Abstract
Since its introduction, endoscopic ultrasound (EUS) guided fine needle aspiration and fine needle biopsy have become an indispensable tool for the diagnosis of lesions within the gastrointestinal tract and surrounding organs. It has proved to be an effective diagnostic method with high accuracy and low complication rates. Several factors can influence the accuracy and the diagnostic yield of this procedure including experience of the endosonographer, availability of onsite cytopathology services, the method of cytopathology preparation, the location and physical characteristics of the lesion, sampling techniques and the type and size of the needle used. In this review we will outline the recent studies evaluating EUS-guided tissue acquisition and will provide practical recommendations to maximize tissue yield.
Keywords: Endoscopic ultrasound, Fine needle aspiration, Endoscopic ultrasound guided sampling techniques, Cytological diagnosis, Core biopsy device, Gastrointestinal endoscopy
Core tip: The impact of the type and size of needles used for endoscopic ultrasound guided-guided tissue acquisition have been the center of recent studies aiming at maximizing tissue yield. In addition to needles, several other variables impact the final outcome of tissue acquisition including the location and characteristics of the lesion, the fine needle aspiration technique, and the availability of on-site cytopathology services. In this review we outline the results of these studies and summarize the recent advances in this field.
INTRODUCTION
Endoscopic ultrasound guided fine needle aspiration (EUS-FNA) was initially described in 1992[1] and soon became the procedure of choice to obtain diagnostic samples from lesions within the GI tract and regional orga[2-4]. EUS-FNA is highly accurate, sensitive and specific with estimates reaching 80%, 90% and 100% respectively for cytological diagnoses[5-8]. However, the diagnostic accuracy of EUS-FNA can be influenced by several factors including the experience of the endosonographer, the availability of onsite cytopathology review, the method of cytopathology preparation, the location and physical characteristics of the lesion, and type and size of the needle[9-13]. Currently, three needle sizes are commercially available including 19-G, 22-G and 25-G[2]. To choose a particular needle size, one should consider the location and the type of lesion targeted for sampling, in addition to the type of sample desired; whether a cytological or histological sample is necessary to establish the diagnosis. Currently, the 22-G needles are probably the most widely used; however, a recent trend toward increased utilization of the smaller 25-G needle has been observed in many centers, particularly in scenarios where a transduodenal sampling is considered. Theoretically, larger needles can provide larger size tissue samples; however, technical difficulties are more frequently encountered with larger needles. This is largely related to the stiffness of the needle, leading to sampling failures of lesions located in areas that require significant angulation of the echoendoscope. Larger needles also carry higher risk of complications[14] and could increase the “bloodiness” of sample, which can make the diagnosis by cytology more challenging.
The type of lesion also impacts the choice of needle to be used. For example, stromal tumors and lymphomas can be difficult to diagnose by cytology alone, and sometimes require samples with preserved tissue architecture to make a diagnosis[15,16]. Obtaining an adequate histological sample is theoretically difficult with smaller needles. To overcome this, a Trucut biopsy needle (TCB: Cook Medical, Bloomington, IN, United States) was developed using larger size 19 G needles[17]. However, this needle was limited by difficulties encountered with larger needles such as stiffness, reduced maneuverability, and failure of the spring-loading mechanism, and therefore failed to establish itself when transduodenal approach for sampling was required[18]. More recently, a new generation of core biopsy devices of various sizes (ProCore, Cook Medical, Winston-Salem, NC, United States) in addition to another flexible 19-G needle made of nitinol (Expect 19-gauge Flex, Boston Scientific, Natick, MA, United States) were introduced to obtain histological samples including those from lesions that require transduodenal approach with promising results[19].
In addition to needle size, sampling technique can influence the quality of a specimen. Variations in sampling techniques utilized by endosonographers include the use of suction versus no suction and fanning technique to obtain specimens. Reinserting the stylet versus air flushing are techniques employed to express the sample prior to cytopathology exam.
In the following sections we will focus on recent literature comparing various needle types and sizes and their impact on quality of specimens in relation to the location and type of lesion. We will also review the different sampling techniques used by endosonographers and how such techniques may affect the quality of samples.
SAMPLING METHODS AND TECHNIQUES
Variations in technique of EUS-guided FNA have been recently assessed to identify the sampling method with the highest yield. Common examples to such methods include the use of suction, adopting the fanning technique, use of stylet, and expressing samples using air flushing or by reinserting the stylet.
Use of suction
The traditional FNA technique that relies on suction utilization was recently questioned. Lee et al[20] compared the quality and diagnostic yield of samples obtained with and without suction in 81 patients with pancreatic masses. In this study, each patient had specimens taken with and without applying suction. The number of diagnostic samples, cellularity, and accuracy were found to be higher in the suction group. In another trial with similar results[21], 52 patients with solid mass lesions were randomized to FNA with either suction or no suction. Sensitivity and negative predictive values were higher in the suction group compared to the non-suction group (P = 0.05).
Practically speaking, the decision to use suction should depend on the nature of the targeted lesion. In highly vascular lesions such as lymph nodes, a non-suction technique may result in a better quality and less bloody sample, particularly for the on-site cytopathologist to be able to render a preliminary diagnosis. On the other hand, applying suction when aspirating a fibrotic malignant lesion of the pancreas (Figure 1) or in the setting of chronic pancreatitis may provide a superior sample quality[22-24]. We recommend applying suction during the first pass and then tailoring the use of suction and the amount of based on feedback from the cytopathologist. It is always recommended that aspiration should be applied in cystic pancreatic lesions to obtain sufficient fluid for cytology and tumor markers.
Figure 1.
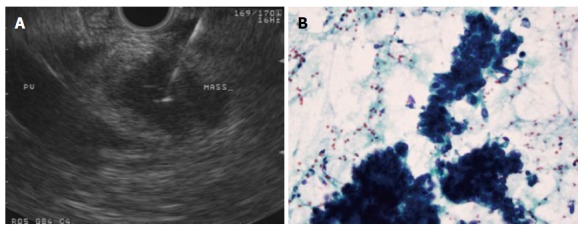
A 25-G needle is used to aspirate a 2 cm pancreatic head mass that does not appear to encase the portal vein (A), adenocarcinoma confirmed on wet smears obtained from the first pass in the case above (B). Papanicolaou stain, × 40.
Use of stylet and fanning technique
It’s widely assumed that using a stylet while going through the gut wall during the initial puncture helps prevent clogging of the needle’s lumen by tissue from the wall, and potentially reduces the contamination of lesional tissue with GI wall components. Therefore, it remains a common practice to re-insert the stylet before every pass. Data comparing the adequacy of EUS-FNA with and without stylet remain limited. Wani et al[25] retrospectively compared EUS-FNA specimens obtained using the stylet to those obtained without using a stylet in terms of cellularity, contamination, adequacy, amount of blood and the diagnostic yield. No difference between the two techniques was found in relation to the variables studied. The authors’ recommendation was against the use of stylet. The use of stylet is considered to be labor intensive and time consuming (particularly with 25-G needles), which could prolong procedure time.
