Abstract
The clinical utility of cisplatin is limited by nephrotoxicity. So et al report that β-lapachone prevents this nephrotoxicity but not cisplatin’s cytotoxicity for cancers. In addition to its potential clinical importance, the beneficial effect of β-lapachone on cisplatin AKI may illustrate fundamental processes that ordinarily link alterations in nutrient availability and intracellular ROS on the one hand, with inflammation and cell death on the other hand.
In this issue of Kidney International, So et al 1 show that β-lapachone exerts its salutary effects on cisplatin nephrotoxicity by being reduced by NAD(P)H: quinone oxidoreductase 1 (NQO1). This results in an increased the ratio of NAD+/ NADH (nicotinamide adenine dinucleotide). The increased NAD+ mimics that seen during caloric restriction, and may mediate the beneficial effects of caloric restriction, without malnutrition, on inappropriate inflammation and apoptosis 2. In other words, and as discussed in greater detail below, the increased NAD+, induced by β-lapachone, activates the deacetylase SIRT1 and may thus “fool” the cell into detecting caloric restriction when there is none; this, in turn, may affect the cell’s “decisions” about its own life and death, and its production of proinflammatory molecules. Consistent with this idea is the known beneficial effect of caloric restriction on cisplatin nephrotoxicity that is indeed mediated by NAD+ - activated SIRT1 expression 3. Thus, the major components of the beneficial effects of β-lapachone are NQO1, NAD+, and SIRT1. We discuss each below.
NQO1
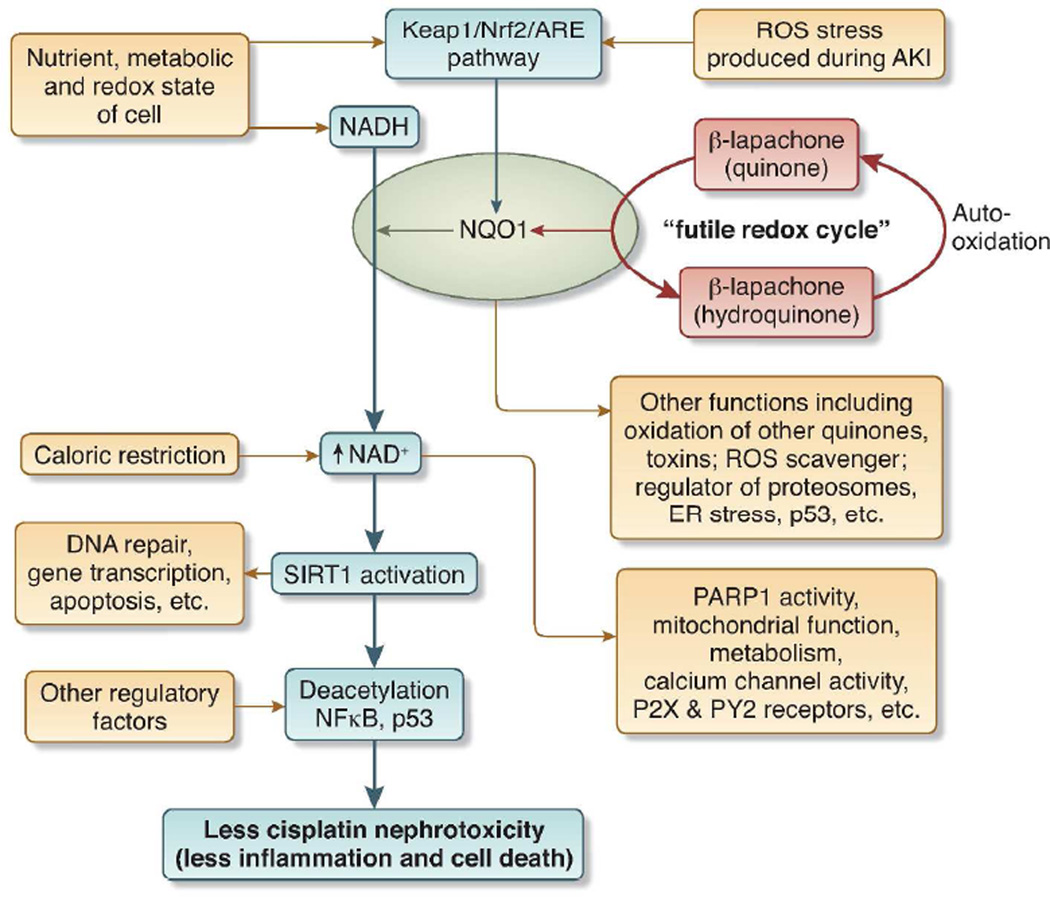
The increased NAD+ seen after administration of β-lapachone requires NQO1. This enzyme transfers hydrides from NADH to beta-lapachone. This produces unusually large amounts of NAD+ because the hydroquinone form of beta-lapachone is autooxidized back to the parent quinone form; this is reduced again by NQO1, and so-on in a futile cycle. Because each time beta-lapachone is reduced, NADH is converted to NAD+, a large quantity of NAD+ should be produced 4. This increase in NAD+ activates SIRT1, which is sirtuin 1 or silent information regulator 2 [Sir2] protein 1. So et al 1 suggest that the increased SIRT1 activity exerts its beneficial effects through its deacetylation (inactivation) of the transcription factors NFκ B and p65. Inactivation of the former should decrease inflammation; inactivation of the latter should decrease apoptosis. See Figure 1.
