Abstract
Minimally invasive distal pancreatectomy with splenectomy has been regarded as a safe and effective treatment for benign and borderline malignant pancreatic lesions. However, its application for left-sided pancreatic cancer is still being debated. The clinical evidence for radical antegrade modular pancreatosplenectomy (RAMPS)-based minimally invasive approaches for left-sided pancreatic cancer was reviewed. Potential indications and surgical concepts for minimally invasive RAMPS were suggested. Despite the limited clinical evidence for minimally invasive distal pancreatectomy in left-sided pancreatic cancer, the currently available clinical evidence supports the use of laparoscopic distal pancreatectomy under oncologic principles in well-selected left sided pancreatic cancers. A pancreas-confined tumor with an intact fascia layer between the pancreas and left adrenal gland/kidney positioned more than 1 or 2 cm away from the celiac axis is thought to constitute a good condition for the use of margin-negative minimally invasive RAMPS. The use of minimally invasive (laparoscopic or robotic) anterior RAMPS is feasible and safe for margin-negative resection in well-selected left-sided pancreatic cancer. The oncologic feasibility of the procedure remains to be determined; however, the currently available interim results indicate that even oncologic outcomes will not be inferior to those of open radical distal pancreatosplenectomy.
Keywords: Pancreatic cancer; Laparoscopic pancreatectomy, Robotic pancreatectomy
Core tip: Minimally invasive (laparoscopic or robotic) radical distal pancreatosplenectomy is technically feasible and safe for margin-negative resection in well-selected left sided pancreatic cancer. Generally acceptable potential indications are proposed to include the following: (1) pancreas-confined tumors; (2) intact fascia layer between the distal pancreas and left adrenal gland/kidney; and (3) tumor 1-2 cm from celiac axis. The long-term oncologic feasibility remains to be discerned, but the currently available interim results are encouraging. Further clinical experience with this minimally invasive approach for left-sided pancreatic cancer should be accumulated by experienced surgeons. In the near future, surgical approaches should be specified according to the conditions of the individual pancreatic cancer case.
INTRODUCTION
With recent advancements in laparoscopic experience, techniques, and instruments, laparoscopic surgery has replaced conventional open surgery in most general surgical fields, even in cancer surgery. Despite the potential limitations of conventional laparoscopic surgery, many studies have proven the oncologic feasibility and rationale for laparoscopic surgery in various malignant diseases, such as cancers of the esophagus[1,2], stomach[3,4], liver[5], colon[6,7], etc. However, it remains controversial whether minimally invasive surgery should be applied to treat pancreatic cancer.
Pancreatic cancer is known to be one of the most lethal gastrointestinal malignancies. As a monotherapy, margin-negative pancreatectomy can provide the essential clinical conditions for cure, but the resection rate is very low due to the advanced cancer stages that are usually present at the initial diagnosis. In addition, surgical techniques for margin-negative radical pancreatectomy are very difficult and complex procedures, even in the conventional open approach. Therefore, many surgeons greatly fear that the risk of incomplete surgery might arise when applying minimally invasive techniques to treat pancreatic cancers. Moreover, the lack of more advanced laparoscopic techniques and the limited amount of clinical evidence are some of the biggest obstacles to the use of laparoscopic approaches in the treatment of pancreatic cancer.
Still, several currently available studies have suggested that patients with pancreatic cancer may have appropriate backgrounds for the use of a minimally invasive approach to treat well-selected left-sided pancreatic cancers. First, unlike laparoscopic pancreaticoduodenectomy, laparoscopic distal pancreatectomy is generally regarded as a safe and effective treatment modality in benign and borderline malignant diseases[8]. Second, even laparoscopic subtotal (or extended) distal pancreatectomy can be feasible and safe[9]. Third, many laparoscopic gastric surgeons have already proven the oncologic safety and feasibility of laparoscopic perigastric lymph node dissection in the treatment of gastric cancer[10]. Fourth, the concept of radical antegrade modular pancreatosplenectomy (RAMPS)[11] is thought to be a reasonable approach for margin-negative and systemic lymph node clearance in left-sided pancreatic cancer. Fifth, the early detection of small and asymptomatic pancreatic cancer is expected to increase in the near future due to frequent routine medical check-ups. Finally, even though the data remain limited, a few encouraging studies have been published on the feasibility of a minimally invasive approach to pancreatic cancer[12-14].
Various types of minimally invasive pancreatectomy are currently feasible; however, in this review, we will address distal pancreatosplenectomy in the treatment of pancreatic cancer because this surgical procedure is popular and generally regarded as safe. Therefore, it is thought that laparoscopic distal pancreatectomy with splenectomy could be the initial step for generalizing the concept of a minimally invasive approach to well-selected pancreatic cancers.
CONCEPT OF RAMPS AS A MINIMALLY INVASIVE (LAPAROSCOPIC OR ROBOTIC) APPROACH
Strasberg et al[11,15] presented this modified distal pancreatosplenectomy technique in pancreatic cancer. In this method, dissection proceeds from right to left after an early division of the pancreatic neck on one of the two posterior dissection planes to achieve negative posterior resection margins. The plane of dissection runs posteriorly in the sagittal plane along the superior mesenteric artery and celiac artery to the level of the aorta and then laterally, either anterior or posterior to the adrenal gland, for tangential margin clearance. The accompanying N1 lymph node dissection is based on the established anatomy of lymphatic drainage of the pancreas. The posterior dissection plane can be actively placed for tangential margin clearance. According to the posterior dissection plane of the pancreas, three types of RAMPS can be generally classified (Figure 1). Compared to the usual conventional technique for distal pancreatosplenectomy (dissection from left to right first and vascular control later[16]), RAMPS is thought to be more in line with general oncologic concepts, such as early vascular control and no-touch isolation with en bloc surgical resection. Therefore, when applying minimally invasive approaches to left-sided pancreatic cancer, the principles behind RAMPS should be incorporated, although the generally acceptable extent to which minimally invasive RMAPS can be applied must be determined first.
Figure 1.

Mode of radical antegrade modular pancreatosplenectomy. A: Anterior radical antegrade modular pancreatosplenectomy (RAMPS); B: Posterior RAMPS 1; C: Posterior RAMPS 2. Dissection plane (yellow line) should be changed for clear tangential margin a ccording to tumor condition (red circle).
