a weak independent predictor (Stefan et al., 2008). A pilot study suggested that omentectomy might improve metabolic function in those undergoing the bariatric surgery procedure of laparoscopic banding (Thörne et al., 2002). Subsequent studies have failed to confirm that removal of omental fat from obese, diabetic humans improved glucose tolerance after 4 months or provided added benefit to patients undergoing bariatric surgery (Fabbrini et al., 2010; Herrera et al., 2010). However, removal of small amounts of omental fat from healthy, nonobese dogs might improve insulin sensitivity (Lottati et al., 2009). Removal of intra-abdominal fat from young rats increased lifespan and prevented age-related insulin resistance (Ben-Shlomo et al., 2012; Muzumdar et al., 2008). Removing subcutaneous fat from hamsters had the opposite effect (Weber et al., 2000).
The lack of a clear benefit from removing omental fat from obese, metabolically compromised humans despite pronounced effects of removing intra-abdominal fat from rodents could be due to species differences. Also, intra-abdominal fat may need to be removed before rather than after the development of obesity or metabolic dysfunction to have beneficial effects. The association between metabolic dysfunction and visceral obesity could be due to underlying processes that predispose to both visceral obesity and metabolic dysfunction, rather than constituting a direct causal relationship (Fabbrini et al., 2010). Alternatively, inherent differences among fat depots in fat cell lineage characteristics, such as cytokine secretory profiles, may be responsible (Heilbronn et al., 2004).
Conclusions
Fat is not homogeneous. Depot-dependent differences among preadipocytes, from which new fat cells arise, appear to be inherent. Regional differences are apparent in rats, mice, and humans, indicating evolutionary conservation. Given that multiple cell-dynamic processes and developmental gene expression vary among depots, it appears preadipocytes from different depots are effectively distinct cell subtypes. These inherent mechanisms, combined with local variation in fat-depot cellular composition, circulation, and neurological and other factors, probably account for regional differences in fat-tissue size and function. Sex, obesity, or other factors also have fat-depot-dependent effects on cellular composition, the paracrine micro-environment, and adipose function. Thus, different fat depots are separate miniorgans.
Further research is needed for defining the mechanisms responsible for regional differences in fat-tissue function, determining whether and how regional differences cause systemic dysfunction and disease, and developing mechanism-based interventions accordingly. Immediate questions include whether visceral obesity is a manifestation of a general process that leads to systemic metabolic dysfunction, whether it is causal, or whether subcutaneous fat dysfunction initiates both visceral obesity and metabolic disease. Evidence is mounting in favor of the latter, but this requires more investigation. Furthermore, the roles of particular visceral and subcutaneous depots need to be determined (e.g., mesenteric versus omental and femoral versus abdominal subcutaneous).
Some currently available interventions target specific fat depots. Liposuction and cosmetic surgery can be used to reduce areas of subcutaneous fat but induce increased visceral fat (Mauer et al., 2001). It may be feasible to develop antiobesity drugs with region-specific effects, given that existing agents can do so, including TZDs, sex steroids, and glucocorticoids (reviewed in Thomou et al., 2010). Risks associated with central obesity might be reduced if pharmacological or transplantation approaches could be developed that would restore the function of abnormal subcutaneous fat, convert visceral fat depots into fat with properties more closely resembling those of healthy subcutaneous depots, or, if intra-abdominal depots (e.g., mesenteric fat) do indeed prove to be causally linked to metabolic syndrome, to target these depots. To aid in the rational development of such interventions, we must understand the mechanisms responsible for regional obesity and its link to metabolic dysfunction in greater depth.
Subcutaneous fat tissue dysfunction, with failed adipogenesis, decreased lipid-storage capacity, and inflammation, could lead to ectopic fat deposition, with expansion of visceral fat as an indicator rather than the cause of lipotoxicity. Therefore, enhancing subcutaneous fat tissue function is potentially a better approach for treating metabolic syndrome than eliminating visceral fat. Indeed, TZDs may work this way. If further experimental results indicate that this speculation has merit, screening for compounds that enhance subcutaneous preadipocyte replication and adipogenesis might lead to effective treatments for preventing complications of metabolic syndrome.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful for the administrative support of L. Wadum and J. Armstrong. This work was funded by NIH grants AG41122 (J.K.), AG13925 (J.K.), AG31736 (J.K.), DK50456 (J.K. and M.D.J.), DK40484 (M.D.J.), DK45343 (M.D.J.), DK47343 (C.P.), DK60729 (C.P.), and DK86150 (C.P.); the Noaber, Ellison, and Broad Medical Foundations; and the Crohn’s and Colitis Foundation of America.
Footnotes
SUPPLEMENTAL INFORMATION Supplemental Information includes a note on the nomenclature of fat cell progenitors and Supplemental References and can be found with this article online at http://dx.doi.org/10.1016/j.cmet.2013.03.008.
