Abstract
AIM: To study the ileal endocrine cell types in irritable bowel syndrome (IBS) patients.
METHODS: Ninety-eight patients with IBS (77 females and 21 males; mean age 35 years, range 18-66 years) were included, of which 35 patients had diarrhea (IBS-D), 31 patients had a mixture of both diarrhea and constipation (IBS-M), and 32 patients had constipation (IBS-C) as the predominant symptoms. The controls were 38 subjects (26 females and 12 males; mean age 40 years, range 18-65 years) who had submitted to colonoscopy for the following reasons: gastrointestinal bleeding, where the source of bleeding was identified as hemorrhoids (n = 24) or angiodysplasia (n = 3), and health worries resulting from a relative being diagnosed with colon carcinoma (n = 11). The patients were asked to complete the: Birmingham IBS symptom questionnaire. Ileal biopsy specimens from all subjects were immunostained using the avidin-biotin-complex method for serotonin, peptide YY (PYY), pancreatic polypeptide (PP), enteroglucagon, and somatostatin cells. The cell densities were quantified by computerized image analysis, using Olympus cellSens imaging software.
RESULTS: The gender and age distributions did not differ significantly between the patients and the controls (P = 0.27 and P = 0.18, respectively). The total score of Birmingham IBS symptom questionnaire was 21 ± 0.8, and the three underlying dimensions: pain, diarrhea, and constipation were 7.2 ± 0.4, 6.6 ± 0.4, and 7.2 ± 0.4, respectively. The density of serotonin cells in the ileum was 40.6 ± 3.6 cells/mm2 in the controls, and 11.5 ± 1.2, 10.7 ± 5.6, 10.0 ± 1.9, and 13.9 ± 1.4 cells/mm2 in the all IBS patients (IBS-total), IBS-D, IBS-M, and IBS-C patients, respectively. The density in the controls differed significantly from those in the IBS-total, IBS-D, IBS-M, and IBS-C groups (P < 0.0001, P = 0.0001, P = 0.0001, and P < 0.0001, respectively). There was a significant inverse correlation between the serotonin cell density and the pain dimension of Birmingham IBS symptom questionnaire (r = -0.6, P = 0.0002). The density of PYY cells was 26.7 ± 1.6 cells/mm2 in the controls, and 33.1 ± 1.4, 27.5 ± 1.4, 34.1 ± 2.5, and 41.7 ± 3.1 cells/mm2 in the IBS-total, IBS-D, IBS-M, and IBS-C patients, respectively. This density differed significantly between patients with IBS-total and IBS-C and the controls (P = 0.03 and < 0.0001, respectively), but not between controls and, IBS-D, and IBS-M patients (P = 0.8, and P = 0.1, respectively). The density of PYY cells correlated significantly with the degree of constipation as recorded by the Birmingham IBS symptom questionnaire (r = 0.6, P = 0.0002). There were few PP-, enteroglucagon-, and somatostatin-immunoreactive cells in the biopsy material examined, which made it impossible to reliably quantify these cells.
CONCLUSION: The decrease of ileal serotonin cells is associated with the visceral hypersensitivity seen in all IBS subtypes. The increased density of PYY cells in IBS-C might contribute to the constipation experienced by these patients.
Keywords: Computer image analysis, Irritable bowel syndrome, Ileum, Peptide YY, Serotonin
Core tip: The present study investigated for the first time the ileal endocrine cells in patients with irritable bowel syndrome (IBS). It included a relatively a large cohort of patients and comprising all the IBS subtypes, namely diarrhea (IBS-D), a mixture of both diarrhea and constipation (IBS-M) and constipation (IBS-C). It showed that the density of serotonin cells is reduced in patients with IBS, regardless of the subtype. On the other hand the density of peptide YY (PYY) cells in the ileum of IBS-D and IBS-M patients did not differ from that of controls, but was significantly elevated in those with IBS-C. It was concluded that the reduction of ileal serotonin cells may be connected to the visceral hypersensitivity seen in all IBS subtypes and that increase in the PYY cell density in IBS-C, would slow the intestinal transit and cause constipation.
INTRODUCTION
Irritable bowel syndrome (IBS) is a common chronic gastrointestinal functional disorder that is characterized by abdominal discomfort or pain, altered bowel habits, and bloating/abdominal distension[1,2]. IBS reportedly has a prevalence of 5%-20% and an incidence of about 200 per 100000 of the adult population[2-9]. IBS reduces the quality of life considerably in IBS patients and is an economic burden to society for various reasons, including overconsumption of healthcare resources and increased sick leave[10]. However, IBS is not known to be associated with the development of serious disease or with increased mortality[11,12].
The diagnosis of IBS is based on the assessment of symptoms and detailed, accurate, and clinically useful definitions of the syndrome that have been elaborated by the working parties responsible for producing the latest Rome III Criteria[13,14]. In addition to these criteria, warning symptoms (so-called red flags) such as age > 50 years, short history of symptoms, nocturnal symptoms, weight loss, rectal bleeding, anemia, and the presence of markers for inflammation or infections should be excluded. IBS patients are sub-grouped on the basis of differences in the predominant bowel symptoms into diarrhea (IBS-D), constipation (IBS-C), both diarrhea and constipation (IBS-M), and un-subtyped IBS in patients with insufficient abnormality of stool consistency to meet criteria for IBS-C, -D, or -M[13,14]. Because of the overlap of symptomology with celiac disease and microscopic colitis, some gastroenterologists (including the present authors) believe that these disorders should be excluded in addition to applying the Rome Criteria[15].
Abnormalities in the endocrine cells in the stomach, duodenum, colon, and rectum have been reported in patients with IBS[16-29], but the ileal endocrine cells have not been investigated previously. The endocrine cell types differ markedly between the distal and proximal small intestine, probably due to the quite different functions performed by these two parts of the intestine. The proximal small intestine contains serotonin, secretin, cholecystokinin (CCK), gastric inhibitory polypeptide (GIP), and somatostatin cells, while the ileum has the same endocrine cell types as in the large intestine, namely serotonin, peptide YY (PYY), pancreatic polypeptide (PP), enteroglucagon, and somatostatin cells[30].
A recent study observed endocrine cell depletion in the ileum of patients with sporadic IBS[31]. This depletion was detected by chromogranin A, which is a common marker for endocrine cells[32-34]. The aim of the present study was to clarify the affected endocrine cell types by examining various ileal endocrine cells in the same cohort of IBS patients investigated using chromogranin A.