When puncturing a lesion, endosonographers should attempt to sample multiple areas within the same lesion during every pass, a technique referred to as fanning. During this technique, the needle track is slightly altered during every from movement by modulating the up and down dial of the echoendoscope or by using elevator. Bang et al[26] compared this technique to the standard one in sampling 54 solid pancreatic masses, and found fanning to be superior by establishing diagnosis in fewer numbers of passes, and resulted in higher first pass diagnostic rate of (85.7% vs 57.7 %, P = 0.02).
Number of passes
To date, no definite number of passes to achieve the highest diagnostic yield has been established yet for various lesions. Nevertheless, increasing the number of passes has been associated with higher diagnostic yield[27]. Most studies have shown that 5-7 passes would be adequate in solid lesions[27,28]. In general, fewer passes are required when sampling highly vascular lesions such as lymph nodes compared to solid masses like pancreatic tumors[27,29]. For example, seven passes have resulted in a sensitivity and specificity of 83% and 100% respectively in solid pancreatic masses, while, in the case of lymph nodes, five passes provided a sensitivity and specificity of 77% and 100% respectively[30]. For cystic lesions, it is recommended that the lesion be completely aspirated until it collapses via a single puncture.
On-site cytopathology evaluation
The availability of rapid on-site cytopathology evaluation can improve the sampling process by reducing the number of passes needed and the frequency of inconclusive diagnoses. A feedback from an on-site cytopathologist can dictate whether additional passes are needed to procure a better quality specimen[31]. To evaluate the effect of an on-site cytopathology examination on sample quality and the need to repeat the procedure, Collins et al[32] compared cytological outcomes from pancreatic mass FNAs done in the presence of an on-site cytopathologist with those without. The presence of an immediate on-site cytopathology exam resulted in a significant impact on the diagnostic yield; where only 2.9% of the procedures needed to be repeated compared to 5.8% when an on-site cytopathologist was unavailable. Additionally, definitive diagnosis in the repeated procedures was achieved more frequently in procedures where an onsite cytopathology evaluation was present (67% vs 27%). Several other studies supported the presence of on-site cytopathology evaluation and showed improved adequacy of samples[33-35]. In comparison, fewer studies showed that the presence of an on-site cytopathology failed to translate into significant improvement to EUS-FNA outcomes[36,37].
Method of sample expression
The traditional method of expressing FNA samples is via air flushing the needle. Recently, this was compared to the method of reinserting the stylet in a study by Lee et al[20] Samples expressed by the two techniques were compared in terms of quality, cellularity and bloodiness. Bloodiness was less in the air-flushed group compared to the reinserting-the-stylet group (P = 0.02), but quality of samples and cellularity were similar in both groups. Similar results were reported by Rastogi et al[38] in a randomized controlled trial as well as by Sahai et al[39].
Reinserting the stylet remains a common practice despite being time consuming and could potentially be associated with increased risk of needle stick injury[2,9,38,39]. Based on the results of the above studies, stylet reinsertion could be reserved to conditions when the sample is dry or clotted and cannot be expressed by air flushing[20,33], which is not infrequent with 25-G needles.
Key points: Application of suction during sampling can increase the cellularity and the diagnostic yield particularly in solid fibrotic lesions. In highly vascular lesions a non-suction technique can reduce the bloodiness of the sample. Fanning technique can reduce the number of passes required to reach a diagnosis. Expression techniques have minimal impact on the sample’s quality; however, air flushing seems to be less labor intensive. Use of stylet during the initial puncture or to express the sample has not been associated with improved specimen quality.
Needle size: FNA needles are commercially available in 19, 22 and 25-G sizes. Among all the variables that could impact the diagnostic outcome of EUS-FNA, needle size remains probably the most exhaustively evaluated. The most commonly used needle is the 22-G, although recently the 25-G needles have gained popularity in many centers due to their ease of use and recent data showing diagnostic equivalence compared to 22-G needles. In the following section we will discuss in details the choice of a particular needle size as it relates to the location of the lesion, type of the specimen and overall the quality of sample.
Location of the lesion
The location of the lesion can direct the sampling approach, and in some instances the size of the needle to be used. Approximately 65% of pancreatic cancers are found in the head or uncinate process[40]. Such tumors are best visualized and sampled through the duodenum, whereas lesions in the pancreatic body and tail are best evaluated through the stomach. In the duodenum, angulation of the echoendoscope tip is often required to maintain apposition with the mass. This position creates more resistance and makes the use of stiffer, larger 19-G needles challenging.
Itoi et al[41] objectively evaluated the resistance of 19-G Tru-cut, traditional 19-G, 22-G and 25-G aspiration needles during insertion and advancement under variable conditions of the echoendoscope (straight and angulated endoscope position, endoscope tip angulation, and while using of the elevator). In this trial, lower resistance was encountered with the 22-G and 25-G needles under almost every position compared to the conventional 19-G needle and 19-G Tru-cut needle. Additionally, the maneuverability of the scope itself was found to be reduced when using the 19-G needles.
To minimize resistance during transduodenal sampling, it is recommended to maintain the scope in a short position whenever possible. While the scope might be less stable at this position, it facilitates needle advancement out of the scope and penetration of the lesion. To improve stability of the scope, air suction or an inflated balloon could help bring the duodenal wall closer to the probe and further stabilize the tip of the scope during FNA.
Type of specimen
EUS-FNA remains the standard procedure for sampling pancreatic masses, in addition to other lesions like subepithelial tumors and enlarged lymph nodes. In most cases, cytology alone is adequate to reach a diagnosis. However, EUS-FNA has certain limitations. First, on-site cytopathology is not available in many centers. Second, certain conditions such as lymphoma, mesenchymal tumors, and well-differentiated adenocarcinomas can be difficult to diagnose by cytology alone[28,42], and a core biopsy (Trucut biopsy; TCB) with well-preserved tissue architecture may be essential for the diagnosis[43-45]. Third, the negative predictive value of EUS-FNA is relatively low, and therefore does not exclude malignancy in all negative specimens. In the last decade, a spring-loaded 19-TCB needle (Quick-core; Cook Medical, Winston-Salem, NC, United States) has been developed to overcome the limitations of FNA and procure larger quantities of tissue for histologic analysis[43]. Larghi et al[46] evaluated this needle in 23 patients with pancreatic masses. The overall success rate was 74% (17/23), however the success rate was significantly lower in transduodenal approach compared with transgastric approach. A larger study by Thomas et al[47] included 113 patients and showed a diagnostic accuracy of EUS-TCB of 68%. In this study there was no significant difference in the diagnostic yield for lesions procured by either transduodenal or transgastric approach. Based on a decade of experience, the use of this device has been mainly recommended in certain conditions where prior studies have shown better diagnostic compared to FNA yield such as lymphoma[48] and autoimmune pancreatitis[49]. The routine use of such device as an adjunct to FNA sampling was limited by the difficulty in operating it due to its stiffness and mechanical failures associated with its spring-loading mechanism.