REFERENCES
- Adams M, Montague CT, Prins JB, Holder JC, Smith SA, Sanders L, Digby JE, Sewter CP, Lazar MA, Chatterjee VK, O’Rahilly S. Activators of peroxisome proliferator-activated receptor gamma have depot-specific effects on human preadipocyte differentiation. J. Clin. Invest. 1997;100:3149–3153. doi: 10.1172/JCI119870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfadda AA, Sallam RM, Chishti MA, Moustafa AS, Fatma S, Alomaim WS, Al-Naami MY, Bassas AF, Chrousos GP, Jo H. Differential patterns of serum concentration and adipose tissue expression of chemerin in obesity: adipose depot specificity and gender dimorphism. Mol. Cells. 2012;33:591–596. doi: 10.1007/s10059-012-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AH, Koutsari C, Mundi M, Stegall MD, Heimbach JK, Taler SJ, Nygren J, Thorell A, Bogachus LD, Turcotte LP, et al. Free fatty acid storage in human visceral and subcutaneous adipose tissue: role of adipocyte proteins. Diabetes. 2011;60:2300–2307. doi: 10.2337/db11-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardilouze JL, Sotorník R, Dennis LA, Fielding BA, Frayn KN, Karpe F. Failure to increase postprandial blood flow in subcutaneous adipose tissue is associated with tissue resistance to adrenergic stimulation. Diabetes Metab. 2012;38:27–33. doi: 10.1016/j.diabet.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Arner P. Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract. Res. Clin. Endocrinol. Metab. 2005;19:471–482. doi: 10.1016/j.beem.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shlomo S, Einstein FH, Zvibel I, Atias D, Shlomai A, Halpern Z, Barzilai N, Fishman S. Perinephric and epididymal fat affect hepatic metabolism in rats. Obesity (Silver Spring) 2012;20:151–156. doi: 10.1038/oby.2011.261. [DOI] [PubMed] [Google Scholar]
- Bergman RN, Kim SP, Catalano KJ, Hsu IR, Chiu JD, Kabir M, Hucking K, Ader M. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity (Silver Spring) 2006;14(Suppl 1):16S–19S. doi: 10.1038/oby.2006.277. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell. 2007;128:669–681. doi: 10.1016/j.cell.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Blüher M, Patti ME, Gesta S, Kahn BB, Kahn CR. Intrinsic heterogeneity in adipose tissue of fat-specific insulin receptor knock-out mice is associated with differences in patterns of gene expression. J. Biol. Chem. 2004;279:31891–31901. doi: 10.1074/jbc.M404569200. [DOI] [PubMed] [Google Scholar]
- Burguera B, Proctor D, Dietz N, Guo Z, Joyner M, Jensen MD. Leg free fatty acid kinetics during exercise in men and women. Am. J. Physiol. Endocrinol. Metab. 2000;278:E113–E117. doi: 10.1152/ajpendo.2000.278.1.E113. [DOI] [PubMed] [Google Scholar]
- Cancello R, Tordjman J, Poitou C, Guilhem G, Bouillot JL, Hugol D, Coussieu C, Basdevant A, Bar Hen A, Bedossa P, et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes. 2006;55:1554–1561. doi: 10.2337/db06-0133. [DOI] [PubMed] [Google Scholar]
- Cartier A, Lemieux I, Alméras N, Tremblay A, Bergeron J, Després JP. Visceral obesity and plasma glucose-insulin homeostasis: contributions of interleukin-6 and tumor necrosis factor-alpha in men. J. Clin. Endocrinol. Metab. 2008;93:1931–1938. doi: 10.1210/jc.2007-2191. [DOI] [PubMed] [Google Scholar]
- Cartwright MJ, Schlauch K, Lenburg ME, Tchkonia T, Pirtskhalava T, Cartwright A, Thomou T, Kirkland JL. Aging, depot origin, and preadipocyte gene expression. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65:242–251. doi: 10.1093/gerona/glp213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta F, Tchkonia T, Civelek VN, Prentki M, Brown NF, McGarry JD, Forse RA, Corkey BE, Hamilton JA, Kirkland JL. Fat depot origin affects fatty acid handling in cultured rat and human preadipocytes. Am. J. Physiol. Endocrinol. Metab. 2001;280:E238–E247. doi: 10.1152/ajpendo.2001.280.2.E238. [DOI] [PubMed] [Google Scholar]
- Catalano KJ, Stefanovski D, Bergman RN. Critical role of the mesenteric depot versus other intra-abdominal adipose depots in the development of insulin resistance in young rats. Diabetes. 2010;59:1416–1423. doi: 10.2337/db08-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J. Lipid Res. 2012;53:227–246. doi: 10.1194/jlr.R021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrière G, Cousin B, Arnaud E, André M, Bacou F, Penicaud L, Casteilla L. Preadipocyte conversion to macrophage. Evidence of plasticity. J. Biol. Chem. 2003;278:9850–9855. doi: 10.1074/jbc.M210811200. [DOI] [PubMed] [Google Scholar]
- Chung S, Lapoint K, Martinez K, Kennedy A, Boysen Sandberg M, McIntosh MK. Preadipocytes mediate lipopolysaccharide-induced inflammation and insulin resistance in primary cultures of newly differentiated human adipocytes. Endocrinology. 2006;147:5340–5351. doi: 10.1210/en.2006-0536. [DOI] [PubMed] [Google Scholar]