Figure 1. β-lapachone, NQO1, NAD+, and SIRT1 at the crossroads of metabolism, injury, and inflammation during cisplatin nephrotoxicity.
Blue arrows and blue box show processes elucidated by So et al. 1 Black arrows show additional processes that may be involved in cisplatin nephrotoxicity. Abbreviations: ARE = antioxidant response element; Keap = Kelch-like ECH-associated protein 1; NADH = nicotinamide adenine dinucleotide; NQO1 = NAD(P)H: quinone oxidoreductase 1; Nrf2 = nuclear factor-erythorid 2 related factor 2; PARP1 = poly (ADP-ribose) polymerase-1; ROS = reactive oxygen species; SIRT1 = sirtuin 1 = silent information regulator 2 [Sir2] protein 1.
Paradoxically, NQO1 is also required for β-lapachone’s opposite effects on cancers versus renal cells – cytotoxic for the former, but cytoprotective for the latter. The effects of β-lapachone on cancer have been explored by others 5. This commentary is focused on this drug’s beneficial effects on cisplatin nephrotoxicity.
In addition to reducing β-lapachone, NQO1 is an anti-oxidant enzyme with multiple cytoprotective functions when the cell is challenged by oxidative stress. Such stress may be in the form of exogenous polyaromatic toxins and carcinogens, or in the form of either exogenous or endogenous superoxides and peroxides. NQO1 responds to these oxidative stresses by catalyzing the two electron reduction of a variety of quinones to hydroquinones by using NADPH or NADH as the hydride donor. Many of the hydroquinone products are metabolized to glucuronide and sulfate conjugates and excreted. NQO1 also may directly scavenge superoxide. It also has non-catalytic function in regulating the 20S proteasome and thus ER stress and the half life of p53 6.
Oxidative stress is a factor in the complications of metabolic syndrome, hypertension, atherosclerosis, and autoimmunity, and NQO1 may have a salutary role in these diseases. Furthermore, oxidative stress elicits immune responses and increased NQO1 activity may ameliorate some experimental autoimmune diseases 6.
NQO1 is an inducible anti-oxidant enzyme. Its abundance is increased several orders of magnitude in response to the anti-oxidant Keap1/ Nrf2/ ARE pathway. Indeed, NQO1 is the prototypic member of the family of over 100 cytoprotective genes that are regulated by the antioxidant response element (ARE) of DNA. Activation of ARE (ROS) results in the transcription of genes for anti-oxidant proteins. ARE are activated by the heterodimer of a small Maf protein and Nrf2 (nuclear factor-erythroid 2-related factor 2). In the absence of oxidative stress, Nrf2 is bound to Keap1 (Kelch-like ECH-associated protein 1) that inactivates it and promotes its proteasomal degradation. In the presence of excess ROS, Nrf2 is released from Keap 1, moves to the nucleus and activates NQO1 and other antioxidant genes 6, 7.
Previous studies showed that pharmacologic stimulation of the Keap1/ Nrf2/ ARE pathway protects the kidney from cisplatin, in part because this pathway increased the expression NQO1, and other anti-oxidant enzymes. These neutralize the increased ROS produced during cisplatin nephrotoxicity 3. See review 8.
NAD+
So et al 1 show that the end result of the reduction of β-lapachone by NQO1 is increased NAD+. Similar increased NAD+ is seen after caloric restriction. Increased NAD+ should have profound effects on the cell because the redox pair of NAD+/ NADH participate in and/or regulate a number of critical processes in physiology and pathophysiology. NADH is a substrate for the mitochondrial electron transport chain that mediates the production of ATP and ROS from oxygen. NAD+ is a cofactor for enzymes that generate energy from glucose and fat breakdown. NAD+ is also converted to metabolites by enzymes such as such as NAD+ glycohydrolases (CD38/ CD157), and ADP-ribosyltransferases (ART). These metabolites activate the the non-selective cation channel TRPM2 (transient receptor potential melastatin-related channel 2) that causes calcium influx across cell membrane, P2X/ P2Y receptors, and have other effects. For more details, the reader is referred to a number of excellent recent reviews on NAD+ 2.
