Abstract
AIM: To investigate whether palatable sweet foods have a beneficial effect on chronic stress-induced colonic motility and inflammatory cytokines.
METHODS: Adult male rats were divided into 3 groups: control (CON, n = 5), chronic variable stress with chow (CVS-A, n = 6), and chronic variable stress with chow and sweet food (CVS-B, n = 6). The rats were fed standard rodent chow as the chow food and/or AIN-76A as the sweet food. A food preference test for AIN-76A was performed in another group of normal rats (n = 10) for twelve days. Fecal pellet output (FPO) was measured for 6 wk during water bedding stress in the CVS groups. The weight of the adrenal glands, adrenocorticotropic hormone (ACTH) and corticosterone levels in plasma were measured. The expression levels of transforming growth factor-β, interleukin (IL)-2, and interferon-gamma (IFN-γ) were measured in the distal part of colonic tissues and plasma using Western blot analysis.
RESULTS: In sweet preference test, all rats initially preferred sweet food to chow food. However, the consumption rate of sweet food gradually decreased and reduced to below 50% of total intake eight days after sweet food feeding. Accumulated FPO was higher in the CVS-A group compared with the CVS-B group over time. All stress groups showed significant increases in the adrenal to body weight ratio (CVS-A, 0.14 ± 0.01; CVS-B, 0.14 ± 0.01) compared with the control group (0.12 ± 0.01, P < 0.05). The plasma corticosterone and ACTH levels were significantly higher in the CVS-A (537.42 ± 32.95, 44.44 ± 6.54 pg/mL) and CVS-B (655.07 ± 30.82, 65.46 ± 4.44 pg/mL) groups than in the control group (46.96 ± 13.29, 8.51 ± 1.35 pg/mL, P < 0.05). Notably, the ratio of corticosterone to ACTH was significantly increased in the CVS-A group only. Rats exposed to CVS displayed significantly increased expression of IL-2 and IFN-γ in the plasma and distal colon compared to the control group, whereas this effect was significantly attenuated in the CVS-B group.
CONCLUSION: These results suggest that concurrent sweet food ingestion during CVS might have an effect on the reduction of stress-induced colonic hyper-motility and pro-inflammatory cytokine production in rats.
Keywords: Irritable bowel syndrome, Colon, Stress, Adrenal hormones, Cytokines, Rat
Core tip: Stress has an important role in the pathogenesis of irritable bowel syndrome (IBS), and palatable foods have been used as an ameliorator for psychological stress. Several reports have supported the hypothesis that palatable foods are used for consolation from psychological stress. Thus we hypothesized that hypothalamic-pituitary-adrenal axis function and immune status in the plasma and colon could be altered by chronic stress, and these changes could be attenuated by concurrent ingestion of sweet food. These results imply that reducing the effect of stress appropriately by any means is important for preventing induces gastrointestinal symptoms in a patient with IBS.
INTRODUCTION
It is generally accepted that psychological stress induces gastrointestinal (GI) symptoms, including dyspepsia, abdominal pain and increased colonic motility[1]. Stress has an important role in the pathogenesis of irritable bowel syndrome (IBS) as a risk, trigger and perpetuating factor[2]. Although the underlying pathogenesis of IBS still remains unclear, dysregulation of the brain-gut-axis[3] and low grade GI inflammation have recently received attention as potential causes[4].
A patient with IBS exhibits over-activation of the hypothalamic-pituitary-adrenal (HPA) axis to corticotropin-releasing factor (CRF) stimulation or visceral stimulation and exhibits increased pro-inflammatory cytokines in the blood[5,6]. There has been growing evidence regarding the association between IBS and low grade inflammation, such as increased expression of immune and mast cells[7,8] cytokine imbalance in the mucosa[9], and elevated circulating levels of the pro-inflammatory cytokine in IBS patients[10]. Concerning the function of cortisol, such as its role in reducing the number of leukocytes[11] and suppressing the release of interferon-gamma (IFN-γ)[12] and interleukin (IL)-2[13], it can be assumed that stress and the associated HPA axis dysfunction act in concert to contribute to the alteration of GI immune function in the pathogenesis of IBS.
Eating behavior is closely related to stress, and some people preferentially consume palatable food such as chocolate or ice cream during stressful situations[14]. Several reports have supported the hypothesis that palatable foods are used for consolation from psychological stress through the stabilization of CRF in the hypothalamus[15-17]. Therefore, it appears that the consumption of sweet food has beneficial effects on stress-induced physiologic dysfunction. However, it has never been reported whether eating sweet food has an effect on the disordered GI motility and immune status in chronically stressed rats.
In the present study, we hypothesized that the HPA axis function and the immune status in the plasma and colon could be altered by chronic stress, and these changes could be attenuated by concurrent ingestion of sweet food. Therefore, the aim of this study was to investigate the effect of the concurrent eating of sweet food on colonic motility, HPA axis status, and the levels of inflammatory cytokines in the plasma and colon in chronic variable stress (CVS) rats.