MATERIALS AND METHODS
Patients and controls
Ninety-eight patients (77 females and 21 males; mean age 35 years, range 18-66 years) with IBS according to Rome III Criteria were included in the study[13,14]. The IBS subtypes were distributed as follows: 35 patients with IBS-D, 31 patients with IBS-M, and 32 patients with IBS-C. Symptoms had been present in all of the patients for many years, and the onset of their IBS symptoms could not be associated with any events, in particular gastrointestinal or other infections. All patients underwent a complete physical examination and were investigated using the following blood tests: full blood count, electrolytes, inflammatory markers, liver tests, and thyroid function tests. They also underwent further gastroscopy with duodenal biopsies, which were used to exclude celiac disease. All of the patients had been tested previously (i.e., before they were referred to us) for lactose intolerance and the presence of intestinal infectious agents including parasites in the stool; the results of all of these tests were negative.
For comparison, the control group comprised 38 subjects (26 females and 12 males; mean age 40 years, range 18-65 years) who had submitted to colonoscopy for the following reasons: gastrointestinal bleeding, where the source of bleeding was identified as hemorrhoids (n = 24) or angiodysplasia (n = 3), and health worries resulting from a relative being diagnosed with colon carcinoma (n = 11).
The patients were asked to complete the: Birmingham IBS symptom questionnaire. The Birmingham IBS symptom score questionnaire is a disease specific score to measure the symptoms of patients with IBS. It has been developed to be suitable for self-completion and has been found to be acceptable to patients. Its dimensions have good reliability, external validity and sensitivity[35]. The questionnaire comprises 11 questions based on the frequency of IBS related symptoms. Each question has a standard response scale with symptoms all being measured on a 5-point Likert scale ranging from 0 ("none of the time") to 5 ("all of the time"). There are three underlying dimensions: pain (3 items), diarrhea (5 items) and constipation (3 items)[35].
The study was performed in accordance with the Declaration of Helsinki and was approved by the Regional Committee for Medical and Health Research Ethics West, Bergen, Norway. All subjects gave oral and written consent to participate.
Colonoscopy, histopathology, and immunohistochemistry
Colonoscopy was performed on both the patients and the controls, segmental biopsy specimens were taken from the colon and rectum, and four biopsy samples were taken from the ileum of each subject. The biopsy samples were fixed overnight in 4% buffered paraformaldehyde, embedded in paraffin, and cut into 5-m sections. The sections were stained with hematoxylin-eosin, and immunostained by the avidin-biotin complex (ABC) method using the Vectastain ABC kit (Vector Laboratories). The sections were hydrated and immersed in 0.01% hydrogen peroxide in PBS buffer (pH 7.4) for 10 min to inhibit endogenous peroxidase. After washing in buffer, the sections were treated with 1% bovine serum albumin for 30 min to block the nonspecific binding sites, and then incubated with the primary antiserum/antibody at room temperature for 2 h. The sections were then washed in PBS buffer and incubated with biotinylated swine anti-mouse (in the case of monoclonal antibodies) or anti-rabbit IgG (in the case of polyclonal antibodies) diluted 1:200 for 30 min at room temperature. After washing the slides in PBS buffer, the sections were incubated for 30 min with avidin-biotin-peroxidase complex diluted 1:100, and then immersed in 3,3’-diaminobenzidine (DAB) peroxidase substrate (Vector laboratories), followed by counterstaining in hematoxylin[18]. The following primary antisera/antibodies were used: monoclonal mouse anti-serotonin (Dako, code no. 5HT-209), polyclonal anti-porcine peptide PYY (Alpha-Dagnostica, code PYY 11A), polyclonal rabbit anti-synthetic-human PP (Diagnostic Biosystems, code No. 114), polyclonal rabbit anti-porcine glicentin/glucagon (Acris Antibodies, code BP508), and polyclonal rabbit anti-synthetic-human somatostatin (Dako, code no. A566); these antibodies were used at dilutions of 1:1500, 1:1000, 1:800, 1:400, and 1:200, respectively.
Computerized image analysis
Quantification of the endocrine cells was done as described previously[36,37]. Measurements were performed using Olympus cellSens imaging software (version 1.7) on a computer linked to an Olympus microscope type BX 43 with an Olympus camera (DP 26). The number of immunoreactive cells and the area of the epithelial cells were measured. The numbers of endocrine cells in each field were counted manually by pointing and clicking the computer mouse, and the areas of the epithelium containing these cells were drawn manually using the computer mouse. A × 40 objective was used, for which each frame (field) on the monitor represented a tissue area of 0.14 mm2. Each individual and peptide hormone was measured in ten randomly chosen fields. Immunostained sections from the IBS patients and controls were coded and mixed, and measurements were made by the same person (El-Salhy M) who was blind to the identity of the sections. The data from the fields were tabulated, and the cell density of the epithelium (in cells/mm2) was computed and statistically analyzed.
Statistical analysis
The gender difference between patients and controls was tested by Fisher’s exact test, and the age difference was tested by the Mann-Whitney nonparametric test. Differences between controls, all IBS patients (IBS-total), IBS-D, IBS-M, and IBS-C patients were tested by the Kruskal-Wallis nonparametric test with Dunn’s post-test. Correlation was done by Spearman nonparametric test. The data are presented as mean ± SE values, and differences with P < 0.05 were considered to be statistically significant.
RESULTS
Gender and age characteristics of patients and controls
The gender and age distributions did not differ significantly between the patients and the controls (P = 0.27 and P = 0.18, respectively). The total score of Birmingham IBS symptom questionnaire was 21 ± 0.8, and the three underlying dimensions: pain, diarrhea, and constipation were 7.2 ± 0.4, 6.6 ± 0.4, and 7.2 ± 0.4, respectively.
Endoscopy, histopathology, and immunohistochemistry
The colon and rectum of both the patients and the control subjects were macroscopically normal. The ileum was macroscopically normal in all except in one control subject and three patients, in whom lymphoid hyperplasia was observed; this condition is a common finding in young individuals and has no pathological relevance. The results of histopathological examinations of the ileum, colon, and rectum were normal in both the patients and the controls.
Serotonin-, PYY-, PP-, enteroglucagon-, and somatostatin-immunoreactive cells were found in the ileum of all of the subjects (i.e., patients and controls), mostly in the crypts. These cells were basket- or flask-shaped.
