Abstract
Increasing contamination and higher enrichment ratio of non-essential heavy metal cadmium (Cd) induce various toxic responses in plants when accumulated above the threshold level. These effects and growth responses are genotype and Cd level dependent. An experiment was conducted to analyze the effect of Cd toxicity in Brassica juncea [L] Czern and Coss by selecting its two varieties Varuna and RH-30. Cadmium (0, 25, 50 or 100 mg CdCl2 kg−1 of soil) fed to soil decreased the values of growth characteristics, activity of nitrate reductase and leaf water potential, whereas activities of antioxidant enzymes and proline content increased with the increasing concentration of Cd, observed at 30 and 60 day stages of growth, in both the varieties. Moreover, Cd uptake by the roots was higher in RH-30 than Varuna. Also the activity of antioxidant enzymes and proline accumulation were higher in Varuna with increasing soil level of Cd. Out of the two varieties, Varuna was more tolerant than RH-30 to Cd stress.
Keywords: Antioxidation, Cadmium toxicity, Leaf water potential, Nitrate reductase, Varietal difference
1. Introduction
Untreated sewage sludge, industrial waste water or inappropriate use of phosphate fertilizers to agricultural fields are progressively increasing the soil Cd level. Therefore, metal accumulator crop species take up and store Cd in their tissues at high concentration which not only poses threat to plant survival but also induces several toxic responses and raises the danger of food adulteration in crop plants. Cd competes with the uptake of other essential minerals and causes desiccation stress (Hernandez et al., 1996). When taken up in the cellular environment it binds with membranes and enzymes interfering with their functions and stability (Karcz and Kurtyka, 2007). Plant species generate a range of defense mechanisms to resist Cd induced toxicity and to recover the subsequent damages (Meharg, 1993; Mohamed et al., 2012) eliciting their genotype based biochemical responses. However, the resistance response relies on the interaction of genotype with dose of toxicity to show comparative resistance. The excessive uptake of nonessential bivalent cations to the aerial plant parts shifts its cellular phosphorylation state, eliciting oxidative stress and a range of physiological disturbances (Meharg, 1993; Akhtar and Macfie, 2012).
To counter this oxidative stress plants have an efficient system of stress enzymes and antioxidant non-enzyme molecules, that is termed as antioxidant system. Among these enzymes, superoxide dismutase (SOD) is the first line of defense against ROS, dismutating to oxygen molecule and H2O2. Another enzyme is catalase (CAT), that breaks H2O2 to water and oxygen while peroxidase (POX) scavenges H2O2 in chloroplast and cytosol of plant cells (Gill and Tuteja, 2010; Gill et al., 2011). The metabolite proline serves multiple functions in plant stress adaptions. It works as protein-compatible hydrotope, osmo-protectant, ROS scavenger and regulator of cellular redox status. Proline regulates the redox signal governing the metabolite pool and expression of several genes that affect plant growth and development (Kavi Kishor et al., 2005; Szabados and Savoure, 2010; Hayat et al., 2012).
Species of mustard are good accumulators of sufficient quantities of Cd in their tissues. The brown mustard or Brassica juncea [L] Czern and Coss is economically very important crop, primarily used to harvest edible oil and also as a vegetable. However, Cd toxicity responses of different varieties vary greatly and are dependent on the interaction of the genotype with the type of metal and its concentration. The varieties of B. juncea could be classified as sensitive or resistant based on their responses to Cd toxicity. The objective of the study is to assess the level of oxidative stress, internal Cd level and the efficiency of antioxidant enzymes which might play a regulatory role against Cd induced metabolic shift.
2. Materials and methods
Seeds of B. juncea; Varuna and RH-30, procured from the National Seed Corporation, New Delhi, India, were surface sterilized (with 0.01% HgCl2) followed by repeated washings with double distilled water (DDW). A completely randomized block design experiment was arranged in the net house of Department of Botany of Aligarh Muslim University, Aligarh, India during September-February 2009–2010 under the ambient environmental conditions with optimum temperature that varied from 10 to 30 °C.