MATERIALS AND METHODS
Animals and study design
Animal use protocols (No. WKU09-112) were approved by the Institutional Guidelines of the Committee on Animal Research at the Wonkwang University, and all efforts were taken to minimize animal suffering and to reduce the number of animals necessary according to the guideline recommendations. Seventeen male Sprague-Dawley rats purchased from Samtaco Co., Ltd. (Pyeongtaek, South Korea), weighing approximately 270 g each, were housed in individual cages for ten days for acclimatization. Rats were provided ad libitum access to food and water in 12 h/12 h light-dark cycles at 24 °C.
The animals were randomly divided into 3 groups: no stress with chow food (CON, n = 5), CVS with chow food (CVS-A, n = 6), and CVS with chow + sweet food (CVS-B, n = 6).
Supplied food and preference test
We used commercial standard rodent chow (60% carbohydrate, 20% protein, 4.5% fat, Purina Mills Inc., St. Louis, MO, United States) as the chow food and AIN-76A (66% carbohydrate, 20% protein, 5% fat, Research Diets Inc., New Brunswick, United States) as the sweet food. Not only does AIN-76A contain a higher carbohydrate percentage, but the source of carbohydrate is also sucrose, which is sweeter than glucose, dextrose and lactose. AIN-76A contains more sucrose than any other purified food (AIN-93G, AIN-93M) or cereal-based chow food. Before the stress experiment, a food preference test for AIN-76A was performed in another group of normal rats (n = 10) for twelve days. To diminish neophobia to the novel foods, CVS-B rats were exposed to AIN-76A in advance for 3 d before the experiment.
CVS protocol
The CVS protocol used in this study was modified from the previous study[18]. This protocol was demonstrated to induce visceral hypersensitivity and is suggested as an IBS animal model[18]. The weekly protocol consisted of placement in a small cage (confinement), water bedding, exposure to white noise, a stroboscope, light at night (illumination), and irregular vibration (each rat cage was placed on a wooden panel and a vibration device was contacting the panel). Each period of exposure to the stressors lasted 2 to 16 h each week (Table 1). Fecal pellet output (FPO) was measured 3 times per week during the water bedding stress session of the CVS protocol to investigate stress-induced colonic motility changes. For the water bedding stress, rats in the CVS group were placed in the empty cage with only room temperature water for 1 h in the morning of Monday, Wednesday, and Friday each week. The height of the water was approximately 1-2 cm, enough to cover their feet. Fecal pellets found in the cage were counted at the end of each water bedding stress session.
Table 1.
Experimental schedule for chronic variable stress
| Schedule | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday |
| 09:00-12:00 | Water bedding | Vibration | Water bedding | Confinement | Water bedding | Vibration |
| (1 h) | (3 h) | (1 h) | (2 h) | (1 h) | (3 h) | |
| 12:00-18:00 | Confinement | |||||
| (2 h) | ||||||
| 18:00-06:00 | Stroboscope | Illumination | White noise | Stroboscope | Illumination | |
| (1 h) | (12 h) | (15 h) | (15 h) | (12 h) |
Measurement of adrenal weight and radioimmunoassay of adrenocorticotropic hormone and corticosterone
All rats were killed by decapitation and blood samples were obtained. The wet weight of adrenal glands was measured. Plasma adrenocorticotropic hormone (ACTH) and corticosterone levels were measured with a commercially available kit (ELSA-ACTH, CIS Bio international, Gif-sur-Yvette Cedex, France; Coat-a-Count rat corticosterone, Diagnostic Product Cooperation, LA, CA, United States) by radioimmunoassay (RIA). The sensitivity of the assay was 2.0 pg/mL for ACTH and 5.7 ng/mL for corticosterone. The intra-/interassay coefficient of variation was 5.3%/6.1% for ACTH and 12.2%/14.9% for corticosterone.
Western blot analysis
The expression levels of transforming growth factor-β (TGF-β), IL-2, and IFN-γ were measured in the distal part of colonic tissues and plasma using western blot analysis. The distal parts of the colonic tissues were washed with Tris buffered saline (TBS), and approximately 5 g of tissue was added to lysis buffer [25 mmol/L Tris-Cl, 1 mmol/L ethylene glycol bis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, 1 mmol/L dithiothreitol, 0.1% Triton X-100, protease inhibitor cocktail, and phosphatase inhibitor cocktail; pH 7.4] and homogenized. The samples were then centrifuged at 12000 g for 30 min, and the cytosolic fractions were obtained. Whole blood samples were centrifuged at 3000 r/min for 15 min, and the plasma was obtained and diluted with TBS. The protein concentration of both samples from the plasma and colonic tissues was estimated using bovine serum albumin as a standard and adjusted to examine identical amounts of total protein. The sample buffer was added to the samples, the mixture was boiled at 100 °C for 5 min, and electrophoresis was performed at 100 V for 2 h using a minigel electrophoresis apparatus (mini-PROTEIN Tetra cell; Bio-Rad Laboratories, Inc., Hercules, CA, United States). After electrophoresis, the gels were stained with Coomassie brilliant blue R-250 for 1 h. Following staining, the samples were destained with 10% acetic acid and 10% methanol, and the protein bands were observed. The proteins were transferred to 0.45 μm polyvinylidenedifluoride membranes (Roche Diagnostics GmbH, Mannheim, Germany) using a protein transfer apparatus (mini Transblot cell; Bio-Rad Laboratories) at 100 V for 90 min. To prevent non-specific binding of the primary antibody, the polyvinylidenedifluoride membranes were incubated with blocking buffer (5% skim milk in Tris buffer saline with 0.05% tween 20 (TBST, pH 7.6) for 1 h. The membranes were washed 3 times with TBST and incubated overnight at 4 °C with primary antibodies (TGF-β, IL-2, and IFN-γ) diluted to 1:1000 with TBS containing 3% bovine serum albumin. The membranes were then washed 4 times with TBST. Next, the membranes were incubated for 1 h with a horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (Enzo Life Science International Inc., Plymouth Meeting, PA) (1:5000). The membranes were washed 4 times with TBST, incubated with Immobilon Western chemiluminescent horseradish peroxidase substrate (Millipore., MA, United States), and exposed to a chemiluminescence film in a dark room. The expression levels of TGF-β, IL-2, and IFN-γ were compared. In addition, as a control experiment, double staining for glyceraldehyde-3-phosphate dehydrogenase was performed with TGF-β, IL-2, and IFN-γ under identical conditions.
