Abstract
Depolarisation-induced Ca2+ influx into electrically excitable cells is determined by the density of voltage-gated Ca2+ channels at the cell surface. Surface expression is modulated by physiological stimuli as well as by drugs and can be altered under pathological conditions. Extracellular epitope-tagging of channel subunits allows to quantify their surface expression and to distinguish surface channels from those in intracellular compartments. Here we report the first systematic characterisation of extracellularly epitope-tagged CaV2.1 channels. We identified a permissive region in the pore-loop of repeat IV within the CaV2.1 α1 subunit, which allowed integration of several different tags (hemagluttinine [HA], double HA; 6-histidine tag [His], 9-His, bungarotoxin-binding site) without compromising α1 subunit protein expression (in transfected tsA-201 cells) and function (after expression in X. laevis oocytes). Immunofluorescence studies revealed that the double-HA tagged construct (1722-HAGHA) was targeted to presynaptic sites in transfected cultured hippocampal neurons as expected for CaV2.1 channels. We also demonstrate that introduction of tags into this permissive position creates artificial sites for channel modulation. This was demonstrated by partial inhibition of 1722-HA channel currents with anti-HA antibodies and the concentration-dependent stimulation or partial inhibition by Ni-nitrilo triacetic acid (NTA) and novel bulkier derivatives (Ni-trisNTA, Ni-tetrakisNTA, Ni-nitro-o-phenyl-bisNTA, Ni-nitro-p-phenyl-bisNTA). Therefore our data also provide evidence for the concept that artificial modulatory sites for small ligands can be introduced into voltage-gated Ca2+ channel for their selective modulation.
Keywords: voltage-gated calcium channels, extracellular epitope-tagging, calcium channel blockers, nickel-NTA, immunocytochemistry, live cell staining
Introduction
The family of voltage-gated calcium channels (VGCCs) comprises 10 different isoforms that fulfil important functions in a wide range of physiological situations like muscle contraction, neurotransmitter release and hormone secretion.1-3 Immunohistochemical localisation studies were an important prerequisite to reveal the expression of the different isoforms in various tissues and subcellular compartments. Artificial peptide sequences specifically recognised by antibodies (epitope tags) have been introduced into extracellularly accessible positions of α1 subunits to biochemically quantify the channel population mediating Ca2+ influx on the cell surface,4,5 to study their membrane turnover6 and to distinguish them from intracellular channel pools.7,8 Based on current folding models, less than 20% of amino acids of CaV1 and CaV2 Ca2+ channels face the extracellular space and are thus suitable for tag insertion. The vast majority of those regions comprise the pore-forming S5–S6 linkers in each homologous repeat.1,9 Tag insertion into these regions may disturb channel function and/or expression making experimental results obtained with such constructs difficult to interpret. Up to now, there was no report about a systematic analysis of different insertion regions to gain a fully functional tagged CaV2.1 voltage-gated calcium channel. CaV2.1 channels are the major isoform expressed in synapses, where they control fast neurotransmitter release. Monitoring the trafficking and localisation pattern of CaV2.1 channels may allow clearer insight into their functioning and regulation and help to clarify the pathophysiological mechanisms of neurological diseases caused by mutations in CaV2.1 α1 subunits.10-12
Regions permissive for tag insertion (especially within pore-forming regions) may also serve as artificial binding sites for ligands that modulate channel function, allowing channel activation or inhibition despite the absence of specific non-peptide modulators, as is the case for CaV2.1 channels. This concept would apply for cell culture systems as well as for mutant mouse strains, which could be used to predict potential therapeutic actions of CaV2.1-specific modulators. There have been attempts to generate specific ion channel blockers by engineering antibodies selectively binding to the third extracellular region (E3; the sequence stretch extending from the extracellular end of segments S5 to the selectivity filter) of an α1 subunit channel domain. Indeed, partial block of TRPV5- and NaV1.5 channels by this approach has been reported.13
In this study we provide a systematic analysis of the functional effects of tagging CaV2.1 α1 subunits at multiple extracellular locations and identify functionally silent positions. Thereby we also provide a proof-of-concept for the introducing artificial modulatory sites into voltage-gated calcium channels (VGCCs).
Results
Identification of a permissive region for insertion of epitope tag sequences in CaV2.1 α1
To identify regions in the α1 subunit of CaV2.1 allowing introduction of extracellular tags not interfering with function and protein expression we generated a series of tagged subunits using random insertion of HA tag by a Tn5-transposon based approach as well as restriction enzyme cloning.
