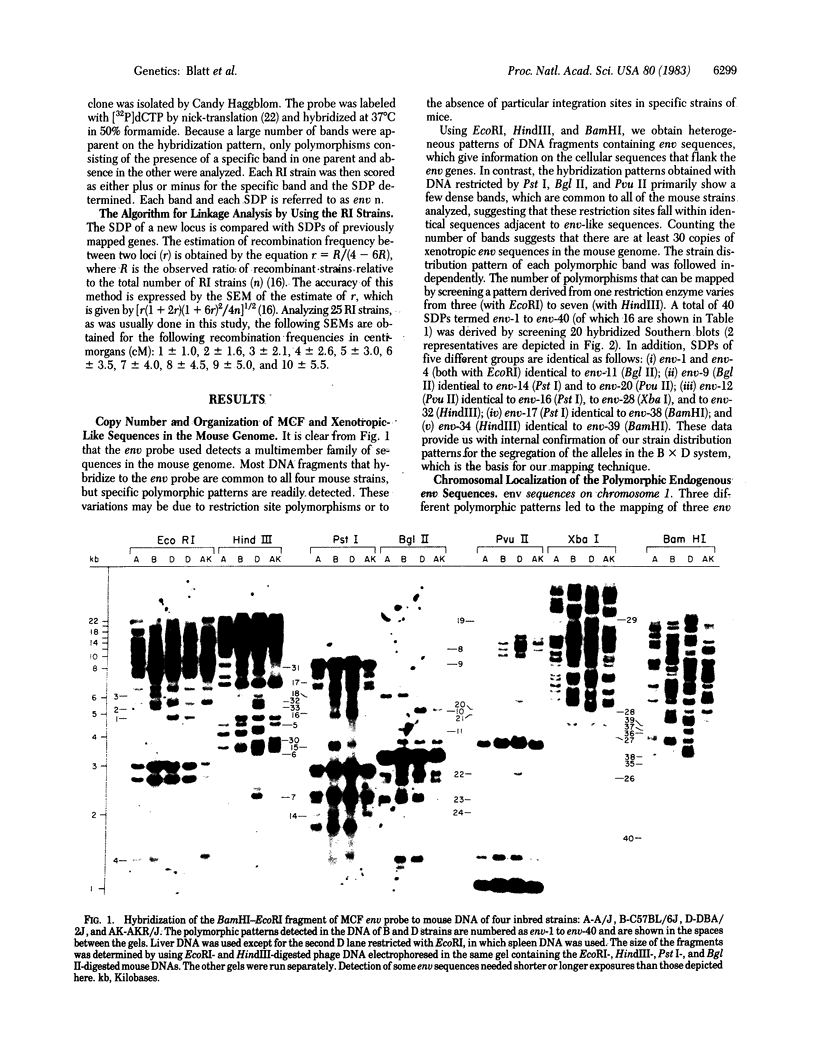
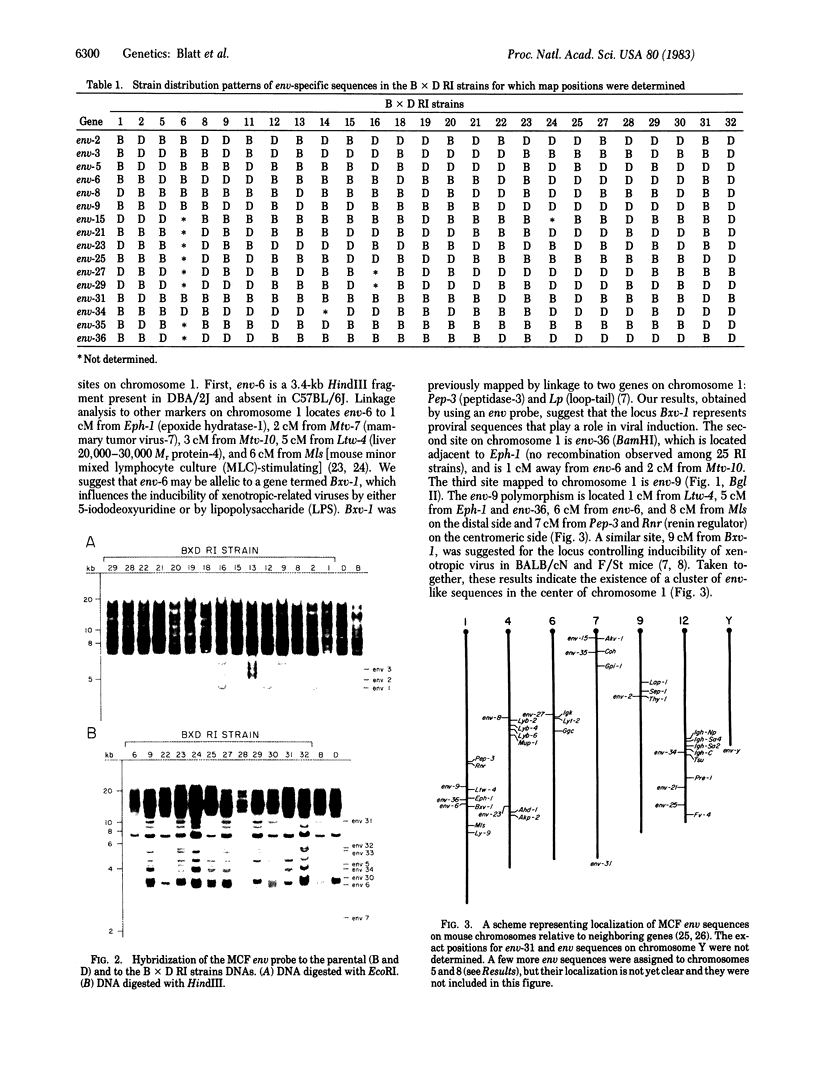
Abstract
Chromosomal locations of members of the xenotropic-related env gene family in the mouse genome have been determined. Endonuclease restriction site polymorphisms detected by molecular hybridization were used to study the inheritance of mink cell-focus inducing and xenotropic env gene-related sequences in recombinant inbred strains of mice. Some of the endogenous env sequences appear to be closely linked to genes determining leukemia virus induction and to genes involved in the immune response, such as the heavy and light chains of the immunoglobulin molecules or allotypic determinants on B and T lymphocytes. The use of probes that detect restriction fragment length polymorphisms in a small family of dispersed sequences promises to yield a large number of markers that can be used together with recombinant inbred strains for efficient mapping of the mouse genome.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey D. W. Recombinant-inbred strains. An aid to finding identity, linkage, and function of histocompatibility and other genes. Transplantation. 1971 Mar;11(3):325–327. doi: 10.1097/00007890-197103000-00013. [DOI] [PubMed] [Google Scholar]
- Bosselman R. A., van Straaten F., Van Beveren C., Verma I. M., Vogt M. Analysis of the env gene of a molecularly cloned and biologically active Moloney mink cell focus-forming proviral DNA. J Virol. 1982 Oct;44(1):19–31. doi: 10.1128/jvi.44.1.19-31.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calame K., Kim S., Lalley P., Hill R., Davis M., Hood L. Molecular cloning of translocations involving chromosome 15 and the immunoglobulin C alpha gene from chromosome 12 in two murine plasmacytomas. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6994–6998. doi: 10.1073/pnas.79.22.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Cloyd M. W., Linemeyer D. L., Lander M. R., Rands E., Lowy D. R. Cellular origin and role of mink cell focus-forming viruses in murine thymic lymphomas. Nature. 1982 Jan 7;295(5844):25–31. doi: 10.1038/295025a0. [DOI] [PubMed] [Google Scholar]
- Chien Y. H., Verma I. M., Shih T. Y., Scolnick E. M., Davidson N. Heteroduplex analysis of the sequence relations between the RNAs of mink cell focus-inducing and murine leukemia viruses. J Virol. 1978 Oct;28(1):352–360. doi: 10.1128/jvi.28.1.352-360.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas T. C., Dawson P. E. Location of the gene for theta antigen in the mouse. III. The position of Thy-1 relative to Lap-1 and Mpi-1. J Hered. 1979 Jul-Aug;70(4):250–254. doi: 10.1093/oxfordjournals.jhered.a109248. