Abstract
Up to a quarter of polyps and adenomas are missed during colonoscopy due to poor visualization behind folds and the inner curves of flexures, and the presence of flat lesions that are difficult to detect. These numbers may however be conservative because they mainly come from back-to-back studies performed with standard colonoscopes, which are unable to visualize the entire mucosal surface. In the past several years, new endoscopic techniques have been introduced to improve the detection of polyps and adenomas. The introduction of high definition colonoscopes and visual image enhancement technologies have been suggested to lead to better recognition of flat and small lesions, but the absolute increase in diagnostic yield seems limited. Cap assisted colonoscopy and water-exchange colonoscopy are methods to facilitate cecal intubation and increase patients comfort, but show only a marginal or no benefit on polyp and adenoma detection. Retroflexion is routinely used in the rectum for the inspection of the dentate line, but withdrawal in retroflexion in the colon is in general not recommended due to the risk of perforation. In contrast, colonoscopy with the Third-Eye Retroscope® may result in considerable lower miss rates compared to standard colonoscopy, but this technique is not practical in case of polypectomy and is more time consuming. The recently introduced Full Spectrum Endoscopy™ colonoscopes maintains the technical capabilities of standard colonoscopes and provides a much wider view of 330 degrees compared to the 170 degrees with standard colonoscopes. Remarkable lower adenoma miss rates with this new technique were recently demonstrated in the first randomized study. Nonetheless, more studies are required to determine the exact additional diagnostic yield in clinical practice. Optimizing the efficacy of colorectal cancer screening and surveillance requires high definition colonoscopes with improved virtual chromoendoscopy technology that visualize the whole colon mucosa while maintaining optimal washing, suction and therapeutic capabilities, and keeping the procedural time as low and patient discomfort as optimal as possible.
Keywords: Colonoscopy, Endoscopic innovations, Adenoma detection, Polyp detection, Gastrointestinal endoscopy
Core tip: Up to a quarter of polyps and adenomas are missed during colonoscopy due to poor visualization behind folds and the inner curves of flexures, and the presence of flat lesions that are difficult to detect. In the past several years, new endoscopic techniques have been introduced to improve the detection of polyps and adenomas. Optimizing the efficacy of colorectal cancer screening and surveillance requires high definition colonoscopes with improved virtual chromoendoscopy technology that visualize the whole colon mucosa while maintaining optimal washing, suction and therapeutic capabilities, and keeping the procedural time as low and patient discomfort as optimal as possible.
INTRODUCTION
Colonoscopy is considered the gold standard for the detection and removal of polyps and adenomas in the colorectum. There is strong evidence that the removal of polyps and adenomas by colonoscopy lowers colorectal cancer (CRC) incidence and mortality[1,2]. However, in recent years there has been an increasing concern about the effectiveness of colonoscopy the detection of adenomas, early-stage CRC and especially right-sided cancers[3]. Population-based studies have reported that 3%-8% of patients with CRC had a colonoscopy within 3-5 years prior to CRC diagnosis[4-6]. Retrospective studies revealed that these so-called interval or post-colonoscopy cancers can mainly be attributed to missed lesions or inadequate examination[5]. Indeed, a considerable proportion of polyps and adenomas are being missed with colonoscopy, with overall polyp and adenoma miss rates being estimated between 20%-25% in most back-to-back colonoscopy studies[7].
The main factors thought to be responsible for missing lesions, besides endoscopist dependent factors, include the relative difficulty to visualize polyps at the proximal side of haustral folds and internal curves of flexures[8,9], the presence of flat lesions[10] and poor bowel preparation[11]. In addition, especially right-sided advanced adenomas are more often diminutive in size or non-polypoid in appearance compared to left-sided advanced adenomas and may therefore be more easily overlooked[10,12]. Surface visualization with standard 140 and 170 degrees colonoscopes is approximately between 87% and 92% in a clean colon, which illustrates the limitation of standard colonoscopes to adequately visualize the entire mucosa[13]. As a result, premalignant lesions can be missed and it been shown that two-thirds of the non-rectal ≥ 6 mm lesions that are missed during colonoscopy are located on the proximal side of folds[9].
In more recent years, new endoscopic technologies aimed to increase polyp detection rates (PDR) and adenoma detection rate (ADR) have been developed. In this review we will discuss these endoscopic innovations and their potential to improve the detection of premalignant lesions during colonoscopy (Table 1).
Table 1.
Endoscopic innovations to improve the adenoma detection during colonoscopy
| Technique | Colonoscopy technology | Diagnostic yield | Clinical applicability |
| High definition | High definition monitor with more images per second and high resolution | Marginal increase in number of polyps and adenomas, mostly small, flat, right-sided lesions. approximation 3.5% increase in ADR | High quality images with reduced artifacts and more natural appearance |
| Narrow band imaging (NBI) | Narrow band filters increase blue (415 nm) and green (540 nm) wavelengths and enhance the visualization of mucosal blood vessels | Small increase in flat and small serrated lesions, but higher detection rates when combined with high definition | Possibly improving the detection of subtle lesions, but insufficient brightness and dark appearing bile and stool prohibit optimal pan-colonic use |
| Fujinon intelligent color enhancement | Computed spectral estimation technology enhances the visibility of mucosal and vascular details by narrowing the bandwidth of light | Very few randomized studies but polyp and adenoma detection seems similar compared to white light colonoscopy | Like with NBI, images are to dark to advice routine pan-colonic use |
| Autofluorescence imaging (AFI) | Tissue is exposured to light of short wavelength, which leads to the excitation of endogenous substances and the emission of autofluorescent light | AFI has lower adenoma miss rates (absolute difference of approximation 20%) when compared to white light colonoscopy, especially for flat and depressed lesions | Not advised for routine practice in colonoscopy due to low resolution images, few images per second and artifacts due to residual fecal fluids |
| Water-immersion colonoscopy | Infusion of water, combinated with air-insufflation, during insertion of the colonoscope. Water and remaining fecal content are removed during withdrawal | No difference in ADR between water-immersion and air-insufflated colonoscopy | Reduces pain scores, need for sedation and general intolerability, but only studied in highly experienced hands |
| Water-exchange colonoscopy | Water containing residual feces is removed and “exchanged” for clean water during insertion in lieu of air-insufflation | ADR is reported to be approximation 10% higher with water-exchange colonoscopy compared to standard air-insufflated colonoscopy | Provides extra cleansing of the mucosa but is more time consuming and is thus far only studied in highly experienced hands |
| Cap-assisted colonoscopy | Can be used to depress colonic folds to improve the visualization of proximal aspects of these folds | Contradicting results with approximation 10% higher detection rates for small polyps and adenomas in some studies, but no beneficial results in others | Easy to use, can assist during mucosectomies and facilitates introduction of the colonoscope, but probably has a limited effect on diagnostic yield |
| Retroflexion | Withdrawal in retroflexion is possible in the proximal colon due to the large diameter of this segment and may improve the visualization of the proximal aspects of folds | No additional diagnostic yield in the proximal colon and questionable in the rectum | Routine withdrawal in retroflexion is not recommended but may facilitate the removal of large sessile polyps |
| Third-eye retroscope | The retroscope is retroflexed 180 degrees after being advanced through the working channel and enhances the visualization behind folds | Limited number of studies, but polyp and adenoma detection are reported to be 15%-25% higher compared to standard colonoscopy | Increases diagnostic yield, but reduces suctioning capacity when in position and needs to be removed from working channel in case of polypectomy |
| Full spectrum endoscopy (FUSE) | Three imagers positioned at the front and both sides of the tip provide a 330 degrees view, which improve the visualization of the internal lining of flexures and proximal aspects of folds | One randomized tandem study thus far, which showed considerably lower miss rates for polyps (9.7% vs 43.%) and adenomas (7.5% vs 40.8%) compared to standard colonoscopy | Provides a comprehensive view while maintaining technical capabilities of standard colonoscopes. Requires little training |
ADR: Adenoma detection rate.