Tn5 transposon-mediated random cloning resulted in the insertion of a 27 amino acid peptide consisting of the HA tag (nine amino acids) flanked on either side by nine amino acid transposase recognition sites (for details see Materials and Methods). Random integration of this sequence into the coding sequence of CaV2.1 α1 yielded nineteen individual positions of the HA tag (Fig. 1A, filled dots), with an apparent hot spot of integration at 5′ codon 1908. This is most likely due to sequence similarity at this position to a previously reported target consensus sequence for Tn5 insertion (see inset Fig. 1A).14 As shown in Figure 1A four of the nineteen insertion sites were located in putatively extracellular regions of CaV2.1 α1: TP-280 and TP-336 in the pore loop of domain I, TP-685 in the pore loop of domain II, and TP-1269 in the S1–S2 linker of domain III. Immunoblot analysis of all four extracellularly tagged clones and of three internally tagged control constructs (TP-916, TP-953 and TP-1886, for location see Fig. 1A) is shown in Figure 1B. Both TP-280 and TP-336 showed protein expression comparable to wild-type levels when tested with an antibody against the α1 subunit of CaV2.1 (rabbit anti-CaV2.11141-1156,16 upper lane) and a strong signal with an antibody against the HA tag (lower lane). This was also observed for the three intracellularly tagged clones tested (Fig. 1B). In contrast, expression levels of the other two extracellularly tagged α1 subunits were much lower than wild-type (TP-1269) or even absent (TP-685).
Figure 1. Location of HA tags in the α1 subunit of CaV2.1 inserted by a Tn5 transposon-based approach and rationally designed tag positions as well as protein expression of various tested constructs.
(A) Topography of CaV2.1 α1 subunit shown in an open conformation visualising the four domain structure and 24 transmembrane segments. N- and C-terminus reside in the cytosol. HA tags introduced via a random Tn5 transposon-based approach are indicated by filled dots. Note that 4 of the 19 HA tags lie at positions putatively accessible from the extracellular space. Positions of rationally designed tags at positions 1722, 1727 and 1732 are indicated by open circles. Inset: Alignment between preferred integration site found in CaV2.1 α1 for six of 25 transposon insertions (upper sequence) and a reported target consensus sequence for Tn5 integration (lower sequence).14 (B) Western blot analysis of seven randomly HA-tagged constructs (four of which are at putatively extracellular positions) in expression vectors pCMV15 or pβAeGFP7 after heterologous transfection into tsA201 cells (together with α2δ-1 and β3). Membrane preparation and immuno-blotting was performed as described under Materials and Methods. Upper lanes stained with rabbit antibody anti-CaV2.11141-1156;16 lower lanes with mouse anti-HA antibody (clone 12CA5, Roche). All subunits migrated at the expected molecular mass as indicated by an arrow. With the exception of TP-1886 which was blotted only twice, one representative experiment of at least three is shown.
Since protein expression similar to wild-type was a predefined criterion for our further functional characterisation, only TP-280 and TP-336 qualified for further electrophysiological analysis in Xenopus laevis oocytes. Neither of the two constructs was able to conduct measurable currents (n > 18, at least three independent cRNA injections in which efficient wild-type CaV2.1 currents were measured in control experiments; data not shown). Therefore none of these extracellularly tagged constructs was suitable for further characterisation.
We also identified an insertion site on a more rational basis and used conventional cloning techniques to reduce insert length. Alignment of all the α1 subunits of the CaV1 and CaV2 family revealed that CaV2.1 α1 contains an eight amino acid stretch (position 1722–1729) in the pore loop of domain IV that solely appears in this isoform (Fig. 2A) and may indicate some structural flexibility in this region. We therefore tested if HA tag insertion into this region yields functional CaV2.1 channels by exchanging amino acids 1722 to 1729 with the sequence for a single HA tag (1722-HA, Fig. 2B). In addition, a tandem HA construct (two HA tags linked by a glycine residue) was generated (1722-HAGHA, Fig. 2B). Like TP-280 and TP-336, 1722-HAGHA and 1722-HA were positive for HA-immunoreactivity in immunoblot analysis and expressed at protein levels comparable to wild-type (Fig. 3A). We therefore tested if their functional properties were affected by tag insertion by co-expressing CaV2.1 α1 or 1722-HA or 1722-HAGHA cRNA together with β3 and α2δ-1 in Xenopus laevis oocytes. For both the single and tandem HA-tagged α1 subunit no statistically significant functional changes were found for the voltage of half-maximal activation (V0.5;act), the voltage of IBa (Vmax), the steepness of the activation curve (k0.5;act) (Fig. 3B, Table 1), IBa inactivation during trains of short pulses (Fig. 3C, Table 1) and during 3-s depolarisations to Vmax (Fig. 3D, Table 2). Current densities of 1722-HA (−0.68 ± 0.34 μA, n = 16) or 1722-HAGHA (−0.78 ± 0.29 μA, n = 15) were also indistinguishable from wild-type (−0.73 ± 0.28 μA, n = 14) channels. 1722-HA channels current properties did also not differ from wild-type when heterologously expressed in tsA-201 cells. No changes were found in the voltage-dependence of activation (wild-type vs. 1722-HA: V0.5;act: −4.9 ± 0.6 mV, −6.7 ± 1.1 mV; kact: −4.5 ± 0.1 mV, −4.3 ± 0.4 mV, n = 5) and inactivation (V0.5;inact: −46.5 ± 1.7 mV, −45.9 ± 1.1 mV; kinact: −6.2 ± 0.3, −5.4 ± 0.2, n = 9–11), in the activation and inactivation time courses (n = 9–11; not illustrated) and in current densities (110.4 ± 86.2 pA/pF, n = 13; 115.7 ± 70.8 pA/pF, n = 11).