Computerized image analysis
Serotonin cell density: The density of serotonin cells in the ileum was 40.6 ± 3.6 cells/mm2 in the controls, and 11.5 ± 1.2, 10.7 ± 5.6, 10.0 ± 1.9, and 13.9 ± 1.4 cells/mm2 in the IBS-total, IBS-D, IBS-M, and IBS-C patients, respectively. The serotonin cell density differed significantly between the controls and the IBS-total and IBS subgroups (P < 0.0001). Posttests showed that the density in the controls differed significantly from those in the IBS-total, IBS-D, IBS-M, and IBS-C groups (P < 0.0001, P = 0.0001, P = 0.0001, and P < 0.0001, respectively) (Figures 1 and 2). There was a significant inverse (negative) correlation between the serotonin cell density and the pain dimension of Birmingham IBS symptom questionnaire (r = -0.6, P = 0.0002). There was no significant correlation between the total score of Birmingham IBS symptom questionnaire, the diarrhea or constipation dimension (r = -0.05, P = 0.8; r = -0.4, P = 0.8; and r = -0.2, P = 0.2 respectively). The density of PYY cells was 26.7 ± 1.6 cells/mm2 in the controls, and 33.1 ± 1.4, 27.5 ± 1.4, 34.1 ± 2.5, and 41.7 ± 3.1 cells/mm2 in the IBS-total, IBS-D, IBS-M, and IBS-C patients, respectively. The PYY cell density differed significantly between the controls and the IBS-total and IBS subgroups (P < 0.0001). This density differed significantly between patients with IBS-total and IBS-C and the controls (P = 0.03 and < 0.0001, respectively), but not between controls and, IBS-D, and IBS-M patients (P = 0.8, and P = 0.1, respectively) (Figures 3 and 4). The density of PYY cells correlated significantly with the degree of constipation as recorded by the Birmingham IBS symptom questionnaire (r = 0.6, P = 0.0002). There was not significant correlation between the cell density of PYY and the total Birmingham IBS symptom questionnaire, the pain-, or diarrhea dimension (r = 0.2, P = 0.2; r = 0.2, P = 0.06; and r = 0.1, P = 0.5 respectively).
Figure 1.
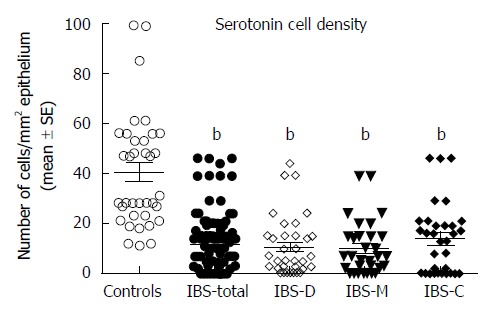
Serotonin cell densities in the ileum of controls and in all patients with irritable bowel syndrome, patients with diarrhea as the predominant symptom, patients with both diarrhea and constipation, and patients with constipation as the predominant symptom patients. bP < 0.01 vs controls. IBS-total: All patients with irritable bowel syndrome; IBS-D: Patients with diarrhea as the predominant symptom; IBS-M: Patients with both diarrhea and constipation; IBS-C: Patients with constipation as the predominant symptom.
Figure 2.
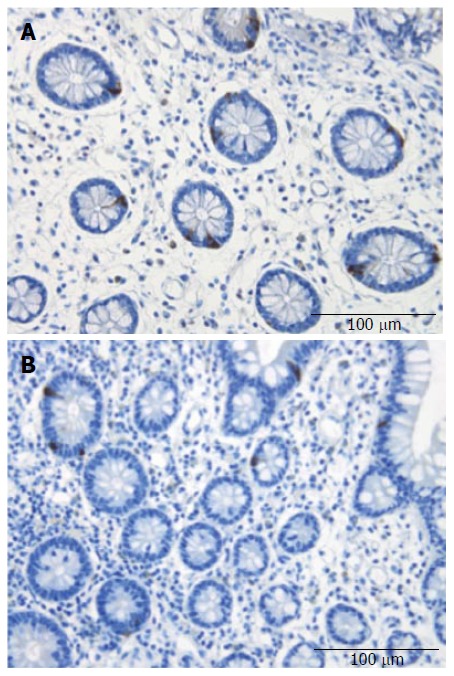
Ileal serotonin-immunoreactive cells in a control subject (A) and a patient with irritable bowel syndrome (B).
Figure 3.
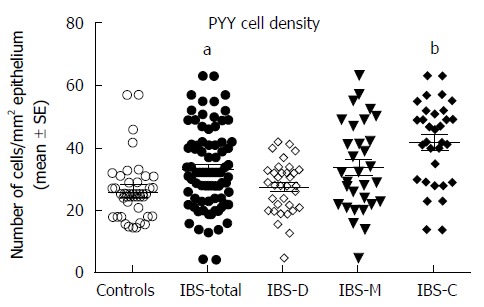
Peptide YY cell densities in the ileum of controls and in all patients with irritable bowel syndrome, patients with diarrhea as the predominant symptom, patients with both diarrhea and constipation, and patients with constipation as the predominant symptom patients. aP < 0.05, bP < 0.01 vs controls. PYY: Peptide YY; IBS-total: All patients with irritable bowel syndrome; IBS-D: Patients with diarrhea as the predominant symptom; IBS-M: Patients with both diarrhea and constipation; IBS-C: Patients with constipation as the predominant symptom.
Figure 4.
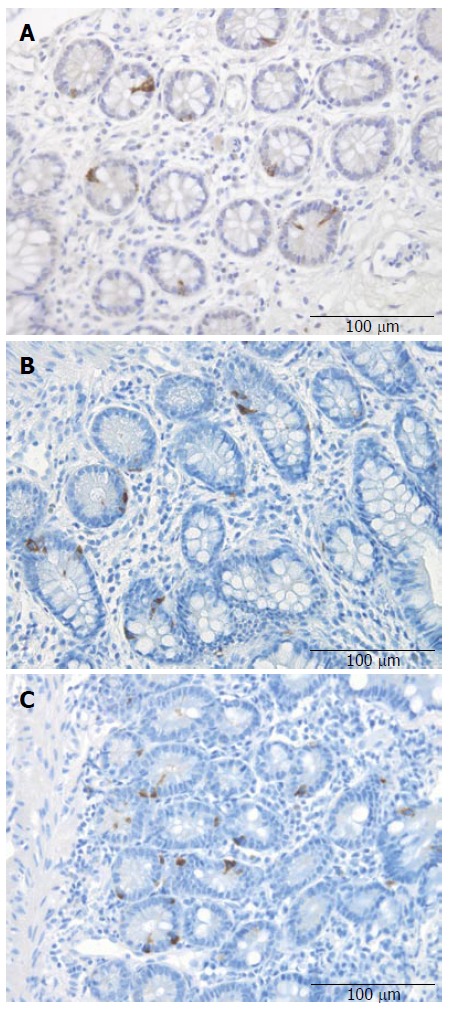
Peptide YY cells in the ileum of a control subject (A), a patient with irritable bowel syndrome into diarrhea (B), and a patient with irritable bowel syndrome into constipation (C).