To overcome some of the limitations associated with obtaining TCBs, a new series of fine needle biopsy needles has been developed (Procore, Cook Endoscopy, Winston Salem, NC) with reverse bevel technology to improve tissue acquisition. Iglesias-Garcia et al[44] evaluated the 19 G Procore needle of this series in a study that included 114 lesions. Adequate histological samples were obtained in 89% of lesions with overall diagnostic accuracy of over 85%. Transduodenal biopsy was successful in 33 of 35 cases (94%). However, technical difficulties were encountered when the needle was used transduodenally and in many cases the needle had to be advanced out of the scope in the stomach before reaching the duodenum. A prospective trial is currently underway to compare the performance and diagnostic yield of this and the older TCB device (Quick-core).
In another study, Bang et al[45] compared FNA using standard 22-G aspiration needle 22-G Procore needle for sampling solid pancreatic masses in 56 patients (Table 1). The trial showed no significant difference between the two devices in procurement of the core tissue (100% vs 83.3%, P = 0.26) or the presence of diagnostic histologic specimens (66.7% vs 80%, P = 0.66). No difficulty in performing transgastric or transduodenal biopsies was reported in this study using the ProCore needle. In another trial by Larghi et al[50], adequate histological samples were obtained in 54 out of 61 patients (88.5%) using the Procore needle.
Table 1.
Rate of histologically adequate specimens procured using the 3 common size fine needles in recent literature
| Ref. | Needle size | Number of patients | Histological adequacy | Location of biopsy |
| Bang et al[45] | 22-G FNA | 28 | 66.7% | Pancreas |
| 22-G FNB | 28 | |||
| Yasuda et al[52] | 19-G | 104 | 98.0% | Lymph nodes |
| Rong et al[51] | 22-G | 54 | 70.4% | Pancreas |
| 25-G | 54 | 61.1% | Pancreas | |
| 22-G | 27 | 74.1% | Submucosal tumors | |
| 25-G | 27 | 55.6% | Submucosal tumors | |
| Larghi et al[53] | 19-G | 120 | 97.5% | Various |
| Varadarajulu et al[19] | 19-G1 | 38 | 94.7% | Subepithelial masses |
| Pancreatic (head and uncinate lesions) |
A flexible Nitinol based needle was used in all procedures. FNA: Fine needle aspiration; FNB: Fine needle biopsy.
The ability of standard FNA needles to provide adequate histological samples has been evaluated as well. In one trial, Rong et al[51] found that histologic adequacy of the standard 22-G needle was superior to the 25-G one in sampling pancreatic masses (70.4% vs 61.1%, P = 0.33) and submucosal tumors (74.1% vs 55.6%, P = 0.18). However, diagnostic accuracy did not differ between the two needles (80.0% vs 78.9%) when both needles were used on the same patient.
In evaluating the ability of the 19-G needle to obtain histological specimens, Yasuda et al[52] reported 98% accuracy of EUS-FNB using the conventional 19-G needle in diagnosing patients with unknown lymphadenopathy. In patients with lymphoma, the needle provided sufficient tissue to classify their lymphomas in accordance with the World Health Organization classifications in 88% of them. In another trial, adequate histological samples from solid masses were obtained in 97.5% of patients using the 19-G needle[53]. However, this study excluded patients with pancreatic head and uncinate masses that required transduodenal approach for sampling.
The stiffness of 19-G needles has been recently reduced in a new needle made of nitinol (Expect 19-gauge Flex, Boston Scientific, Natick, MA, United States). Nitinol is an alloy made of Nickel and Titanium, used in the construction of biliary endoprosthesis. The properties of this needle include resistance to deformation and high elasticity, which facilitate tissue sampling when the tip of the echoendoscope is in an angulated position. Two recent studies evaluated the clinical performance of this flexible 19-G needle (Figure 2)[19,54]. The first study by Varadarajulu et al[19] included 38 patients in whom 32 had pancreatic head or uncinate lesions. There were no technical failures reported in this trial, and histological samples were satisfactory in 94.7% of patients. In another multi-center trial published in abstract form, the needle was used in a variety of applications, with similar high technical success and histological adequacy rates[54].
Figure 2.
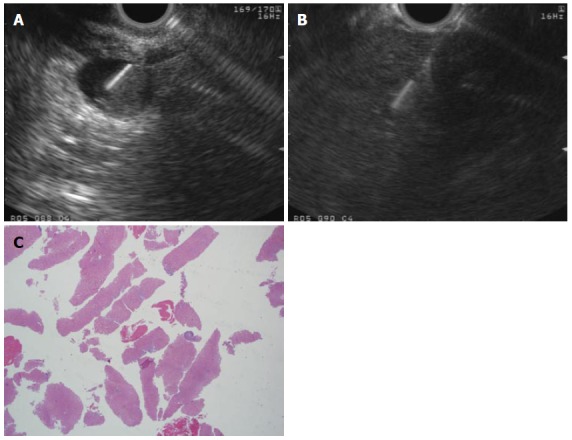
Flexible 19-G needle. A: 2 cm rectal subepithelial lesion was found to originate from the muscularis propria on endoscopic ultrasound guided and is sampled using a flexible 19-G needle in this figure; B: A core liver biopsy was obtained using a flexible 19-G needle is a patient with elevated transaminases; C: Histopathological assessment of the core biopsy obtained in the case above confirmed steatohepatitis without significant fibrosis. Adequate histopathology sample was obtained and stained positively for CD-117, confirming gastrointestinal stromal tumor; H and E stain, × 2.
Key points: Histological specimens are necessary for the diagnosis and appropriate classification of certain conditions such as lymphoma, stromal cell tumors and well-differentiated cancers. New generation of fine needle core biopsy devices of various sizes facilitate transduodenal sampling and have been associated with high technical success rates and adequate histological sampling.
Quality of sample
Sample quality depends on several variables, including experience of the endosonographer, the availability of onsite cytopathology review, the method of cytopathology preparation, the location and physical characteristics of the lesion, and size of the needle[9-13]. Among all these factors, needle size continues to receive most attention as an independent factor that could impact the diagnostic yield of EUS-FNA. Comparing the quality of samples and diagnostic yield of the different needle sizes have been the focus of recent studies, most of which compared 22-G with 25-G needles[55-60] with fewer studies including 19-G needles[56,57] (Table 2).
Table 2.