Seeds were sown in earthen pots (25 × 25 cm) filled with 5 kg of soil containing sandy loam soil and farmyard manure (6:1 v/v), urea, single superphosphate and muriate of potash were added at 40, 138 and 26 mg kg−1 of soil, respectively. Soil in the selected pots was mixed with Cd (0, 25, 50 or 100 mg CdCl2 kg−1 of soil) and watered on alternate days. Both the varieties were sampled at two growth stages (30 and 60 DAS). The plants were removed from the pots along with the soil and were dipped in a bucket filled with tap water. The plants were gently moved to remove the adhering soil particles.
2.1. Growth analysis
The length and fresh mass of roots and shoots were measured using a meter scale and an electronic balance, respectively. The leaf area was measured manually using a graph sheet, where the squares covered by the leaf were counted. The plants were then placed in an oven at 80 °C for 72 h. The dried plants were then weighed to record plant dry mass.
2.2. Nitrate reductase activity
Nitrate reductase (NR) activity was measured by the method of Jaworski (1971) in fresh leaf samples that were cut into small pieces. The absorbance was read at 540 nm and the activity of NR [n mole NO2 g−1 (FM) s−1] was calculated.
2.3. Antioxidant enzyme activities
The activity of peroxidase (POX) and catalase (CAT) were assayed following the procedure described by Chance and Maehly (1955). The activity of superoxide dismutase (SOD) was assayed by measuring its ability to inhibit the photochemical reduction of nitroblue tetrazolium (NBT) using the method of Beauchamp and Fridovich (1971). The amount of enzyme which causes 50% inhibition in photochemical reduction of NBT was considered as one enzyme unit.
2.4. Leaf water potential and proline content
Leaf water potential, was measured in fresh, detached leaves of the sampled plants by using PSYPRO, leaf water potential system (WESCOR, Inc. Longman, USA). The proline content in fresh leaf samples was determined by the method of Bates et al. (1973). The absorbance of the toluene layer was read at 528 nm, on a spectrophotometer (Milton & Roy, USA).
2.5. Cd accumulation in root and shoot
The root and shoot samples were placed for 10 min in ice cold 5 mM CaCl2 solution to displace extracellular Cd, rinsed with DDW and then oven dried (Meuwly and Rauser, 1992). Cd concentration in tissues was estimated after digesting the samples in nitric acid:perchloric acid (3:1, v/v). Cd concentration was determined by an atomic absorption spectrophotometer (Perkin-Elmer A, Analyst, 300).
2.6. Statistical analysis
The experiment was conducted according to simple randomized block design. Each treatment was replicated five times and three plants were maintained in each pot, representing a replicate. Treatment means were compared by the analysis of variance (ANOVA) using SPSS 17.0 for Windows (SPSS, Chicago, IL, USA). Least Significant Difference (LSD) between treatment means was calculated at the 5% level of probability.
3. Results
3.1. Growth parameters
Cd (0, 25, 50 or 100 mg kg−1) administered through the soil significantly declined the growth (length, fresh mass, dry mass of root and shoot and leaf area) parameters in both the varieties in a concentration dependent manner both at 30 and 60 DAS (Fig. 1A–G). The highest concentration of Cd (100 mg kg−1) caused maximum damage and decreased the root and shoot length by 65% and 39%, fresh mass 67% and 55%, dry mass 69% and 65%, and leaf area 54%, respectively, as compared to control plants of RH-30, at 30 DAS. The reduction was higher in RH-30 than Varuna at both the growth stages (30 and 60 DAS). However, per cent loss was more at 30 DAS.
Figure 1.