DETERMINING THE EXTENT OF MINIMALLY INVASIVE RAMPS AND POTENTIAL INDICATIONS
According to our surgical experiences with left-side pancreatic cancer, bloodless and margin-negative resection is an important factor in treating left-sided pancreatic cancer[14]; other reports have also supported this finding[17,18]. However, the use of combined adjacent organ resection has been associated with large amounts of intraoperative bleeding, transfusion, morbidity, and increased risks of a positive resection margin[19,20].
When correlating between the RAMPS surgical mode and the potential tumor behavior, several relationships can be identified (Figure 2, solid line). For example, in the case where posterior RAMPS 2 is selected for margin-negative resection, as opposed to anterior RAMPS, there is a high probability of a large tumor size, combined resection of adjacent organs, large amounts of intraoperative bleeding, and perioperative transfusions, as well as technically demanding, more aggressive tumor behaviors, such as actual margin positivity, peritoneal seeding, or hidden distant metastasis. In contrast, when considering the current technical feasibility of minimally invasive distal pancreatosplenectomy for bloodless and margin-negative resections, minimally invasive anterior RAMPS is well accepted; however, it would be very technically difficult to obtain margin-negative and bloodless resections in the case of minimally invasive posterior RAMPS 1 or RAMPS 2 (Figure 2, dotted line). Certainly, minimally invasive posterior RAMPS 1 and RAMPS 2 are also feasible [Figure 2, areas (B) and (C)], but it is thought that only a few expert laparoscopic surgeons can be fully responsible for those demanding surgical procedures[21]. Therefore, it is generally recommended that open aggressive pancreatectomy only be performed for patients requiring posterior RAMPS 1 and 2. Consequently, when generalizing the concept of minimally invasive approaches to left-sided pancreatic cancer, it would be wise to limit the procedure to anterior RMAPS alone [Figure 2, area (A)][22]. This surgical extent will cover following potential tumor conditions: (1) pancreas-confined tumors; (2) intact fascia layer between the distal pancreas and left adrenal gland/kidney; and (3) tumor 1-2 cm from celiac axis (Figures 3 and 4).
Figure 2.
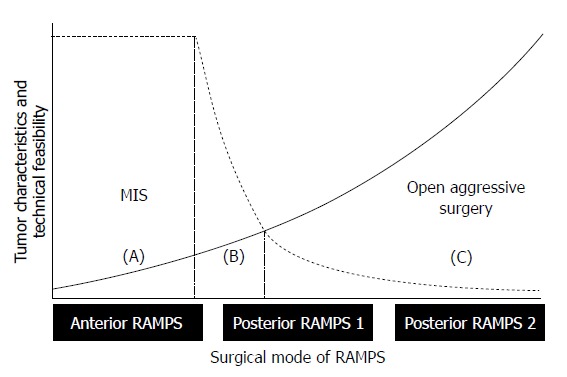
Determining the extent of minimally invasive radical antegrade modular pancreatosplenectomy. The dotted line shows the technical feasibility of bloodless and margin-negative radical antegrade modular pancreatosplenectomy (RAMPS) by a minimally invasive approach, and the solid line represents the biological aggressiveness of tumors, according to the appropriate mode of RAMPS for margin-negative resection. Tentatively, minimally invasive anterior RAMPS is thought to represent a generally acceptable surgical extent for bloodless and margin-negative resections. Oncologically safe posterior RAMPS 1 and 2 might be difficult to perform using a minimally invasive approach. Note the marginal zone of (B). Only a few expert laparoscopic surgeons can be fully responsible for this region. Future directions include widening the area of (B) by means of technical evolution (shifting of the dotted line to the left) and improving early tumor detection (attenuating the slope of solid line). MIS: Minimally invasive surgery.
Figure 3.
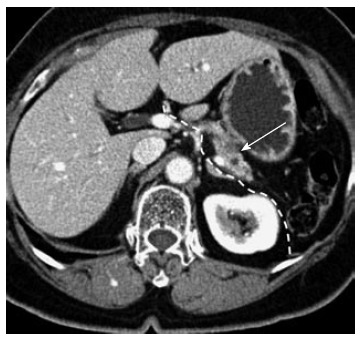
Potential indication for minimally invasive anterior radical antegrade modular pancreatosplenectomy. A 76-year-old female. A relatively pancreas-confined low density mass lesion is noted (arrow). The dotted white line indicates the dissection plane for minimally invasive anterior radical antegrade modular pancreatosplenectomy (RAMPS). The intact fascia layer between the pancreas and left adrenal gland/kidney can facilitate posterior margin clearance when removing the surgical specimen. The tumor is separated from the origin of the splenic artery, necessary for safe vascular control by introducing a minimally invasive technique. The patient underwent laparoscopic anterior RAMPS and has been followed for more than 1 year without evidence of tumor recurrence.
Figure 4.
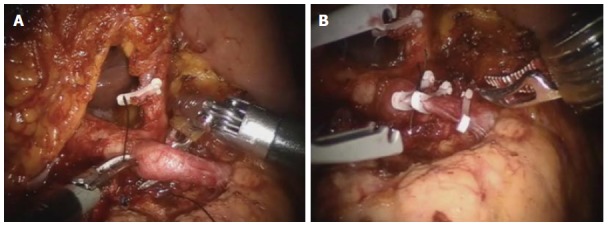
Adequate distance between celiac axis and tumor. Robotic anterior radical antegrade modular pancreatosplenectomy. The origin of the splenic artery is isolated (A) and ligated (B) by the robotic surgical system. For technically and oncologically safe minimally invasive vascular control, some cancer-free space is extremely necessary.