Quality of samples using different needle sizes as reported by recent studies
| Ref. | Needle size | Number of patients | Location of lesion | Result |
| Lee et al[55] | 22-G and 25-G | 12 | Pancreas and peripancreatic | No difference between the two needles in terms of cellularity (P = 0.84) |
| Siddiqui et al[56] | 22-G and 25-G | 131 | Pancreas | No significant difference in diagnostic yield (P = 0.18) |
| (22-G = 64 patients) | ||||
| (25-G = 67 patients) | ||||
| Fabbri et al[57] | 22-G and 25-G | 50 | Pancreas | No significant difference in diagnostic accuracy 94% vs 86% |
| Imazu et al[58] | 22-G and 25-G | 43 | Pancreas, lymph nodes, submucosal tumors | Similar overall diagnostic yield |
| 22 > 25 in submucosal lesions (80% vs 60%) | ||||
| 25-G > 22-G in pancreatic lesions (91.5% vs 75%) | ||||
| Camellini et al[59] | 22-G and 25-G | 127 | Pancreatic, lymph nodes and subepithelial tumors | No significant difference in sample adequacy overall (77.8% vs 78.1%) |
| Pancreatic lesions: 25-G > 22-G (87.8% vs 76.7%) | ||||
| Subepithelial lesions: 22-G > 25-G (55.5% vs 20%) | ||||
| Lymph nodes: 22-G > 25-G (100% vs 60%) | ||||
| Sakamoto et al[18] | 19-G, 22-G and 25-G | 24 | Pancreas | 19-G and 22-G > 25-G in adequacy of samples for histological diagnosis |
| 25-G had better diagnostic accuracy in pancreatic head and uncinate lesions | ||||
| Song et al[61] | 19-G and 22-G | 117 | Pancreatic and peripancreatic lesions | Sample quality and cellular material: 19-G > 22-G (P = 0.03) |
Lee et al[55] (12 patients), Siddiqui et al [56] (131 patients) and Fabbri et al[57] (50 patients) compared the quality of samples and the diagnostic yield of the 22-G and 25-G needles in pancreatic lesions[55-57] and peri-pancreatic lesions[55]. The studies by Lee et al[55] and Fabbri et al[57] provided a comparison of the two needles in the same lesion. In all three studies, there was no significant difference in diagnostic yield between the two needles.
On the other hand, Imazu et al[58] found that the quantity of specimens obtained by the 22-G needle was overall higher than the 25-G needle, which resulted in a higher diagnostic yield in patients with submucosal tumors, where histological sample is essential for diagnosis. However, in patents with pancreatic lesions, the diagnostic yield of the 25-G needle was higher. This was believed to be the result of the tissue characteristics of solid pancreatic lesions which are typically hard in consistency and a smaller needle may provide an easier puncture and lead to better sample quality and less technical failures.
In a large prospective randomized study, Camellini et al[59] compared 22 with 25-G needles in 127 solid lesions with salvage crossover for inadequate passes or upon failure of puncturing the lesion. The number of passes made and specimen adequacy was not different between the 2 needles. More cross overs from 22 to 25-G needles were observed in uncinate process masses due to technical failures. This study suggested superiority of the 25-G needle in obtaining samples through transduodenal approach.
Fewer studies included 19-G needles for assessment of specimen quality. Sakamoto et al[18] included 19-G TCB needle in their study that compared it to 25-G and 22-G needles in 24 patients with solid pancreatic masses. The 22-G and 19-G TCB needles were superior to the 25-G needle (P < 0.05) in providing adequate histological diagnosis in technically successful cases. However, the 25-G needle was superior in overall diagnostic accuracy particularly in lesions in the pancreatic head and uncinate process. In another randomized study including 117 patients with pancreatic and peripancreatic masses[61], the traditional 19-G and 22-G needles were compared. Technical failure occurred in 5 out of 60 patients who were randomized to the 19-G needle, all of which arose when sampling pancreatic head or uncinate process lesions. Those masses were successfully sampled once crossed over to 22-G needles. Excluding those with technical failures, the overall diagnostic accuracy was higher in the 19-G group (94.5% vs 78.9% P = 0.02). Sample quality was also superior in 19-G needle group (P = 0.03).
Key points: Needle size can influence the quality of samples of EUS-FNA. 22-G and 25-G needles appear to be equivalent in sampling capabilities. 19-G needles appear to be capable of providing superior tissue quantity and quality compared to 22 and 25-G needles, but carry a higher chance of technical failure when utilized for transduodenal sampling.
COMPLICATIONS OF EUS-GUIDED TISSUE ACQUISITION
Complications of EUS-FNA are rare and include bleeding, infection, and acute pancreatitis, which collectively occurs in about 2%-3% or less of procedures[62-63]. Additionally, there have been few case reports of tumor seeding after FNA although this remains very rare[63-67]. Theoretically, the larger the needle size and the higher the number of passes made, the higher the likelihood of complications. Nevertheless, a recent meta-analysis[68] that included 51 studies failed to demonstrate a statistical difference in rate of adverse events associated with 19-G needle as compared to 22 and 25-G needles.
To date, few studies have evaluated the specific factors associated with increased incidence of adverse events. One study of 316 patients[65] found that post-procedural adverse events are higher when accessing pancreatic lesions smaller than 20 mm in size. This can be explained by the fact that smaller lesions can be more difficult to access, and require longer time of penetration and higher number of passes, all of which can increase the risk of damaging the normal surrounding tissues. The same study showed that certain cancers such as neuroendocrine tumors are associated with higher incidence of complications after EUS-FNA, probably due to the highly vascular nature of such lesions. Other variables such as patients’ age, needle size, lesion location and number of passes did not have an impact on the incidence of adverse events. It should be noted that due to the very low overall rate of EUS-FNA related complications, a rather large sample size is required to demonstrate any potential increase in complication rates when using larger size needles. In addition, certain complications like bleeding following FNA remain subclinical and rarely result in hospitalizations or further interventions. In general, it is always recommended that the diagnosis be made with smaller needles and minimal number of passes in order to avoid any unnecessary risks.
RECENT ADVANCES IN EUS-GUIDED TISSUE ACQUISITION
The recent technological developments in EUS equipment employ physical concepts of ultrasound in an attempt to improve the diagnostic yield of this procedure while maintaining its high safety profile. One glaring example is the use of real-time sono-elastography, which is a technique that measures tissue elasticity through calculation of tissue strain[69]. Due to the fact that tissue elasticity is often altered when replaced by cancer, EUS elastography can detect small tumors and malignant lymph nodes and this can direct FNA to high yield sites. This can be particularly useful in the setting of chronic pancreatitis, which is estimated to be present in up to 20%-35% of patients undergoing FNA for pancreatic lesions[13,70,71]. This also can be of value when EUS-FNA is negative for malignancy when the suspicion of cancer remains strong[71-75]. Other recent advances such as tridimensional (3D) EUS, contrast-enhanced EUS and EUS spectrum analysis have minimal or no role in tissue acquisition but can provide better visualization of pancreatic masses. This can positively impact the FNA outcome due to better differentiation of malignancies from other inflammatory conditions.