Statistical analysis
All values are represented as the mean ± SE. Statistical analyses were performed out with SPSS software (V.18.0 SPSS Inc., Chicago, IL, United States). One-way analysis of variance (ANOVA) was performed, followed by Bonferroni post-hoc tests to analyze changes in all parameters between all groups. For comparing fecal pellet output between the CVS-A and CVS-B groups, the data were analyzed using repeated measures ANOVA and unpaired Student’s t tests. Null hypotheses of no differences were rejected if P values were less than 0.05.
RESULTS
Sweet food preference test
All rats initially preferred sweet food to chow food at first and some rats even consumed 100% sweet food. However, the consumption rate of sweet food was gradually decreased and reduced to below 50% of total intake eight days after sweet food feeding (Figure 1).
Figure 1.
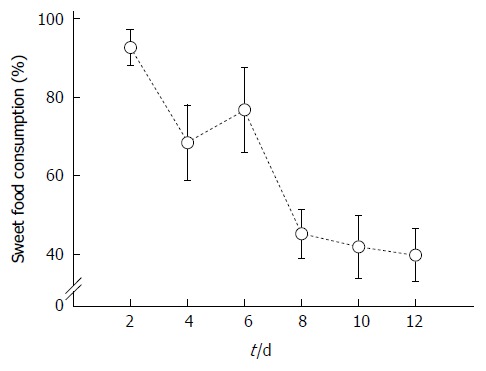
Time-dependent changes of sweet food consumption in normal rats for 12 d. The percentage of sweet food consumption decreased to below 50% 8 d after sweet food feeding.
Changes in body weight and food intake
At the beginning of the stress experiment, no significant differences were observed in body weight between all groups (CON, 292.50 ± 5.16 g; CVS-A, 291.08 ± 4.73 g; CVS-B, 292.07 ± 2.06 g). However, one week after beginning the stress protocol, there was a significant difference in body weight and food intake between the CON and other groups (Figure 2A, B).
Figure 2.
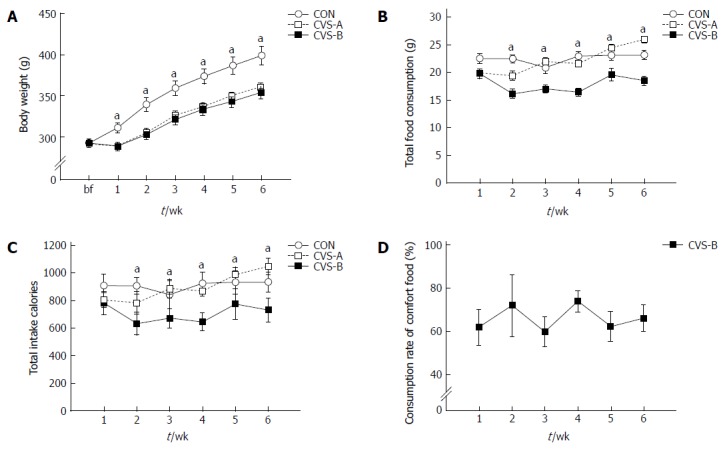
Time-dependent changes of body weight (A), food consumption (B), caloric efficiency (C), and consumption rate of sweet food (D) in each group for 6 wk. There were significant differences in body weight between control and treated groups. Total food consumption and caloric intake were significantly reduced in the chronic variable stress-B (CVS-B) group only. Denotes significant differences among groups based on one-way analysis of variance and the Bonferroni test (aP < 0.05 vs other groups).
The body weight (BW) of the stress groups (CVS-A, 361.08 ± 4.77 g; CVS-B, 354.13 ± 7.76 g) were significantly lower than that of the control group (399.22 ± 11.12 g) throughout the remainder of the experimental period (Figure 2A, P < 0.05). However, there was no difference in BW between the CVS-A and CVS-B groups (Figure 2A). From the second week onward, the amount of consumed food and total calorie intake became significantly lower in the CVS-B group compared with the other groups (Figure 2B, C).
In contrast to the result of the preferential test in normal rats, the total consumption rate of sweet food was maintained between 60% and 80% in the CVS-B group until the end of the experimental period (Figure 2D).