Furthermore, NAD+ is an essential cofactor for, the poly-(ADP-ribose) polymerases (PARPs). These enzymes attach negatively charged polymers of ADP-ribose to proteins and change their function; in this way, PARP1 regulates apoptosis, DNA repair, necrosis, and gene transcription, including transcription of proinflammatory genes 9. This regulation is germaine for this discussion because PARP1 is required for cisplatin nephrotoxicity 10.
SIRT1
Finally, and most importantly for our discussion, increased NAD+ activates SIRT1 [sirtuin 1 or SIRT1. SIRT1 is the most extensively studied of the 7 member family of mammalian sirtuins. SIRT1 couples NAD+ hydrolysis with protein deacylation. It is a member of the Class III family of histone deacylases, and regulates DNA repair, and gene transcription. SIRT1 has profound beneficial effects on renal diseases including renal aging, diabetic nephropathy, and the response to unilateral ureteral obstruction 11–13. Furthermore, SIRT1 has recently received much attention because it has salutary effects on metabolic syndrome and may mediate some of the life-prolonging effects of caloric restriction 2.
So et al 1 show that beta-lapachone increases NAD+. This was correlated with increased SIRT1 enzymatic activity, and deacylation of NFκB and p53. This was associated with less nephrotoxicity and less renal cytokine/ chemokine production after cisplatin treatment. Others have shown that renal tubule--specific overexpression of SIRT1 ameliorated cisplatin nephrotoxicity 3, and inactivated the NFκB p65 subunit. In addition, activation of SIRT1 by resveratrol 14 resulted in the deacylation (deactivation) of p53 and ameliorated cisplatin nephrotoxicity.
More complexity to our story
Like other important provocative observations, the studies of So et al raise new questions. Would other ways of increasing NAD+ similarly protect against nephrotoxicity? These include a number of drugs under development, and old drugs, such as niacin that is a precursor of NAD+ and is already approved for the treatment of hyperlipidemia 2. Can the beneficial effects of β-lapachone on cisplatin nephrotoxicity be extended to other types of renal injury? In other words does this drug affect a final common pathway for acute kidney injury, or are its effect specific for cisplatin nephrotoxicity.
Although the major beneficial effect of β-lapachone may be increases in NAD+, the ensuing SIRT1 activation, and deacylation of NFκB and p53, the story is likely to be more complex. As reviewed above, increased NAD+ should have other effects, in addition to SIRT1 activation; and SIRT1 activation should have other effects in addition to deacylation of NFκB and p53.
Furthermore, the activation of SIRT1 and other members of its family are likely to be regulated by factors in addition to NAD+. A multiple hit hypothesis of activation involving NAD+ and other regulators has been proposed; this would allow differential regulation of SIRT1 and the various other SIRTs in different types of cells and in different microenvironments of the same cells 2. This differential regulation would, for example, allow fine specificity of which NFκB regulated genes are inhibited by SIRT1. Thus, the proinflammatory genes regulated by NFκB studied by So et al might be inhibited in the kidney after cisplatin exposure, but pro-survival genes regulated by NFκB might not 15.
A growing number of agents may ameliorate cisplatin nephrotoxicity without decreasing cisplatin’s anti-tumor effectiveness. Further research may elucidate the relationship between the salutary effects of β-lapachone and protection mediated by inhibiting protein kinase C delta 16, and the growing number of other nephroprotective agents 17.
So et al studied the effects of β-lapachone on acute cisplatin nephrotoxicity. Like other forms of AKI 18–20, cisplatin nephrotoxicity may progress to chronic kidney disease. This progression may be due to chronic ischemia and the ensuing increased ROS that triggers continued injury and fibrosis 21. Insofar as NAD+, NQO1, and SIRT1 might ameliorate the response to ROS 11–13, would β-lapachone ameliorate CKD resulting from acute cisplatin nephrotoxicity and other forms of AKI?
Conclusion
So et al show that β-lapachone prevents cisplatin nephrotoxicity but not tumor cytotoxicity in a murine model. In addition to its potential clinical importance, this observation may illustrate the fundamental contributions of NAD+, NQO1, and SIRT1 to AKI.
Acknowledgements
CYL supported by NIH RO1DK069633, the Beecherl Foundation, and the UT Southwestern O’Brien Kidney Research Core Center (NIH DK079328).
Footnotes
Disclosures: CYL supported by an unrestricted grant from Reata Pharmaceuticals.