PP, enteroglucagon, and somatostatin cell densities: There were few PP-, enteroglucagon-, and somatostatin-immunoreactive cells in the biopsy material examined, which made it impossible to reliably quantify these cells.
DISCUSSION
The present study showed that the density of serotonin cells in the ileum is reduced in patients with sporadic (nonspecific) IBS, regardless of the subtype. This finding is similar to that reported in the colon of IBS patients[19]. On the other hand the density of PYY cells in the ileum of IBS-D and IBS-M patients did not differ from that of controls, but was significantly elevated in those with IBS-C. The observations made here on PYY cell density differ from those reported in the colon, where the density of PYY cells was reduced in IBS patients.
Abnormalities in the endocrine cells in the stomach, duodenum, colon, and rectum have been reported in patients with IBS[16-29]. The density of ghrelin cells in the stomach is lower in IBS-C and higher in IBS-D than in healthy controls[16]. In the duodenum, the cell densities of GIP and somatostatin are decreased in both IBS-D and IBS-C[18]. The densities of duodenal secretin and CCK cells are decreased in IBS-D but unchanged in IBS-C[18]. The duodenal serotonin cells are not affected in both IBS-D and IBS-C[18]. Postinfectious IBS was found to be associated with increased numbers of duodenal CCK cells but decreased numbers of serotonin cells[17]. Colonic serotonin and PYY cell densities have been found to be low in both IBS-D and IBS-C[19]. In the rectum of patients with sporadic (nonspecific) IBS, the densities of PYY and enteroglucagon cells were significantly lower and that of somatostatin cells was significantly higher in both IBS-D and IBS-C than in the controls, whereas the serotonin cell density in these patients did not differ from that in healthy controls[21,38]. Rectal serotonin and PYY cell densities in postinfectious IBS have been reported to be elevated[23,25,27,39,40].
Serotonin cells are the predominant endocrine cell type in the ileum, which could account for the general reduction of ileal endocrine cells reported elsewhere[31]. Each intestinal crypt contains four to six pluripotent stem cells that differentiate through a series of cellular precursors (progenitors) into all epithelial cell types including enterocytes, goblet cells, Paneth cells, and endocrine cells[41-57]. It is possible that the reduction in ileal serotonin cells in IBS patients is due to abnormal cell differentiation from stem cell. This assumption gets support from the findings that depletions of endocrine cell in rejected ileum transplants are associated with marked depression in the expression of neurogenin-3 (NEUROG3) which is a early progenitor for endocrine cells and NeuroD, which is a transcription factor expressed by cells derived from NEUROG3[58]. Furthermore, a mutant NEUROG3 has been described in patients with congenital malabsorption diarrhea and lack of intestinal endocrine cells[59].
Serotonin activates the submucosal sensory branch of the enteric nervous system that conveys sensation from the gut to the central nervous system[60,61]. Serotonin modulates is known to modulate visceral sensitivity of the gastrointestinal tract[62,63]. It is therefore conceivable to conclude that the reduction of ileal serotonin cells may be connected to the visceral hypersensitivity seen in all IBS subtypes. It is difficult to establish whether the reduction in these ileal serotonin cells is primary or secondary to the visceral hypersensitivity. However, it is tempting to speculate that this abnormality is secondary to the visceral hypersensitivity and represents an adaptation mechanism for reducing the sensation conveyed from the gut to the central nervous system by serotonin. This assumption gets support from the present finding that serotonin cell density is correlated inversely to the pain score obtained by the Birmingham IBS symptom questionnaire. Speaking against this assumption are the findings that tryptophan hydroxylase (TPH)-1, which is the limiting enzyme for the synthesis in serotonin cells, and serotonin transporter (SERT) mRNA levels have been reported to be lower in the rectum and sigmoid colon of IBS patients than control subjects[64]. There was no difference, however, in the levels of (TPH)-1 and SERT between IBS patients with rectal hypersensitivity and those without[64].
PYY stimulates the absorption of water and electrolytes, and is a major regulator of the “ileal brake”[65-70]. Furthermore, PYY inhibits prostaglandin E2 and vasoactive intestinal polypeptide (VIP), which stimulate intestinal fluid secretion[71-73]. Administration of PYY inhibits diarrhea in experimental mouse models by reducing intestinal fluid secretion and slowing colonic transit[74]. It is thus possible that the increase in the PYY cell density in IBS-C patients, and consequently the increase in PYY, would slow the intestinal transit by strengthening the ileal brake, increasing the absorption of water, and decreasing the secretion of the intestinal fluid in the distal small intestine via inhibiting VIP and prostaglandin E2. These effects would in turn cause constipation. In support of this conclusion is the observation that the density PYY cell correlated to the constipation score calculated from the Birmingham IBS symptom questionnaire.
The above summary indicates that most of the reported abnormalities in gastrointestinal endocrine cells are similar in all IBS subtypes[18,20,28-31]. However, in IBS-C the density of ghrelin cells in the oxyntic mucosa of the stomach was significantly lower than in healthy controls[16]. In addition to its role in regulating appetite and energy metabolism, ghrelin accelerates gastric and small- and large-intestinal motility[75-88]. It can be speculated that the low density of ghrelin cells previously reported and the high density of PYY cells observed in the present study explain why constipation predominates in the IBS-C subtype. On the other hand, in IBS-D patients the density of ghrelin cells in the stomach was significantly higher and the densities of duodenal secretin and CCK were lower than healthy volunteers[16,18]. Secretin inhibits gastric emptying and intestinal motility, and stimulates pancreatic bicarbonate and fluid secretions[30,89,90]. The secretion of pancreatic bicarbonate increases the pH of gut contents, which are highly acidic after leaving the stomach, and this is essential for lipid digestion as pancreatic lipase is irreversibly inactivated below pH 4.0[91]. CCK relaxes the proximal stomach in order to increase its reservoir capacity, inhibits gastric emptying and stimulates gall bladder contractions and pancreatic exocrine secretions of digestive enzymes from pancreatic exocrine glands[91-94]. The low densities of secretin and CCK cells in IBS-diarrhea patients could cause a rapid gastric emptying and acceleration of intestinal motility and ultimately diarrhea in these patients.