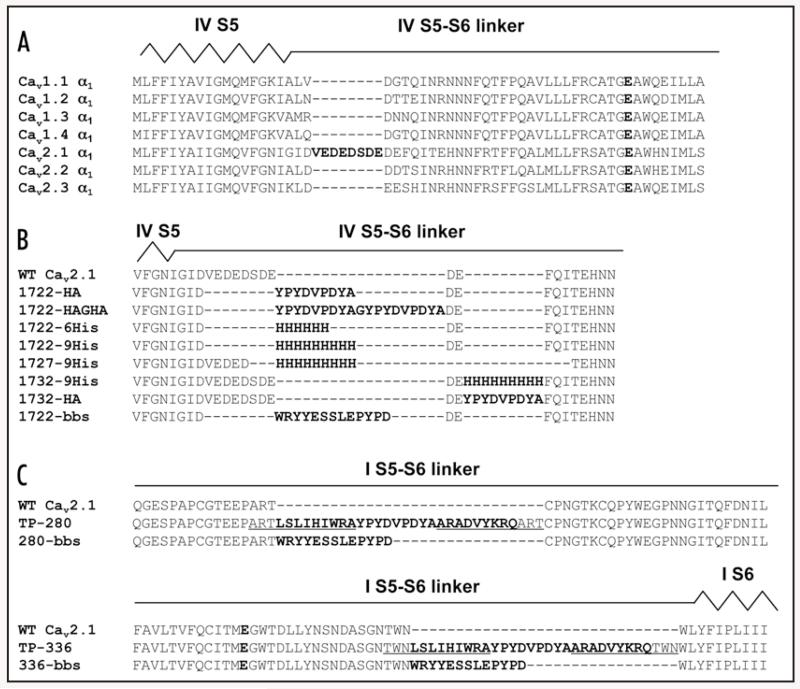
Figure 2. Sequences of tag insertion in extraccellular positions in CaV2.1 α1.
(A) Amino acid sequence alignment of α1 subunits of the CaV1 and CaV2 family from 1702 to 1765 (numbering according to CaV2.1 α1). Accession numbers of all α1 sequences given in section Materials and Methods. Putative locations of domain IV segment S5 (IV S5) and the linker connecting domain IV S5 and S6 segment (IV S5–S6 linker) are indicated above the alignment. The glutamate residue responsible for calcium selectivity is shown as bold letter. (B) Amino acid sequence alignment of wild-type CaV2.1 α1 subunit (residues 1714 to 1739) with 1722-HA, 1722-HAGHA, 1722-6His, 1722-9His, 1727-9His, 1732-9His, 1732-HA and 1722-bbs. Putative locations of domain IV segment S5 (IV S5) and the linker connecting domain IV S5 and S6 segment (IV S5-S6 linker) are indicated in analogy to Figure 2A. In 1722-HA amino acids 1722 to 1729 have been replaced with the nine amino acid sequence of the HA tag (YPYDVPDYA) by SOE PCR. For 1722-HAGHA an additional HA tag plus a single glycine to space the two tags have been inserted into 1722-HA. For CaV2.1 α1 with 6 histidines at position 1722 (1722-6His), CaV2.1 α1 with 9 histidines at position 1722 (1722-9His), and CaV2.1 α1 with a bungarotoxin-binding site (bbs) at position 1722 (1722-bbs) amino acids 1722 to 1729 were exchanged with 6 histidines, 9 histidines, or bbs, respectively. For 1727-9His amino acids 1727 to 1734 were exchanged with 9 histidines, whereas 1732-9His harbours 9 histidines at position 1732 without deletion of any wild-type sequences. The analogue construct to 1732-9His, 1732-HA, harbours an HA-tag instead of the 9 histidines. All epitope sequences are shown in bold letters. All primers are listed in Materials and Methods. (C) Amino acid sequence alignment of wild-type CaV2.1 α1 subunit (residues 264 to 304) with 280-bbs, and wild-type CaV2.1 α1 subunit (residues 305 to 345) with 336-bbs. Putative locations of the linker connecting domain I S5 and S6 segment (I S5-S6 linker) and the transmembrane segment S6 (I S6) are indicated above the alignments. The glutamate residue responsible for calcium selectivity is shown as bold letter. In 280-bbs and 336-bbs, bbs was introduced without deletion of any wild-type sequences either at position 280 (280-bbs) corresponding to the random HA integration site of TP-280 located in the pore loop of domain I or at position 336 (336-bbs) corresponding to the random HA integration site of TP-336 located in the pore loop of domain I. Underlined amino acids in TP-280 and TP-336 have been replicated in the transposase reaction (see Materials and Methods). Amino acids translated from transposase recognition sequences (mosaic ends, ME) are depicted as underlined and bold. All primers are listed in Materials and Methods.