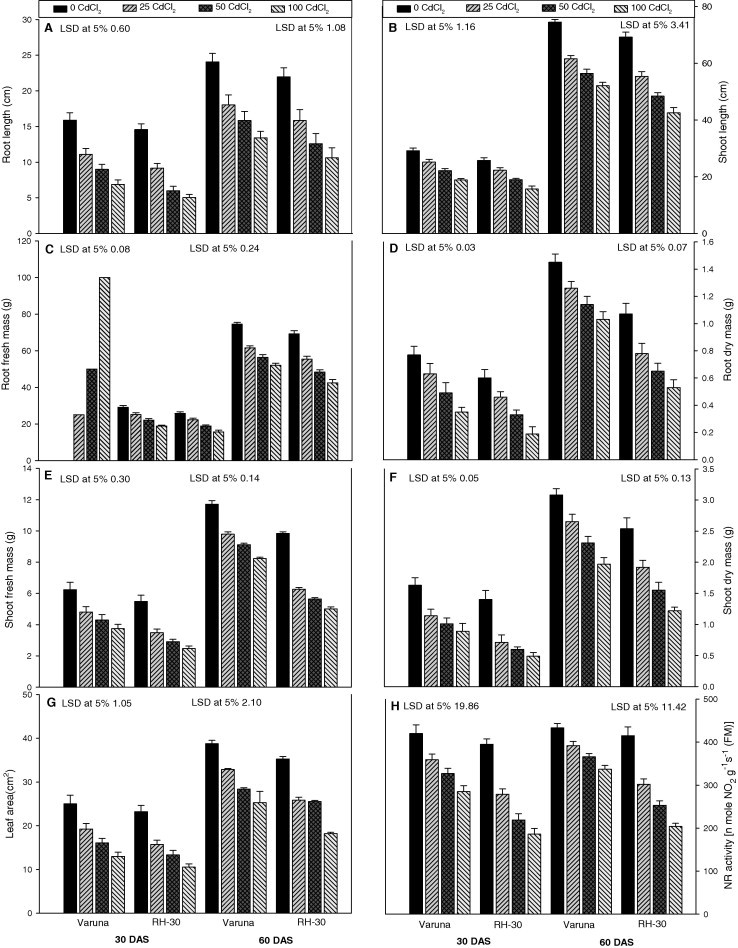
Effect of soil amended cadmium (CdCl2; 0, 25, 50 or 100 mg Kg−1 of soil) induced changes in the (A) root length, (B) shoot length, (C) root fresh mass, (D) root dry mass, (E) shoot fresh mass, (F) shoot dry mass, (G) leaf area, (H) NR activity of Varuna and RH-30 varieties of Brassica juncea L. plants at 30 and 60 DAS.
3.2. Nitrate reductase activity
As depicted in Fig. 1H, nitrate reductase (NR) activity decreased significantly as the concentration of soil Cd increased in both the varieties at 30 and 60 DAS. The maximum decline was observed at a concentration of 100 mg kg−1 of Cd which reduced the activity by 32% and 22% in Varuna and 53% and 51% in RH-30 at 30 and 60 DAS, respectively, as compared to their control plants. The loss in the activity was more prominent in RH-30 than Varuna. The per cent activity increased with the age of plants from 30 to 60 DAS.
3.3. Antioxidant enzyme activity
The activity of antioxidant enzymes; [peroxidase (POX), catalase (CAT) and superoxide dismutase (SOD)] in the leaves of plants increased significantly in response to Cd in a concentration dependent manner at both the growth stages (Fig. 2A–C). The highest Cd level (100 mg kg−1) caused maximum increase in enzyme activity that was 57% and 55% (POX), 36% and 23% (CAT) and 81% and 71% (SOD) in Varuna and RH-30, respectively, as compared to the control, at 30 DAS. Per cent enzyme activity decreased with the growth advancement from 30 to 60 DAS. Varuna possessed more enzyme activity than RH-30 at both the growth stages.
Figure 2.