COMMENTS
Background
Irritable bowel syndrome (IBS) is a common chronic gastrointestinal disorder that is characterized by intermittent abdominal discomfort or pain, altered bowel habits, and bloating/abdominal distension. IBS reduces the quality of life considerably, but it is not known to be associated with the development of serious disease or with an increased mortality rates. Abnormal endocrine cells have been reported in the stomach, duodenum, colon, and rectum in patients with IBS, but the ileal endocrine cells have not been investigated previously.
Research frontiers
This study showed for the first time the ileal endocrine cells in patients with IBS are abnormal. It included a large cohort of patients and comprising all the IBS subtypes. It showed that the density of serotonin cells is reduced in patients with IBS, regardless of the subtype. Moreover, it revealed that the density of peptide Y (PYY) cells in the ileum of patients with IBS-D and IBS-, did not differ from that of controls, but was significantly elevated in IBS-C. It was concluded that the reduction of ileal serotonin cells may play a role in visceral hypersensitivity seen in IBS and that increase in the PYY cell density in IBS-C, may affect the intestinal transit and cause constipation.
Innovations and breakthroughs
The present study showed that the endocrine cells of the ileum of patients with IBS are abnormal. This together with the previously published results in the duodenum as well as in the stomach and large intestine of these patients indicate that the endocrine cells in all the segments of the gastrointestinal tract are affected. The gastrointestinal endocrine cells have specialized microvilli that project into the lumen and function as sensors for the luminal content and respond to luminal stimuli by releasing hormones into the lamina propria, which starts a chain reactions that progress throughout the entire neuroendocrine system. It is possible, therefore, that abnormalities the gut endocrine cells play a central role in the pathogenesis of IBS.
Applications
Identifying abnormalities in the gut endocrine cells may provide an effective tool in the treatment of IBS. Actually, a serotonin agonist is available in the market, which is approved for the treatment of chronic constipation.
Peer review
The study addresses the interesting areas of endocrine cells in IBS. The authors present a well conducted and written histologic study of enteroendocrine cell types in the ileum of IBS patients, correlating cell densities with symptoms and comparing them to control subjects.
Footnotes
Supported by A Grant from Helse-Fonna
P- Reviewers: Fishbein TM, Khan WI S- Editor: Cui XM L- Editor: A E- Editor: Wang CH
References
- 1.Thompson WG. A world view of IBS. In: Camilleri M, Spiller RC, editors. Irritable bowel syndrome: diagnosis and treatment. Philadelphia and London: Saunders; 2002. pp. 17–26. [Google Scholar]
- 2.Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Thompson WG, Whitehead WE, Janssens J, Funch-Jensen P, Corazziari E. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–1580. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 3.El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Irritable bowel syndrome: diagnosis, pathogenesis and treatment options. New York: Nova Science Publishers, Inc; 2012. [Google Scholar]
- 4.Ford AC, Vandvik PO. Irritable bowel syndrome. Clin Evid (Online) 2012;2012:pii: 0410. [PMC free article] [PubMed] [Google Scholar]
- 5.Quigley EM, Locke GR, Mueller-Lissner S, Paulo LG, Tytgat GN, Helfrich I, Schaefer E. Prevalence and management of abdominal cramping and pain: a multinational survey. Aliment Pharmacol Ther. 2006;24:411–419. doi: 10.1111/j.1365-2036.2006.02989.x. [DOI] [PubMed] [Google Scholar]
- 6.Vandvik PO, Lydersen S, Farup PG. Prevalence, comorbidity and impact of irritable bowel syndrome in Norway. Scand J Gastroenterol. 2006;41:650–656. doi: 10.1080/00365520500442542. [DOI] [PubMed] [Google Scholar]
- 7.Saito YA, Schoenfeld P, Locke GR. The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol. 2002;97:1910–1915. doi: 10.1111/j.1572-0241.2002.05913.x. [DOI] [PubMed] [Google Scholar]
- 8.Dakin CL, Small CJ, Batterham RL, Neary NM, Cohen MA, Patterson M, Ghatei MA, Bloom SR. Peripheral oxyntomodulin reduces food intake and body weight gain in rats. Endocrinology. 2004;145:2687–2695. doi: 10.1210/en.2003-1338. [DOI] [PubMed] [Google Scholar]
- 9.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–721.e4. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 10.El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Chromogranin A cell density as a diagnostic marker for lymphocytic colitis. Dig Dis Sci. 2012;57:3154–3159. doi: 10.1007/s10620-012-2249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvey RF, Mauad EC, Brown AM. Prognosis in the irritable bowel syndrome: a 5-year prospective study. Lancet. 1987;1:963–965. doi: 10.1016/s0140-6736(87)90304-7. [DOI] [PubMed] [Google Scholar]
- 12.Nørgaard M, Farkas DK, Pedersen L, Erichsen R, de la Cour ZD, Gregersen H, Sørensen HT. Irritable bowel syndrome and risk of colorectal cancer: a Danish nationwide cohort study. Br J Cancer. 2011;104:1202–1206. doi: 10.1038/bjc.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 14.Spiller R, Aziz Q, Creed F, Emmanuel A, Houghton L, Hungin P, Jones R, Kumar D, Rubin G, Trudgill N, et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut. 2007;56:1770–1798. doi: 10.1136/gut.2007.119446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Salhy M. Irritable bowel syndrome: diagnosis and pathogenesis. World J Gastroenterol. 2012;18:5151–5163. doi: 10.3748/wjg.v18.i37.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Salhy M, Lillebø E, Reinemo A, Salmelid L. Ghrelin in patients with irritable bowel syndrome. Int J Mol Med. 2009;23:703–707. doi: 10.3892/ijmm_00000183. [DOI] [PubMed] [Google Scholar]