- Cleveland-Donovan K, Maile LA, Tsiaras WG, Tchkonia T, Kirkland JL, Boney CM. IGF-I activation of the AKT pathway is impaired in visceral but not subcutaneous preadipocytes from obese subjects. Endocrinology. 2010;151:3752–3763. doi: 10.1210/en.2010-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin B, Munoz O, Andre M, Fontanilles AM, Dani C, Cousin JL, Laharrague P, Casteilla L, Pénicaud L. A role for preadipocytes as macrophage-like cells. FASEB J. 1999;13:305–312. doi: 10.1096/fasebj.13.2.305. [DOI] [PubMed] [Google Scholar]
- Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011;12:722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossno JT, Jr., Majka SM, Grazia T, Gill RG, Klemm DJ. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J. Clin. Invest. 2006;116:3220–3228. doi: 10.1172/JCI28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankel SN, Fadnes DJ, Stavrum AK, Stansberg C, Holdhus R, Hoang T, Veum VL, Christensen BJ, Våge V, Sagen JV, et al. Switch from stress response to homeobox transcription factors in adipose tissue after profound fat loss. PLoS ONE. 2010;5:e11033. doi: 10.1371/journal.pone.0011033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbois AP, Smith VJ. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010;85:1629–1642. doi: 10.1007/s00253-009-2355-3. [DOI] [PubMed] [Google Scholar]
- Digby JE, Crowley VE, Sewter CP, Whitehead JP, Prins JB, O’Rahilly S. Depot-related and thiazolidinedione-responsive expression of uncoupling protein 2 (UCP2) in human adipocytes. Int. J. Obes. Relat. Metab. Disord. 2000;24:585–592. doi: 10.1038/sj.ijo.0801201. [DOI] [PubMed] [Google Scholar]
- DiGirolamo M, Fine JB, Tagra K, Rossmanith R. Qualitative regional differences in adipose tissue growth and cellularity in male Wistar rats fed ad libitum. Am. J. Physiol. 1998;274:R1460–R1467. doi: 10.1152/ajpregu.1998.274.5.R1460. [DOI] [PubMed] [Google Scholar]
- Duffaut C, Zakaroff-Girard A, Bourlier V, Decaunes P, Maumus M, Chiotasso P, Sengenès C, Lafontan M, Galitzky J, Bouloumié A. Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arterioscler. Thromb. Vasc. Biol. 2009;29:1608–1614. doi: 10.1161/ATVBAHA.109.192583. [DOI] [PubMed] [Google Scholar]
- Dusserre E, Moulin P, Vidal H. Differences in mRNA expression of the proteins secreted by the adipocytes in human subcutaneous and visceral adipose tissues. Biochim. Biophys. Acta. 2000;1500:88–96. doi: 10.1016/s0925-4439(99)00091-5. [DOI] [PubMed] [Google Scholar]
- Edens NK, Fried SK, Kral JG, Hirsch J, Leibel RL. In vitro lipid synthesis in human adipose tissue from three abdominal sites. Am. J. Physiol. 1993;265:E374–E379. doi: 10.1152/ajpendo.1993.265.3.E374. [DOI] [PubMed] [Google Scholar]
- Einstein FH, Huffman DM, Fishman S, Jerschow E, Heo HJ, Atzmon G, Schechter C, Barzilai N, Muzumdar RH. Aging per se increases the susceptibility to free fatty acid-induced insulin resistance. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65:800–808. doi: 10.1093/gerona/glq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrini E, Tamboli RA, Magkos F, Marks-Shulman PA, Eckhauser AW, Richards WO, Klein S, Abumrad NN. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology. 2010;139:448–455. doi: 10.1053/j.gastro.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faty A, Ferré P, Commans S. The acute phase protein Serum Amyloid A induces lipolysis and inflammation in human adipocytes through distinct pathways. PLoS ONE. 2012;7:e34031. doi: 10.1371/journal.pone.0034031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink C, Karagiannides I, Bakirtzi K, Pothoulakis C. Adipose tissue and inflammatory bowel disease pathogenesis. Inflamm. Bowel Dis. 2012;18:1550–1557. doi: 10.1002/ibd.22893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol. Med. 2010;16:238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried SK, Leibel RL, Edens NK, Kral JG. Lipolysis in intraabdominal adipose tissues of obese women and men. Obes. Res. 1993;1:443–448. doi: 10.1002/j.1550-8528.1993.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Funada J, Dennis AL, Roberts R, Karpe F, Frayn KN. Regulation of subcutaneous adipose tissue blood flow is related to measures of vascular and autonomic function. Clin. Sci. 2010;119:313–322. doi: 10.1042/CS20100066. [DOI] [PubMed] [Google Scholar]
- Gesta S, Blüher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, Boucher J, Lewis C, Kahn CR. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc. Natl. Acad. Sci. USA. 2006;103:6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesta S, Bezy O, Mori MA, Macotela Y, Lee KY, Kahn CR. Mesodermal developmental gene Tbx15 impairs adipocyte differentiation and mitochondrial respiration. Proc. Natl. Acad. Sci. USA. 2011;108:2771–2776. doi: 10.1073/pnas.1019704108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross K, Karagiannides I, Thomou T, Koon HW, Bowe C, Kim H, Giorgadze N, Tchkonia T, Pirtskhalava T, Kirkland JL, Pothoulakis C. Substance P promotes expansion of human mesenteric preadipocytes through proliferative and antiapoptotic pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G1012–G1019. doi: 10.1152/ajpgi.90351.