CURRENT CLINICAL PRACTICE OF THE MINIMALLY INVASIVE APPROACH TO LEFT-SIDED PANCREATIC CANCER
Primitive evidence
Until now, many studies have proven the clinical benefit of laparoscopic distal pancreatectomy, with or without splenectomy, in benign and borderline malignant pancreatic disease. However, only a few previous studies have reported the laparoscopic approach for left-sided pancreatic cancer with available long-term survival outcomes[12,23-27]. Since Gagner et al[28] first reported laparoscopic distal pancreatectomy, with the advance of laparoscopic techniques and experiences, several other studies have been published, showing the technical feasibility, safety, and clinical benefit of laparoscopic distal pancreatectomy over open distal pancreatectomy. However, most reported cases of pancreatic cancer (ductal adenocarcinoma) treated by laparoscopic distal pancreatectomy were incidentally included in those series. As a result, we cannot fully assess the surgical quality based on relevant oncologic concepts. In addition, the lack of information on tumor characteristics, such as pT stage, pN stage, number of retrieved lymph nodes, margin status, and survival outcomes, creates difficulties in determining the oncologic feasibility of the laparoscopic approach to the left-sided pancreatic cancer[24-27,29,30]. For example, in one collective review performed in 2009[31], a final diagnosis of pancreatic ductal adenocarcinoma was found in 51 patients (9.8%, 51 out of 588 patients). However, the margin status was available in only 20 patients (39%). In addition, the number of retrieved lymph nodes in patients with pancreatic cancer was reported in only three articles[26,32,33] (12.5%, 3 of 24 articles identified). Not surprisingly, there is still a lack of long-term survival outcomes. Despite the efforts of several surgeons to perform laparoscopic distal pancreatectomy for pancreatic cancer, it was found that there is a substantial lack of evidence on the oncologic outcomes and surgical quality. Consequently, for the last several decades, we were uncertain whether the minimally invasive approach to left-sided pancreatic cancer was appropriate.
Intermediate evidence
Recently, several studies have been published that focused on the question of whether laparoscopic distal pancreatectomy is oncologically feasible.
DiNorcia et al[34] reported their experiences with laparoscopic distal pancreatectomy between 1991 and 2009. Seventy-one patients underwent laparoscopic distal pancreatectomy, and only 9 patients (12.7%) were reported to have malignant pathologies, including 3 cases of pancreatic ductal adenocarcinoma. Long-term survival outcomes were not analyzed; however, the margin-negative resection rate (2.8% vs 13%, P < 0.01) and mean number of retrieved lymph nodes [6 (range: 2.5-12.0) vs 8 ( range: 3.0-13.0), P = 0.29] were shown to be comparable with those in a conventional open approach.
A recent multicenter analysis reported by Kooby et al[13] has provided the most encouraging and impressive evidence, considering the lack of long-term oncologic evidence of laparoscopic approaches to left-sided pancreatic cancer. This study showed that laparoscopic distal pancreatectomy is able to provide similar short- and long-term oncologic outcomes to those obtained with open distal pancreatectomy and suggested that laparoscopic distal pancreatectomy is an acceptable approach for the resection of the left-sided pancreatic cancer in selected patients. In the matched analysis of the overall survival for the patients undergoing an open (n = 70) versus a laparoscopic distal pancreatectomy (n = 23) for pancreatic cancer, the median survival was comparable among the two the groups (median 16 mo, P = 0.71).
In addition, Kim et al[35] also published the long-term outcomes of patients who were postoperatively diagnosed with malignancies after laparoscopic distal pancreatectomy. Of the 88 patients who underwent a laparoscopic distal pancreatectomy, 11 (12.5%) were subsequently diagnosed with malignancies in their postoperative pathologic reports. Pancreatic ductal adenocarcinoma was the most common (5 out of 11 patients), followed by invasive intraductal papillary mucinous neoplasm (n = 3), neuroendocrine carcinoma (n = 1), and so forth. During the follow-up period (range, 3-60 mo), they reported only 1 patient who died of cancer; all others were still alive. Thus, the authors carefully concluded that the postoperative outcomes among patients who were diagnosed postoperatively with malignant pancreatic disease are acceptable.
Although these retrospective studies were not able to suggest either standardized surgical procedures or proper indications, they did suggest potential oncologic outcomes and verify the technical feasibility of the laparoscopic approach to left-sided pancreatic cancer.
Recent advance evidence
More encouraging clinical data with intent-to-treat for minimally invasive approach to left-sided pancreatic cancer has been published. When considering RAMPS-based laparoscopic approaches to treating left-sided pancreatic cancer, only 5 studies investigated the interim or long-term follow up results on the safety and feasibility of minimally invasive distal pancreatectomy for pancreatic cancer (Table 1). A group in Barcelona[12] reported that the median survival of pancreatic cancers treated by laparoscopic RAMPS was 14 mo, comparable to the usual pancreatic cancers that were treated with an open surgery. This report is thought to be first to reveal the technical and oncologic feasibility of laparoscopic RAMPS in left-sided pancreatic cancer. In addition, despite the limited follow-up period, current interim results[36-38] strongly suggest that the possible oncologic outcomes following minimally invasive RAMPS will not be inferior to those of conventional open surgery in well-selected left-sided pancreatic cancer.
Table 1.