HIGH-DEFINITION COLONOSCOPY
High-definition colonoscopy uses a high definition monitor that enables more images per second to be shown. Moreover, the images have a higher resolution as compared to standard definition colonoscopy. Although high definition colonoscopy provides much better imaging, studies evaluating polyp detection with high definition as compared to standard definition colonoscopes are scarce and show conflicting results[14]. Two randomized trials[15,16] found no significant differences in ADR and PDR between both techniques. In contrast, one randomized study reported a higher PDR (64% vs 53%, P = 0.03) and mean number of small hyperplastic polyps per subject (0.10 vs 0.25, P = 0.003) with high definition colonoscopy[17], while in another randomized multicenter study[18] high definition colonoscopy yielded more adenomas per subject (1.12 vs 0.69, P = 0.02) and especially flat adenomas and right-sided adenomas (both P < 0.01). Furthermore, East et al[19] reported in a prospective non-randomized study more diminutive (< 6 mm), non-flat adenomas with high definition colonoscopy, although no significant differences in ADR and PDR could be demonstrated. Similar results were found in a retrospective study by Buchner et al[20] including 1226 patients undergoing standard definition colonoscopy and 1204 patients undergoing high definition colonoscopy. Both ADR (28.8% vs 24.3%) and PDR (42.2% vs 37.8%) were statistically significantly higher with high definition colonoscopy but this mainly concerned smaller lesions.
Hence, the use of high definition colonoscopy leads to high quality images and a marginal increase in ADR and PDR compared to standard definition colonoscopy. The absolute increase in ADR is however small and is estimated to be approximately 3.5% according to a meta-analysis with pooled data of five studies in 4422 patients[21]. The additional value of high definition colonoscopy seems mainly limited to small lesions and, according to one study, flat lesions in the right colon. However, caution is required when interpreting the results because marked heterogeneity exists with differences in study design and the type of population included.
VIRTUAL CHROMOENDOSCOPY
Virtual chromoendoscopy uses a narrow spectrum of wavelengths with a decreased penetration depth to enhance visualization of the colon mucosa and has been developed as an alternative to dye assisted chromoendoscopy. Light of short wavelengths increases the vascular contrast of the mucosa, allowing improved visualization of the colonic mucosal surface. Manufactures have developed multiple techniques including Narrow Band Imaging (NBI), Fuji Intelligent Color Enhancement (FICE) and Autofluorescence Imaging (AFI), which can easily be switched on during colonoscopy. These techniques have been suggested to improve the detection of (subtle) mucosal lesions[22-24].
Narrow band imaging
NBI (Figure 1) is one of the most widely used and extensively studied image enhancement technologies and is aimed to improve adenoma detection and differentiation. Narrow band filters placed behind the light source eliminate red light and increase the contribution of blue (415 nm) and green (540 nm) wavelengths. The 415 nm light enhances the visualization of superficial mucosal capillaries while the 540 nm light increases the visibility of submucosal and deeper mucosal vessels.
Figure 1.
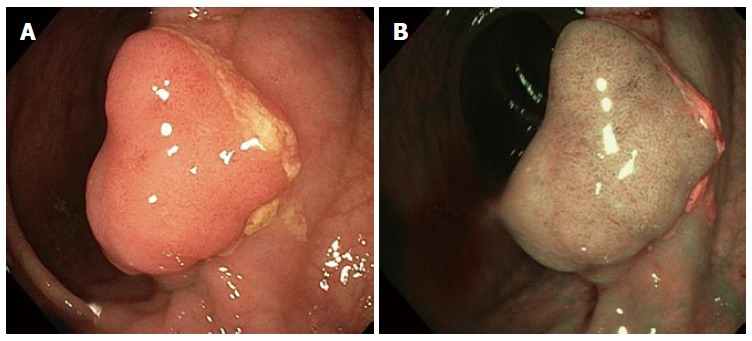
View of adenomatous polyp with white light and narrow band imaging. A: Adenoma view with white light; B: Adenoma view with narrow band imaging.
Studies investigating the additional yield of pan-colonic NBI are somewhat conflicting. A meta-analysis including six randomized trials with a total of 2284 patients[23] reported no significant differences between high definition NBI and high definition white light colonoscopy for the detection of total, flat and < 10 mm adenomas or polyps. Furthermore, no differences in adenoma or polyp miss rates were observed between both techniques. These findings were recently confirmed in a large randomized study by Chung et al[25]. In contrast, studies in which high definition NBI was compared to standard definition colonoscopy have shown differences in detection and miss rates of polyps and adenomas[18,26,27]. In a randomized back-to-back study by Gross et al[27] comparing high definition NBI and standard definition colonoscopy, significant lower miss rates for polyps (31% vs 57%, P = 0.005) and adenomas (27% vs 49%, P = 0.036) were observed. However, the same group reported similar results in a retrospective study comparing high definition white light to standard definition white light colonoscopy, which suggests that the additional yield obtained with high definition NBI may be related to the high definition component and not to the use of NBI[20]. This is further supported by a study of Rastogi et al[18] in which more adenomas per subjects were found with high definition NBI (1.13) compared to standard definition white light (1.13 vs 0.69, P = 0.01) but not to high definition white light colonoscopy (1.13 vs 1.12, P > 0.05). In the latter study, high definition NBI detected significantly more flat and right sided lesions compared to standard definition colonoscopy but a similar number compared to high definition colonoscopy. A back-to-back study including patients with hyperplastic polyposis syndrome also reported a lower polyp miss rate, in particular for flat polyps and sessile serrated adenomas, when high resolution NBI colonoscopy was compared to white light colonoscopy[28]. Two randomized studies that compared high definition NBI with high definition white light colonoscopy[29,30] reported no difference in adenoma detection, but high definition NBI yielded more flat adenomas[29,30] and hyperplastic polyps[29].
In summary, polyp and adenoma detection seem to be higher with high definition NBI compared to standard definition white light colonoscopy, but the additional value of high definition NBI over high definition white light colonoscopy may be limited to the detection of subtle lesions such as small serrated lesions and flat adenomas. It has been suggested that the limited value of high definition NBI over high definition white light colonoscopy may be related to the potential learning effect that is induced by NBI during colonoscopy, i.e., the introduction of NBI may have improved the recognition of polyps and adenomas with white light colonoscopy[23]. In this regard, it is of interest that East et al[31-33] showed that the improvement in adenoma detection rate by high definition NBI colonoscopy over high definition white light colonoscopy declined from 61% in the first 52 patients to 45% and only 8% in a second and third group of 91 and 214 patients. A similar effect was observed in a study by Adler et al[34] with consecutive groups of 100 patients undergoing white light colonoscopy; the ADR of 8% in the first group increased to 26% in the last group of patients, compared to an ADR of 25% with NBI which remained unchanged during the course of the study.
FICE
FICE is a computed spectral estimation technology that enhances the visibility of mucosal and vascular details by narrowing the bandwidth of light. FICE enables the endoscopist to choose between different wavelengths for optimal examination of the colon mucosa[24]. Only a limited number of studies have evaluated FICE colonoscopy for its proposed increased capability to detect of adenomas and polyps. In the reported randomized back-to-back studies that compared FICE with white light colonoscopy[25,35] or NBI[36] no significant benefit of FICE was demonstrated. Furthermore, in an earlier randomized study[37] the ADR and mean number of adenomas were similar with FICE compared to targeted indigocarmine chromoendoscopy.
AFI
Real-time pseudo-color images produced with AFI are created by a rotating filter producing a short wavelength light. The exposure of tissue to this specific light leads to the excitation of some endogenous substances and subsequently the emission of fluorescent light. The autofluorescent image produced with AFI is created by a green filter, which exposes the tissue to the remaining blue and red light. The reflected blue light is blocked by a second filter while the reflected red light and the emitted green autofluorescence from the tissue are used to obtain a pseudo-color image[22,38]. AFI colonoscopy colors neoplastic lesions red-purple while non-neoplastic mucosa appears green.
Three back-to-back studies reported lower adenoma miss rates with AFI colonoscopy compared to white light colonoscopy with an absolute difference of approximately 20%[39-41]. In one of these studies[39], the location, size, macroscopic appearance and histopathology of the lesions detected with AFI and white light colonoscopy were not different, but the lesions that were histologically graded as dysplastic were less frequently missed with AFI (30% vs 49%, P = 0.01). Another study by Moriichi et al[40] compared AFI with high resolution white light colonoscopy and reported a higher ADR (26.1% vs 18.2%, P < 0.05) and more specifically a higher detection rate of flat and depressed adenomas (9.1% vs 3.4%, P < 0.05). In the same study, an increased ADR with AFI was only observed when used by less experienced endoscopists. One study investigated the diagnostic yield of high resolution colonoscopy using Endoscopic Trimodal Imaging technology[42]. These colonoscopes have both AFI and NBI technology incorporated in the endoscope. The high resolution and AFI technology in these colonoscopes can be used to detect lesions (“red flag”), whereas NBI can be used to differentiate between different types of lesions. The study was performed in six non-academic centers and showed no differences in ADR or adenoma miss rate compared to standard white light colonoscopy.