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Gautsch J. W., Jensen F. C., Lerner R. A., Hartley J. W., Rowe W. P. Biochemical evidence that MCF murine leukemia viruses are envelope (env) gene recombinants. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4676–4680. doi: 10.1073/pnas.74.10.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Jensen F. C., Bryant M. L., Lerner R. A. Polymorphism of the major envelope glycoprotein (gp70) of murine C-type viruses: virion associated and differentiation antigens encoded by a multi-gene family. Nature. 1977 May 5;267(5606):23–28. doi: 10.1038/267023a0. [DOI] [PubMed] [Google Scholar]
- Hara I., Izui S., McConahey P. J., Elder J. H., Jensen F. C., Dixon F. J. Induction of high serum levels of retroviral env gene products (gp70) in mice by bacterial lipopolysaccharide. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4397–4401. doi: 10.1073/pnas.78.7.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggan M. D., Buckler C. E., Sears J. F., Rowe W. P., Martin M. A. Organization and stability of endogenous xenotropic murine leukemia virus proviral DNA in mouse genomes. J Virol. 1983 Jan;45(1):473–477. doi: 10.1128/jvi.45.1.473-477.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Joseph D. R., Domotor J. J., Jr Genetic linkage of C3H/HeJ and BALB/c endogenous ecotropic C-type viruses to phosphoglucomutase-1 on chromosome 5. Science. 1979 Apr 6;204(4388):71–73. doi: 10.1126/science.219476. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Dilute (d) coat colour mutation of DBA/2J mice is associated with the site of integration of an ecotropic MuLV genome. Nature. 1981 Oct 1;293(5831):370–374. doi: 10.1038/293370a0. [DOI] [PubMed] [Google Scholar]
- Kemp M. C., Famulari N. G., Compans R. W. Glycoproteins of murine leukemia viruses. III. Glycosylation of env precursor glycoproteins. J Virol. 1981 Aug;39(2):463–470. doi: 10.1128/jvi.39.2.463-470.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C. A., Rowe W. P. Genetic mapping of xenotropic murine leukemia virus-inducing loci in five mouse strains. J Exp Med. 1980 Jul 1;152(1):219–228. doi: 10.1084/jem.152.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni C., Matter A., Stoye J. P., Monckton R. P., Delamarter J. F., Schumann G. Concanavalin A promotes bromodeoxyuridine induction of enodgenous C-Type virus in B cells. Cell Immunol. 1980 Aug 15;54(1):107–114. doi: 10.1016/0008-8749(80)90194-x. [DOI] [PubMed] [Google Scholar]