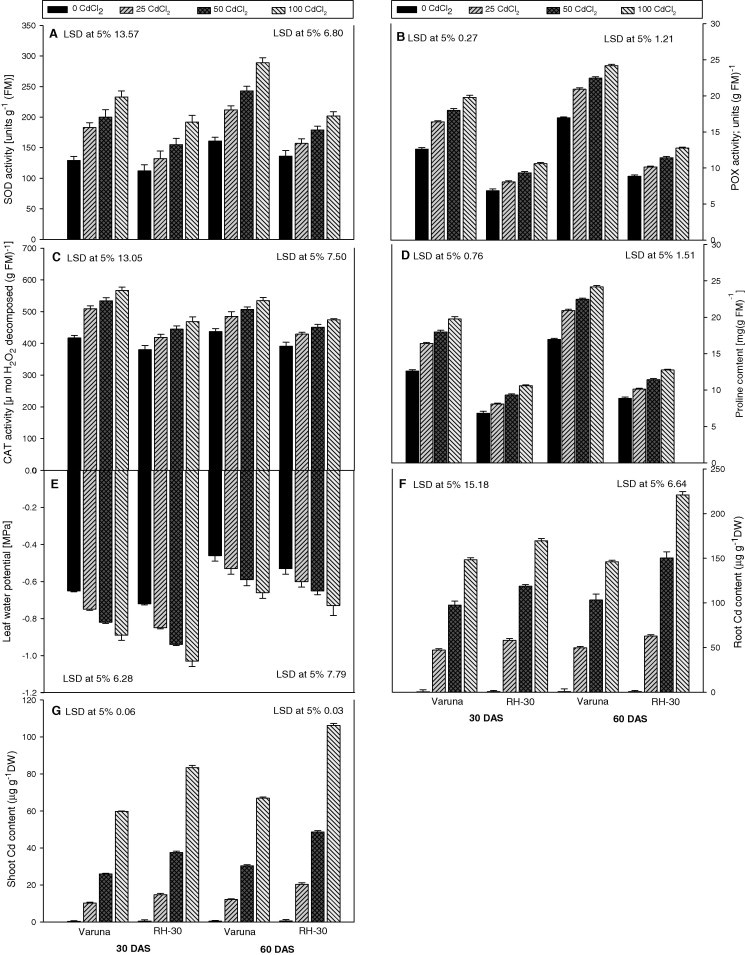
Effect of soil amended cadmium (CdCl2; 0, 25, 50 or 100 mg Kg−1 soil) induced changes in the (A) SOD activity, (B) POX activity, (C) CAT activity, (D) proline content, (E) leaf water potential, (F) root Cd content, (G) shoot Cd content of Varuna and RH-30 varieties of Brassica juncea L. plants at 30 and 60 DAS.
3.4. Proline content
It is evident from Fig. 2D that soil amended with Cd caused a significant increase in the proline content in a concentration (0, 25, 50 or 100 mg CdCl2 kg−1) dependent manner in Varuna and RH-30 at both the growth stages. The highest concentration (100 mg kg−1) of Cd caused maximum accumulation of proline that was 82% and 66% in Varuna and 75% and 63% in RH-30, as compared to their control plants at 30 and 60 DAS, respectively. Varuna accumulated more proline as compared to RH-30 in response to all the treatments.
3.5. Leaf water potential (ψ)
The leaf water potential (LWP) decreased with an increase in the Cd level in the soil in both the varieties at 30 and 60 DAS (Fig. 2E). The highest level of Cd caused maximum reduction that was 37% and 43% in Varuna and RH-30 at 60 DAS, respectively, compared to the control plants. RH-30 was more vulnerable to Cd stress than Varuna at both the growth stages. The degree of Cd toxicity was more at early stage (30 DAS) of the growth than at latter stage (60 DAS).
3.6. Cd accumulation in root and shoot
An increasing trend of Cd accumulation was recorded both in root and shoot tissues with the increase of CdCl2 in the soil (Fig. 2F and G). Shoot comparatively accumulated lesser quantities of Cd than root in both the mustard varieties. The percent increase in the Cd accumulation was higher in RH-30 than Varuna at 30 DAS. At the highest concentration of Cd (100 mg kg−1) Varuna accumulated 148 μg and 60 μg Cd and RH-30 170 μg and 83 μg Cd g−1 of root and shoot dry mass, respectively, at 30 DAS.