In summary, the effect of pan-colonic virtual chromoendoscopy on adenoma and polyp detection seems limited and virtual chromoendoscopy probably only has a minor benefit on the detection of small and flat lesions. These somewhat disappointing results are most likely due to technical issues inherent to virtual chromoendoscopy, in that the brightness of the virtual image with high-definition technology remains insufficient to allow optimal visualization of the colonic mucosa in a large diameter colon lumen. In addition, a good inspection of the colon mucosa with virtual chromoendoscopy is only possible in a colon that is really optimally prepared because remaining bile fluid and stool appear red and dark in virtual images, hindering an optimal view of the mucosa[43]. In our opinion, virtual chromoendoscopy is most optimally used as an add-on technology to differentiate between neoplastic and non-neoplastic lesions. This could allow a “resect-and-discard” or “leave-in-situ” approach to reduce the risk of complications and costs associated with unnecessary removal of polyps. However, accuracy rates should exceed well above 90% to consider such an approach. In experienced hands, high accuracy rates for a “resect-and-discard” policy have been reported for NBI[44-46], FICE[47,48] and AFI[49], ranging between 85%-92% when used with high magnification, but these rates are lower when used by non-experts[42,44,50]. Good training may improve the detection and differentiation of lesions, but before the routine use of pan-colonic virtual chromoendoscopy can be justified, new generation devices with higher light intensity are required.
WATER-INFUSION TECHNIQUES
Colonoscopy techniques combining or replacing air-insufflation with water infusion were initially designed to facilitate cecal intubation, reduce colonic spasms, lower patient discomfort and need for sedation[51,52]. The infusion of water during the insertion of the colonoscope causes the colon to distend and can be combined with air-insufflation (water-immersion method) or be performed without air-insufflation (water-exchange method)[53,54]. Similarly to standard air-insufflated colonoscopy, air is also insufflated during withdrawal of the colonoscope irrespective of the type of water infusion technique used. The water-immersion technique allows the water to flow in the direction of the lumen which may aid in finding the correct direction for intubation. The infused water and remaining fecal contents are mainly removed during withdrawal of the colonoscope.
This method has been shown to reduce pain scores[55-61], need for sedation[55,59,62] and general intolerability[55,59,60] in most studies, but concerns have been raised about an impaired ability to detect lesions due to contaminated water impairing visibility. A recent systematic review[53] in which the results of six studies were combined, reported no differences in ADR between water-immersion and air-insufflated colonoscopy. In contrast, the recently developed water-exchange method was reported to increase ADR compared to air-insufflation colonoscopy in the first observational study (water-exchange 36.5% vs air 25.8%, P = 0.18)[63], in a subsequent retrospective cohort study (water-exchange 34.9% vs air 26.9%, P = 0.003)[64] and in a head-to-head comparison study (water-exchange 57.1% vs air 46.1%, P = 0.04)[65]. In two randomized controlled trials[62,66], ADR was higher with the water-exchange method but this difference was not statistically significant. The water-exchange method is a technique in which water containing residual feces is removed and “exchanged” for clean water in lieu of air-insufflation. The exchange of large volumes of water during the insertion of the colonoscope results in additional cleansing of the mucosa, which has been proposed to improve the detection of adenomas[53]. An alternative hypothesis is that the improved cleansing during colonoscope insertion allows more time for inspection during withdrawal since less time needs to be spent on colonic cleansing. Nonetheless, several attempts have been made to improve the efficacy of water-exchange colonoscopy. In a group of 50 consecutive US veterans undergoing water-exchange colonoscopy, indigocarmine was added to the infused water (concentration 0.008%)[67]. The ADR was significantly higher in the indigocarmine group in comparison with a historical cohort of patients who had undergone standard water-exchange colonoscopy (62% vs 40%, P < 0.05) or air-insufflation colonoscopy (62% vs 36%, P < 0.05). In a pilot study by Yen et al[68], the water-exchange method was combined with cap assisted colonoscopy in 50 consecutive patients. The results were compared to a control group of 101 consecutive patients undergoing air-insufflation colonoscopy. It was demonstrated that the mean number of adenomas was higher with the water-exchange cap assisted colonoscopy method compared to air-insufflated colonoscopy (3.08 vs 1.50, P = 0.002), although the ADR was not statistically significantly higher (70.0% vs 59.4%, P = 0.22).
Although water-exchange colonoscopy improves the detection of adenomas, the benefit of water-infusion colonoscopy methods seems particularly be due to improving patient comfort. In addition, the majority of studies published so far were performed by endoscopists that were highly experienced with water-infusion colonoscopy. This raises the question whether the same results can be achieved when performed by less experienced endoscopists. Especially when considering the prolonged insertion time due to the time consuming suction and exchange of water, it remains to be further elucidated whether water-exchange colonoscopy will indeed be one of the preferred techniques in daily clinical colonoscopy practice.
CAP-ASSISTED COLONOSCOPY
Transparent caps attached to the distal tip of the colonoscope were first designed to assist during endoscopic mucosal resection but they have also been suggested to be of help in depressing colonic folds to improve visualization of their proximal aspects. A potential disadvantage of cap-assisted colonoscopy is that fecal debris may accumulate in the cap, requiring removal by water irrigation and drainage through the side holes present in some cap models. Several studies have reported reduced cecal intubation times[69-71] and improved cecal intubation rates for trainees using cap-assisted colonoscopy[70]. The same accounted for procedures in patients in whom cecal intubation initially failed with standard colonoscopy[72,73]. Randomized controlled trials that evaluated the additional diagnostic yield of cap-assisted colonoscopy were mostly performed in Asian countries and have in general shown mixed results[74].
In a study by Kondo et al[70], 684 subjects were randomized to colonoscopy with a 4-mm transparent cap or a 2-mm rubber cap or to colonoscopy without a cap. PDR for colonoscopies with the transparent cap, rubber cap and no cap were 49.3%, 44.7% and 39.1%, respectively, with only the difference between the transparent 4-mm cap and no cap being statistically significant. In a recent study reporting on 2502 procedures performed by trainees[75], a statistically significant higher overall PDR was found for cap-assisted colonoscopy compared to standard colonoscopy (47.0% vs 42.6%). Subgroup analyses showed that this difference was particularly due to an improved detection of small (≤ 5 mm) polyps. In a randomized controlled trial by Rastogi et al[71], ADR was 13% higher with cap-assisted colonoscopy compared to standard colonoscopy, but similarly to the previous study, this was only observed for small (≤ 5 mm) adenomas. Horiuchi et al[76] studied a retractable transparent device that can be extended up to a maximum length of 7 mm by injection of air. The mean number of adenomas detected was statistically significantly higher with the retractable extension device compared to standard colonoscopy (0.48 vs 0.36, P = 0.04) while the ADR was comparable between both groups. In contrast, in the single largest randomized trial[73] published thus far (1000 patients included), a lower ADR (30.5% vs 37.5%) and mean number of adenomas per subject was reported with cap-assisted colonoscopy compared to standard colonoscopy. Furthermore, three later studies, including the largest published multicenter trial thus far, reported no higher overall[69,77,78] and small polyp[69,77,78] detection rates with cap-assisted colonoscopy.
Taken together, cap-assisted colonoscopy may be of benefit in reducing cecal intubation time, but has limited or no benefit on polyp detection, which is confirmed by the results of a recent meta-analysis including 16 randomized controlled trials[74]. In this study, a marginally higher proportion of subjects with polyps was found with cap-assisted colonoscopy (RR = 1.08, 95%CI: 1.00-1.17) while no statistically significant difference in ADR was found. Of note, subgroup analysis showed that both expert and trainee endoscopists had reduced cecal intubation times and improved polyp detection rate, highlighting that it is unlikely that especially trainees should benefit from cap-assisted colonoscopy.
RETROFLEXION
Retroflexion is commonly used in the rectum for the inspection of the dentate line, though the additional diagnostic yield is questionable[79]. Due to its relatively large diameter, the retroflexion technique has also been suggested to be useful in the proximal colon to improve the visualization on the proximal aspects of folds and to facilitate the removal of proximally located large sessile polyps. This was shown in a retrospective observational study in 59 patients[80].