REFERENCES
- 1.Oh GS, Kim H, Choi J, et al. NQO1 activation increases NAD+ levels, which consequently attenuates cisplatin-mediated acute kidney injury in mice. Kidney Int. 2013 doi: 10.1038/ki.2013.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houtkooper RH, Canto C, Wanders RJ, et al. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr Rev. 2010;31:194–223. doi: 10.1210/er.2009-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasegawa K, Wakino S, Yoshioka K, et al. Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J Biol Chem. 2010;285:13045–13056. doi: 10.1074/jbc.M109.067728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pink JJ, Planchon SM, Tagliarino C, et al. NAD(P)H:Quinone oxidoreductase activity is the principal determinant of beta-lapachone cytotoxicity. J Biol Chem. 2000;275:5416–5424. doi: 10.1074/jbc.275.8.5416. [DOI] [PubMed] [Google Scholar]
- 5.Bey EA, Bentle MS, Reinicke KE, et al. An NQO1- and PARP-1-mediated cell death pathway induced in non-small-cell lung cancer cells by beta-lapachone. Proc Natl Acad Sci U S A. 2007;104:11832–11837. doi: 10.1073/pnas.0702176104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu H, Li Y. NAD(P)H: quinone oxidoreductase 1 and its potential protective role in cardiovascular diseases and related conditions. Cardiovasc Toxicol. 2012;12:39–45. doi: 10.1007/s12012-011-9136-9. [DOI] [PubMed] [Google Scholar]
- 7.Copple IM. The Keap1-Nrf2 cell defense pathway--a promising therapeutic target? Adv Pharmacol. 2012;63:43–79. doi: 10.1016/B978-0-12-398339-8.00002-1. [DOI] [PubMed] [Google Scholar]
- 8.Shelton LM, Park BK, Copple IM. Role of Nrf2 in protection against acute kidney injury. Kidney Int. 2013 doi: 10.1038/ki.2013.248. [DOI] [PubMed] [Google Scholar]
- 9.Schreiber V, Dantzer F, Ame JC, et al. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7:517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Long KE, Tang K, et al. Poly(ADP-ribose) polymerase 1 activation is required for cisplatin nephrotoxicity. Kidney Int. 2012;82:193–203. doi: 10.1038/ki.2012.64. [DOI] [PubMed] [Google Scholar]
- 11.Nath KA. The role of Sirt1 in renal rejuvenation and resistance to stress. J Clin Invest. 2010;120:1026–1028. doi: 10.1172/JCI42184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hao CM, Haase VH. Sirtuins and their relevance to the kidney. J Am Soc Nephrol. 2010;21:1620–1627. doi: 10.1681/ASN.2010010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitada M, Kume S, Takeda-Watanabe A, et al. Sirtuins and renal diseases: relationship with aging and diabetic nephropathy. Clin Sci (Lond) 2013;124:153–164. doi: 10.1042/CS20120190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim DH, Jung YJ, Lee JE, et al. SIRT1 activation by resveratrol ameliorates cisplatin-induced renal injury through deacetylation of p53. Am J Physiol Renal Physiol. 2011;301:F427–F435. doi: 10.1152/ajprenal.00258.2010. [DOI] [PubMed] [Google Scholar]
- 15.Pasparakis M. Role of NF-kappaB in epithelial biology. Immunol Rev. 2012;246:346–358. doi: 10.1111/j.1600-065X.2012.01109.x. [DOI] [PubMed] [Google Scholar]
- 16.Pabla N, Dong G, Jiang M, et al. Inhibition of PKCdelta reduces cisplatin-induced nephrotoxicity without blocking chemotherapeutic efficacy in mouse models of cancer. J Clin Invest. 2011;121:2709–2722. doi: 10.1172/JCI45586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.dos Santos NA, Carvalho Rodrigues MA, Martins NM, et al. Cisplatin-induced nephrotoxicity and targets of nephroprotection: an update. Archives of toxicology. 2012;86:1233–1250. doi: 10.1007/s00204-012-0821-7. [DOI] [PubMed] [Google Scholar]
- 18.Hsu CY. Yes, AKI Truly Leads to CKD. J Am Soc Nephrol. 2012;23:967–969. doi: 10.1681/ASN.2012030222. [DOI] [PubMed] [Google Scholar]
- 19.Venkatachalam MA, Griffin KA, Lan R, et al. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol. 2010;298:F1078. doi: 10.1152/ajprenal.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82:516–524. doi: 10.1038/ki.2012.208. [DOI] [PubMed] [Google Scholar]
- 21.Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int. 2008;74:867–872. doi: 10.1038/ki.2008.350. [DOI] [PubMed] [Google Scholar]