4. Discussion
Plant genotypes differ in their ability to take up and translocate soil-amended Cd from roots to shoots (Metwally et al., 2005). The ability to check root uptake and aerial distribution of Cd depends on its binding to extracellular matrix, root efflux, intracellular detoxification and its transport efficiency (Marchiol et al., 1996; Akhtar and Macfie, 2012; Meng et al., 2012). In this study Varuna, compared with RH-30, accumulated lesser quantity of Cd both in root and shoot tissues (Fig. 2F and G) because of the reasons mentioned earlier. The absorbed Cd accumulates preferably in plant roots followed by shoots, which often restricts the uptake and distribution of other nutrients (Gomes et al., 2013 and Fig. 2F and G). This study indicates, the level of the metal increased with a progressive increase in the soil Cd content (0, 25, 50 or 100 mg kg−1) both in root and shoot.
Cadmium uptake at toxic level causes mineral deficiency, desiccation and cellular metabolic disturbances (Marshner, 2012; Gomes et al., 2013) in plants. Cadmium alters the membrane permeability and hence cellular LWP (Fig. 2D). Cd affected membrane potential and proton pump activity could restrict the growth of maize plants (Karcz and Kurtyka, 2007). Moreover, Cd brought about aquaporin mediated reduction in maize root hydraulic conductivity that reduced the cellular turgor and leaf elongation even without changing transpiration (Ehlert et al., 2009). Therefore, an increase in Cd concentration both in root and shoot (Fig. 2F and G) partially damaged the membrane which resulted in decreased LWP (Fig. 2D). However, proline accumulation is an adaptive mechanism to counter osmotic stress caused by decreased LWP; therefore, it reestablishes the LWP and augments the loss of cellular osmoticum (Alia and Saradhi, 1991; Albert et al., 2012). Varuna accumulated more proline than RH-30 which could have favored the maintenance of LWP in this cultivar (Fig. 2D and E).
The Cd binding to root epidermal membrane affects the functioning of transporter proteins either through direct binding to the ion transporters or via membrane assisted ROS production. The competitive exclusion of the substrate () potentially impeded the NR activity (Hernandez et al., 1996; Campbell; 1999; Fig. 1H) or, alternatively, the metal could have bound with the –SH group, directly affecting the enzyme structure and its functions (Choudhary and Singh, 2000). Cadmium induced supra-optimal generation of ROS could interfere with the active state of NR rendering it inactive. The NR activity exhibited a progressive decline in response to increasing dose of Cd (Fig. 1H). However, proline protects membranes and subcellular structures, hydrates the enzymes to restore their activity and neutralizes reactive oxygen/nitrogen species (Hare and Cress, 1997; Kavi Kishor et al., 2005), its increased detoxification capacity (Fig. 2A–C) may have potentially protected the NR activity more effectively in Varuna as compared to RH-30.
Besides proline, antioxidant enzymes are also the key players in maintaining cellular redox status and stress induced plant tolerance (Kavi Kishor et al., 2005; Gill and Tuteja, 2010; Gill et al., 2011; Hayat et al., 2012). The higher activity of antioxidant enzymes (i.e. POX, CAT and SOD) was in proportion to the progressive increase in the concentration of Cd (CdCl2; 0, 25, 50 or 100 mg Kg−1 of soil; Fig. 2A–C). Moreover, the per cent increase in antioxidant enzymes was more in Varuna as compared to RH-30 (Figs. 2A–C). Mohamed et al. (2012) has shown in B. juncea that the higher activity of antioxidative enzymes offers a greater detoxification efficiency which provides better resistance to a plant variety against heavy metal induced oxidative stress.