Harrison et al[81] performed a randomized study in 100 patients who underwent standard forward colonoscopy from the cecum to the splenic flexure with removal of polyps. The cecum was then reintubated and patients were randomized to undergo a second exam of the proximal colon in retroflexion or in forward view. Retroflexion was successfully performed in the ascending and transverse colon in almost all patients. No statistically significant differences were observed between forward view and retroflexion with regard to the detection of additional polyps and adenomas. A more recent observational study in a cohort of 1000 consecutive patients reported an adenoma miss rate of 9.8% in patients first undergoing careful inspection of the proximal colon in forward view and a second inspection in retroflexion[82]. Although this was an observational study, the adenoma miss rate was thought to be comparable to that expected when a second inspection would have been done with forward viewing colonoscopy.
Based on the relatively limited number of studies which demonstrated no clear extra additional polyps being detected, in combination with a possibly increased risk of perforation when withdrawing the colonoscope in retroflexion, we currently do not recommend this technique in routine colonoscopy practice.
THIRD-EYE RETROSCOPE
A device specifically designed to enhance the visualization behind the proximal aspects of colonic folds is the Third-Eye Retroscope® (Avantis Medical Systems, Inc) (Figure 2). This device consists of a video processor, a single-use polarizing filter cap for the colonoscope light source, and a 3.5 mm flexible single-use catheter with a camera and diode light source at the tip. The retroscope is retroflexed 180 degrees after being advanced through the working channel of the colonoscope and provides a 135 degrees retrograde view of the colon. In simulated colonoscopies using CT-colonography software, it was shown that the Third-Eye Retroscope improves the visualization of the colonic surface area from 87% with standard 140 degrees view colonoscopes to 99%[13].
Figure 2.
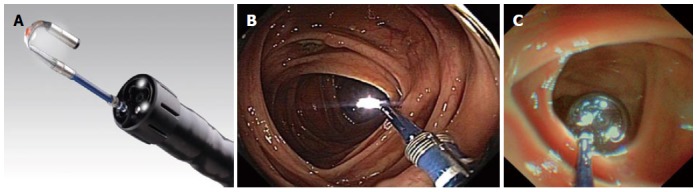
Colonoscopy with the Third-Eye Retroscope. A: Detailed view of the Third-Eye Retroscope; B, C: Colon view with the Third-Eye Retroscope.
The efficacy of the Third-Eye Retroscope was initially studied in three colon models with simulated polyps[83]. Standard colonoscopy detected 12% of the polyps located on the proximal aspects of folds, while 81% of these polyps were detected with the Third-Eye Retroscope. The first pilot study[84] in 24 patients resulted in an 11.8% increase in diagnostic yield, with 34 polyps detected in the antegrade view and 4 additional polyps in the retrograde view. In two non-randomized studies[85,86], the additional diagnostic yield of the Third-Eye Retroscope was investigated by evaluating whether polyps detected with the Third-Eye Retroscope could also been seen with the antegrade view of the colonoscope alone. In the first study, 182 polyps in 298 subjects were found with the antegrade view and 27 additional polyps were found with the Third-Eye Retroscope, resulting in a 14.8% increase in polyp detection and a 16.0% increase in adenoma detection[85]. The second study reported a similar result with a 13.2% increase in polyp detection and a 11.0% increase in adenoma detection[86]. Until now, one randomized back-to-back study has been performed, the TERRACE study[87]. In this multicenter study including 349 patients, a net additional detection rate with the Third-Eye Retroscope of 29.8% for polyps and 23.2% for adenomas was reported. This study was criticized by the fact that the mean withdrawal time was almost two min longer with the Third-Eye Retroscope compared to standard colonoscopy as this may have resulted in some bias in this study. In a post-hoc analysis of the TERRACE study[88], withdrawal time was not significantly associated with the risk of missing adenomas. Interestingly, the Third-Eye Retroscope was shown to be particularly beneficial in patients undergoing colonoscopy for surveillance or diagnostic work-up and not in those undergoing screening colonoscopy.
Studies that investigated the Third-Eye Retroscope have shown a significant additional diagnostic yield when using this technique, but there are some limitations inherent to this device. First, thorough suctioning of colonic debris must be done during insertion of the colonoscope due to a 50% reduced suctioning capacity when the retroscope is in position. A second disadvantage is that the Third-Eye Retroscope needs to be removed from the working channel in case an accessory device is required, such as a biopsy forces or a polypectomy snare, which is bothersome and increases the procedural time.
FULL SPECTRUM ENDOSCOPY
The recently developed Full Spectrum Endoscopy™ (FUSE; EndoChoice®, Alpharetta, Georgia, United States) (Figure 3) colonoscope allows a high resolution 330 degrees “full spectrum” viewing of the colonic lumen while maintaining the standard colonoscope technical features and capabilities of a standard 140 and 170 degrees colonoscope. The FUSE system consists of a main control unit and a video colonoscope with three imagers and LED groups located at the front and both sides of the flexible tip. The video images transmitted from the three cameras on the left-side, front and right-side of the colonoscope are displayed on three contiguous monitors corresponding with each individual camera. The two additional side cameras incorporated in the FUSE colonoscope provide a better and comprehensive view of the total colonic lumen. The frequently encountered blind spots, such as the internal lining of flexures and proximal aspects of folds, should be easily visualized with this system.
Figure 3.
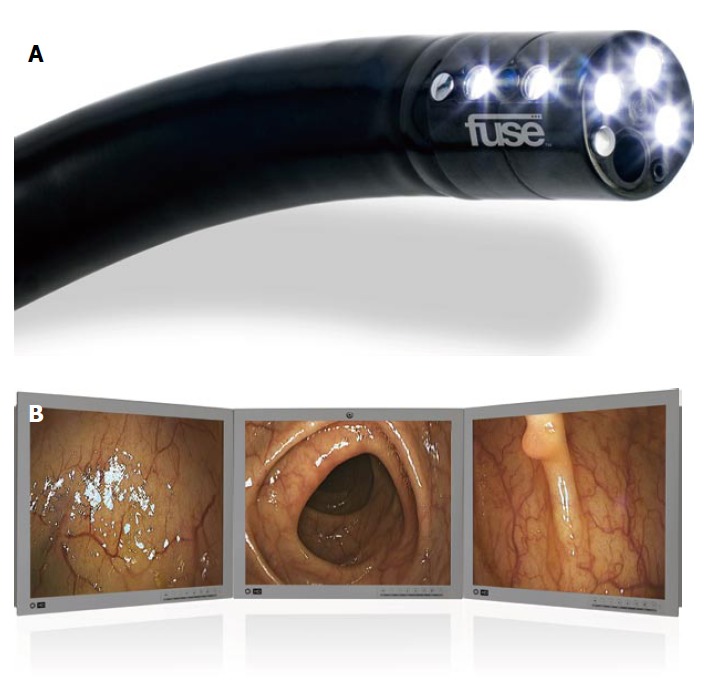
Colonoscopy with the Full Spectrum Endoscopy colonoscope. A: Detailed view of the Full Spectrum Endoscopy colonoscope; B: Colon view with the Full Spectrum Endoscopy colonoscope.
The first published study was performed with an anatomical model of the colon with simulated polyps in a non-randomized setting[89]. Thirty-seven endoscopists performed colonoscopy by using the forward-viewing camera only (160 degrees), followed by a colonoscopy with all three cameras, which increases the field of view to 330 degrees. In total, 85.7% of the polyps were detected with the three cameras compared to 52.9% with only forward-viewing colonoscopy (P < 0.001). Particularly polyps that were “hidden” behind flexures and folds were more frequently detected with FUSE colonoscopy than with forward-viewing colonoscopy (81.9% vs 31.9%). An additional pilot study including 50 patients showed that FUSE colonoscopy was indeed safe and feasible with a 100% cecal intubation rate and a mean cecal intubation time of 3.1 ± 1.5 min. Preliminary results of a randomized, multicenter, back-to-back study presented at the Digestive Disease Week 2013 are promising. Same-day colonoscopies with FUSE and standard colonoscopes were performed in 185 randomized subjects. In 88 subjects undergoing standard colonoscopy first, 50 polyps including 28 adenomas were detected while FUSE yielded 39 additional polyps including 20 adenomas, corresponding with an increase in polyps and adenomas detection of 78.0% and 71.4%, respectively. In 97 subjects undergoing FUSE first, 102 polyps including 61 adenomas were detected while standard colonoscopy yielded 11 additional polyps including 5 adenomas, corresponding with an increase in polyps and adenomas of 10.8% and 8.2%, respectively (FUSE vs standard, P < 0.01). The adenoma miss rate with FUSE was found to be considerably lower than with standard colonoscopy (7.5% vs 40.8%, P < 0.0001). However, the median withdrawal time was approximately half a minute longer with FUSE colonoscopy (5.6 min vs 6.2 min, P < 0.01) and may have caused some bias in the results. More studies will be required before definitive conclusions can be made, but the first results definitely show that FUSE colonoscopy may be an important advancement to improve adenoma detection.