Figure 3. Electrophysiological characterisation of 1722-HA and 1722-HAGHA; modulation of 1722-HA with rat anti-HA antibody.
(A) Western blot analysis of tsA201 cells heterologously transfected with α2δ-1 and β3 cDNAs together with either 1722-HA or 1722-HAGHA. All subunits migrated at the expected molecular mass as indicated by an arrow. One representative experiment of at least three is shown. (B) Wild-type CaV2.1 α1, 1722-HA, or 1722-HAGHA cRNA was co-injected with auxiliary subunits into Xenopus laevis oocytes as described in section Materials and Methods. IBa was measured 1–3 days after injection (10 mM Ba2+ as charge carrier) in the indicated number of cells. Wild-type shown in black, 1722-HA in grey, and 1722-HAGHA in open symbols or dotted line. Current-voltage relationship was determined by depolarising pulses to test potentials ranging from −40 to + 30 mV from a holding potential of −80 mV. (C) Use-dependence protocol elicited 15 pulses of 100 ms in a frequency of 1 Hz from a holding potential of −60 mV to Vmax. Peak currents were normalised to peak IBa of the first pulse and are plotted against the pulse number (means ± SD). (D) Representative traces all normalised to a peak IBa of 1 μA obtained during a 3-s depolarising pulse from holding potential −80 mV to Vmax. (E) Application of 25 nM rabbit IgG as control to wild-type (black bar) and 1722-HA (grey bar), as well as 25 nM rat anti-HA antibody to 1722-HA (open bar) shown as bar graphs for the indicated number of experiments. All data are shown normalised to baseline IBa before drug application. Antisera were applied to the measured oocyte in 0.1 mg/ml BSA in bath solution. A forth experiment also showed a blocking trend but could not be quantified due to a current runup and was therefore not included in the statistical evaluation. Data are means ± SD for the indicated number of experiments. Statistical data were obtained by Student’s t test and are indicated as **p < 0.01.
Table 1. Effects of tag insertions on IBa parameters of activation and inactivation.
| V0.5;act [mV] | k0.5;act [mV] | Vmax [mV] | n | Inactivation after pulse trains [%] | n | |
|---|---|---|---|---|---|---|
| wild-type CaV2.1 | −2.8 ± 2.0 | −3.1 ± 0.6 | 4.9 ± 2.5 | 14 | 41.9 ± 7.0 | 13 |
| 1722-HA | −2.2 ± 2.4 | −3.3 ± 0.8 | 5.6 ± 2.9 | 16 | 37.0 ± 6.1 | 12 |
| wild-type CaV2.1 | −4.3 ± 1.7 | −2.9 ± 0.5 | 3.2 ± 2.3 | 15 | 39.6 ± 4.2 | 15 |
| 1722-HAGHA | −3.9 ± 2.0 | −2.7 ± 0.7 | 3.3 ± 2.7 | 15 | 37.0 ± 4.3 | 15 |
| wild-type CaV2.1 | −3.0 ± 2.5 | −3.1 ± 0.5 | 4.6 ± 2.8 | 45 | 44.5 ± 8.5 | 14 |
| 1722-9His | 1.3 ± 2.9a | −3.9 ± 0.7a | 10.0 ± 3.2a | 54 | 45.9 ± 8.4 | 13 |