Accumulated FPO
The CVS-B group barely defecated except during the first week, whereas the CVS-A group continuously expelled fecal pellets during water bedding throughout the entire experimental period. As a result, there was a significant difference in the accumulated FPO between the CVS-A and CVS-B groups from the second week onward (Figure 3). Data were analyzed with repeated-measures ANOVA. Mauchly’s test of sphericity was significant, and therefore, the Greenhouse-Geisser correction was used. The repeated measures ANOVA revealed highly significant differences in FPO between groups [F (1, 10) = 6.989, P = 0.025] and time [F (1, 10) = 10.657, P = 0.003)] on water bedding stress. The results also demonstrated a significant interaction between group and time [F (1, 10) = 5.154, P = 0.028], thus indicating that the CVS-A rats increased FPO differently than the CVS-B rats (Figure 3).
Figure 3.
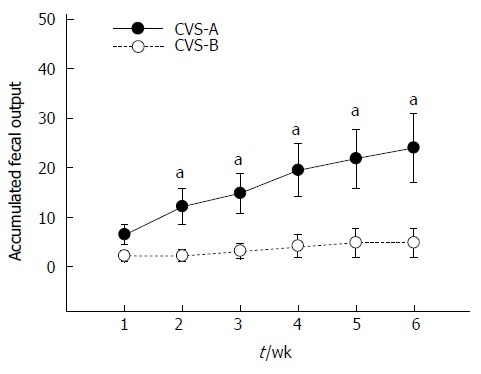
Accumulated fecal pellet output in the chronic variable stress-A and chronic variable stress-B groups. There was no significant difference in the first week, but accumulated fecal output difference gradually increased with time. Statistical analysis was performed using repeated measures ANOVA and unpaired t test in each week between groups (aP < 0.05 vs chronic variable stress (CVS)-B group).
Adrenal gland weight and plasma corticosterone and ACTH levels
The adrenal weight-to-BW ratio was significantly higher in the stress groups (CVS-A, 0.14 ± 0.01; CVS-B, 0.14 ± 0.01) than in the control group (0.12 ± 0.01) (Figure 4A, P < 0.05).
Figure 4.
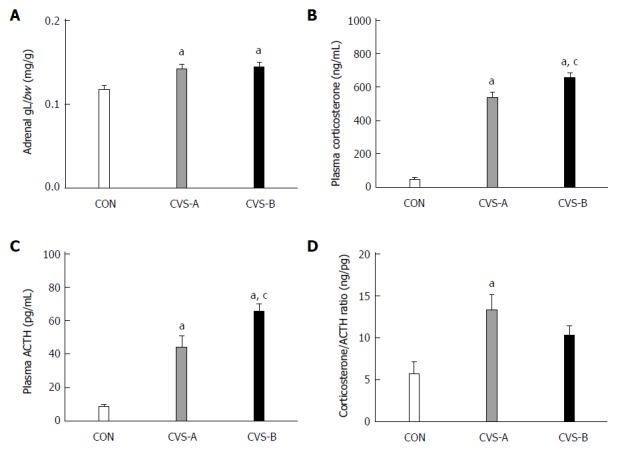
Relative weight of the adrenal gland (A), plasma corticosterone levels (B), ACTH levels (C), and the ratio of corticosterone/ACTH following the 6-wk stress experiment. Denotes significant difference between control and other groups (aP < 0.05 vs control groups). There was a significant difference in plasma corticosterone and adrenocorticotropic hormone (ACTH) concentrations between CON and the other groups. Denotes significant difference between chronic variable stress (CVS)-A and CVS-B group (cP < 0.05 vs CVS-A groups). There was a significant difference in plasma corticosterone and ACTH concentration between CVS-A and CVS-B groups. CVS: Chronic variable stress.
The plasma corticosterone and ACTH levels were significantly higher in the CVS-A (537.42 ± 32.95, 44.44 ± 6.54 pg/mL) and CVS-B (655.07 ± 30.82, 65.46 ± 4.44 pg/mL) groups than in the control group (46.96 ± 13.29, 8.51 ± 1.35 pg/mL) (Figure 4B, C, P < 0.05). Notably, the ratio of corticosterone to ACTH was significantly increased in the CVS-A group only (Figure 4D, P < 0.05).
Western blot analysis of anti- and pro-inflammatory cytokines in the plasma and the distal colon
There was no difference in TGF-β expression in the plasma and colon between all groups. Rats exposed to CVS displayed significantly increased expression of IL-2 and IFN-γ in the plasma and distal colon compared to the control group, whereas this effect was significantly attenuated in the CVS-B group (Figure 5, P < 0.05).
Figure 5.