- 17.Dizdar V, Spiller R, Singh G, Hanevik K, Gilja OH, El-Salhy M, Hausken T. Relative importance of abnormalities of CCK and 5-HT (serotonin) in Giardia-induced post-infectious irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2010;31:883–891. doi: 10.1111/j.1365-2036.2010.04251.x. [DOI] [PubMed] [Google Scholar]
- 18.El-Salhy M, Vaali K, Dizdar V, Hausken T. Abnormal small-intestinal endocrine cells in patients with irritable bowel syndrome. Dig Dis Sci. 2010;55:3508–3513. doi: 10.1007/s10620-010-1169-6. [DOI] [PubMed] [Google Scholar]
- 19.El-Salhy M, Gundersen D, Ostgaard H, Lomholt-Beck B, Hatlebakk JG, Hausken T. Low densities of serotonin and peptide YY cells in the colon of patients with irritable bowel syndrome. Dig Dis Sci. 2012;57:873–878. doi: 10.1007/s10620-011-1948-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Salhy M, Mazzawi T, Gundersen D, Hatlebakk JG, Hausken T. Changes in the symptom pattern and the densities of large-intestinal endocrine cells following Campylobacter infection in irritable bowel syndrome: a case report. BMC Res Notes. 2013;6:391. doi: 10.1186/1756-0500-6-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Wang SH, Dong L, Luo JY, Gong J, Li L, Lu XL, Han SP. Decreased expression of serotonin in the jejunum and increased numbers of mast cells in the terminal ileum in patients with irritable bowel syndrome. World J Gastroenterol. 2007;13:6041–6047. doi: 10.3748/wjg.v13.45.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee KJ, Kim YB, Kim JH, Kwon HC, Kim DK, Cho SW. The alteration of enterochromaffin cell, mast cell, and lamina propria T lymphocyte numbers in irritable bowel syndrome and its relationship with psychological factors. J Gastroenterol Hepatol. 2008;23:1689–1694. doi: 10.1111/j.1440-1746.2008.05574.x. [DOI] [PubMed] [Google Scholar]
- 24.Park JH, Rhee PL, Kim G, Lee JH, Kim YH, Kim JJ, Rhee JC, Song SY. Enteroendocrine cell counts correlate with visceral hypersensitivity in patients with diarrhoea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2006;18:539–546. doi: 10.1111/j.1365-2982.2006.00771.x. [DOI] [PubMed] [Google Scholar]
- 25.Kim HS, Lim JH, Park H, Lee SI. Increased immunoendocrine cells in intestinal mucosa of postinfectious irritable bowel syndrome patients 3 years after acute Shigella infection--an observation in a small case control study. Yonsei Med J. 2010;51:45–51. doi: 10.3349/ymj.2010.51.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunlop SP, Coleman NS, Blackshaw E, Perkins AC, Singh G, Marsden CA, Spiller RC. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:349–357. doi: 10.1016/s1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- 27.Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Salhy M, Lomholt-Beck B, Hausken T. Chromogranin A as a possible tool in the diagnosis of irritable bowel syndrome. Scand J Gastroenterol. 2010;45:1435–1439. doi: 10.3109/00365521.2010.503965. [DOI] [PubMed] [Google Scholar]
- 29.El-Salhy M, Mazzawi T, Gundersen D, Hausken T. Chromogranin A cell density in the rectum of patients with irritable bowel syndrome. Mol Med Rep. 2012;6:1223–1225. doi: 10.3892/mmr.2012.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Salhy M, Seim I, Chopin L, Gundersen D, Hatlebakk JG, Hausken T. Irritable bowel syndrome: the role of gut neuroendocrine peptides. Front Biosci (Elite Ed) 2012;4:2783–2800. doi: 10.2741/e583. [DOI] [PubMed] [Google Scholar]
- 31.El-Salhy M, Wendelbo IH, Gundersen D. Reduced chromogranin A cell density in the ileum of patients with irritable bowel syndrome. Mol Med Rep. 2013;7:1241–1244. doi: 10.3892/mmr.2013.1325. [DOI] [PubMed] [Google Scholar]
- 32.Taupenot L, Harper KL, O’Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- 33.Wiedenmann B, Huttner WB. Synaptophysin and chromogranins/secretogranins--widespread constituents of distinct types of neuroendocrine vesicles and new tools in tumor diagnosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;58:95–121. doi: 10.1007/BF02890062. [DOI] [PubMed] [Google Scholar]
- 34.Deftos LJ. Chromogranin A: its role in endocrine function and as an endocrine and neuroendocrine tumor marker. Endocr Rev. 1991;12:181–187. doi: 10.1210/edrv-12-2-181. [DOI] [PubMed] [Google Scholar]
- 35.Roalfe AK, Roberts LM, Wilson S. Evaluation of the Birmingham IBS symptom questionnaire. BMC Gastroenterol. 2008;8:30. doi: 10.1186/1471-230X-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.el-Salhy M, Sandström O, Näsström E, Mustajbasic M, Zachrisson S. Application of computer image analysis in endocrine cell quantification. Histochem J. 1997;29:249–256. doi: 10.1023/a:1026458027425. [DOI] [PubMed] [Google Scholar]
- 37.El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. High densities of serotonin and peptide YY cells in the colon of patients with lymphocytic colitis. World J Gastroenterol. 2012;18:6070–6075. doi: 10.3748/wjg.v18.i42.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Abnormal rectal endocrine cells in patients with irritable bowel syndrome. Regul Pept. 2013;188C:60–65. doi: 10.1016/j.regpep.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Wang LH, Fang XC, Pan GZ. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut. 2004;53:1096–1101. doi: 10.1136/gut.2003.021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651–1659. doi: 10.1053/j.gastro.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 41.Cardoso WV, Lü J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133:1611–1624. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- 42.Darlington GJ. Molecular mechanisms of liver development and differentiation. Curr Opin Cell Biol. 1999;11:678–682. doi: 10.1016/s0955-0674(99)00035-6. [DOI] [PubMed] [Google Scholar]