The increased uptake and accumulation of heavy metal in plants cause osmotic shift, metabolic alterations and also ROS induced damages (Gill and Tuteja, 2010; Gill et al., 2011) while Cd induced mineral stress could reduce plant dry weight accumulation (Marshner, 2012). Cadmium induced restricted water uptake hampers turgor mediated wall extensibility (Marchiol et al., 1996) which could decrease cell division (Marshner, 2012). Cadmium mediated cumulative effect of these factors caused a decrease in leaf area, fresh and dry mass, and length of root and shoot (Fig. 1A–G). The values for all these growth characteristics decreased in a dose dependent manner of Cd level in the two mustard varieties (Varuna and RH-30). However, the increased activity of antioxidant enzymes and that of proline level presumably protected the metabolic machinery, stabilized the membranes to prevent water loss and supported nutrient uptake to augment growth performance more in Varuna than RH-30. The present findings get additional support from the work of Sharma et al. (2010) and Hasan et al. (2011) in tomato and Hayat et al. (2011) in brassica, respectively, under heavy metal stress.
5. Conclusion
The two mustard varieties (Varuna and RH-30) responded differentially against Cd induced oxidative stress. Varuna was more resistant than RH-30. The increased activity of antioxidant enzymes and leaf proline level protected the plant growth in a genotype dependent manner besides the restricted uptake and transport of Cd.
Acknowledgements
The authors are thankful to Chairman, Department of Botany, Aligarh Muslim University, India for providing necessary facilities and the University Grant Commission, New Delhi, India for non-NET-JRF fellowship.
Footnotes
Peer review under responsibility of King Saud University.
References
- Akhtar M.F., Macfie S.M. Species-specific relationship between transpiration and cadmium translocation in lettuce, barley and radish. J. Plant Stud. 2012;1 [Google Scholar]
- Albert B., Le Cahérec F., Niogret M.F., Faes P., Avice J.C., Leport L., Bouchereau A. Nitrogen availability impacts oilseed rape (Brassica napus L.) plant water status and proline production efficiency under water-limited conditions. Planta. 2012;236:659–676. doi: 10.1007/s00425-012-1636-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alia, Saradhi P.P. Proline accumulation under heavy metal stress. J. Plant Physiol. 1991;138:504–508. [Google Scholar]
- Bates L.S., Waldren R.T., Teare I.D. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase improved assays and assay applicable to acrylamide gels. Ann. Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Campbell W.H. Nitrate reductase structure, function and regulation bridging the gap between biochemistry and physiology. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999;50:277–303. doi: 10.1146/annurev.arplant.50.1.277. [DOI] [PubMed] [Google Scholar]
- Chance B., Maehly A.C. Assay of catalase and peroxidase. Methods Enzymol. 1955;2:764–775. [Google Scholar]
- Choudhary A., Singh R.P. Cadmium induced changes in diamine oxidase activity and polyamines levels in Vigna radiata Wilczek seedlings. J. Plant Physiol. 2000;156:704–710. [Google Scholar]
- Ehlert C., Maurel C., Tardieu F., Simonneau T. Aquaporin-mediated reduction in maize root hydraulic conductivity impacts cell turgor and leaf elongation even without changing transpiration. Plant Physiol. 2009;150:1093–1104. doi: 10.1104/pp.108.131458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S.S., Khan N.A., Tuteja N. Differential cadmium stress tolerance in five Indian mustard (Brassica juncea L.) cultivars, an evaluation of the role of antioxidant machinery. Plant Signal. Behav. 2011;6:293–300. doi: 10.4161/psb.6.2.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S.S., Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Gomes M.P., Marques T.C.L.L.S.M., Soares A.M. Cadmium effects on mineral nutrition of the Cd-hyperaccumulator Pfaffia glomerata. Biologia. 2013;68(2):223–230. [Google Scholar]