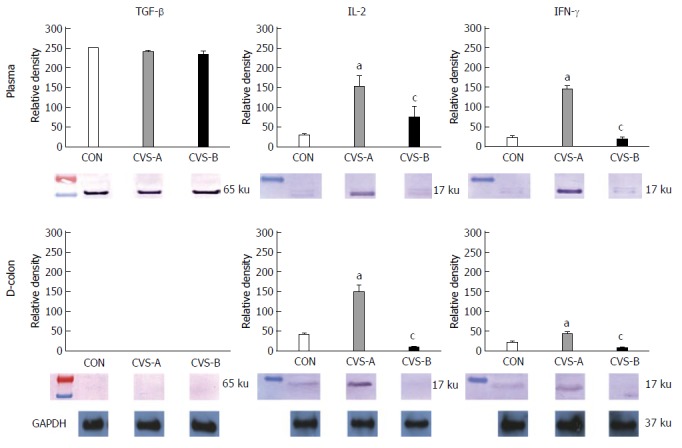
Inflammatory cytokine expressions in the plasma and in the distal colon by western blot analysis following the 6 wk stress experiment. Note the prominent expressions of interleukin-2 (IL-2) and interferon-γ (IFN-γ) in the distal colon and in the plasma in the chronic variable stress (CVS)-A group only. aDenotes significant difference between control and other groups (aP < 0.05). There was a significant difference in the relative density of IL-2 and IFN-γ in the plasma and distal colon between CON and the other groups. bDenotes significant difference between CVS-A and CVS-B group (cP < 0.05 vs CVS-A groups). There was also a significant difference in the relative density of IL-2 and IFN-γ in the plasma and distal colon between CVS-A and CVS-B groups.
DISCUSSION
In humans, chronic stress can induce increased comfort food intake[14]. In addition, chronically stressed animals increase their ingestion of palatable food when given a choice of highly palatable food, such as lard or sugar[15,17,19] possibly decreasing the stress response in the HPA axis[17].
Therefore, we hypothesized that eating sweet food might affect the HPA axis and reduce chronic stress effects through CRF stabilization in the hypothalamus, with beneficial effects on abnormal changes of colonic motility and colonic inflammatory cytokine profiles in rats. The main findings of this study on concurrent sweet food ingestion under chronic stress are that (1) the stress-induced FPO increment reflecting increased colonic motility was reduced; (2) the corticosterone/ACTH ratio was reduced to control levels; and (3) the pro-inflammatory cytokines increased by CVS were attenuated.
Preference for sweet food
The consumption rate of sweet food in the normal rats gradually decreased and reduced below 50% of total intake eight days after sweet food feeding. In contrast to normal rats, the consumption rate of sweet food ranged from 60% to 80% of total intake in stressed rats through the entire experimental period without decrement. The decrement of sucrose intake observed in normal rats in this study may be related to the concomitant increment of insulin and leptin levels with increasing adiposity[20].
A plausible explanation for the increased ingestion of sweet food in the CVS rats may be related to the reduction of anxiety through the activation of the reward system. It has also been suggested that this preference is likely because of a higher level of anxiety in stressed rats because the preference for sweet food in rats under restraint stress could be reversed by diazepam[17]. In addition, eating palatable food, as with drug abuse, can activate the brain reward system, comprised of opioid, dopamine, and endocannabinoid[21].
Accumulated FPO
The monitoring of FPO is used to measure stress response in animal experiments[22,23]. We quantified FPO during water bedding stress in the present study to demonstrate the effect of sweet food on stress-induced colonic motility. Cumulative FPO was significantly higher in the CVS-A group compared with the CVS-B group throughout the entire experimental period except for the first week.
Psychological stress activates some areas in the CNS including the paraventricular nucleus (PVN) and induces rapid transcription of the gene encoding CRF in the PVN, resulting in the stimulation of the sacral parasympathetic nucleus, which innervates the descending colon[1]. Foster et al[24] recently demonstrated that chronic access to sucrose or lard prior to the stress period significantly decreased CRF mRNA expression in the PVN after restraint stress in rats. Based on their report, we can speculate that sweet food might decrease CRF production in the PVN during stress periods and consequently prevent stress-induced colonic hyper-motility. The evidence across these studies leads to the interpretation that the FPO difference between the CVS-A and CVS-B groups in this study may result from a decreased level of CRF in the PVN through the effect of sweet food.
Alteration of the HPA axis
Previous reports regarding alterations of the HPA axis in IBS patients are not consistent because of differing methodologies, patient populations, sexes, and comorbid psychiatric conditions[6]. Additionally, the alteration of ACTH and corticosterone depends on the mode and duration of stress[25]. In our study, both of the CVS groups displayed increased ACTH and corticosterone levels. Because FPO, the well-known stress response, was decreased by sweet food intake in the CVS-B group, we expected a relatively normal function of the HPA axis in the CVS-B group compared with the CVS-A group. Contrary to our expectations, the levels of ACTH and corticosterone were higher in the CVS-B group compared with the CVS-A group. However, the ratio of corticosterone/ACTH increased in the CVS-A group, but was not significantly different between the CVS-B and control groups. Chang et al[6] recently reported that basal levels of plasma ACTH significantly decreased while the level of cortisol tended to increase in IBS patients using 24 h blood sampling. This finding might be interpreted as enhanced adrenocortical sensitivity to ACTH and is similar to the corticosterone/ACTH ratio of the CVS-A group in our experiment. In depressive patients, plasma ACTH responses to stress and CRF administration are blunted, whereas plasma cortisol responses are normal or augmented[26,27], suggesting down-regulation or desensitization of CRF receptors in the pituitary glands and adrenal hyper-responsiveness to ACTH[28,29]. Thus, the higher corticosterone/ACTH ratio in the CVS-A group could be explained by enhanced adrenocortical sensitivity to ACTH or down-regulation of the CRF receptor at the hypothalamus by the high CRF milieu in the CNS, resulting in relatively low ACTH levels[30,31]. The last assumption is supported by the stabilization of FPO during water bedding stress in the CVS-B group in our study and the important role of CRF in the increased colonic motility induced by stress.