Recent studies on minimally invasive radical antegrade modular pancreatosplenectomy and open distal pancreatectomy in left-sided pancreatic cancer n (%)
| Characterisc |
Minimally invasive distal pancreatosplenectomy |
Open distal pancreatosplenectomy |
||||||||
| Fernández-Cruz et al[12] | Song et al[37] | Choi et al[36,38] | Magge et al[40] | Marangos et al[41] | Kanda et al[48] | Kooby et al[23] | Kang et al[14] | Mitchem et al[22] | Okada et al[50] | |
| Surgical technique | RAMPS-based | RAMPS- based | RAMPS- based | RAMPS-based | Conventional technique-based | NA | NA | Conventional technique-based | RAMPS-based | Standard DP |
| Indication | Anatomic dissection No combined resection | No distant metastasis Not locally advanced | Relatively pancreas-confined, intact fascia layer, apart from celiac axis | Allegedly the same as open surgery, even when allowing adjacent organ combined resection | Apparently the same as open surgery, even when allowing adjacent organ combined resection | Resectable, no distal metastasis, no peritoneal seeding, no major vascular invasion | NA | Resectable, no distal metastasis, no peritoneal seeding, no major vascular invasion | Resectable, no distant metastasis, no peritoneal seeding | Resectable, not invading major vessel |
| Patients (n) | 10 | 24 | 9 | 28 | 21 | 51 | 70 | 27 | 47 | 36 |
| Age (yr) | NA | NA | 64.8 (54-76) | 67 (60-75) | 63.1 (49-83) | 62.7 (38-79) | 65.9 ± 11.1 | 60.5 (47-75) | 64.5 ± 10.3 | 68 |
| Gender (male/female) | NA | 16/8 | 5/4 | 9/19 | 6/15 | 34/17 | 27/43 | 20/7 | 20/27 | 23/13 |
| Tumor size (cm) | NA | 2.6 (1.4-10) | 2.5 (1.2-6) | 3.0 (2.2-3.6) | 4.8 (1.0-10) | 7 (< 2 cm) 44 (> 2 cm) | 3.5 ± 1.4 | 3.9 (2.5-5.3) | 4.4 ± 2.1 | NA |
| pT stage (T1/T2/T3/T4) | NA | NA | 0/0/9/0 | NA | NA | 2/5/20/24/01 | NA | 1/4/20/2 | 1/4/41/1 | 1/2/15/17/0/1/1 |
| Retrieved LNs | 14.5 (6-20) | 10.3 ± 8.6 | 10.8 (2-23) | 11 (8-20) | 7.4(0-26) | NA | 12.3 ± 8.3 | 11.3 (5-18) | 18.0 ± 11.7 | NA |
| pN stage (N0/N1) | 5/5 | NA | 2/7 | 12/16 | 14/9 | 25/26 | NA | 15/12 | 26/21 | 19/17 |
| R0(%)/R1/R2 | 9 (90)/1/0 | 22 (91.7)/2/0 | 9 (100)/0/0 | 24 (86)/4 (14)/0 | 19 (90.5)/2/0 | 38 (74.5)/13 | 46/24 | 3/4/2020 | 81%/19%/0% | 29 (80.5%)/6/1 |
| Operation time (min) | 320 (280-330) | 225 (95-360) | 368.3 (180-700) | 260 (220-340) | NA | NA | 216 ± 69 | 274 (97-453) | 243.6 ± 93.5 | 203 (128-276) |
| Blood loss | 720 (300-1300) | NA | 360.1 (trace-1350) | 200 (150-300) | NA | NA | 751 ± 853 | 643.3 (100-1200) | 744.3 ± 570.4 | 700 (10-2850) |
| Hospital stay (d) | 8 (7-11) | 9.5 (5-22) | 9 (4-16) | 6 (4-6) | 5 | NA | 9.4 ± 4.7 | 21.2 (7-24) | 11.3 ± 6.8 | NA |
| Adjuvant chemotherapy | 10 (100) | 17 (70.8) | 9 (100) | 17 (100) | NA | NA | 45 (64) | NA | NA | NA |
| Follow-up period (mo) | NA | 9.95 (1.3-48.5) | 20.7 (5.1-45.9) | NA | 21.1 (0.5-108) | 17.1 | 10 | NA | 26.4 | 25 |
| Oncologic outcome | Median survival 14 mo | 2-yr overall survival, 85.2% | 2-yr disease free survival, 83.3% | NA | Median survival 19 mo | 1, 3, 5-yr overall survival rate, 57.2%, 12.4%, 6.2% | Overall survival 16 mo | Median survival 27.9 mo, 5-yr survival, 28.9% | Median survival 25.9 mo, 5-yr survival, 30.4% | Median survival 32 mo, 2-yr survival 52% |
Data are confined to pancreatic ductal adenocarcinoma alone.
UICC Cancer Staging System. NA: Not available; RAMPS: Radical antegrade modular pancreatosplenectomy.
A group from Pittsburgh recently published their institutional historical experiences of minimally invasive (laparoscopic and robotic) distal pancreatectomy[39]. A total of 27 patients were reported to have undergone minimally invasive distal pancreatectomy for the treatment of pancreatic ductal adenocarcinoma. When excluding conversion cases during laparoscopic distal pancreatectomy, the margin-positive resection rate was reported to be 4%, and the capacity for lymph node retrieval was up to 17 (range 10-19); these results are comparable with those of robotic distal pancreatectomy [R1 resection rate, 0% and nodal harvested, median 19 (range 17-27)], suggesting an acceptable quality of surgery in treating pancreatic cancer. They also analyzed retrospective 62 consecutive patients undergoing open distal pancreatectomy (ODP = 34) and minimally invasive distal pancreatectomy (MIDP = 28 with 5 conversions) for pancreatic ductal adenocarcinoma[40]. It was shown that overall survival after ODP or intended MIDP was similar after adjusting for comorbidity and year of surgery [relative hazard, 1.11 (95%CI: 0.47-2.62)]. These two studies still lack long-term oncologic outcomes (median follow up of 21 mo), however, no evidence was detected that MIDP was inferior to ODP in treating pancreatic cancer.
On the other hand, Marangos et al[41] published an interesting paper about their surgical experiences with laparoscopic distal pancreatosplenectomy for pancreatic exocrine carcinoma. Since 1997, they reported removing all lesions in the body and tail of the pancreas laparoscopically, and 29 patients with pancreatic cancer (11.6%, 29 out of 250 patients) underwent laparoscopic distal pancreatosplenectomy. Their approach was not based on RAMPS but rather on the conventional left-to-right technique. In addition, they did not perform formal lymph node dissection; instead, they only removed the enlarged or suspicious regional lymph nodes. The dissection plane and resection margins were carefully guided by laparoscopic intraoperative ultrasound. They reported an overall 93% R0 resection rate with a median survival of 23 mo (in particular, 19 mo for 21 pancreatic ductal adenocarcinomas), which is also comparable to the best open series[15,42]. It was noted that the median number of retrieved lymph nodes was smaller (5 nodes), but this did not translate into poor oncologic outcomes, again reminding us of the outcomes of previous prospective randomized controlled studies on standard and extended pancreaticoduodenectomy in the treatment of pancreatic head cancer[43-45]. In addition, in comparison with the oncologic outcomes from open radical surgery, perioperative and oncologic outcomes appear to be comparable between the minimally invasive radical distal pancreatectomy and the open approach (Table 1). One of the most significant weak points of the minimally invasive approach to pancreatic cancer is that the oncologic outcomes are still based on a short-term follow-up period, compared to that of open radical pancreatectomy[14,15,46-48]. However, recently, the single-center-based Pittsburgh group[40] reported a comparative analysis, including long-term survival, of 34 patients with open radical pancreatectomy and 34 with minimally invasive distal pancreatectomy in pancreatic ductal adenocarcinoma to determine the oncological safety and efficacy of minimally invasive surgery. They demonstrated no significant difference between two groups in tumor size (3.0 cm vs 3.0 cm), radiologic stage (IA/IB/IIA/IIB, 3/12/10/6 vs 3/11/5/4), margin-negative resection (88% vs 86%), power of lymph node retrieval (12 vs 11), or lymph node metastasis (38% vs 57%) and similar postoperative complications, leading to equivalent survival in propensity score-adjusted overall survival analysis [relative hazard, 1.11 (95%CI: 0.47-2.62), P = 0.80]. Along with the multicenter case-matched analysis by Kooby et al[13], this study provides powerful evidence to support the technical feasibility of minimally invasive radical oncologic surgery. The study further shows that the quality of surgical specimens is quite acceptable and provides encouraging oncologic survival outcomes.