Genetic mutations have been studied as adjunct markers to aid in the diagnosis of pancreatic cancers. The most practical example in relation to FNA of pancreatic cancer is K-ras mutation, which has been the focus of several studies to evaluate its impact on the diagnostic yield of FNA[76-78]. K-ras oncogene is activated by somatic point substitution and considered as an initial event in pancreatic carcinogenesis[79], and K-ras mutation can be found in 90% of patients with this disease. In a recent prospective series including 394 pancreatic masses, Ogura et al[76] found that combining K-ras mutation analysis with cytopathology increased the sensitivity of EUS-FNA from 87% to 93% (P < 0.001) and the accuracy from 89% to 94% (P < 0.001) for the diagnosis of pancreatic ductal adenocarcinoma. In this study, out of the 39 patients who were undiagnosed using cytology, K-ras was detected in 18 patients (46%). In a recent meta-analysis[77]. which included 8 studies with 931 patients undergoing EUS-FNA of pancreatic masses, the pooled sensitivity and specificity of EUS-FNA were 80 and 97% respectively; the estimated sensitivity and specificity of K-ras mutation analysis were 76.8% and 93.3% respectively, and 88.7% and 92% when cytology and K-ras mutation analysis were combined. Overall, K-ras mutation testing applied to cases that were inconclusive by EUS-FNA reduced the false-negative rate by 55.6%, with a false-positive rate of 10.7%. In addition to K-ras oncogene, a number of tumor suppressor genes were found to be affected by genetic alteration in pancreatic cancer such as p53, p16 and DPC4. Those, in addition to K-ras have been shown to increase the sensitivity of pancreatic cancer detection to up to 90%-100% in cases where FNA was inconclusive[78,79]. Due to the relative high diagnostic accuracy of standard EUS-FNA as well as the relatively high cost and limited availability of these genetic tests, the use of genetic testing of EUS-FNA samples has been limited to research protocols and inconclusive cytopathology specimens.
CONCLUSION
EUS-guided tissue acquisition has evolved to become an indispensible tool for the diagnostic work up of gastrointestinal malignancies and other non-malignant disorders. High sensitivity, specificity and diagnostic accuracy coupled with low rates of adverse events have made this procedure more suitable than other invasive ones such as CT-guided biopsies. The quality of sample acquired by EUS is influenced by numerous factors, including needle size and sampling techniques. Recent studies have demonstrated the adequacy of FNA specimens provided by 25-G needle compared to other needles and this should be strongly considered in transduodenal sampling. Larger size needles like 19-G appear to provide better sample quality overall, but can be associated with technical failures in the transduodenal approach and potentially higher rates of complications. New flexible 19-G needles and newly designed core biopsy devices appear capable of delivering adequate histopathology samples when this is needed for the diagnosis.
Footnotes
P- Reviewers: Baghbanian M, Meister T S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
References
- 1.Vilmann P, Jacobsen GK, Henriksen FW, Hancke S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172–173. doi: 10.1016/s0016-5107(92)70385-x. [DOI] [PubMed] [Google Scholar]
- 2.Erickson RA. EUS-guided FNA. Gastrointest Endosc. 2004;60:267–279. doi: 10.1016/s0016-5107(04)01529-9. [DOI] [PubMed] [Google Scholar]
- 3.Mortensen MB, Pless T, Durup J, Ainsworth AP, Plagborg GJ, Hovendal C. Clinical impact of endoscopic ultrasound-guided fine needle aspiration biopsy in patients with upper gastrointestinal tract malignancies. A prospective study. Endoscopy. 2001;33:478–483. doi: 10.1055/s-2001-14966. [DOI] [PubMed] [Google Scholar]
- 4.Shah JN, Ahmad NA, Beilstein MC, Ginsberg GG, Kochman ML. Clinical impact of endoscopic ultrasonography on the management of malignancies. Clin Gastroenterol Hepatol. 2004;2:1069–1073. doi: 10.1016/s1542-3565(04)00444-6. [DOI] [PubMed] [Google Scholar]
- 5.Chang KJ, Katz KD, Durbin TE, Erickson RA, Butler JA, Lin F, Wuerker RB. Endoscopic ultrasound-guided fine-needle aspiration. Gastrointest Endosc. 1994;40:694–699. [PubMed] [Google Scholar]
- 6.Vilmann P, Hancke S, Henriksen FW, Jacobsen GK. Endoscopic ultrasonography-guided fine-needle aspiration biopsy of lesions in the upper gastrointestinal tract. Gastrointest Endosc. 1995;41:230–235. doi: 10.1016/s0016-5107(95)70343-8. [DOI] [PubMed] [Google Scholar]
- 7.Williams DB, Sahai AV, Aabakken L, Penman ID, van Velse A, Webb J, Wilson M, Hoffman BJ, Hawes RH. Endoscopic ultrasound guided fine needle aspiration biopsy: a large single centre experience. Gut. 1999;44:720–726. doi: 10.1136/gut.44.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin HJ, Lahoti S, Sneige N. Endoscopic ultrasound-guided fine-needle aspiration in 179 cases: the MD Anderson cancer center experience. Cancer. 2002;96:174–180. doi: 10.1002/cncr.10614. [DOI] [PubMed] [Google Scholar]
- 9.Savides TJ. Tricks for improving EUS-FNA accuracy and maximizing cellular yield. Gastrointest Endosc. 2009;69:S130–S133. doi: 10.1016/j.gie.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Bentz JS, Kochman ML, Faigel DO, Ginsberg GG, Smith DB, Gupta PK. Endoscopic ultrasound-guided real-time fine-needle aspiration: clinicopathologic features of 60 patients. Diagn Cytopathol. 1998;18:98–109. doi: 10.1002/(sici)1097-0339(199802)18:2<98::aid-dc4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 11.Mertz H, Gautam S. The learning curve for EUS-guided FNA of pancreatic cancer. Gastrointest Endosc. 2004;59:33–37. doi: 10.1016/s0016-5107(03)02028-5. [DOI] [PubMed] [Google Scholar]
- 12.Itoi T, Itokawa F, Sofuni A, Nakamura K, Tsuchida A, Yamao K, Kawai T, Moriyasu F. Puncture of solid pancreatic tumors guided by endoscopic ultrasonography: a pilot study series comparing Trucut and 19-gauge and 22-gauge aspiration needles. Endoscopy. 2005;37:362–366. doi: 10.1055/s-2004-826156. [DOI] [PubMed] [Google Scholar]