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Hensrud DD, Johnson CM, Jensen MD. Regional postprandial fatty acid metabolism in different obesity phenotypes. Diabetes. 1999;48:1586–1592. doi: 10.2337/diabetes.48.8.1586. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Mepani RJ, Kleiner S, Lo JC, Khandekar MJ, Cohen P, Frontini A, Bhowmick DC, Ye L, Cinti S, Spiegelman BM. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab. 2012;15:230–239. doi: 10.1016/j.cmet.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson B, Gogg S, Hedjazifar S, Jenndahl L, Hammarstedt A, Smith U. Inflammation and impaired adipogenesis in hypertrophic obesity in man. Am. J. Physiol. Endocrinol. Metab. 2009;297:E999–E1003. doi: 10.1152/ajpendo.00377.2009. [DOI] [PubMed] [Google Scholar]
- Harman-Boehm I, Blüher M, Redel H, Sion-Vardy N, Ovadia S, Avinoach E, Shai I, Klöting N, Stumvoll M, Bashan N, Rudich A. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J. Clin. Endocrinol. Metab. 2007;92:2240–2247. doi: 10.1210/jc.2006-1811. [DOI] [PubMed] [Google Scholar]
- Hauner H, Wabitsch M, Pfeiffer EF. Differentiation of adipocyte precursor cells from obese and nonobese adult women and from different adipose tissue sites. Horm. Metab. Res. Suppl. 1988;19(Supplemental):35–39. [PubMed] [Google Scholar]
- Heid IM, Jackson AU, Randall JC, Winkler TW, Qi L, Steinthorsdottir V, Thorleifsson G, Zillikens MC, Speliotes EK, Mägi R, et al. MAGIC Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat. Genet. 2010;42:949–960. doi: 10.1038/ng.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn L, Smith SR, Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int. J. Obes. Relat. Metab. Disord. 2004;28(Suppl 4):S12–S21. doi: 10.1038/sj.ijo.0802853. [DOI] [PubMed] [Google Scholar]
- Herrera MF, Pantoja JP, Velázquez-Fernández D, Cabiedes J, Aguilar-Salinas C, García-García E, Rivas A, Villeda C, Hernández-Ramírez DF, Dávila A, Zaraín A. Potential additional effect of omentectomy on metabolic syndrome, acute-phase reactants, and inflammatory mediators in grade III obese patients undergoing laparoscopic Roux-en-Y gastric bypass: a randomized trial. Diabetes Care. 2010;33:1413–1418. doi: 10.2337/dc09-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hida K, Wada J, Eguchi J, Zhang H, Baba M, Seida A, Hashimoto I, Okada T, Yasuhara A, Nakatsuka A, et al. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc. Natl. Acad. Sci. USA. 2005;102:10610–10615. doi: 10.1073/pnas.0504703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstedt J, Arner E, Wahrenberg H, Andersson DP, Qvisth V, Löfgren P, Rydén M, Thörne A, Wirén M, Palmér M, et al. Regional impact of adipose tissue morphology on the metabolic profile in morbid obesity. Diabetologia. 2010;53:2496–2503. doi: 10.1007/s00125-010-1889-3. [DOI] [PubMed] [Google Scholar]
- Huang Y, Sitwala K, Bronstein J, Sanders D, Dandekar M, Collins C, Robertson G, MacDonald J, Cezard T, Bilenky M, et al. Identification and characterization of Hoxa9 binding sites in hematopoietic cells. Blood. 2012;119:388–398. doi: 10.1182/blood-2011-03-341081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Takahashi K, Bujo H, Hashimoto N, Yagui K, Saito Y. Subcutaneous fat modulates insulin sensitivity in mice by regulating TNF-alpha expression in visceral fat. Horm. Metab. Res. 2006;38:631–638. doi: 10.1055/s-2006-954580. [DOI] [PubMed] [Google Scholar]
- Jensen MD. Gender differences in regional fatty acid metabolism before and after meal ingestion. J. Clin. Invest. 1995;96:2297–2303. doi: 10.1172/JCI118285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MD, Sarr MG, Dumesic DA, Southorn PA, Levine JA. Regional uptake of meal fatty acids in humans. Am. J. Physiol. Endocrinol. Metab. 2003;285:E1282–E1288. doi: 10.1152/ajpendo.00220.2003. [DOI] [PubMed] [Google Scholar]
- Julien P, Despres JP, Angel A. Scanning electron microscopy of very small fat cells and mature fat cells in human obesity. J. Lipid Res. 1989;30:293–299. [PubMed] [Google Scholar]
- Kahn SE, Prigeon RL, Schwartz RS, Fujimoto WY, Knopp RH, Brunzell JD, Porte D., Jr Obesity, body fat distribution, insulin sensitivity and Islet β-cell function as explanations for metabolic diversity. J. Nutr. 2001;131:354S–360S. doi: 10.1093/jn/131.2.354S. [DOI] [PubMed] [Google Scholar]