CHALLENGING ISSUES
Combined and vascular resection
Distal pancreatectomy with en bloc celiac axis resection (DPCAR) has been introduced for locally advanced left-sided pancreatic cancer involving the common hepatic artery and/or celiac axis, with perineural invasion in the nerve plexus surrounding these arteries[49,50]. In particular, Okada et al[50] recently concluded that DP-CAR is feasible and should be reserved for patients without tumors infiltrating either the portal venous or arterial system. Considering these circumstances, DP-CAR is suggested to be a safe procedure, similar to standard distal pancreatectomy in well-selected patients. Recent technological innovations and extensive surgical experiences are expanding the clinical applications for laparoscopic distal pancreatectomy. As a result, the technical feasibility of minimally invasive distal pancreatectomy with combined celiac trunk or portal vein resection has also been reported. Cho et al[21] reported the technical feasibility of pure laparoscopic DP-CAR, finding it safe and feasible to achieve R0 resections in selected patients with locally advanced pancreatic cancer. Giulianotti et al[51] and Boggi et al[52] also reported robotic pancreatectomy with vascular resection for locally advanced pancreatic tumors. In addition, Kendrick et al[53] reported 11 patients who underwent total laparoscopic pancreaticoduodenectomy with major venous vascular resection, including laparoscopic end-to-end vascular reconstruction, patch, and renal vein graft. However, patients with left-sided pancreatic cancer invading isolated superior mesenteric vein-splenic vein-portal vein confluence are rare, as most cases of pancreatic cancer are usually associated with celiac axis and superior mesenteric artery invasion[54], which will be determined as locally invasive pancreatic cancer (unresectable). In general, pancreatic surgeons must consider possible combined vascular resection in their surgical approaches to pancreatic cancer and should be prepared to meet this surgical demand. However, how many surgeons can be responsible for this advanced laparoscopic technique How should the educational system be modified to reproduce this surgical skill
Is only RAMPS the ideal approach
The surgical approach of RAMPS has demonstrated favorable oncologic outcomes in treating left-sided pancreatic cancer[11,15,22,55]. The basic concept of RAMPS is, of course, oncologically sound and reasonable; however, it is notable that no randomized controlled studies have tested the oncologic superiority between RAMPS and conventional radical distal pancreatectomy. There are several comparable reports showing similar survival outcomes to RAMPS[14,41,56]. An RCT should be performed to test whether the RAMPS procedure is superior to standard distal pancreatectomy. However, it is very difficult to organize a successful trial. Mitchem et al[22] have already commented on this issue, as follows: “However, the disparity between the number of cases available for study and the number required for a randomized trial makes this goal unattainable”.
FUTURE PERSPECTIVES ON MINIMALLY INVASIVE LEFT-SIDED RADICAL PANCREATECTOMY
As shown in other gastrointestinal cancer surgeries, there has been an increasing clinical effort to apply the laparoscopic approach to left-sided pancreatic cancer. However, procedural standardization and surgical indications have not yet been established. Currently, RAMPS seems to be a reasonable approach, with encouraging oncologic outcomes in the treatment of left-sided pancreatic cancer[22]. Nevertheless, it might be difficult to expand the use of minimally invasive RAMPS to all left-sided pancreatic cancers because these cancers are usually found in advanced cancer stages. However, the clinical conditions required to widen the area (B) in Figure 2, such as technical evolution (right sided-shift of dotted line in Figure 2) and the early detection of the cancer (attenuating slope of the solid line), would facilitate the clinical application of minimally invasive RAMPS in well-selected cases of left-sided pancreatic cancer.
Recently, the use of radical pancreatectomy followed by neoadjuvant chemoradiation therapy has been successfully applied for the treatment of advanced pancreatic cancers[57-59]. In considering the future circumstances of potent chemoradiation therapy for the treatment of pancreatic cancers, minimally invasive RAMPS following neoadjuvant chemoradiation therapy would be another potential option for well-selected patients. In particular, considering the technical advances of combined vascular resection in treating pancreatic cancer, the indications for minimally invasive radical distal pancreatectomy should be expanded in the near future. In addition, many academic institutions seem to be carefully accumulating clinical experience with the minimally invasive resection of left-sided pancreatic cancer. Perhaps in the near future, more relevant clinical evidence with adequate long-term follow-up and qualified oncologic outcomes will become available, leading to the oncologic feasibility of minimally invasive left-sided pancreatectomy in pancreatic cancers. Generally, these conclusions will be influenced by selection bias from the retrospective nature of studies. However, these identified instances of selection bias, in turn, will become potential selection criteria for minimally invasive radical pancreatectomy in distal pancreatic cancers, especially given the difficulty of establishing an RCT in the present circumstances.
CONCLUSION
More than 20 years have passed since the first laparoscopic cholecystectomy was performed in the late 1980s. Tremendous improvements in the surgical techniques, experiences, and new effective instruments have successfully expanded the indications for laparoscopic surgery. Minimally invasive (laparoscopic and robotic) radical pancreatectomy in well-selected left-sided pancreatic cancers is feasible under general oncologic concepts; however, solid clinical evidence is still lacking. Further clinical experience with a minimally invasive approach to left-side pancreatic cancer must be carefully accumulated by experienced surgeons. The oncological feasibility should be addressed in greater detail based on long-term survival outcomes. However, we should not overlook that the currently available interim results demonstrating minimally invasive left-sided radical pancreatectomy are not inferior to those of conventional open radical pancreatectomy.