Inflammatory cytokines
Corticosterone (or glucocorticoids), the end products of HPA axis activity, inhibits lymphocyte proliferation and cytotoxicity and the secretion of TNFα, IL-2, and IFN-γ[13,14]. Corticosterone also enhances the synthesis of TGF-β, another cytokine with potent anti-inflammatory activities in human T cells[32] and increases the secretion of IL-10 and IL-4 that aid in the anti-inflammatory reaction[33-35].
In this study, to investigate the difference of cytokine expression in each group, Western blot of plasma IL-2, IFN-γ and TGF-β were performed in the plasma and distal segment of the colon. Although there was no difference in TGF-β among all groups, there was an observable increase of IL-2 and INF-γ levels in both the plasma and distal colon in the CVS-A group only. The CVS-A group, exposed to CVS regarded as unpredictable and non-adaptable stress with chow food, displayed an increase in pro-inflammatory cytokines that mediate inflammation, implying that corticosterone did not work properly in the CVS-A group.
Corticosterone is responsible for many quantitative and qualitative changes in immune function. The greatest effect of stress on the immune system is related to the suppression of immune functions and the exacerbation of diseases such as asthma, allergic, autoimmune and inflammatory diseases[36-38]. IBS is characterized as increasing HPA activity and the production of pro-inflammatory cytokines in human[5] and animal models[39]. Notably, previous studies have demonstrated that INF-γ increases in the rat colon and blood due to repeated water avoidance stress[39] or maternal separation[40], commonly used stress protocols for IBS animal studies[41]. The CVS-A group in our study also displayed increased INF-γ levels in both the plasma and colonic tissue. It has been suggested that the CRF-induced secretion of cortisol in IBS patients could be linked to the coincident increase in cytokine release and an associated decrease in the sensitivity of glucocorticoid receptors[5]. Thus, stress-induced illnesses such as IBS could be characterized by an over-activation of the HPA axis, an increase of pro-inflammatory cytokines, a change in adrenal responsiveness to ACTH[42] and possibly decreased sensitivity at the level of the glucocorticoid receptor[36,43]. In contrast to the CVS-A group, there was little expression of pro-inflammatory cytokines in the CVS-B group, which was given sweet food. The CVS-B group also exhibited a relatively normal corticosterone/ACTH ratio and colonic motility suggesting stabilization of CRF production in the hypothalamus or modulation of the HPA axis by palatable food ingestion[44]. Therefore, it can be assumed that palatable sweet food could normalize HPA dysfunction during chronic unpredictable stress periods and possibly maintain glucocorticoid receptor sensitivity. As a result, cytokine imbalance did not occur in the CVS-B group.
In conclusion, our results indicate that CVS may increase colonic motility, blunt ACTH response with over-activation of the HPA axis, decrease glucocorticoid sensitivity and increase pro-inflammatory cytokine production. These responses could be reduced by palatable sweet food consumption possibly through CRF stabilization at the central level. These results imply that reducing the effect of stress appropriately by any means is important for preventing GI symptoms in a patient with IBS.
COMMENTS
Background
Eating behavior is closely related to stress and some people preferentially consume palatable food such as chocolate or ice cream during stressful situations. Several reports have supported the hypothesis that palatable foods have been used as an ameliorator for psychological stress through the stabilization of corticotropin-releasing factor (CRF) in the hypothalamus. It seems that the consumption of sweet food has beneficial effects on the stress-induced physiologic dysfunction. However, it has never been reported whether eating sweet food has an effect on the disordered gastrointestinal (GI) motility and immune status in chronically stressed rats.
Research frontiers
They hypothesized that sweet food may have a beneficial effect on stress induced irritable bowel syndrome (IBS)-like symptoms such as hyper-motility in rat model under chronic stress. This is the first report showing that palatable sweet food supplement can reduce colonic hyper-motility and cytokine imbalance during stress period.
Innovations and breakthroughs
This results showed that chronic stress increased colonic motility, blunted adrenocorticotropic hormone (ACTH) response with over-activation of hypothalamic-pituitary-adrenal axis, decreased the glucocorticoid sensitivity and increased pro-inflammatory cytokine production. These responses could be reduced by palatable sweet food eating possibly through CRF stabilization at the central level.
Applications
The present study showed that concurrent palatable sweet food ingestion during chronic stress reduced the stress-induced colonic hyper-motility and pro-inflammatory cytokine productions in rats. These findings provided a therapeutic implication of reducing stress for preventing GI symptoms of functional gastrointestinal disorder.
Terminology
The chronic variable stress (CVS) model (also known as the chronic mild stress model) was originally used in psychiatric research for studying depression. Rats in the CVS model are subjected to a variety of stressors, and the essential feature of this model is the variety and unpredictability of stressors. Because patients with IBS experience levels of depression between groups of psychiatric and healthy controls, CVS protocol may be a good tool for investigating the relationship between chronic stress, depression and IBS.