- 43.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 44.Rawlins EL, Hogan BL. Ciliated epithelial cell lifespan in the mouse trachea and lung. Am J Physiol Lung Cell Mol Physiol. 2008;295:L231–L234. doi: 10.1152/ajplung.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaret KS. Regulatory phases of early liver development: paradigms of organogenesis. Nat Rev Genet. 2002;3:499–512. doi: 10.1038/nrg837. [DOI] [PubMed] [Google Scholar]
- 46.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 47.Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barker N, Clevers H. Tracking down the stem cells of the intestine: strategies to identify adult stem cells. Gastroenterology. 2007;133:1755–1760. doi: 10.1053/j.gastro.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 49.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 50.Fontaine J, Le Lièvre C, Le Douarin NM. What is the developmental fate of the neural crest cells which migrate into the pancreas in the avian embryo. Gen Comp Endocrinol. 1977;33:394–404. doi: 10.1016/0016-6480(77)90055-7. [DOI] [PubMed] [Google Scholar]
- 51.Le Douarin NM, Teillet MA. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973;30:31–48. [PubMed] [Google Scholar]
- 52.Rawdon BB, Andrew A. Origin and differentiation of gut endocrine cells. Histol Histopathol. 1993;8:567–580. [PubMed] [Google Scholar]
- 53.Hoffman J, Kuhnert F, Davis CR, Kuo CJ. Wnts as essential growth factors for the adult small intestine and colon. Cell Cycle. 2004;3:554–557. [PubMed] [Google Scholar]
- 54.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 55.May CL, Kaestner KH. Gut endocrine cell development. Mol Cell Endocrinol. 2010;323:70–75. doi: 10.1016/j.mce.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gunawardene AR, Corfe BM, Staton CA. Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int J Exp Pathol. 2011;92:219–231. doi: 10.1111/j.1365-2613.2011.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee CS, Kaestner KH. Clinical endocrinology and metabolism. Development of gut endocrine cells. Best Pract Res Clin Endocrinol Metab. 2004;18:453–462. doi: 10.1016/j.beem.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 58.Fishbein TM, Novitskiy G, Lough DM, Matsumoto C, Kaufman SS, Shetty K, Zasloff M. Rejection reversibly alters enteroendocrine cell renewal in the transplanted small intestine. Am J Transplant. 2009;9:1620–1628. doi: 10.1111/j.1600-6143.2009.02681.x. [DOI] [PubMed] [Google Scholar]
- 59.Wang J, Cortina G, Wu SV, Tran R, Cho JH, Tsai MJ, Bailey TJ, Jamrich M, Ament ME, Treem WR, et al. Mutant neurogenin-3 in congenital malabsorptive diarrhea. N Engl J Med. 2006;355:270–280. doi: 10.1056/NEJMoa054288. [DOI] [PubMed] [Google Scholar]
- 60.Gershon MD. Plasticity in serotonin control mechanisms in the gut. Curr Opin Pharmacol. 2003;3:600–607. doi: 10.1016/j.coph.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 61.Kellum JM, Albuquerque FC, Stoner MC, Harris RP. Stroking human jejunal mucosa induces 5-HT release and Cl- secretion via afferent neurons and 5-HT4 receptors. Am J Physiol. 1999;277:G515–G520. doi: 10.1152/ajpgi.1999.277.3.G515. [DOI] [PubMed] [Google Scholar]
- 62.Camilleri M. Serotonergic modulation of visceral sensation: lower gut. Gut. 2002;51 Suppl 1:i81–i86. doi: 10.1136/gut.51.suppl_1.i81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tack J, Sarnelli G. Serotonergic modulation of visceral sensation: upper gastrointestinal tract. Gut. 2002;51 Suppl 1:i77–i80. doi: 10.1136/gut.51.suppl_1.i77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kerckhoffs AP, ter Linde JJ, Akkermans LM, Samsom M. SERT and TPH-1 mRNA expression are reduced in irritable bowel syndrome patients regardless of visceral sensitivity state in large intestine. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1053–G1060. doi: 10.1152/ajpgi.00153.2011. [DOI] [PubMed] [Google Scholar]
- 65.Maljaars PW, Keszthelyi D, Masclee AA. An ileal brake-through. Am J Clin Nutr. 2010;92:467–468. doi: 10.3945/ajcn.2010.30180. [DOI] [PubMed] [Google Scholar]
- 66.Van Citters GW, Lin HC. Ileal brake: neuropeptidergic control of intestinal transit. Curr Gastroenterol Rep. 2006;8:367–373. doi: 10.1007/s11894-006-0021-9. [DOI] [PubMed] [Google Scholar]
- 67.Lin HC, Zhao XT, Wang L, Wong H. Fat-induced ileal brake in the dog depends on peptide YY. Gastroenterology. 1996;110:1491–1495. doi: 10.1053/gast.1996.v110.pm8613054. [DOI] [PubMed] [Google Scholar]
- 68.Pironi L, Stanghellini V, Miglioli M, Corinaldesi R, De Giorgio R, Ruggeri E, Tosetti C, Poggioli G, Morselli Labate AM, Monetti N. Fat-induced ileal brake in humans: a dose-dependent phenomenon correlated to the plasma levels of peptide YY. Gastroenterology. 1993;105:733–739. doi: 10.1016/0016-5085(93)90890-o. [DOI] [PubMed] [Google Scholar]
- 69.Spiller RC, Trotman IF, Adrian TE, Bloom SR, Misiewicz JJ, Silk DB. Further characterisation of the ‘ileal brake’ reflex in man--effect of ileal infusion of partial digests of fat, protein, and starch on jejunal motility and release of neurotensin, enteroglucagon, and peptide YY. Gut. 1988;29:1042–1051. doi: 10.1136/gut.29.8.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spiller RC, Trotman IF, Higgins BE, Ghatei MA, Grimble GK, Lee YC, Bloom SR, Misiewicz JJ, Silk DB. The ileal brake--inhibition of jejunal motility after ileal fat perfusion in man. Gut. 1984;25:365–374. doi: 10.1136/gut.25.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goumain M, Voisin T, Lorinet AM, Ducroc R, Tsocas A, Rozé C, Rouet-Benzineb P, Herzog H, Balasubramaniam A, Laburthe M. The peptide YY-preferring receptor mediating inhibition of small intestinal secretion is a peripheral Y(2) receptor: pharmacological evidence and molecular cloning. Mol Pharmacol. 2001;60:124–134. doi: 10.1124/mol.60.1.124. [DOI] [PubMed] [Google Scholar]