Footnotes
P- Reviewers: Camp ER, Nakao A, Stefaniak T S- Editor: Wen LL L- Editor: A E- Editor: Zhang DN
References
- 1.Nguyen NT, Hinojosa MW, Smith BR, Chang KJ, Gray J, Hoyt D. Minimally invasive esophagectomy: lessons learned from 104 operations. Ann Surg. 2008;248:1081–1091. doi: 10.1097/SLA.0b013e31818b72b5. [DOI] [PubMed] [Google Scholar]
- 2.Smithers BM, Gotley DC, Martin I, Thomas JM. Comparison of the outcomes between open and minimally invasive esophagectomy. Ann Surg. 2007;245:232–240. doi: 10.1097/01.sla.0000225093.58071.c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, Ponzano C. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232–237. doi: 10.1097/01.sla.0000151892.35922.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimizu S, Uchiyama A, Mizumoto K, Morisaki T, Nakamura K, Shimura H, Tanaka M. Laparoscopically assisted distal gastrectomy for early gastric cancer: is it superior to open surgery. Surg Endosc. 2000;14:27–31. doi: 10.1007/s004649900005. [DOI] [PubMed] [Google Scholar]
- 5.Gigot JF, Glineur D, Santiago Azagra J, Goergen M, Ceuterick M, Morino M, Etienne J, Marescaux J, Mutter D, van Krunckelsven L, et al. Laparoscopic liver resection for malignant liver tumors: preliminary results of a multicenter European study. Ann Surg. 2002;236:90–97. doi: 10.1097/00000658-200207000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44–52. doi: 10.1016/S1470-2045(08)70310-3. [DOI] [PubMed] [Google Scholar]
- 7.McLeod R. Long-term results of laparoscopic-assisted colectomy are comparable to results after open colectomy. Ann Surg. 2008;248:8–9. doi: 10.1097/SLA.0b013e31817c965d. [DOI] [PubMed] [Google Scholar]
- 8.Merchant NB, Parikh AA, Kooby DA. Should all distal pancreatectomies be performed laparoscopically. Adv Surg. 2009;43:283–300. doi: 10.1016/j.yasu.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Kang CM, Choi SH, Hwang HK, Kim DH, Yoon CI, Lee WJ. Laparoscopic distal pancreatectomy with division of the pancreatic neck for benign and borderline malignant tumor in the proximal body of the pancreas. J Laparoendosc Adv Surg Tech A. 2010;20:581–586. doi: 10.1089/lap.2009.0348. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi H, Ochiai T, Shimada H, Gunji Y. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc. 2005;19:1172–1176. doi: 10.1007/s00464-004-8207-4. [DOI] [PubMed] [Google Scholar]
- 11.Strasberg SM, Drebin JA, Linehan D. Radical antegrade modular pancreatosplenectomy. Surgery. 2003;133:521–527. doi: 10.1067/msy.2003.146. [DOI] [PubMed] [Google Scholar]
- 12.Fernández-Cruz L, Cosa R, Blanco L, Levi S, López-Boado MA, Navarro S. Curative laparoscopic resection for pancreatic neoplasms: a critical analysis from a single institution. J Gastrointest Surg. 2007;11:1607–1621; discussion 1621-1622. doi: 10.1007/s11605-007-0266-0. [DOI] [PubMed] [Google Scholar]
- 13.Kooby DA, Hawkins WG, Schmidt CM, Weber SM, Bentrem DJ, Gillespie TW, Sellers JB, Merchant NB, Scoggins CR, Martin RC, et al. A multicenter analysis of distal pancreatectomy for adenocarcinoma: is laparoscopic resection appropriate. J Am Coll Surg. 2010;210:779–785, 786-787. doi: 10.1016/j.jamcollsurg.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 14.Kang CM, Kim DH, Lee WJ. Ten years of experience with resection of left-sided pancreatic ductal adenocarcinoma: evolution and initial experience to a laparoscopic approach. Surg Endosc. 2010;24:1533–1541. doi: 10.1007/s00464-009-0806-7. [DOI] [PubMed] [Google Scholar]
- 15.Strasberg SM, Linehan DC, Hawkins WG. Radical antegrade modular pancreatosplenectomy procedure for adenocarcinoma of the body and tail of the pancreas: ability to obtain negative tangential margins. J Am Coll Surg. 2007;204:244–249. doi: 10.1016/j.jamcollsurg.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Fernández-Cruz L. Distal pancreatic resection: technical differences between open and laparoscopic approaches. HPB (Oxford) 2006;8:49–56. doi: 10.1080/13651820500468059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH, Lillemoe KD. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 18.Goh BK, Tan YM, Cheow PC, Chung YF, Chow PK, Wong WK, Ooi LL. Outcome of distal pancreatectomy for pancreatic adenocarcinoma. Dig Surg. 2008;25:32–38. doi: 10.1159/000117821. [DOI] [PubMed] [Google Scholar]
- 19.Christein JD, Kendrick ML, Iqbal CW, Nagorney DM, Farnell MB. Distal pancreatectomy for resectable adenocarcinoma of the body and tail of the pancreas. J Gastrointest Surg. 2005;9:922–927. doi: 10.1016/j.gassur.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Shoup M, Conlon KC, Klimstra D, Brennan MF. Is extended resection for adenocarcinoma of the body or tail of the pancreas justified. J Gastrointest Surg. 2003;7:946–952; discussion 952. doi: 10.1016/j.gassur.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Cho A, Yamamoto H, Kainuma O, Ota T, Park S, Ikeda A, Souda H, Nabeya Y, Takiguchi N, Nagata M. Pure laparoscopic distal pancreatectomy with en bloc celiac axis resection. J Laparoendosc Adv Surg Tech A. 2011;21:957–959. doi: 10.1089/lap.2011.0300. [DOI] [PubMed] [Google Scholar]