- Morse H. C., 3rd, Kozak C. A., Yetter R. A., Hartley J. W. Unique features of retrovirus expression in F/St mice. J Virol. 1982 Jul;43(1):1–7. doi: 10.1128/jvi.43.1.1-7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen F. L., Riblet R., Taylor B. A. The T suppressor cell alloantigen Tsud maps near immunoglobulin allotype genes and may be an heavy chain constant-region marker on a T cell receptor. J Exp Med. 1981 Apr 1;153(4):801–810. doi: 10.1084/jem.153.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen F. L., Spurll G. M., Panageas E. Tthyd, a new thymocyte alloantigen linked to Igh-1. Implications for a switch mechanism for T cell antigen receptors. J Exp Med. 1982 Jan 1;155(1):52–60. doi: 10.1084/jem.155.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechavi G., Givol D., Canaani E. Activation of a cellular oncogene by DNA rearrangement: possible involvement of an IS-like element. Nature. 1982 Dec 16;300(5893):607–611. doi: 10.1038/300607a0. [DOI] [PubMed] [Google Scholar]
- Roman J. M., Hirsch J., Readhead C., Levy D., DeOgny L., Dreyer W. J. Heritable differences among gp70-like molecules on C3H ultraviolet light-induced fibrosarcomas. Transplant Proc. 1981 Dec;13(4):1782–1786. [PubMed] [Google Scholar]
- Rommelaere J., Faller D. V., Hopkins N. Characterization and mapping of RNase T1-resistant oligonucleotides derived from the genomes of Akv and MCF murine leukemia viruses. Proc Natl Acad Sci U S A. 1978 Jan;75(1):495–499. doi: 10.1073/pnas.75.1.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Hartley J. W., Bremner T. Genetic mapping of a murine leukemia virus-inducing locus of AKR mice. Science. 1972 Nov 24;178(4063):860–862. doi: 10.1126/science.178.4063.860. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taub R., Kirsch I., Morton C., Lenoir G., Swan D., Tronick S., Aaronson S., Leder P. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7837–7841. doi: 10.1073/pnas.79.24.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. A., Bailey D. W., Cherry M., Riblet R., Weigert M. Genes for immunoglobulin heavy chain and serum prealbumin protein are linked in mouse. Nature. 1975 Aug 21;256(5519):644–646. doi: 10.1038/256644a0. [DOI] [PubMed] [Google Scholar]
- Traina V. L., Taylor B. A., Cohen J. C. Genetic mapping of endogenous mouse mammary tumor viruses: locus characterization, segregation, and chromosomal distribution. J Virol. 1981 Dec;40(3):735–744. doi: 10.1128/jvi.40.3.735-744.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung J. S., Vitetta E. S., Fleissner E., Boyse E. A. Biochemical evidence linking the GIX thymocyte surface antigen to the gp69/71 envelope glycoprotein of murine leukemia virus. J Exp Med. 1975 Jan 1;141(1):198–205. doi: 10.1084/jem.141.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetter R. A., Hartley J. W., Morse H. C., 3rd H-2-linked regulation of xenotropic murine leukemia virus expression. Proc Natl Acad Sci U S A. 1983 Jan;80(2):505–509. doi: 10.1073/pnas.80.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]