- 13.Fritscher-Ravens A, Topalidis T, Bobrowski C, Krause C, Thonke E, Jäckle S, Soehendra N. Endoscopic ultrasound-guided fine-needle aspiration in focal pancreatic lesions: a prospective intraindividual comparison of two needle assemblies. Endoscopy. 2001;33:484–490. doi: 10.1055/s-2001-14970. [DOI] [PubMed] [Google Scholar]
- 14.Buscail L, Faure P, Bournet B, Selves J, Escourrou J. Interventional endoscopic ultrasound in pancreatic diseases. Pancreatology. 2006;6:7–16. doi: 10.1159/000090022. [DOI] [PubMed] [Google Scholar]
- 15.DeWitt J, Emerson RE, Sherman S, Al-Haddad M, McHenry L, Cote GA, Leblanc JK. Endoscopic ultrasound-guided Trucut biopsy of gastrointestinal mesenchymal tumor. Surg Endosc. 2011;25:2192–2202. doi: 10.1007/s00464-010-1522-z. [DOI] [PubMed] [Google Scholar]
- 16.Amador-Ortiz C, Chen L, Hassan A, Frater JL, Burack R, Nguyen TT, Kreisel F. Combined core needle biopsy and fine-needle aspiration with ancillary studies correlate highly with traditional techniques in the diagnosis of nodal-based lymphoma. Am J Clin Pathol. 2011;135:516–524. doi: 10.1309/AJCP3WZ8ZDRJQDOU. [DOI] [PubMed] [Google Scholar]
- 17.Levy MJ. Endoscopic ultrasound-guided trucut biopsy of the pancreas: prospects and problems. Pancreatology. 2007;7:163–166. doi: 10.1159/000104240. [DOI] [PubMed] [Google Scholar]
- 18.Sakamoto H, Kitano M, Komaki T, Noda K, Chikugo T, Dote K, Takeyama Y, Das K, Yamao K, Kudo M. Prospective comparative study of the EUS guided 25-gauge FNA needle with the 19-gauge Trucut needle and 22-gauge FNA needle in patients with solid pancreatic masses. J Gastroenterol Hepatol. 2009;24:384–390. doi: 10.1111/j.1440-1746.2008.05636.x. [DOI] [PubMed] [Google Scholar]
- 19.Varadarajulu S, Bang JY, Hebert-Magee S. Assessment of the technical performance of the flexible 19-gauge EUS-FNA needle. Gastrointest Endosc. 2012;76:336–343. doi: 10.1016/j.gie.2012.04.455. [DOI] [PubMed] [Google Scholar]
- 20.Lee JK, Choi JH, Lee KH, Kim KM, Shin JU, Lee JK, Lee KT, Jang KT. A prospective, comparative trial to optimize sampling techniques in EUS-guided FNA of solid pancreatic masses. Gastrointest Endosc. 2013;77:745–751. doi: 10.1016/j.gie.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Puri R, Vilmann P, Săftoiu A, Skov BG, Linnemann D, Hassan H, Garcia ES, Gorunescu F. Randomized controlled trial of endoscopic ultrasound-guided fine-needle sampling with or without suction for better cytological diagnosis. Scand J Gastroenterol. 2009;44:499–504. doi: 10.1080/00365520802647392. [DOI] [PubMed] [Google Scholar]
- 22.Santos JE, Leiman G. Nonaspiration fine needle cytology. Application of a new technique to nodular thyroid disease. Acta Cytol. 1988;32:353–356. [PubMed] [Google Scholar]
- 23.Kinney TB, Lee MJ, Filomena CA, Krebs TL, Dawson SL, Smith PL, Raafat N, Mueller PR. Fine-needle biopsy: prospective comparison of aspiration versus nonaspiration techniques in the abdomen. Radiology. 1993;186:549–552. doi: 10.1148/radiology.186.2.8421763. [DOI] [PubMed] [Google Scholar]
- 24.Polkowski M, Larghi A, Weynand B, Boustière C, Giovannini M, Pujol B, Dumonceau JM. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy. 2012;44:190–206. doi: 10.1055/s-0031-1291543. [DOI] [PubMed] [Google Scholar]
- 25.Wani S, Gupta N, Gaddam S, Singh V, Ulusarac O, Romanas M, Bansal A, Sharma P, Olyaee MS, Rastogi A. A comparative study of endoscopic ultrasound guided fine needle aspiration with and without a stylet. Dig Dis Sci. 2011;56:2409–2414. doi: 10.1007/s10620-011-1608-z. [DOI] [PubMed] [Google Scholar]
- 26.Bang JY, Magee SH, Ramesh J. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy. 2013;45:445–450. doi: 10.1055/s-0032-1326268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS guided fine needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184–190. doi: 10.1016/s0016-5107(00)70416-0. [DOI] [PubMed] [Google Scholar]
- 28.Wee E, Lakhtakia S, Gupta R, Sekaran A, Kalapala R, Monga A, Arjunan S, Reddy DN. Endoscopic ultrasound guided fine-needle aspiration of lymph nodes and solid masses: factors influencing the cellularity and adequacy of the aspirate. J Clin Gastroenterol. 2012;46:487–493. doi: 10.1097/MCG.0b013e31824432cb. [DOI] [PubMed] [Google Scholar]
- 29.Kulesza P, Eltoum IA. Endoscopic ultrasound-guided fine-needle aspiration: sampling, pitfalls, and quality management. Clin Gastroenterol Hepatol. 2007;5:1248–1254. doi: 10.1016/j.cgh.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 30.LeBlanc JK, Ciaccia D, Al-Assi MT, McGrath K, Imperiale T, Tao LC, Vallery S, DeWitt J, Sherman S, Collins E. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc. 2004;59:475–481. doi: 10.1016/s0016-5107(03)02863-3. [DOI] [PubMed] [Google Scholar]
- 31.Iglesias-Garcia J, Dominquez-Munoz JE, Abdulkader I, Larino-Noia J, Eugenyeva E, Lorenzo-Leon A, Forteza-Vila J. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011;106:1705–1710. doi: 10.1038/ajg.2011.119. [DOI] [PubMed] [Google Scholar]
- 32.Collins BT, Murad FM, Wang JF, Bernadt CT. Rapid on-site evaluation for endoscopic ultrasound-guided fine-needle biopsy of the pancreas decreases the incidence of repeat biopsy procedures. Cancer Cytopathol. 2013;121:518–524. doi: 10.1002/cncy.21340. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt RL, Witt BL, Matynia AP, Barraza G, Layfield LJ, Adler DG. Rapid on-site evaluation increases endoscopic ultrasound-guided fine-needle aspiration adequacy for pancreatic lesions. Dig Dis Sci. 2013;58:872–882. doi: 10.1007/s10620-012-2411-1. [DOI] [PubMed] [Google Scholar]
- 34.Klapman JB, Logrono R, Dye CE, Waxman I. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2003;98:1289–1294. doi: 10.1111/j.1572-0241.2003.07472.x. [DOI] [PubMed] [Google Scholar]
- 35.Jhala NC, Jhala DN, Chhieng DC, Eloubeidi MA, Eltoum IA. Endoscopic ultrasound-guided fine-needle aspiration. A cytopathologist’s perspective. Am J Clin Pathol. 2003;120:351–367. doi: 10.1309/MFRF-J0XY-JLN8-NVDP. [DOI] [PubMed] [Google Scholar]
- 36.Cherian PT, Mohan P, Douiri A, Taniere P, Hejmadi RK, Mahon BS. Role of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis of solid pancreatic and peripancreatic lesions: is onsite cytopathology necessary. HPB (Oxford) 2010;12:389–395. doi: 10.1111/j.1477-2574.2010.00180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nayar MK, Chatterjee S, Wadehra V, Cunningham J, Leeds J, Oppong K. Does on-site adequacy assessment by cytotechnologists improve results of EUS guided FNA of solid pancreaticobiliary lesions. JOP. 2013;14:44–49. doi: 10.6092/1590-8577/1277. [DOI] [PubMed] [Google Scholar]