Peer review
Nice study showing the positive influence of sweet food on stress measured by fecal output and corticosterone/ACTH ratio.
Footnotes
Supported by 2011 Sunmoon University in South Korea
P- Reviewers: Moldovan R, Padin-Iruegas ME, Sijens PE, Tsamis D S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
References
- 1.Taché Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest. 2007;117:33–40. doi: 10.1172/JCI30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut. 2000;47:861–869. doi: 10.1136/gut.47.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones MP, Dilley JB, Drossman D, Crowell MD. Brain-gut connections in functional GI disorders: anatomic and physiologic relationships. Neurogastroenterol Motil. 2006;18:91–103. doi: 10.1111/j.1365-2982.2005.00730.x. [DOI] [PubMed] [Google Scholar]
- 4.Spiller R, Lam C. An Update on Post-infectious Irritable Bowel Syndrome: Role of Genetics, Immune Activation, Serotonin and Altered Microbiome. J Neurogastroenterol Motil. 2012;18:258–268. doi: 10.5056/jnm.2012.18.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinan TG, Quigley EM, Ahmed SM, Scully P, O’Brien S, O’Mahony L, O’Mahony S, Shanahan F, Keeling PW. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker. Gastroenterology. 2006;130:304–311. doi: 10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 6.Chang L, Sundaresh S, Elliott J, Anton PA, Baldi P, Licudine A, Mayer M, Vuong T, Hirano M, Naliboff BD, et al. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol Motil. 2009;21:149–159. doi: 10.1111/j.1365-2982.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–1783. doi: 10.1053/gast.2002.33579. [DOI] [PubMed] [Google Scholar]
- 8.Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 9.Macsharry J, O’Mahony L, Fanning A, Bairead E, Sherlock G, Tiesman J, Fulmer A, Kiely B, Dinan TG, Shanahan F, et al. Mucosal cytokine imbalance in irritable bowel syndrome. Scand J Gastroenterol. 2008;43:1467–1476. doi: 10.1080/00365520802276127. [DOI] [PubMed] [Google Scholar]
- 10.Dinan TG, Clarke G, Quigley EM, Scott LV, Shanahan F, Cryan J, Cooney J, Keeling PW. Enhanced cholinergic-mediated increase in the pro-inflammatory cytokine IL-6 in irritable bowel syndrome: role of muscarinic receptors. Am J Gastroenterol. 2008;103:2570–2576. doi: 10.1111/j.1572-0241.2008.01871.x. [DOI] [PubMed] [Google Scholar]
- 11.Calvano SE, Albert JD, Legaspi A, Organ BC, Tracey KJ, Lowry SF, Shires GT, Antonacci AC. Comparison of numerical and phenotypic leukocyte changes during constant hydrocortisone infusion in normal humans with those in thermally injured patients. Surg Gynecol Obstet. 1987;164:509–520. [PubMed] [Google Scholar]
- 12.Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- 13.Boumpas DT, Chrousos GP, Wilder RL, Cupps TR, Balow JE. Glucocorticoid therapy for immune-mediated diseases: basic and clinical correlates. Ann Intern Med. 1993;119:1198–1208. doi: 10.7326/0003-4819-119-12-199312150-00007. [DOI] [PubMed] [Google Scholar]
- 14.Gibson EL. The psychobiology of comfort eating: implications for neuropharmacological interventions. Behav Pharmacol. 2012;23:442–460. doi: 10.1097/FBP.0b013e328357bd4e. [DOI] [PubMed] [Google Scholar]
- 15.Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19:275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- 17.Laugero KD, Bell ME, Bhatnagar S, Soriano L, Dallman MF. Sucrose ingestion normalizes central expression of corticotropin-releasing-factor messenger ribonucleic acid and energy balance in adrenalectomized rats: a glucocorticoid-metabolic-brain axis. Endocrinology. 2001;142:2796–2804. doi: 10.1210/endo.142.7.8250. [DOI] [PubMed] [Google Scholar]
- 18.Kim YS, Lee MY, Choi CS, Sohn YW, Park BR, Choi MG, Nah YH, Choi SC. The effect of chronic variable stress on bowel habit and adrenal function in rats. J Gastroenterol Hepatol. 2008;23:1840–1846. doi: 10.1111/j.1440-1746.2008.05524.x. [DOI] [PubMed] [Google Scholar]
- 19.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Figlewicz DP, Bennett JL, Naleid AM, Davis C, Grimm JW. Intraventricular insulin and leptin decrease sucrose self-administration in rats. Physiol Behav. 2006;89:611–616. doi: 10.1016/j.physbeh.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 21.Cota D, Tschöp MH, Horvath TL, Levine AS. Cannabinoids, opioids and eating behavior: the molecular face of hedonism. Brain Res Rev. 2006;51:85–107. doi: 10.1016/j.brainresrev.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Barone FC, Deegan JF, Price WJ, Fowler PJ, Fondacaro JD, Ormsbee HS. Cold-restraint stress increases rat fecal pellet output and colonic transit. Am J Physiol. 1990;258:G329–G337. doi: 10.1152/ajpgi.1990.258.3.G329. [DOI] [PubMed] [Google Scholar]