CONCLUSION
A considerable proportion of polyps and adenomas are missed during colonoscopy due to poor visualization behind folds and the inner curves of flexures, and the presence of flat lesions that are known to be difficult to detect. Based on the findings of back-to-back studies with standard colonoscopes, adenoma and polyp miss rates are estimated to be approximately 20% to 25%. However, some recent studies that evaluated new endoscopic technologies have reported even higher miss rates (up to 40%) with standard colonoscopy than previously reported, which suggests that the miss rates with standard colonoscopy may have been previously underestimated.
The introduction of high-definition technology has considerably improved the quality of images during colonoscopy and is likely to stay the standard in the field of endoscopy. Visual image enhancement technologies such as NBI, FICE and AFI have possibly resulted in an increased recognition of flat and small lesions, but the absolute increase in terms of numbers of adenoma is probably limited. Besides, the quality of the images produced with virtual chromoendoscopy technologies requires further improvement before the general application of such technologies can be fully recommended. Cap-assisted colonoscopy and water-exchange colonoscopy were originally designed to facilitate cecal intubation and increase patient comfort, but studies have generally shown a marginal or no benefit at all on polyp and adenoma detection. Furthermore, the applicability of water-infusion methods has only been studied in highly experienced hands and is more time consuming compared to standard colonoscopy. Retroflexion is commonly used in the rectum for the inspection of the dentate line, but its use in the proximal colon has not clearly been demonstrated to improve ADR and may be associated with an increased risk of perforation. Studies evaluating colonoscopy with the Third-Eye Retroscope have shown considerable lower miss rates compared to standard colonoscopy, but this device is inconvenient in case of polypectomy, it impacts suction capabilities and it adds to total colonoscopy time. The recently introduced FUSE colonoscope maintains the technical capabilities of standard colonoscopes and provides a much wider view of 330 degrees compared to 170 degrees with standard colonoscopes. A recent randomized back-to-back study using FUSE colonoscopy has shown remarkable lower adenoma miss rates with this technique. Although the results look promising, more studies investigating the diagnostic yield and the use of three monitors are needed before this device can be recommended for routine practice.
Hence, the majority of the endoscopic innovations that have been introduced in the past few years have only shown little additional diagnostic yield, are more time consuming or are not practical in use. In order to increase the efficacy of screening and surveillance colonoscopies, colonoscopy techniques will be needed that provide an optimal view of the whole colonic mucosa while maintaining optimal washing, suction and therapeutic capabilities and without increasing the procedural time or impairing patients comfort. In this perspective, a combination of high-definition and improved virtual enhancement technologies incorporated in ultra-wide colonoscopes may be the most obvious way to enhance the diagnostic yield of colonoscopy in the next few years.
Footnotes
P- Reviewers: Coskun A, Endo I S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
References
- 1.Jacob BJ, Moineddin R, Sutradhar R, Baxter NN, Urbach DR. Effect of colonoscopy on colorectal cancer incidence and mortality: an instrumental variable analysis. Gastrointest Endosc. 2012;76:355–364.e1. doi: 10.1016/j.gie.2012.03.247. [DOI] [PubMed] [Google Scholar]
- 2.Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lakoff J, Paszat LF, Saskin R, Rabeneck L. Risk of developing proximal versus distal colorectal cancer after a negative colonoscopy: a population-based study. Clin Gastroenterol Hepatol. 2008;6:1117–1121; quiz 1064. doi: 10.1016/j.cgh.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Bressler B, Paszat LF, Chen Z, Rothwell DM, Vinden C, Rabeneck L. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology. 2007;132:96–102. doi: 10.1053/j.gastro.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 5.le Clercq CM, Bouwens MW, Rondagh EJ, Bakker CM, Keulen ET, de Ridder RJ, Winkens B, Masclee AA, Sanduleanu S. Postcolonoscopy colorectal cancers are preventable: a population-based study. Gut. 2013:Epub ahead of print. doi: 10.1136/gutjnl-2013-304880. [DOI] [PubMed] [Google Scholar]
- 6.Singh H, Nugent Z, Demers AA, Bernstein CN. Rate and predictors of early/missed colorectal cancers after colonoscopy in Manitoba: a population-based study. Am J Gastroenterol. 2010;105:2588–2596. doi: 10.1038/ajg.2010.390. [DOI] [PubMed] [Google Scholar]
- 7.van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343–350. doi: 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 8.Pickhardt PJ, Choi JR, Hwang I, Butler JA, Puckett ML, Hildebrandt HA, Wong RK, Nugent PA, Mysliwiec PA, Schindler WR. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191–2200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 9.Pickhardt PJ, Nugent PA, Mysliwiec PA, Choi JR, Schindler WR. Location of adenomas missed by optical colonoscopy. Ann Intern Med. 2004;141:352–359. doi: 10.7326/0003-4819-141-5-200409070-00009. [DOI] [PubMed] [Google Scholar]
- 10.Heresbach D, Barrioz T, Lapalus MG, Coumaros D, Bauret P, Potier P, Sautereau D, Boustière C, Grimaud JC, Barthélémy C, et al. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy. 2008;40:284–290. doi: 10.1055/s-2007-995618. [DOI] [PubMed] [Google Scholar]
- 11.Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61:378–384. doi: 10.1016/s0016-5107(04)02776-2. [DOI] [PubMed] [Google Scholar]
- 12.Rondagh EJ, Bouwens MW, Riedl RG, Winkens B, de Ridder R, Kaltenbach T, Soetikno RM, Masclee AA, Sanduleanu S. Endoscopic appearance of proximal colorectal neoplasms and potential implications for colonoscopy in cancer prevention. Gastrointest Endosc. 2012;75:1218–1225. doi: 10.1016/j.gie.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 13.East JE, Saunders BP, Burling D, Boone D, Halligan S, Taylor SA. Surface visualization at CT colonography simulated colonoscopy: effect of varying field of view and retrograde view. Am J Gastroenterol. 2007;102:2529–2535. doi: 10.1111/j.1572-0241.2007.01429.x. [DOI] [PubMed] [Google Scholar]
- 14.Subramanian V, Ramappa V, Telakis E, Mannath J, Jawhari AU, Hawkey CJ, Ragunath K. Comparison of high definition with standard white light endoscopy for detection of dysplastic lesions during surveillance colonoscopy in patients with colonic inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:350–355. doi: 10.1002/ibd.23002. [DOI] [PubMed] [Google Scholar]
- 15.Longcroft-Wheaton G, Brown J, Cowlishaw D, Higgins B, Bhandari P. High-definition vs. standard-definition colonoscopy in the characterization of small colonic polyps: results from a randomized trial. Endoscopy. 2012;44:905–910. doi: 10.1055/s-0032-1310004. [DOI] [PubMed] [Google Scholar]
- 16.Pellisé M, Fernández-Esparrach G, Cárdenas A, Sendino O, Ricart E, Vaquero E, Gimeno-García AZ, de Miguel CR, Zabalza M, Ginès A, et al. Impact of wide-angle, high-definition endoscopy in the diagnosis of colorectal neoplasia: a randomized controlled trial. Gastroenterology. 2008;135:1062–1068. doi: 10.1053/j.gastro.2008.06.090. [DOI] [PubMed] [Google Scholar]