- 38.Rastogi A, Wani S, Gupta N, Singh V, Gaddam S, Reddymasu S, Ulusarac O, Fan F, Romanas M, Dennis KL, et al. A prospective, single-blind, randomized, controlled trial of EUS-guided FNA with and without a stylet. Gastrointest Endosc. 2011;74:58–64. doi: 10.1016/j.gie.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 39.Sahai AV, Paquin SC, Gariépy G. A prospective comparison of endoscopic ultrasound-guided fine needle aspiration results obtained in the same lesion, with and without the needle stylet. Endoscopy. 2010;42:900–903. doi: 10.1055/s-0030-1255676. [DOI] [PubMed] [Google Scholar]
- 40.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 41.Itoi T, Itokawa F, Kurihara T, Sofuni A, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, Kawai T, Moriyasu F. Experimental endoscopy: objective evaluation of EUS needles. Gastrointest Endosc. 2009;69:509–516. doi: 10.1016/j.gie.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 42.Ribeiro A, Vazquez-Sequeiros E, Wiersema LM, Wang KK, Clain JE, Wiersema MJ. EUS-guided fine-needle aspiration combined with flow cytometry and immunocytochemistry in the diagnosis of lymphoma. Gastrointest Endosc. 2001;53:485–491. doi: 10.1067/mge.2001.112841. [DOI] [PubMed] [Google Scholar]
- 43.Levy MJ, Jondal ML, Clain J, Wiersema MJ. Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc. 2003;57:101–106. doi: 10.1067/mge.2003.49. [DOI] [PubMed] [Google Scholar]
- 44.Iglesias-Garcia J, Poley JW, Larghi A, Giovannini M, Petrone MC, Abdulkader I, Monges G, Costamagna G, Arcidiacono P, Biermann K, et al. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011;73:1189–1196. doi: 10.1016/j.gie.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 45.Bang JY, Hebert-Magee S, Trevino J, Ramesh J, Varadarajulu S. Randomized trial comparing the 22-gauge aspiration and 22-gauge biopsy needles for EUS-guided sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2012;76:321–327. doi: 10.1016/j.gie.2012.03.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larghi A, Verna EC, Stavropoulos SN, Rotterdam H, Lightdale CJ, Stevens PD. EUS-guided trucut needle biopsies in patients with solid pancreatic masses: a prospective study. Gastrointest Endosc. 2004;59:185–190. doi: 10.1016/s0016-5107(03)02538-0. [DOI] [PubMed] [Google Scholar]
- 47.Thomas T, Kaye PV, Ragunath K, Aithal G. Efficacy, safety, and predictive factors for a positive yield of EUS-guided Trucut biopsy: a large tertiary referral center experience. Am J Gastroenterol. 2009;104:584–591. doi: 10.1038/ajg.2008.97. [DOI] [PubMed] [Google Scholar]
- 48.Eloubeidi MA, Mehra M, Bean SM. EUS-guided 19-gauge trucut needle biopsy for diagnosis of lymphoma missed by EUS-guided FNA. Gastrointest Endosc. 2007;65:937–939. doi: 10.1016/j.gie.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 49.Levy MJ, Reddy RP, Wiersema MJ, Smyrk TC, Clain JE, Harewood GC, Pearson RK, Rajan E, Topazian MD, Yusuf TE, et al. EUS-guided trucut biopsy in establishing autoimmune pancreatitis as the cause of obstructive jaundice. Gastrointest Endosc. 2005;61:467–472. doi: 10.1016/s0016-5107(04)02802-0. [DOI] [PubMed] [Google Scholar]
- 50.Larghi A, Iglesias-Garcia J, Poley JW, Monges G, Petrone MC, Rindi G, Abdulkader I, Arcidiacono PG, Costamagna G, Biermann K, et al. Feasibility and yield of a novel 22-gauge histology EUS needle in patients with pancreatic masses: a multicenter prospective cohort study. Surg Endosc. 2013;27:3733–3738. doi: 10.1007/s00464-013-2957-9. [DOI] [PubMed] [Google Scholar]
- 51.Rong L, Kida M, Yamauchi H, Okuwaki K, Miyazawa S, Iwai T, Kikuchi H, Watanabe M, Imaizumi H, Koizumi W. Factors affecting the diagnostic accuracy of endoscopic ultrasonography-guided fine-needle aspiration (EUS-FNA) for upper gastrointestinal submucosal or extraluminal solid mass lesions. Dig Endosc. 2012;24:358–363. doi: 10.1111/j.1443-1661.2012.01243.x. [DOI] [PubMed] [Google Scholar]
- 52.Yasuda I, Tsurumi H, Omar S, Iwashita T, Kojima Y, Yamada T, Sawada M, Takami T, Moriwaki H, Soehendra N. Endoscopic ultrasound-guided fine-needle aspiration biopsy for lymphadenopathy of unknown origin. Endoscopy. 2006;38:919–924. doi: 10.1055/s-2006-944665. [DOI] [PubMed] [Google Scholar]
- 53.Larghi A, Verna EC, Ricci R, Seerden TC, Galasso D, Carnuccio A, Uchida N, Rindi G, Costamagna G. EUS-guided fine-needle tissue acquisition by using a 19-gauge needle in a selected patient population: a prospective study. Gastrointest Endosc. 2011;74:504–510. doi: 10.1016/j.gie.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 54.Al-Haddad M, Ashish A, Aman A. EUS-Guided Biopsy with a Novel 19-gauge Fine Needle Biopsy (FNB) Device: Multi-Center Experience. Gastrointest Endosc. 2013;77:AB403–AB404. [Google Scholar]
- 55.Lee JH, Stewart J, Ross WA, Anandasabapathy S, Xiao L, Staerkel G. Blinded prospective comparison of the performance of 22-gauge and 25-gauge needles in endoscopic ultrasound-guided fine needle aspiration of the pancreas and peri-pancreatic lesions. Dig Dis Sci. 2009;54:2274–2281. doi: 10.1007/s10620-009-0906-1. [DOI] [PubMed] [Google Scholar]
- 56.Siddiqui UD, Rossi F, Rosenthal LS, Padda MS, Murali-Dharan V, Aslanian HR. EUS-guided FNA of solid pancreatic masses: a prospective, randomized trial comparing 22-gauge and 25-gauge needles. Gastrointest Endosc. 2009;70:1093–1097. doi: 10.1016/j.gie.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 57.Fabbri C, Polifemo AM, Luigiano C, Cennamo V, Baccarini P, Collina G, Fornelli A, Macchia S, Zanini N, Jovine E, et al. Endoscopic ultrasound-guided fine needle aspiration with 22- and 25-gauge needles in solid pancreatic masses: a prospective comparative study with randomisation of needle sequence. Dig Liver Dis. 2011;43:647–652. doi: 10.1016/j.dld.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 58.Imazu H, Uchiyama Y, Kakutani H, Ikeda K, Sumiyama K, Kaise M, Omar S, Ang TL, Tajiri H. A prospective comparison of EUS-guided FNA using 25-gauge and 22-gauge needles. Gastroenterol Res Pract. 2009;2009:546390. doi: 10.1155/2009/546390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Camellini L, Carlinfante G, Azzolini F, Iori V, Cavina M, Sereni G, Decembrino F, Gallo C, Tamagnini I, Valli R, et al. A randomized clinical trial comparing 22G and 25G needles in endoscopic ultrasound-guided fine-needle aspiration of solid lesions. Endoscopy. 2011;43:709–715. doi: 10.1055/s-0030-1256482. [DOI] [PubMed] [Google Scholar]