- Karagiannides I, Kokkotou E, Tansky M, Tchkonia T, Giorgadze N, O’Brien M, Leeman SE, Kirkland JL, Pothoulakis C. Induction of colitis causes inflammatory responses in fat depots: evidence for substance P pathways in human mesenteric preadipocytes. Proc. Natl. Acad. Sci. USA. 2006;103:5207–5212. doi: 10.1073/pnas.0600821103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannides I, Bakirtzi K, Kokkotou E, Stavrakis D, Margolis KG, Thomou T, Giorgadze N, Kirkland JL, Pothoulakis C. Role of substance P in the regulation of glucose metabolism via insulin signaling-associated pathways. Endocrinology. 2011a;152:4571–4580. doi: 10.1210/en.2011-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannides I, Stavrakis D, Bakirtzi K, Kokkotou E, Pirtskhalava T, Nayeb-Hashemi H, Bowe C, Bugni JM, Nuño M, Lu B, et al. Substance P (SP)-neurokinin-1 receptor (NK-1R) alters adipose tissue responses to high-fat diet and insulin action. Endocrinology. 2011b;152:2197–2205. doi: 10.1210/en.2010-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karastergiou K, Fried SK, Xie H, Lee MJ, Divoux A, Rosencrantz MA, Chang RJ, Smith SR. Distinct developmental signatures of human abdominal and gluteal subcutaneous adipose tissue depots. J. Clin. Endocrinol. Metab. 2013;98:362–371. doi: 10.1210/jc.2012-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2000;278:E941–E948. doi: 10.1152/ajpendo.2000.278.5.E941. [DOI] [PubMed] [Google Scholar]
- Kirkland JL, Hollenberg CH, Gillon WS. Age, anatomic site, and the replication and differentiation of adipocyte precursors. Am. J. Physiol. 1990;258:C206–C210. doi: 10.1152/ajpcell.1990.258.2.C206. [DOI] [PubMed] [Google Scholar]
- Kirkland JL, Hollenberg CH, Gillon W, Kindler S. Effect of hypophysectomy on rat preadipocyte replication and differentiation. Endocrinology. 1992;131:2769–2773. doi: 10.1210/endo.131.6.1446615. [DOI] [PubMed] [Google Scholar]
- Kirkland JL, Hollenberg CH, Gillon WS. Effects of fat depot site on differentiation-dependent gene expression in rat preadipocytes. Int. J. Obes. Relat. Metab. Disord. 1996;20(Suppl 3):S102–S107. [PubMed] [Google Scholar]
- Kirkland JL, Tchkonia T, Giorgadze N, Pirtskhalava T. Adipose tissue as an endocrine organ: Regional differences in adipocyte endocrine function. Prog. Obesity Res. 2003;9:87–95. [Google Scholar]
- Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, Mohammed BS. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N. Engl. J. Med. 2004;350:2549–2557. doi: 10.1056/NEJMoa033179. [DOI] [PubMed] [Google Scholar]
- Klöting N, Berndt J, Kralisch S, Kovacs P, Fasshauer M, Schön MR, Stumvoll M, Blüher M. Vaspin gene expression in human adipose tissue: association with obesity and type 2 diabetes. Biochem. Biophys. Res. Commun. 2006;339:430–436. doi: 10.1016/j.bbrc.2005.11.039. [DOI] [PubMed] [Google Scholar]
- Koon HW, Kim YS, Xu H, Kumar A, Zhao D, Karagiannides I, Dobner PR, Pothoulakis C. Neurotensin induces IL-6 secretion in mouse preadipocytes and adipose tissues during 2,4,6,-trinitrobenzensulphonic acid-induced colitis. Proc. Natl. Acad. Sci. USA. 2009;106:8766–8771. doi: 10.1073/pnas.0903499106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos K, Wong S, Tan BK, Kerrigan D, Randeva HS, Pinkney JH, Wilding JP. Human RBP4 adipose tissue expression is gender specific and influenced by leptin. Clin. Endocrinol. (Oxf.) 2011;74:197–205. doi: 10.1111/j.1365-2265.2010.03892.x. [DOI] [PubMed] [Google Scholar]
- Koster A, Stenholm S, Alley DE, Kim LJ, Simonsick EM, Kanaya AM, Visser M, Houston DK, Nicklas BJ, Tylavsky FA, et al. Health ABC Study Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity (Silver Spring) 2010;18:2354–2361. doi: 10.1038/oby.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsari C, Ali AH, Mundi MS, Jensen MD. Storage of circulating free fatty acid in adipose tissue of postabsorptive humans: quantitative measures and implications for body fat distribution. Diabetes. 2011;60:2032–2040. doi: 10.2337/db11-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsari C, Mundi MS, Ali AH, Jensen MD. Storage rates of circulating free fatty acid into adipose tissue during eating or walking in humans. Diabetes. 2012;61:329–338. doi: 10.2337/db11-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacova Z, Tencerova M, Roussel B, Wedellova Z, Rossmeislova L, Langin D, Polak J, Stich V. The impact of obesity on secretion of adiponectin multimeric isoforms differs in visceral and subcutaneous adipose tissue. Int J Obes (Lond) 2012;36:1360–1365. doi: 10.1038/ijo.2011.223. [DOI] [PubMed] [Google Scholar]
- Lacasa D, Garcia E, Agli B, Giudicelli Y. Control of rat preadipocyte adipose conversion by ovarian status: regional specificity and possible involvement of the mitogen-activated protein kinase-dependent and c-fos signaling pathways. Endocrinology. 1997;138:2729–2734. doi: 10.1210/endo.138.7.5246. [DOI] [PubMed] [Google Scholar]
- Lacasa D, Taleb S, Keophiphath M, Miranville A, Clement K. Macrophage-secreted factors impair human adipogenesis: involvement of proinflammatory state in preadipocytes. Endocrinology. 2007;148:868–877. doi: 10.1210/en.2006-0687. [DOI] [PubMed] [Google Scholar]
- Laurencikiene J, Skurk T, Kulyté A, Hedén P, Aström G, Sjölin E, Rydén M, Hauner H, Arner P. Regulation of lipolysis in small and large fat cells of the same subject. J. Clin. Endocrinol. Metab. 2011;96:E2045–E2049. doi: 10.1210/jc.2011-1702. [DOI] [PubMed] [Google Scholar]
- Liu J, Divoux A, Sun J, Zhang J, Clément K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat. Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KH, Chan YL, Chan WB, Chan JC, Chu CW. Mesenteric fat thickness is an independent determinant of metabolic syndrome and identifies subjects with increased carotid intima-media thickness. Diabetes Care. 2006;29:379–384. doi: 10.2337/diacare.29.02.06.dc05-1578. [DOI] [PubMed] [Google Scholar]