- 17.Tribonias G, Theodoropoulou A, Konstantinidis K, Vardas E, Karmiris K, Chroniaris N, Chlouverakis G, Paspatis GA. Comparison of standard vs high-definition, wide-angle colonoscopy for polyp detection: a randomized controlled trial. Colorectal Dis. 2010;12:e260–e266. doi: 10.1111/j.1463-1318.2009.02145.x. [DOI] [PubMed] [Google Scholar]
- 18.Rastogi A, Early DS, Gupta N, Bansal A, Singh V, Ansstas M, Jonnalagadda SS, Hovis CE, Gaddam S, Wani SB, et al. Randomized, controlled trial of standard-definition white-light, high-definition white-light, and narrow-band imaging colonoscopy for the detection of colon polyps and prediction of polyp histology. Gastrointest Endosc. 2011;74:593–602. doi: 10.1016/j.gie.2011.04.050. [DOI] [PubMed] [Google Scholar]
- 19.East JE, Stavrindis M, Thomas-Gibson S, Guenther T, Tekkis PP, Saunders BP. A comparative study of standard vs. high definition colonoscopy for adenoma and hyperplastic polyp detection with optimized withdrawal technique. Aliment Pharmacol Ther. 2008;28:768–776. doi: 10.1111/j.1365-2036.2008.03789.x. [DOI] [PubMed] [Google Scholar]
- 20.Buchner AM, Shahid MW, Heckman MG, McNeil RB, Cleveland P, Gill KR, Schore A, Ghabril M, Raimondo M, Gross SA, et al. High-definition colonoscopy detects colorectal polyps at a higher rate than standard white-light colonoscopy. Clin Gastroenterol Hepatol. 2010;8:364–370. doi: 10.1016/j.cgh.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Subramanian V, Mannath J, Hawkey CJ, Ragunath K. High definition colonoscopy vs. standard video endoscopy for the detection of colonic polyps: a meta-analysis. Endoscopy. 2011;43:499–505. doi: 10.1055/s-0030-1256207. [DOI] [PubMed] [Google Scholar]
- 22.Haringsma J, Tytgat GN, Yano H, Iishi H, Tatsuta M, Ogihara T, Watanabe H, Sato N, Marcon N, Wilson BC, et al. Autofluorescence endoscopy: feasibility of detection of GI neoplasms unapparent to white light endoscopy with an evolving technology. Gastrointest Endosc. 2001;53:642–650. doi: 10.1067/mge.2001.114419. [DOI] [PubMed] [Google Scholar]
- 23.Pasha SF, Leighton JA, Das A, Harrison ME, Gurudu SR, Ramirez FC, Fleischer DE, Sharma VK. Comparison of the yield and miss rate of narrow band imaging and white light endoscopy in patients undergoing screening or surveillance colonoscopy: a meta-analysis. Am J Gastroenterol. 2012;107:363–370; quiz 371. doi: 10.1038/ajg.2011.436. [DOI] [PubMed] [Google Scholar]
- 24.Pohl J, May A, Rabenstein T, Pech O, Ell C. Computed virtual chromoendoscopy: a new tool for enhancing tissue surface structures. Endoscopy. 2007;39:80–83. doi: 10.1055/s-2006-945045. [DOI] [PubMed] [Google Scholar]
- 25.Chung SJ, Kim D, Song JH, Kang HY, Chung GE, Choi J, Kim YS, Park MJ, Kim JS. Comparison of detection and miss rates of narrow band imaging, flexible spectral imaging chromoendoscopy and white light at screening colonoscopy: a randomised controlled back-to-back study. Gut. 2013:Epub ahead of print. doi: 10.1136/gutjnl-2013-304578. [DOI] [PubMed] [Google Scholar]
- 26.Adler A, Aminalai A, Aschenbeck J, Drossel R, Mayr M, Scheel M, Schröder A, Yenerim T, Wiedenmann B, Gauger U, et al. Latest generation, wide-angle, high-definition colonoscopes increase adenoma detection rate. Clin Gastroenterol Hepatol. 2012;10:155–159. doi: 10.1016/j.cgh.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Gross SA, Buchner AM, Crook JE, Cangemi JR, Picco MF, Wolfsen HC, DeVault KR, Loeb DS, Raimondo M, Woodward TA, et al. A comparison of high definition-image enhanced colonoscopy and standard white-light colonoscopy for colorectal polyp detection. Endoscopy. 2011;43:1045–1051. doi: 10.1055/s-0030-1256894. [DOI] [PubMed] [Google Scholar]
- 28.Boparai KS, van den Broek FJ, van Eeden S, Fockens P, Dekker E. Increased polyp detection using narrow band imaging compared with high resolution endoscopy in patients with hyperplastic polyposis syndrome. Endoscopy. 2011;43:676–682. doi: 10.1055/s-0030-1256447. [DOI] [PubMed] [Google Scholar]
- 29.Adler A, Aschenbeck J, Yenerim T, Mayr M, Aminalai A, Drossel R, Schröder A, Scheel M, Wiedenmann B, Rösch T. Narrow-band versus white-light high definition television endoscopic imaging for screening colonoscopy: a prospective randomized trial. Gastroenterology. 2009;136:410–416.e1; quiz 715. doi: 10.1053/j.gastro.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 30.Paggi S, Radaelli F, Amato A, Meucci G, Mandelli G, Imperiali G, Spinzi G, Terreni N, Lenoci N, Terruzzi V. The impact of narrow band imaging in screening colonoscopy: a randomized controlled trial. Clin Gastroenterol Hepatol. 2009;7:1049–1054. doi: 10.1016/j.cgh.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 31.East JE, Suzuki N, Stavrindis M, Palmer N, Guenther T, Saunders BP. Randomized trial of narrow band imaging (NBI) for polyp and adenoma detection in the colon: interim results. Gut. 2006;55 Suppl 2:A17. [Google Scholar]
- 32.East JE, Suzuki N, Stavrindis M, Palmer N, Guenther T, Saunders BP. Narrow Band Imaging Improves Adenoma Detection in Patients At High Risk for Adenoma: A Randomised Trial. Gastrointest Endosc. 2007;65:AB95. [Google Scholar]
- 33.East JE, Ignjatovic A, Suzuki N, Guenther T, Bassett P, Tekkis PP, Saunders BP. A randomized, controlled trial of narrow-band imaging vs high-definition white light for adenoma detection in patients at high risk of adenomas. Colorectal Dis. 2012;14:e771–e778. doi: 10.1111/codi.12014. [DOI] [PubMed] [Google Scholar]
- 34.Adler A, Pohl H, Papanikolaou IS, Abou-Rebyeh H, Schachschal G, Veltzke-Schlieker W, Khalifa AC, Setka E, Koch M, Wiedenmann B, et al. A prospective randomised study on narrow-band imaging versus conventional colonoscopy for adenoma detection: does narrow-band imaging induce a learning effect. Gut. 2008;57:59–64. doi: 10.1136/gut.2007.123539. [DOI] [PubMed] [Google Scholar]
- 35.Chung SJ, Kim D, Song JH, Park MJ, Kim YS, Kim JS, Jung HC, Song IS. Efficacy of computed virtual chromoendoscopy on colorectal cancer screening: a prospective, randomized, back-to-back trial of Fuji Intelligent Color Enhancement versus conventional colonoscopy to compare adenoma miss rates. Gastrointest Endosc. 2010;72:136–142. doi: 10.1016/j.gie.2010.01.055. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida Y, Matsuda K, Sumiyama K, Kawahara Y, Yoshizawa K, Ishiguro H, Tajiri H. A randomized crossover open trial of the adenoma miss rate for narrow band imaging (NBI) versus flexible spectral imaging color enhancement (FICE) Int J Colorectal Dis. 2013;28:1511–1516. doi: 10.1007/s00384-013-1735-4. [DOI] [PubMed] [Google Scholar]
- 37.Pohl J, Lotterer E, Balzer C, Sackmann M, Schmidt KD, Gossner L, Schaab C, Frieling T, Medve M, Mayer G, et al. Computed virtual chromoendoscopy versus standard colonoscopy with targeted indigocarmine chromoscopy: a randomised multicentre trial. Gut. 2009;58:73–78. doi: 10.1136/gut.2008.153601. [DOI] [PubMed] [Google Scholar]
- 38.Haringsma J, Tytgat GN. Fluorescence and autofluorescence. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:1–10. doi: 10.1053/bega.1999.0003. [DOI] [PubMed] [Google Scholar]