- 22.Mitchem JB, Hamilton N, Gao F, Hawkins WG, Linehan DC, Strasberg SM. Long-term results of resection of adenocarcinoma of the body and tail of the pancreas using radical antegrade modular pancreatosplenectomy procedure. J Am Coll Surg. 2012;214:46–52. doi: 10.1016/j.jamcollsurg.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Kooby DA, Gillespie T, Bentrem D, Nakeeb A, Schmidt MC, Merchant NB, Parikh AA, Martin RC, Scoggins CR, Ahmad S, et al. Left-sided pancreatectomy: a multicenter comparison of laparoscopic and open approaches. Ann Surg. 2008;248:438–446. doi: 10.1097/SLA.0b013e318185a990. [DOI] [PubMed] [Google Scholar]
- 24.Laxa BU, Carbonell AM, Cobb WS, Rosen MJ, Hardacre JM, Mekeel KL, Harold KL. Laparoscopic and hand-assisted distal pancreatectomy. Am Surg. 2008;74:481–486; discussion 486-487. [PubMed] [Google Scholar]
- 25.Lebedyev A, Zmora O, Kuriansky J, Rosin D, Khaikin M, Shabtai M, Ayalon A. Laparoscopic distal pancreatectomy. Surg Endosc. 2004;18:1427–1430. doi: 10.1007/s00464-003-8221-y. [DOI] [PubMed] [Google Scholar]
- 26.Melotti G, Butturini G, Piccoli M, Casetti L, Bassi C, Mullineris B, Lazzaretti MG, Pederzoli P. Laparoscopic distal pancreatectomy: results on a consecutive series of 58 patients. Ann Surg. 2007;246:77–82. doi: 10.1097/01.sla.0000258607.17194.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor C, O’Rourke N, Nathanson L, Martin I, Hopkins G, Layani L, Ghusn M, Fielding G. Laparoscopic distal pancreatectomy: the Brisbane experience of forty-six cases. HPB (Oxford) 2008;10:38–42. doi: 10.1080/13651820701802312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gagner M, Pomp A, Herrera MF. Early experience with laparoscopic resections of islet cell tumors. Surgery. 1996;120:1051–1054. doi: 10.1016/s0039-6060(96)80054-7. [DOI] [PubMed] [Google Scholar]
- 29.Eom BW, Jang JY, Lee SE, Han HS, Yoon YS, Kim SW. Clinical outcomes compared between laparoscopic and open distal pancreatectomy. Surg Endosc. 2008;22:1334–1338. doi: 10.1007/s00464-007-9660-7. [DOI] [PubMed] [Google Scholar]
- 30.Jayaraman S, Gonen M, Brennan MF, D’Angelica MI, DeMatteo RP, Fong Y, Jarnagin WR, Allen PJ. Laparoscopic distal pancreatectomy: evolution of a technique at a single institution. J Am Coll Surg. 2010;211:503–509. doi: 10.1016/j.jamcollsurg.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Borja-Cacho D, Al-Refaie WB, Vickers SM, Tuttle TM, Jensen EH. Laparoscopic distal pancreatectomy. J Am Coll Surg. 2009;209:758–765; quiz 800. doi: 10.1016/j.jamcollsurg.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 32.D’Angelica M, Are C, Jarnagin W, DeGregoris G, Coit D, Jaques D, Brennan M, Fong Y. Initial experience with hand-assisted laparoscopic distal pancreatectomy. Surg Endosc. 2006;20:142–148. doi: 10.1007/s00464-005-0209-3. [DOI] [PubMed] [Google Scholar]
- 33.Dulucq JL, Wintringer P, Stabilini C, Feryn T, Perissat J, Mahajna A. Are major laparoscopic pancreatic resections worthwhile A prospective study of 32 patients in a single institution. Surg Endosc. 2005;19:1028–1034. doi: 10.1007/s00464-004-2182-7. [DOI] [PubMed] [Google Scholar]
- 34.DiNorcia J, Schrope BA, Lee MK, Reavey PL, Rosen SJ, Lee JA, Chabot JA, Allendorf JD. Laparoscopic distal pancreatectomy offers shorter hospital stays with fewer complications. J Gastrointest Surg. 2010;14:1804–1812. doi: 10.1007/s11605-010-1264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J, Han HS, Yoon YS, Cho JY, Ahn KS, Kwon Y. Outcomes of the patients who were postoperatively diagnosed as malignancy after laparoscopic distal pancreatectomy. Surg Laparosc Endosc Percutan Tech. 2012;22:467–470. doi: 10.1097/SLE.0b013e3182632833. [DOI] [PubMed] [Google Scholar]
- 36.Choi SH, Kang CM, Lee WJ, Chi HS. Multimedia article. Laparoscopic modified anterior RAMPS in well-selected left-sided pancreatic cancer: technical feasibility and interim results. Surg Endosc. 2011;25:2360–2361. doi: 10.1007/s00464-010-1556-2. [DOI] [PubMed] [Google Scholar]
- 37.Song KB, Kim SC, Park JB, Kim YH, Jung YS, Kim MH, Lee SK, Seo DW, Lee SS, Park do H, et al. Single-center experience of laparoscopic left pancreatic resection in 359 consecutive patients: changing the surgical paradigm of left pancreatic resection. Surg Endosc. 2011;25:3364–3372. doi: 10.1007/s00464-011-1727-9. [DOI] [PubMed] [Google Scholar]
- 38.Choi SH, Kang CM, Hwang HK, Lee WJ, Chi HS. Robotic anterior RAMPS in well-selected left-sided pancreatic cancer. J Gastrointest Surg. 2012;16:868–869. doi: 10.1007/s11605-012-1825-6. [DOI] [PubMed] [Google Scholar]
- 39.Daouadi M, Zureikat AH, Zenati MS, Choudry H, Tsung A, Bartlett DL, Hughes SJ, Lee KK, Moser AJ, Zeh HJ. Robot-assisted minimally invasive distal pancreatectomy is superior to the laparoscopic technique. Ann Surg. 2013;257:128–132. doi: 10.1097/SLA.0b013e31825fff08. [DOI] [PubMed] [Google Scholar]
- 40.Magge D, Gooding W, Choudry H, Steve J, Steel J, Zureikat A, Krasinskas A, Daouadi M, Lee KK, Hughes SJ, et al. Comparative effectiveness of minimally invasive and open distal pancreatectomy for ductal adenocarcinoma. JAMA Surg. 2013;148:525–531. doi: 10.1001/jamasurg.2013.1673. [DOI] [PubMed] [Google Scholar]