- Lottati M, Kolka CM, Stefanovski D, Kirkman EL, Bergman RN. Greater omentectomy improves insulin sensitivity in nonobese dogs. Obesity (Silver Spring) 2009;17:674–680. doi: 10.1038/oby.2008.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macotela Y, Emanuelli B, Mori MA, Gesta S, Schulz TJ, Tseng YH, Kahn CR. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes. 2012;61:1691–1699. doi: 10.2337/db11-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madani R, Karastergiou K, Ogston NC, Miheisi N, Bhome R, Haloob N, Tan GD, Karpe F, Malone-Lee J, Hashemi M, et al. RANTES release by human adipose tissue in vivo and evidence for depot-specific differences. Am. J. Physiol. Endocrinol. Metab. 2009;296:E1262–E1268. doi: 10.1152/ajpendo.90511.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer MM, Harris RB, Bartness TJ. The regulation of total body fat: lessons learned from lipectomy studies. Neurosci. Biobehav. Rev. 2001;25:15–28. doi: 10.1016/s0149-7634(00)00047-6. [DOI] [PubMed] [Google Scholar]
- Meek SE, Nair KS, Jensen MD. Insulin regulation of regional free fatty acid metabolism. Diabetes. 1999;48:10–14. doi: 10.2337/diabetes.48.1.10. [DOI] [PubMed] [Google Scholar]
- Miller NE, Michel CC, Nanjee MN, Olszewski WL, Miller IP, Hazell M, Olivecrona G, Sutton P, Humphreys SM, Frayn KN. Secretion of adipokines by human adipose tissue in vivo: partitioning between capillary and lymphatic transport. Am. J. Physiol. Endocrinol. Metab. 2011;301:E659–E667. doi: 10.1152/ajpendo.00058.2011. [DOI] [PubMed] [Google Scholar]
- Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, Nojima A, Nabetani A, Oike Y, Matsubara H, et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat. Med. 2009;15:1082–1087. doi: 10.1038/nm.2014. [DOI] [PubMed] [Google Scholar]
- Montague CT, Prins JB, Sanders L, Zhang J, Sewter CP, Digby J, Byrne CD, O’Rahilly S. Depot-related gene expression in human subcutaneous and omental adipocytes. Diabetes. 1998;47:1384–1391. doi: 10.2337/diabetes.47.9.1384. [DOI] [PubMed] [Google Scholar]
- Mori Y, Murakawa Y, Okada K, Horikoshi H, Yokoyama J, Tajima N, Ikeda Y. Effect of troglitazone on body fat distribution in type 2 diabetic patients. Diabetes Care. 1999;22:908–912. doi: 10.2337/diacare.22.6.908. [DOI] [PubMed] [Google Scholar]
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar R, Allison DB, Huffman DM, Ma X, Atzmon G, Einstein FH, Fishman S, Poduval AD, McVei T, Keith SW, Barzilai N. Visceral adipose tissue modulates mammalian longevity. Aging Cell. 2008;7:438–440. doi: 10.1111/j.1474-9726.2008.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RH, Basu R, Johnson CM, Rizza RA, Miles JM. Splanchnic spillover of extracellular lipase-generated fatty acids in overweight and obese humans. Diabetes. 2007;56:2878–2884. doi: 10.2337/db07-0812. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J. Clin. Invest. 2004;113:1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesler CU, Siddle K, Prins JB. Human preadipocytes display a depot-specific susceptibility to apoptosis. Diabetes. 1998;47:1365–1368. doi: 10.2337/diab.47.8.1365. [DOI] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- Pinnick KE, Neville MJ, Fielding BA, Frayn KN, Karpe F, Hodson L. Gluteofemoral adipose tissue plays a major role in production of the lipokine palmitoleate in humans. Diabetes. 2012;61:1399–1403. doi: 10.2337/db11-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prunet-Marcassus B, Cousin B, Caton D, André M, Pénicaud L, Casteilla L. From heterogeneity to plasticity in adipose tissues: site-specific differences. Exp. Cell Res. 2006;312:727–736. doi: 10.1016/j.yexcr.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri N, Raychaudhuri S, Thamotharan M, Devaskar SU. Histone code modifications repress glucose transporter 4 expression in the intrauterine growth-restricted offspring. J. Biol. Chem. 2008;283:13611–13626. doi: 10.1074/jbc.M800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI. Mechanism of free fatty acid-induced insulin resistance in humans. J. Clin. Invest. 1996;97:2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AM, Elabd C, Delteil F, Astier J, Vernochet C, Saint-Marc P, Guesnet J, Guezennec A, Amri EZ, Dani C, Ailhaud G. Adipocyte differentiation of multipotent cells established from human adipose tissue. Biochem. Biophys. Res. Commun. 2004;315:255–263. doi: 10.1016/j.bbrc.2004.01.053. [DOI] [PubMed] [Google Scholar]
- Romanski SA, Nelson RM, Jensen MD. Meal fatty acid uptake in adipose tissue: gender effects in nonobese humans. Am. J. Physiol. Endocrinol. Metab. 2000;279:E455–E462. doi: 10.1152/ajpendo.2000.279.2.E455. [DOI] [PubMed] [Google Scholar]