- 23.Okano S, Nagaya H, Inatomi N. Novelty stress increases fecal pellet output in mongolian gerbils: effects of several drugs. J Pharmacol Sci. 2005;98:411–418. doi: 10.1254/jphs.fp0050353. [DOI] [PubMed] [Google Scholar]
- 24.Foster MT, Warne JP, Ginsberg AB, Horneman HF, Pecoraro NC, Akana SF, Dallman MF. Palatable foods, stress, and energy stores sculpt corticotropin-releasing factor, adrenocorticotropin, and corticosterone concentrations after restraint. Endocrinology. 2009;150:2325–2333. doi: 10.1210/en.2008-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marin MT, Cruz FC, Planeta CS. Chronic restraint or variable stresses differently affect the behavior, corticosterone secretion and body weight in rats. Physiol Behav. 2007;90:29–35. doi: 10.1016/j.physbeh.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 26.Gold PW, Loriaux DL, Roy A, Kling MA, Calabrese JR, Kellner CH, Nieman LK, Post RM, Pickar D, Gallucci W. Responses to corticotropin-releasing hormone in the hypercortisolism of depression and Cushing’s disease. Pathophysiologic and diagnostic implications. N Engl J Med. 1986;314:1329–1335. doi: 10.1056/NEJM198605223142101. [DOI] [PubMed] [Google Scholar]
- 27.Charlton BG, Ferrier IN. Hypothalamo-pituitary-adrenal axis abnormalities in depression: a review and a model. Psychol Med. 1989;19:331–336. doi: 10.1017/s003329170001237x. [DOI] [PubMed] [Google Scholar]
- 28.Jaeckle RS, Kathol RG, Lopez JF, Meller WH, Krummel SJ. Enhanced adrenal sensitivity to exogenous cosyntropin (ACTH alpha 1-24) stimulation in major depression. Relationship to dexamethasone suppression test results. Arch Gen Psychiatry. 1987;44:233–240. doi: 10.1001/archpsyc.1987.01800150041006. [DOI] [PubMed] [Google Scholar]
- 29.Näsman B, Olsson T, Fagerlund M, Eriksson S, Viitanen M, Carlström K. Blunted adrenocorticotropin and increased adrenal steroid response to human corticotropin-releasing hormone in Alzheimer’s disease. Biol Psychiatry. 1996;39:311–318. doi: 10.1016/0006-3223(95)00173-5. [DOI] [PubMed] [Google Scholar]
- 30.Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 31.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 32.Batuman OA, Ferrero A, Cupp C, Jimenez SA, Khalili K. Differential regulation of transforming growth factor beta-1 gene expression by glucocorticoids in human T and glial cells. J Immunol. 1995;155:4397–4405. [PubMed] [Google Scholar]
- 33.Sternberg EM. Neuroendocrine regulation of autoimmune/inflammatory disease. J Endocrinol. 2001;169:429–435. doi: 10.1677/joe.0.1690429. [DOI] [PubMed] [Google Scholar]
- 34.Franchimont D. Overview of the actions of glucocorticoids on the immune response: a good model to characterize new pathways of immunosuppression for new treatment strategies. Ann N Y Acad Sci. 2004;1024:124–137. doi: 10.1196/annals.1321.009. [DOI] [PubMed] [Google Scholar]
- 35.Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann N Y Acad Sci. 2002;966:290–303. doi: 10.1111/j.1749-6632.2002.tb04229.x. [DOI] [PubMed] [Google Scholar]
- 36.Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci USA. 2012;109:5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salleh MR. Life event, stress and illness. Malays J Med Sci. 2008;15:9–18. [PMC free article] [PubMed] [Google Scholar]
- 38.Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300–317. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, Pothoulakis C, McRoberts JA, Mayer EA. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289:G42–G53. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- 40.O'Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, Cryan JF, Dinan TG. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65:263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 41.Larauche M, Mulak A, Taché Y. Stress-related alterations of visceral sensation: animal models for irritable bowel syndrome study. J Neurogastroenterol Motil. 2011;17:213–234. doi: 10.5056/jnm.2011.17.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ulrich-La YM, Engeland WC. Sympatho-adrenal activity and hypothalmic pituitary-adrenal axis regulation. In: Steckler T, Kalin NH, Reul JMHM, editors. Handbook of stress and the brain. Part1. The neurobiology of stress. Amsterdam: Elsevier; 2005. pp. 419–435. [Google Scholar]
- 43.O'Connor TM, O’Halloran DJ, Shanahan F. The stress response and the hypothalamic-pituitary-adrenal axis: from molecule to melancholia. QJM. 2000;93:323–333. doi: 10.1093/qjmed/93.6.323. [DOI] [PubMed] [Google Scholar]
- 44.Laugero KD. A new perspective on glucocorticoid feedback: relation to stress, carbohydrate feeding and feeling better. J Neuroendocrinol. 2001;13:827–835. doi: 10.1046/j.1365-2826.2001.00706.x. [DOI] [PubMed] [Google Scholar]