- 39.Matsuda T, Saito Y, Fu KI, Uraoka T, Kobayashi N, Nakajima T, Ikehara H, Mashimo Y, Shimoda T, Murakami Y, et al. Does autofluorescence imaging videoendoscopy system improve the colonoscopic polyp detection rate--a pilot study. Am J Gastroenterol. 2008;103:1926–1932. doi: 10.1111/j.1572-0241.2008.01931.x. [DOI] [PubMed] [Google Scholar]
- 40.Moriichi K, Fujiya M, Sato R, Watari J, Nomura Y, Nata T, Ueno N, Maeda S, Kashima S, Itabashi K, et al. Back-to-back comparison of auto-fluorescence imaging (AFI) versus high resolution white light colonoscopy for adenoma detection. BMC Gastroenterol. 2012;12:75. doi: 10.1186/1471-230X-12-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramsoekh D, Haringsma J, Poley JW, van Putten P, van Dekken H, Steyerberg EW, van Leerdam ME, Kuipers EJ. A back-to-back comparison of white light video endoscopy with autofluorescence endoscopy for adenoma detection in high-risk subjects. Gut. 2010;59:785–793. doi: 10.1136/gut.2008.151589. [DOI] [PubMed] [Google Scholar]
- 42.Kuiper T, van den Broek FJ, Naber AH, van Soest EJ, Scholten P, Mallant-Hent RCh, van den Brande J, Jansen JM, van Oijen AH, Marsman WA, et al. Endoscopic trimodal imaging detects colonic neoplasia as well as standard video endoscopy. Gastroenterology. 2011;140:1887–1894. doi: 10.1053/j.gastro.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 43.Ng SC, Lau JY. Narrow-band imaging in the colon: limitations and potentials. J Gastroenterol Hepatol. 2011;26:1589–1596. doi: 10.1111/j.1440-1746.2011.06877.x. [DOI] [PubMed] [Google Scholar]
- 44.Gross S, Trautwein C, Behrens A, Winograd R, Palm S, Lutz HH, Schirin-Sokhan R, Hecker H, Aach T, Tischendorf JJ. Computer-based classification of small colorectal polyps by using narrow-band imaging with optical magnification. Gastrointest Endosc. 2011;74:1354–1359. doi: 10.1016/j.gie.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Gupta N, Bansal A, Rao D, Early DS, Jonnalagadda S, Edmundowicz SA, Sharma P, Rastogi A. Accuracy of in vivo optical diagnosis of colon polyp histology by narrow-band imaging in predicting colonoscopy surveillance intervals. Gastrointest Endosc. 2012;75:494–502. doi: 10.1016/j.gie.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 46.van den Broek FJ, Reitsma JB, Curvers WL, Fockens P, Dekker E. Systematic review of narrow-band imaging for the detection and differentiation of neoplastic and nonneoplastic lesions in the colon (with videos) Gastrointest Endosc. 2009;69:124–135. doi: 10.1016/j.gie.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 47.Longcroft-Wheaton GR, Higgins B, Bhandari P. Flexible spectral imaging color enhancement and indigo carmine in neoplasia diagnosis during colonoscopy: a large prospective UK series. Eur J Gastroenterol Hepatol. 2011;23:903–911. doi: 10.1097/MEG.0b013e328349e276. [DOI] [PubMed] [Google Scholar]
- 48.Pohl J, Nguyen-Tat M, Pech O, May A, Rabenstein T, Ell C. Computed virtual chromoendoscopy for classification of small colorectal lesions: a prospective comparative study. Am J Gastroenterol. 2008;103:562–569. doi: 10.1111/j.1572-0241.2007.01670.x. [DOI] [PubMed] [Google Scholar]
- 49.Sato R, Fujiya M, Watari J, Ueno N, Moriichi K, Kashima S, Maeda S, Ando K, Kawabata H, Sugiyama R, et al. The diagnostic accuracy of high-resolution endoscopy, autofluorescence imaging and narrow-band imaging for differentially diagnosing colon adenoma. Endoscopy. 2011;43:862–868. doi: 10.1055/s-0030-1256510. [DOI] [PubMed] [Google Scholar]
- 50.Ignjatovic A, East JE, Guenther T, Hoare J, Morris J, Ragunath K, Shonde A, Simmons J, Suzuki N, Thomas-Gibson S, et al. What is the most reliable imaging modality for small colonic polyp characterization Study of white-light, autofluorescence, and narrow-band imaging. Endoscopy. 2011;43:94–99. doi: 10.1055/s-0030-1256074. [DOI] [PubMed] [Google Scholar]
- 51.Baumann UA. Water intubation of the sigmoid colon: water instillation speeds up left-sided colonoscopy. Endoscopy. 1999;31:314–317. doi: 10.1055/s-1999-23. [DOI] [PubMed] [Google Scholar]
- 52.Church JM. Warm water irrigation for dealing with spasm during colonoscopy: simple, inexpensive, and effective. Gastrointest Endosc. 2002;56:672–674. doi: 10.1067/mge.2002.128916. [DOI] [PubMed] [Google Scholar]
- 53.Leung FW, Amato A, Ell C, Friedland S, Harker JO, Hsieh YH, Leung JW, Mann SK, Paggi S, Pohl J, et al. Water-aided colonoscopy: a systematic review. Gastrointest Endosc. 2012;76:657–666. doi: 10.1016/j.gie.2012.04.467. [DOI] [PubMed] [Google Scholar]
- 54.Rabenstein T, Radaelli F, Zolk O. Warm water infusion colonoscopy: a review and meta-analysis. Endoscopy. 2012;44:940–951. doi: 10.1055/s-0032-1310157. [DOI] [PubMed] [Google Scholar]
- 55.Amato A, Radaelli F, Paggi S, Baccarin A, Spinzi G, Terruzzi V. Carbon dioxide insufflation or warm-water infusion versus standard air insufflation for unsedated colonoscopy: a randomized controlled trial. Dis Colon Rectum. 2013;56:511–518. doi: 10.1097/DCR.0b013e318279addd. [DOI] [PubMed] [Google Scholar]
- 56.Hsieh YH, Lin HJ, Tseng KC. Limited water infusion decreases pain during minimally sedated colonoscopy. World J Gastroenterol. 2011;17:2236–2240. doi: 10.3748/wjg.v17.i17.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hsieh YH, Tseng KC, Hsieh JJ, Tseng CW, Hung TH, Leung FW. Feasibility of colonoscopy with water infusion in minimally sedated patients in an Asian Community Setting. J Interv Gastroenterol. 2011;1:185–190. doi: 10.4161/jig.1.4.19961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leung CW, Kaltenbach T, Soetikno R, Wu KK, Leung FW, Friedland S. Water immersion versus standard colonoscopy insertion technique: randomized trial shows promise for minimal sedation. Endoscopy. 2010;42:557–563. doi: 10.1055/s-0029-1244231. [DOI] [PubMed] [Google Scholar]
- 59.Pohl J, Messer I, Behrens A, Kaiser G, Mayer G, Ell C. Water infusion for cecal intubation increases patient tolerance, but does not improve intubation of unsedated colonoscopies. Clin Gastroenterol Hepatol. 2011;9:1039–1043.e1. doi: 10.1016/j.cgh.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 60.Radaelli F, Paggi S, Amato A, Terruzzi V. Warm water infusion versus air insufflation for unsedated colonoscopy: a randomized, controlled trial. Gastrointest Endosc. 2010;72:701–709. doi: 10.1016/j.gie.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 61.Ransibrahmanakul K, Leung JW, Mann SK, Siao-Salera R, Lim BS, Hasyagar C. Comparative effectiveness of water vs air methods in minimal sedation colonoscopy performed by supervised trainees in the US - a RCT. Am J Clin Med. 2010;7:113–118. [Google Scholar]