- Santosa S, Hensrud DD, Votruba SB, Jensen MD. The influence of sex and obesity phenotype on meal fatty acid metabolism before and after weight loss. Am. J. Clin. Nutr. 2008;88:1134–1141. doi: 10.1093/ajcn/88.4.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B, Liebenberg V, Dietrich D, Schlegel T, Kneip C, Seegebarth A, Flemming N, Seemann S, Distler J, Lewin J, et al. SHOX2 DNA methylation is a biomarker for the diagnosis of lung cancer based on bronchial aspirates. BMC Cancer. 2010;10:600. doi: 10.1186/1471-2407-10-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, Cerletti M, McDougall LE, Giorgadze N, Tchkonia T, et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc. Natl. Acad. Sci. USA. 2011;108:143–148. doi: 10.1073/pnas.1010929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Kajimura S, Spiegelman BM. Transcriptional control of brown adipocyte development and physiological function—of mice and men. Genes Dev. 2009;23:788–797. doi: 10.1101/gad.1779209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadid S, Jensen MD. Pioglitazone increases non-esterified fatty acid clearance in upper body obesity. Diabetologia. 2006;49:149–157. doi: 10.1007/s00125-005-0051-0. [DOI] [PubMed] [Google Scholar]
- Shahparaki A, Grunder L, Sorisky A. Comparison of human abdominal subcutaneous versus omental preadipocyte differentiation in primary culture. Metabolism. 2002;51:1211–1215. doi: 10.1053/meta.2002.34037. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Wu Y, Steinbergs N, Crowley ML, Hanson AS, Casero RA, Woster PM. (Bis)urea and (bis)thiourea inhibitors of lysine-specific demethylase 1 as epigenetic modulators. J. Med. Chem. 2010;53:5197–5212. doi: 10.1021/jm100217a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster A, Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br. J. Radiol. 2012;85:1–10. doi: 10.1259/bjr/38447238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Somers VK, Romero-Corral A, Sert-Kuniyoshi FH, Pusalavidyasagar S, Davison DE, Jensen MD. Effects of weight gain and weight loss on regional fat distribution. Am. J. Clin. Nutr. 2012;96:229–233. doi: 10.3945/ajcn.111.033829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Näslund E, Britton T, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Häing HU. Identification and characterization of metabolically benign obesity in humans. Arch. Intern. Med. 2008;168:1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- Tang QQ, Lane MD. Adipogenesis: from stem cell to adipocyte. Annu. Rev. Biochem. 2012;81:715–736. doi: 10.1146/annurev-biochem-052110-115718. [DOI] [PubMed] [Google Scholar]
- Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Giorgadze N, Pirtskhalava T, Tchoukalova Y, Karagiannides I, Forse RA, DePonte M, Stevenson M, Guo W, Han J, et al. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R1286–R1296. doi: 10.1152/ajpregu.00653.2001. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Tchoukalova YD, Giorgadze N, Pirtskhalava T, Karagiannides I, Forse RA, Koo A, Stevenson M, Chinnappan D, Cartwright A, et al. Abundance of two human preadipocyte subtypes with distinct capacities for replication, adipogenesis, and apoptosis varies among fat depots. Am. J. Physiol. Endocrinol. Metab. 2005;288:E267–E277. doi: 10.1152/ajpendo.00265.2004. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Corkey BE, Kirkland JL. Current Views of the Fat Cell as an Endocrine Cell: Lipotoxicity. In: Bray GA, Ryan DH, editors. Endocrine Updates. Vol. 26: Overweight and the Metabolic Syndrome. Springer; New York: 2006a. pp. 105–118. [Google Scholar]
- Tchkonia T, Giorgadze N, Pirtskhalava T, Thomou T, DePonte M, Koo A, Forse RA, Chinnappan D, Martin-Ruiz C, von Zglinicki T, Kirkland JL. Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes. 2006b;55:2571–2578. doi: 10.2337/db06-0540. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Lenburg M, Thomou T, Giorgadze N, Frampton G, Pirtskhalava T, Cartwright A, Cartwright M, Flanagan J, Karagiannides I, et al. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am. J. Physiol. Endocrinol. Metab. 2007;292:E298–E307. doi: 10.1152/ajpendo.00202.2006. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Giorgadze N, Pirtskhalava T, Thomou T, Villaret A, Bouloumie A, von Zglinicki T, Kirkland JL. Cellular senescence and inflammation in obesity. Obesity (Silver Spring) 2009;17(Supplemental 2):S57. [Google Scholar]
- Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD, Kirkland JL. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchoukalova YD, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc. Natl. Acad. Sci. USA. 2010;107:18226–18231. doi: 10.1073/pnas.1005259107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomou T, Tchkonia T, Kirkland JL. Cellular and molecular basis of functional differences among fat depots. In: Leff TA, Granneman JG, editors. Adipose Tissue in Health and Disease. Wiley-VCH; Weinheim, Germany: 2010. pp. 21–47. [Google Scholar]