- Hare P.D., Cress W.A. Metabolic implications of stress induced proline accumulation in plants. Plant Growth Regul. 1997;21:79–102. [Google Scholar]
- Hernandez L.E., Carpena-Ruiz R., Garate A. Alterations in the mineral nutrition of pea seedlings in exposed to cadmium. J. Plant Nutr. 1996;19:1581–1586. [Google Scholar]
- Hasan S.A., Hayat S., Ahmad A. Brassinosteroids protect photosynthetic machinery against the cadmium induced oxidative stress in two tomato cultivars. Chemosphere. 2011;84:1446–1451. doi: 10.1016/j.chemosphere.2011.04.047. [DOI] [PubMed] [Google Scholar]
- Hayat S., Hasan S.A., Ahmad A. Growth, nitrate reductase activity and antioxidant system in cadmium stressed tomato (Lycopersicon esculentum Mill) cultivars. Biotech. Agron. Soc. Environ. 2011;15:401–414. [Google Scholar]
- Hayat S., Hayat Q., Alyemeni M.N., Wani A.S., Pichtel J., Ahmad A. Role of proline under changing environments: a review. Plant Signal. Behav. 2012;7:1456–1466. doi: 10.4161/psb.21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski E.G. Nitrate reductase assay in intact plant tissues. Biochem. Biophys. Res. Comm. 1971;43:1274–1279. doi: 10.1016/s0006-291x(71)80010-4. [DOI] [PubMed] [Google Scholar]
- Karcz W., Kurtyka R. Effect of cadmium on growth, proton extrusion and membrane potential in maize coleoptile segments. Biol. Plant. 2007;51:713–719. [Google Scholar]
- Kavi Kishor P.B., Sangam S., Amrutha R.N., Sri Laxmi P., Naidu K.R., Rao K.R.S.S., Rao S., Reddy K.J., Theriappan P., Sreenivasulu N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005;88:424–438. [Google Scholar]
- Marchiol L., Leita L., Martin M., Peressotti A., Zerbi G. Physiological responses of two soybean cultivars to cadmium. J. Environ. Qual. 1996;25:562–566. [Google Scholar]
- Marshner P. third ed. Academic Press; London, UK: 2012. Marschner’s Mineral Nutrition of Higher Plants. [Google Scholar]
- Meharg A.A. Integrated tolerance mechanisms: constitutive and adaptive plant response to elevated metal concentrations in the environment. Plant Cell Environ. 1993;17:989–993. [Google Scholar]
- Meng H., Hua S., Shamsi I.H., Jilani G., Li Y., Jiang L. Cadmium-induced stress on the seed germination and seedling growth of Brassica napus L. and its alleviation through exogenous plant growth regulators. Plant Growth Regul. 2012;58:47–59. [Google Scholar]
- Metwally A., Safronova V.I., Belimov A.A., Dietz K.J. Genotypic variation of the response to cadmium toxicity in Pisum sativum L. J. Exp. Bot. 2005;409:167–178. doi: 10.1093/jxb/eri017. [DOI] [PubMed] [Google Scholar]
- Meuwly P., Rauser W.E. Alteration of thiol pools in roots and shoots of maize seedlings exposed to cadmium. Adaptation and developmental cost. Plant Physiol. 1992;99:8–15. doi: 10.1104/pp.99.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed A.A., Castagna A., Ranieri A., Sanità di Toppi L. Cadmium tolerance in Brassica juncea roots and shoots is affected by antioxidant status and phytochelatin biosynthesis. Plant Physiol. Biochem. Pp. 2012;1–8 doi: 10.1016/j.plaphy.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Sharma A., Sainger M., Dwivedi S., Srivastava S., Tripathi R.D., Singh R.P. Genotypic variation in Brassica juncea (L.) Czern. cultivars in growth, nitrate assimilation, antioxidant responses and phytoremediation potential during cadmium stress. J. Environ. Biol. 2010;31:773–780. [Google Scholar]
- Szabados L., Savoure A. Proline: a multifunctional amino acid. Trends Plant Sci. 2010;15:89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]