- 41.Marangos IP, Buanes T, Røsok BI, Kazaryan AM, Rosseland AR, Grzyb K, Villanger O, Mathisen Ø, Gladhaug IP, Edwin B. Laparoscopic resection of exocrine carcinoma in central and distal pancreas results in a high rate of radical resections and long postoperative survival. Surgery. 2012;151:717–723. doi: 10.1016/j.surg.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 42.Shimada K, Sakamoto Y, Sano T, Kosuge T. Prognostic factors after distal pancreatectomy with extended lymphadenectomy for invasive pancreatic adenocarcinoma of the body and tail. Surgery. 2006;139:288–295. doi: 10.1016/j.surg.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Farnell MB, Pearson RK, Sarr MG, DiMagno EP, Burgart LJ, Dahl TR, Foster N, Sargent DJ. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery. 2005;138:618–628; discussion 628-630. doi: 10.1016/j.surg.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 44.Pedrazzoli S, DiCarlo V, Dionigi R, Mosca F, Pederzoli P, Pasquali C, Klöppel G, Dhaene K, Michelassi F. Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: a multicenter, prospective, randomized study. Lymphadenectomy Study Group. Ann Surg. 1998;228:508–517. doi: 10.1097/00000658-199810000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK, Coleman J, Abrams RA, Hruban RH. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355–366; discussion 366-368. doi: 10.1097/00000658-200209000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamamura K, Nakao A, Fujii T, Yamada S, Sugimoto H, Kasuya H, Nomoto S, Kodera Y, Nakamura S, Morita S, et al. Clinicopathologic study of intrapancreatic cancer spread in carcinoma of the body and tail of the pancreas. Pancreas. 2012;41:753–758. doi: 10.1097/MPA.0b013e31823b12a4. [DOI] [PubMed] [Google Scholar]
- 47.Nakao A, Fujii T, Sugimoto H, Kanazumi N, Nomoto S, Kodera Y, Inoue S, Takeda S. Oncological problems in pancreatic cancer surgery. World J Gastroenterol. 2006;12:4466–4472. doi: 10.3748/wjg.v12.i28.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanda M, Fujii T, Sahin TT, Kanzaki A, Nagai S, Yamada S, Sugimoto H, Nomoto S, Takeda S, Kodera Y, et al. Invasion of the splenic artery is a crucial prognostic factor in carcinoma of the body and tail of the pancreas. Ann Surg. 2010;251:483–487. doi: 10.1097/SLA.0b013e3181cf9171. [DOI] [PubMed] [Google Scholar]
- 49.Hirano S, Kondo S, Hara T, Ambo Y, Tanaka E, Shichinohe T, Suzuki O, Hazama K. Distal pancreatectomy with en bloc celiac axis resection for locally advanced pancreatic body cancer: long-term results. Ann Surg. 2007;246:46–51. doi: 10.1097/01.sla.0000258608.52615.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okada K, Kawai M, Tani M, Hirono S, Miyazawa M, Shimizu A, Kitahata Y, Yamaue H. Surgical strategy for patients with pancreatic body/tail carcinoma: who should undergo distal pancreatectomy with en-bloc celiac axis resection. Surgery. 2013;153:365–372. doi: 10.1016/j.surg.2012.07.036. [DOI] [PubMed] [Google Scholar]
- 51.Giulianotti PC, Addeo P, Buchs NC, Ayloo SM, Bianco FM. Robotic extended pancreatectomy with vascular resection for locally advanced pancreatic tumors. Pancreas. 2011;40:1264–1270. doi: 10.1097/MPA.0b013e318220e3a4. [DOI] [PubMed] [Google Scholar]
- 52.Boggi U, Signori S, De Lio N, Perrone VG, Vistoli F, Belluomini M, Cappelli C, Amorese G, Mosca F. Feasibility of robotic pancreaticoduodenectomy. Br J Surg. 2013;100:917–925. doi: 10.1002/bjs.9135. [DOI] [PubMed] [Google Scholar]
- 53.Kendrick ML, Sclabas GM. Major venous resection during total laparoscopic pancreaticoduodenectomy. HPB (Oxford) 2011;13:454–458. doi: 10.1111/j.1477-2574.2011.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang CM, Hwang HK, Choi SH, Lee WJ. Controversial issues of neoadjuvant treatment in borderline resectable pancreatic cancer. Surg Oncol. 2013;22:123–131. doi: 10.1016/j.suronc.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Chang YR, Han SS, Park SJ, Lee SD, Yoo TS, Kim YK, Kim TH, Woo SM, Lee WJ, Hong EK. Surgical outcome of pancreatic cancer using radical antegrade modular pancreatosplenectomy procedure. World J Gastroenterol. 2012;18:5595–5600. doi: 10.3748/wjg.v18.i39.5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamamoto J, Saiura A, Koga R, Seki M, Katori M, Kato Y, Sakamoto Y, Kokudo N, Yamaguchi T. Improved survival of left-sided pancreas cancer after surgery. Jpn J Clin Oncol. 2010;40:530–536. doi: 10.1093/jjco/hyq015. [DOI] [PubMed] [Google Scholar]
- 57.Takahashi S, Kinoshita T, Konishi M, Gotohda N, Kato Y, Kinoshita T, Kobayashi T, Mitsunaga S, Nakachi K, Ikeda M. Borderline resectable pancreatic cancer: rationale for multidisciplinary treatment. J Hepatobiliary Pancreat Sci. 2011;18:567–574. doi: 10.1007/s00534-011-0371-z. [DOI] [PubMed] [Google Scholar]
- 58.Stokes JB, Nolan NJ, Stelow EB, Walters DM, Weiss GR, de Lange EE, Rich TA, Adams RB, Bauer TW. Preoperative capecitabine and concurrent radiation for borderline resectable pancreatic cancer. Ann Surg Oncol. 2011;18:619–627. doi: 10.1245/s10434-010-1456-7. [DOI] [PubMed] [Google Scholar]
- 59.Kang CM, Chung YE, Park JY, Sung JS, Hwang HK, Choi HJ, Kim H, Song SY, Lee WJ. Potential contribution of preoperative neoadjuvant concurrent chemoradiation therapy on margin-negative resection in borderline resectable pancreatic cancer. J Gastrointest Surg. 2012;16:509–517. doi: 10.1007/s11605-011-1784-3. [DOI] [PubMed] [Google Scholar]