- 72.Souli A, Chariot J, Voisin T, Presset O, Tsocas A, Balasubramaniam A, Laburthe M, Rozé C. Several receptors mediate the antisecretory effect of peptide YY, neuropeptide Y, and pancreatic polypeptide on VIP-induced fluid secretion in the rat jejunum in vivo. Peptides. 1997;18:551–557. doi: 10.1016/s0196-9781(97)00069-7. [DOI] [PubMed] [Google Scholar]
- 73.Whang EE, Hines OJ, Reeve JR, Grandt D, Moser JA, Bilchik AJ, Zinner MJ, McFadden DW, Ashley SW. Antisecretory mechanisms of peptide YY in rat distal colon. Dig Dis Sci. 1997;42:1121–1127. doi: 10.1023/a:1018869116284. [DOI] [PubMed] [Google Scholar]
- 74.Moriya R, Shirakura T, Hirose H, Kanno T, Suzuki J, Kanatani A. NPY Y2 receptor agonist PYY(3-36) inhibits diarrhea by reducing intestinal fluid secretion and slowing colonic transit in mice. Peptides. 2010;31:671–675. doi: 10.1016/j.peptides.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 75.Hataya Y, Akamizu T, Takaya K, Kanamoto N, Ariyasu H, Saijo M, Moriyama K, Shimatsu A, Kojima M, Kangawa K, et al. A low dose of ghrelin stimulates growth hormone (GH) release synergistically with GH-releasing hormone in humans. J Clin Endocrinol Metab. 2001;86:4552. doi: 10.1210/jcem.86.9.8002. [DOI] [PubMed] [Google Scholar]
- 76.Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 77.Hosoda H, Kojima M, Kangawa K. Ghrelin and the regulation of food intake and energy balance. Mol Interv. 2002;2:494–503. doi: 10.1124/mi.2.8.494. [DOI] [PubMed] [Google Scholar]
- 78.Masuda Y, Tanaka T, Inomata N, Ohnuma N, Tanaka S, Itoh Z, Hosoda H, Kojima M, Kangawa K. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun. 2000;276:905–908. doi: 10.1006/bbrc.2000.3568. [DOI] [PubMed] [Google Scholar]
- 79.Fujino K, Inui A, Asakawa A, Kihara N, Fujimura M, Fujimiya M. Ghrelin induces fasted motor activity of the gastrointestinal tract in conscious fed rats. J Physiol. 2003;550:227–240. doi: 10.1113/jphysiol.2003.040600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dornonville de la Cour C, Lindström E, Norlén P, Håkanson R. Ghrelin stimulates gastric emptying but is without effect on acid secretion and gastric endocrine cells. Regul Pept. 2004;120:23–32. doi: 10.1016/j.regpep.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 81.Fukuda H, Mizuta Y, Isomoto H, Takeshima F, Ohnita K, Ohba K, Omagari K, Taniyama K, Kohno S. Ghrelin enhances gastric motility through direct stimulation of intrinsic neural pathways and capsaicin-sensitive afferent neurones in rats. Scand J Gastroenterol. 2004;39:1209–1214. [PubMed] [Google Scholar]
- 82.Edholm T, Levin F, Hellström PM, Schmidt PT. Ghrelin stimulates motility in the small intestine of rats through intrinsic cholinergic neurons. Regul Pept. 2004;121:25–30. doi: 10.1016/j.regpep.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 83.Levin F, Edholm T, Schmidt PT, Grybäck P, Jacobsson H, Degerblad M, Höybye C, Holst JJ, Rehfeld JF, Hellström PM, et al. Ghrelin stimulates gastric emptying and hunger in normal-weight humans. J Clin Endocrinol Metab. 2006;91:3296–3302. doi: 10.1210/jc.2005-2638. [DOI] [PubMed] [Google Scholar]
- 84.Tack J, Depoortere I, Bisschops R, Delporte C, Coulie B, Meulemans A, Janssens J, Peeters T. Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut. 2006;55:327–333. doi: 10.1136/gut.2004.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ariga H, Nakade Y, Tsukamoto K, Imai K, Chen C, Mantyh C, Pappas TN, Takahashi T. Ghrelin accelerates gastric emptying via early manifestation of antro-pyloric coordination in conscious rats. Regul Pept. 2008;146:112–116. doi: 10.1016/j.regpep.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 86.Ariga H, Tsukamoto K, Chen C, Mantyh C, Pappas TN, Takahashi T. Endogenous acyl ghrelin is involved in mediating spontaneous phase III-like contractions of the rat stomach. Neurogastroenterol Motil. 2007;19:675–680. doi: 10.1111/j.1365-2982.2007.00945.x. [DOI] [PubMed] [Google Scholar]
- 87.Tümer C, Oflazoğlu HD, Obay BD, Kelle M, Taşdemir E. Effect of ghrelin on gastric myoelectric activity and gastric emptying in rats. Regul Pept. 2008;146:26–32. doi: 10.1016/j.regpep.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 88.Tebbe JJ, Mronga S, Tebbe CG, Ortmann E, Arnold R, Schäfer MK. Ghrelin-induced stimulation of colonic propulsion is dependent on hypothalamic neuropeptide Y1- and corticotrophin-releasing factor 1 receptor activation. J Neuroendocrinol. 2005;17:570–576. doi: 10.1111/j.1365-2826.2005.01340.x. [DOI] [PubMed] [Google Scholar]
- 89.Goyal RK, Hirano I. The enteric nervous system. N Engl J Med. 1996;334:1106–1115. doi: 10.1056/NEJM199604253341707. [DOI] [PubMed] [Google Scholar]
- 90.El-Salhy M, Mazzawi T, Gundersen D, Hatlebakk JG, Hausken T. The role of peptide YY in gastrointestinal diseases and disorders (review) Int J Mol Med. 2013;31:275–282. doi: 10.3892/ijmm.2012.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Camilleri M. Integrated upper gastrointestinal response to food intake. Gastroenterology. 2006;131:640–658. doi: 10.1053/j.gastro.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 92.Lal S, McLaughlin J, Barlow J, D’Amato M, Giacovelli G, Varro A, Dockray GJ, Thompson DG. Cholecystokinin pathways modulate sensations induced by gastric distension in humans. Am J Physiol Gastrointest Liver Physiol. 2004;287:G72–G79. doi: 10.1152/ajpgi.00351.2003. [DOI] [PubMed] [Google Scholar]
- 93.Moran TH, Ladenheim EE, Schwartz GJ. Within-meal gut feedback signaling. Int J Obes Relat Metab Disord. 2001;25 Suppl 5:S39–S41. doi: 10.1038/sj.ijo.0801910. [DOI] [PubMed] [Google Scholar]
- 94.Smith GP, Falasco J, Moran TH, Joyner KM, Gibbs J. CCK-8 decreases food intake and gastric emptying after pylorectomy or pyloroplasty. Am J Physiol. 1988;255:R113–R116. doi: 10.1152/ajpregu.1988.255.1.R113. [DOI] [PubMed] [Google Scholar]