- 60.Madhoun MF, Wani SB, Rastogi A, Early D, Gaddam S, Tierney WM, Maple JT. The diagnostic accuracy of 22-gauge and 25-gauge needles in endoscopic ultrasound-guided fine needle aspiration of solid pancreatic lesions: a meta-analysis. Endoscopy. 2013;45:86–92. doi: 10.1055/s-0032-1325992. [DOI] [PubMed] [Google Scholar]
- 61.Song TJ, Kim JH, Lee SS, Eum JB, Moon SH, Park do H, Seo DW, Lee SK, Jang SJ, Yun SC, et al. The prospective randomized, controlled trial of endoscopic ultrasound-guided fine-needle aspiration using 22G and 19G aspiration needles for solid pancreatic or peripancreatic masses. Am J Gastroenterol. 2010;105:1739–1745. doi: 10.1038/ajg.2010.108. [DOI] [PubMed] [Google Scholar]
- 62.O'Toole D, Palazzo L, Arotçarena R, Dancour A, Aubert A, Hammel P, Amaris J, Ruszniewski P. Assessment of complications of EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:470–474. doi: 10.1067/mge.2001.112839. [DOI] [PubMed] [Google Scholar]
- 63.Adler DG, Jacobson BC, Davila RE, Hirota WK, Leighton JA, Qureshi WA, Rajan E, Zuckerman MJ, Fanelli RD, Baron TH, et al. ASGE guideline: complications of EUS. Gastrointest Endosc. 2005;61:8–12. doi: 10.1016/s0016-5107(04)02393-4. [DOI] [PubMed] [Google Scholar]
- 64.Doi S, Yasuda I, Iwashita T, Ibuka T, Fukushima H, Araki H, Hirose Y, Moriwaki H. Needle tract implantation on the esophageal wall after EUS-guided FNA of metastatic mediastinal lymphadenopathy. Gastrointest Endosc. 2008;67:988–990. doi: 10.1016/j.gie.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 65.Katanuma A, Maguchi H, Yane K, Hashigo S, Kin T, Kaneko M, Kato S, Kato R, Harada R, Osanai M, et al. Factors predictive of adverse events associated with endoscopic ultrasound-guided fine needle aspiration of pancreatic solid lesions. Dig Dis Sci. 2013;58:2093–2099. doi: 10.1007/s10620-013-2590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paquin SC, Gariépy G, Lepanto L, Bourdages R, Raymond G, Sahai AV. A first report of tumor seeding because of EUS-guided FNA of a pancreatic adenocarcinoma. Gastrointest Endosc. 2005;61:610–611. doi: 10.1016/s0016-5107(05)00082-9. [DOI] [PubMed] [Google Scholar]
- 67.Micames C, Jowell PS, White R, Paulson E, Nelson R, Morse M, Hurwitz H, Pappas T, Tyler D, McGrath K. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003;58:690–695. doi: 10.1016/s0016-5107(03)02009-1. [DOI] [PubMed] [Google Scholar]
- 68.Varadarajulu S, Ginnetti L, Peetermans J, Rousseau M, Hasan M, Hawes R. Meta-Analysis Comparing Rates of Complications between the standard 19G and 22/25G needles for EUS-Guided FNA of Pancreatic Lesions. Gastrointest Endosc. 2013;77 Suppl:AB405. [Google Scholar]
- 69.Frey H. [Realtime elastography. A new ultrasound procedure for the reconstruction of tissue elasticity] Radiologe. 2003;43:850–855. doi: 10.1007/s00117-003-0943-2. [DOI] [PubMed] [Google Scholar]
- 70.Fritscher-Ravens A, Brand L, Knöfel WT, Bobrowski C, Topalidis T, Thonke F, de Werth A, Soehendra N. Comparison of endoscopic ultrasound-guided fine needle aspiration for focal pancreatic lesions in patients with normal parenchyma and chronic pancreatitis. Am J Gastroenterol. 2002;97:2768–2775. doi: 10.1111/j.1572-0241.2002.07020.x. [DOI] [PubMed] [Google Scholar]
- 71.Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728–736; quiz 751, 753. doi: 10.1016/j.gie.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 72.Janssen J, Schlörer E, Greiner L. EUS elastography of the pancreas: feasibility and pattern description of the normal pancreas, chronic pancreatitis, and focal pancreatic lesions. Gastrointest Endosc. 2007;65:971–978. doi: 10.1016/j.gie.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 73.Săftoiu A, Vilmann P, Gorunescu F, Gheonea DI, Gorunescu M, Ciurea T, Popescu GL, Iordache A, Hassan H, Iordache S. Neural network analysis of dynamic sequences of EUS elastography used for the differential diagnosis of chronic pancreatitis and pancreatic cancer. Gastrointest Endosc. 2008;68:1086–1094. doi: 10.1016/j.gie.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 74.Hirche TO, Ignee A, Barreiros AP, Schreiber-Dietrich D, Jungblut S, Ott M, Hirche H, Dietrich CF. Indications and limitations of endoscopic ultrasound elastography for evaluation of focal pancreatic lesions. Endoscopy. 2008;40:910–917. doi: 10.1055/s-2008-1077726. [DOI] [PubMed] [Google Scholar]
- 75.Iglesias-Garcia J, Larino-Noia J, Abdulkader I, Forteza J, Dominguez-Munoz JE. EUS elastography for the characterization of solid pancreatic masses. Gastrointest Endosc. 2009;70:1101–1108. doi: 10.1016/j.gie.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 76.Ogura T, Yamao K, Sawaki A, Mizuno N, Hara K, Hijioka S, Niwa Y, Tajika M, Kondo S, Shimizu Y, et al. Clinical impact of K-ras mutation analysis in EUS-guided FNA specimens from pancreatic masses. Gastrointest Endosc. 2012;75:769–774. doi: 10.1016/j.gie.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 77.Fuccio L, Hassan C, Laterza L, Correale L, Pagano N, Bocus P, Fabbri C, Maimone A, Cennamo V, Repici A, et al. The role of K-ras gene mutation analysis in EUS-guided FNA cytology specimens for the differential diagnosis of pancreatic solid masses: a meta-analysis of prospective studies. Gastrointest Endosc. 2013;78:596–608. doi: 10.1016/j.gie.2013.04.162. [DOI] [PubMed] [Google Scholar]
- 78.Salek C, Benesova L, Zavoral M, Nosek V, Kasperova L, Ryska M, Strnad R, Traboulsi E, Minarik M. Evaluation of clinical relevance of examining K-ras, p16 and p53 mutations along with allelic losses at 9p and 18q in EUS-guided fine needle aspiration samples of patients with chronic pancreatitis and pancreatic cancer. World J Gastroenterol. 2007;13:3714–3720. doi: 10.3748/wjg.v13.i27.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]