- Thörne A, Lönnqvist F, Apelman J, Hellers G, Arner P. A pilot study of long-term effects of a novel obesity treatment: omentectomy in connection with adjustable gastric banding. Int. J. Obes. Relat. Metab. Disord. 2002;26:193–199. doi: 10.1038/sj.ijo.0801871. [DOI] [PubMed] [Google Scholar]
- Tran KV, Gealekman O, Frontini A, Zingaretti MC, Morroni M, Giordano A, Smorlesi A, Perugini J, De Matteis R, Sbarbati A, et al. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab. 2012;15:222–229. doi: 10.1016/j.cmet.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harmelen V, Elizalde M, Ariapart P, Bergstedt-Lindqvist S, Reynisdottir S, Hoffstedt J, Lundkvist I, Bringman S, Arner P. The association of human adipose angiotensinogen gene expression with abdominal fat distribution in obesity. Int. J. Obes. Relat. Metab. Disord. 2000;24:673–678. doi: 10.1038/sj.ijo.0801217. [DOI] [PubMed] [Google Scholar]
- Van Harmelen V, Röhrig K, Hauner H. Comparison of proliferation and differentiation capacity of human adipocyte precursor cells from the omental and subcutaneous adipose tissue depot of obese subjects. Metabolism. 2004;53:632–637. doi: 10.1016/j.metabol.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Villaret A, Galitzky J, Decaunes P, Estève D, Marques MA, Sengenès C, Chiotasso P, Tchkonia T, Lafontan M, Kirkland JL, Bouloumié A. Adipose tissue endothelial cells from obese human subjects: differences among depots in angiogenic, metabolic, and inflammatory gene expression and cellular senescence. Diabetes. 2010;59:2755–2763. doi: 10.2337/db10-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen KA, Lönnroth P, Parkkola R, Peltoniemi P, Asola M, Viljanen T, Tolvanen T, Knuuti J, Rönnemaa T, Huupponen R, Nuutila P. Glucose uptake and perfusion in subcutaneous and visceral adipose tissue during insulin stimulation in nonobese and obese humans. J. Clin. Endocrinol. Metab. 2002;87:3902–3910. doi: 10.1210/jcem.87.8.8761. [DOI] [PubMed] [Google Scholar]
- Vitseva OI, Tanriverdi K, Tchkonia TT, Kirkland JL, McDonnell ME, Apovian CM, Freedman J, Gokce N. Inducible Toll-like receptor and NF-kappaB regulatory pathway expression in human adipose tissue. Obesity (Silver Spring) 2008;16:932–937. doi: 10.1038/oby.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votruba SB, Jensen MD. Sex-specific differences in leg fat up-take are revealed with a high-fat meal. Am. J. Physiol. Endocrinol. Metab. 2006;291:E1115–E1123. doi: 10.1152/ajpendo.00196.2006. [DOI] [PubMed] [Google Scholar]
- Votruba SB, Mattison RS, Dumesic DA, Koutsari C, Jensen MD. Meal fatty acid uptake in visceral fat in women. Diabetes. 2007;56:2589–2597. doi: 10.2337/db07-0439. [DOI] [PubMed] [Google Scholar]
- Wamil M, Battle JH, Turban S, Kipari T, Seguret D, de Sousa Peixoto R, Nelson YB, Nowakowska D, Ferenbach D, Ramage L, et al. Novel fat depot-specific mechanisms underlie resistance to visceral obesity and inflammation in 11 β-hydroxysteroid dehydrogenase type 1-deficient mice. Diabetes. 2011;60:1158–1167. doi: 10.2337/db10-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Kirkland JL, Hollenberg CH. Varying capacities for replication of rat adipocyte precursor clones and adipose tissue growth. J. Clin. Invest. 1989;83:1741–1746. doi: 10.1172/JCI114075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber RV, Buckley MC, Fried SK, Kral JG. Subcutaneous lipectomy causes a metabolic syndrome in hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R936–R943. doi: 10.1152/ajpregu.2000.279.3.R936. [DOI] [PubMed] [Google Scholar]
- Wiest R, Moleda L, Farkas S, Scherer M, Kopp A, Wönckhaus U, Büchler C, Schölmerich J, Schäffler A. Splanchnic concentrations and postprandial release of visceral adipokines. Metabolism. 2010;59:664–670. doi: 10.1016/j.metabol.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Xu XJ, Gauthier MS, Hess DT, Apovian CM, Cacicedo JM, Gokce N, Farb M, Valentine RJ, Ruderman NB. Insulin sensitive and resistant obesity in humans: AMPK activity, oxidative stress, and depot-specific changes in gene expression in adipose tissue. J. Lipid Res. 2012;53:792–801. doi: 10.1194/jlr.P022905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang RZ, Lee MJ, Hu H, Pray J, Wu HB, Hansen BC, Shuldiner AR, Fried SK, McLenithan JC, Gong DW. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am. J. Physiol. Endocrinol. Metab. 2006;290:E1253–E1261. doi: 10.1152/ajpendo.00572.2004. [DOI] [PubMed] [Google Scholar]
- Zhang B, Berger J, Hu E, Szalkowski D, White-Carrington S, Spiegel-man BM, Moller DE. Negative regulation of peroxisome proliferator-activated receptor-gamma gene expression contributes to the antiadipogenic effects of tumor necrosis factor-alpha. Mol. Endocrinol. 1996;10:1457–1466. doi: 10.1210/mend.10.11.8923470. [DOI] [PubMed] [Google Scholar]
- Zhang HM, Chen LL, Wang L, Xu S, Wang X, Yi LL, Chen D, Wu ZH, Zhang JY, Liao YF, Shang J. Macrophage infiltrates with high levels of Toll-like receptor 4 expression in white adipose tissues of male Chinese. Nutr. Metab. Cardiovasc. Dis. 2009;19:736–743. doi: 10.1016/j.numecd.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.