- 62.Leung J, Mann S, Siao-Salera R, Ransibrahmanakul K, Lim B, Canete W, Samson L, Gutierrez R, Leung FW. A randomized, controlled trial to confirm the beneficial effects of the water method on U.S. veterans undergoing colonoscopy with the option of on-demand sedation. Gastrointest Endosc. 2011;73:103–110. doi: 10.1016/j.gie.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 63.Leung FW, Aharonian HS, Leung JW, Guth PH, Jackson G. Impact of a novel water method on scheduled unsedated colonoscopy in U.S. veterans. Gastrointest Endosc. 2009;69:546–550. doi: 10.1016/j.gie.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 64.Leung JW, Do LD, Siao-Salera RM, Ngo C, Parikh DA, Mann SK, Leung FW. Retrospective analysis showing the water method increased adenoma detection rate - a hypothesis generating observation. J Interv Gastroenterol. 2011;1:3–7. doi: 10.4161/jig.1.1.14585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramirez FC, Leung FW. A head-to-head comparison of the water vs. air method in patients undergoing screening colonoscopy. J Interv Gastroenterol. 2011;1:130–135. doi: 10.4161/jig.1.3.18512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leung FW, Harker JO, Jackson G, Okamoto KE, Behbahani OM, Jamgotchian NJ, Aharonian HS, Guth PH, Mann SK, Leung JW. A proof-of-principle, prospective, randomized, controlled trial demonstrating improved outcomes in scheduled unsedated colonoscopy by the water method. Gastrointest Endosc. 2010;72:693–700. doi: 10.1016/j.gie.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 67.Leung J, Mann S, Siao-Salera R, Ngo C, McCreery R, Canete W, Leung F. Indigocarmine added to the water exchange method enhances adenoma detection - a RCT. J Interv Gastroenterol. 2012;2:106–111. doi: 10.4161/jig.23728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yen AW, Leung JW, Leung FW. A new method for screening and surveillance colonoscopy: Combined water-exchange and cap-assisted colonoscopy. J Interv Gastroenterol. 2012;2:114–119. doi: 10.4161/jig.23730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Wijkerslooth TR, Stoop EM, Bossuyt PM, Mathus-Vliegen EM, Dees J, Tytgat KM, van Leerdam ME, Fockens P, Kuipers EJ, Dekker E. Adenoma detection with cap-assisted colonoscopy versus regular colonoscopy: a randomised controlled trial. Gut. 2012;61:1426–1434. doi: 10.1136/gutjnl-2011-301327. [DOI] [PubMed] [Google Scholar]
- 70.Kondo S, Yamaji Y, Watabe H, Yamada A, Sugimoto T, Ohta M, Ogura K, Okamoto M, Yoshida H, Kawabe T, et al. A randomized controlled trial evaluating the usefulness of a transparent hood attached to the tip of the colonoscope. Am J Gastroenterol. 2007;102:75–81. doi: 10.1111/j.1572-0241.2006.00897.x. [DOI] [PubMed] [Google Scholar]
- 71.Rastogi A, Bansal A, Rao DS, Gupta N, Wani SB, Shipe T, Gaddam S, Singh V, Sharma P. Higher adenoma detection rates with cap-assisted colonoscopy: a randomised controlled trial. Gut. 2012;61:402–408. doi: 10.1136/gutjnl-2011-300187. [DOI] [PubMed] [Google Scholar]
- 72.Lee YT, Hui AJ, Wong VW, Hung LC, Sung JJ. Improved colonoscopy success rate with a distally attached mucosectomy cap. Endoscopy. 2006;38:739–742. doi: 10.1055/s-2006-925238. [DOI] [PubMed] [Google Scholar]
- 73.Lee YT, Lai LH, Hui AJ, Wong VW, Ching JY, Wong GL, Wu JC, Chan HL, Leung WK, Lau JY, et al. Efficacy of cap-assisted colonoscopy in comparison with regular colonoscopy: a randomized controlled trial. Am J Gastroenterol. 2009;104:41–46. doi: 10.1038/ajg.2008.56. [DOI] [PubMed] [Google Scholar]
- 74.Ng SC, Tsoi KK, Hirai HW, Lee YT, Wu JC, Sung JJ, Chan FK, Lau JY. The efficacy of cap-assisted colonoscopy in polyp detection and cecal intubation: a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2012;107:1165–1173. doi: 10.1038/ajg.2012.135. [DOI] [PubMed] [Google Scholar]
- 75.Takano N, Yamaji Y, Kajiwara H, Sugimoto T, Yamada A, Akanuma M, Togo G, Ogura K, Okamoto M, Yoshida H, Kawabe T, Omata M. A Randomized Controlled Trial of the Usefulness of Cap-Fitted Colonoscopy On the Polyp Detection. Gastrointest Endosc. 2008;67:AB303. [Google Scholar]
- 76.Horiuchi A, Nakayama Y. Improved colorectal adenoma detection with a transparent retractable extension device. Am J Gastroenterol. 2008;103:341–345. doi: 10.1111/j.1572-0241.2007.01555.x. [DOI] [PubMed] [Google Scholar]
- 77.Harada Y, Hirasawa D, Fujita N, Noda Y, Kobayashi G, Ishida K, Yonechi M, Ito K, Suzuki T, Sugawara T, Horaguchi J, Takasawa O, Obana T, Oohira T, Onochi K, Kanno Y, Kuroha M, Iwai W. Impact of a transparent hood on the performance of total colonoscopy: a randomized controlled trial. Gastrointest Endosc. 2009;69:637–644. doi: 10.1016/j.gie.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 78.Tee HP, Corte C, Al-Ghamdi H, Prakoso E, Darke J, Chettiar R, Rahman W, Davison S, Griffin SP, Selby WS, et al. Prospective randomized controlled trial evaluating cap-assisted colonoscopy vs standard colonoscopy. World J Gastroenterol. 2010;16:3905–3910. doi: 10.3748/wjg.v16.i31.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saad A, Rex DK. Routine rectal retroflexion during colonoscopy has a low yield for neoplasia. World J Gastroenterol. 2008;14:6503–6505. doi: 10.3748/wjg.14.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rex DK, Khashab M. Colonoscopic polypectomy in retroflexion. Gastrointest Endosc. 2006;63:144–148. doi: 10.1016/j.gie.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 81.Harrison M, Singh N, Rex DK. Impact of proximal colon retroflexion on adenoma miss rates. Am J Gastroenterol. 2004;99:519–522. doi: 10.1111/j.1572-0241.2004.04070.x. [DOI] [PubMed] [Google Scholar]
- 82.Hewett DG, Rex DK. Miss rate of right-sided colon examination during colonoscopy defined by retroflexion: an observational study. Gastrointest Endosc. 2011;74:246–252. doi: 10.1016/j.gie.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 83.Triadafilopoulos G, Watts HD, Higgins J, Van Dam J. A novel retrograde-viewing auxiliary imaging device (Third Eye Retroscope) improves the detection of simulated polyps in anatomic models of the colon. Gastrointest Endosc. 2007;65:139–144. doi: 10.1016/j.gie.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 84.Triadafilopoulos G, Li J. A pilot study to assess the safety and efficacy of the Third Eye retrograde auxiliary imaging system during colonoscopy. Endoscopy. 2008;40:478–482. doi: 10.1055/s-2007-995811. [DOI] [PubMed] [Google Scholar]
- 85.DeMarco DC, Odstrcil E, Lara LF, Bass D, Herdman C, Kinney T, Gupta K, Wolf L, Dewar T, Deas TM, et al. Impact of experience with a retrograde-viewing device on adenoma detection rates and withdrawal times during colonoscopy: the Third Eye Retroscope study group. Gastrointest Endosc. 2010;71:542–550. doi: 10.1016/j.gie.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 86.Waye JD, Heigh RI, Fleischer DE, Leighton JA, Gurudu S, Aldrich LB, Li J, Ramrakhiani S, Edmundowicz SA, Early DS, et al. A retrograde-viewing device improves detection of adenomas in the colon: a prospective efficacy evaluation (with videos) Gastrointest Endosc. 2010;71:551–556. doi: 10.1016/j.gie.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 87.Leufkens AM, DeMarco DC, Rastogi A, Akerman PA, Azzouzi K, Rothstein RI, Vleggaar FP, Repici A, Rando G, Okolo PI, et al. Effect of a retrograde-viewing device on adenoma detection rate during colonoscopy: the TERRACE study. Gastrointest Endosc. 2011;73:480–489. doi: 10.1016/j.gie.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 88.Siersema PD, Rastogi A, Leufkens AM, Akerman PA, Azzouzi K, Rothstein RI, Vleggaar FP, Repici A, Rando G, Okolo PI, et al. Retrograde-viewing device improves adenoma detection rate in colonoscopies for surveillance and diagnostic workup. World J Gastroenterol. 2012;18:3400–3408. doi: 10.3748/wjg.v18.i26.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gralnek IM, Carr-Locke DL, Segol O, Halpern Z, Siersema PD, Sloyer A, Fenster J, Lewis BS, Santo E, Suissa A, et al. Comparison of standard forward-viewing mode versus ultrawide-viewing mode of a novel colonoscopy platform: a prospective, multicenter study in the detection of simulated polyps in an in vitro colon model (with video) Gastrointest Endosc. 2013;77:472–479. doi: 10.1016/j.gie.2012.12.011. [DOI] [PubMed] [Google Scholar]