| wild-type CaV2.1 | −4.2 ± 2.0 | −2.9 ± 0.6 | 3.4 ± 2.6 | 21 | 38.1 ± 4.9 | 12 |
| 1727-9His | −0.4 ± 1.8a | −3.8 ± 0.9a | 8.0 ± 2.5a | 21 | 28.5 ± 2.6a | 13 |
| 1732-9His | 0.7 ± 2.1a | −4.3 ± 0.6a | 9.7 ± 2.2a | 21 | 36.1 ± 3.7 | 12 |
| 1732-HA | −4.5 ± 1.8 | −2.7 ± 0.6 | 2.7 ± 2.3 | 13 | 36.8 ± 4.1 | 13 |
| wild-type CaV2.1 | −2.9 ± 1.9 | −3.1 ± 0.6 | 4.8 ± 2.4 | 17 | 41.9 ± 7.0 | 13 |
| 280-bbs | 1.7 ± 3.1a | −3.7 ± 0.8c | 9.7 ± 3.5a | 14 | 35.4 ± 3.0c | 9 |
| wild-type CaV2.1 | −3.6 ± 1.1 | −3.2 ± 0.4 | 4.3 ± 1.4 | 12 | 41.9 ± 7.0 | 13 |
| 1722-bbs | −2.3 ± 1.6c | −3.5 ± 0.4 | 5.8 ± 1.7c | 13 | 42.0 ± 6.7 | 9 |
The half-maximal voltage of activation (V0.5;act) and the steepness of the curve at V0.5;act (k0.5;act) were obtained by Boltzmann equation fitting of the mean data for wild-type, 1722-HA, 1722-HAGHA, 1722-9His, 1727-9His, 1732-9H is, 1732-HA, 280-bbs and 1722-bbs. Vmax was determined by magnification of the curve and placing the cursor manually to the curve peak. Inactivation was calculated as a ratio between first and last pulse of the 15 pulses to Vmax. Data are means ± SD for the indicated number of experiments. Statistical data were obtained by Student’s t test and are indicated as
p < 0.001
p < 0.01
p < 0.05.
Wild-type data from experiments carried out with the same batches of injected oocytes are given as controls for each construct.
Table 2. Effects of tag insertions on IBa parameters during 3-s depolarising pulses.
| τfast [ms] | τslow [ms] | %τfast | n | 350 ms [%] | 1000 ms [%] | 2000 ms [%] | n | |
|---|---|---|---|---|---|---|---|---|
| wild-type CaV2.1 | 166.3 ± 25.4 | 758.3 ± 78.7 | 84.2 ± 4.6 | 6 | 78.6 ± 3.2 | 94.6 ± 1.6 | 97.3 ± 1.9 | 11 |
| 1722-HA | 168.2 ± 29.1 | 749.7 ± 89.3 | 83.4 ± 3.6 | 10 | 79.4 ± 4.6 | 95.0 ± 1.2 | 97.8 ± 1.2 | 12 |
| wild-type CaV2.1 | 172.7 ± 21.8 | 801.0 ± 152.2 | 86.2 ± 4.6 | 8 | 79.8 ± 4.1 | 95.1 ± 1.5 | 97.6 ± 1.4 | 15 |
| 1722-HAGHA | 161.3 ± 20.8 | 880.1 ± 166.5 | 88.1 ± 4.9 | 8 | 80.1 ± 6.4 | 95.6 ± 1.6 | 97.9 ± 1.0 | 15 |
| wild-type CaV2.1 | 183.1 ± 33.4 | 875.6 ± 219.7 | 84.4 ± 4.7 | 30 | 77.8 ± 4.5 | 94.3 ± 1.7 | 97.3 ± 1.7 | 58 |
| 1722-9His | 184.7 ± 45.1 | 863.6 ± 144.7 | 82.2 ± 6.6 | 43 | 80.7 ± 4.8b | 95.9 ± 2.5a | 99.0 ± 2.1a | 41 |
| wild-type CaV2.1 | 173.0 ± 17.6 | 793.9 ± 148.1 | 85.9 ± 5.5 | 5 | 79.7 ± 4.1 | 95.1 ± 2.2 | 97.5 ± 1.9 | 12 |
| 1727-9His | 124.4 ± 20.2a | 743.1 ± 136.1 | 88.1 ± 2.5 | 11 | 87.2 ± 2.7a | 97.0 ± 1.0c | 99.0 ± 1.3c | 13 |
| 1732-9His | 126.1 ± 12.6a | 772.7 ± 93.8 | 87.1 ± 1.9 | 11 | 86.3 ± 1.6a | 97.0 ± 1.5c | 99.4 ± 1.7c | 12 |
| 1732-HA | 157.8 ± 18.3 | 815.9 ± 141.5 | 88.5 ± 2.4 | 6 | 80.0 ± 4.4 | 95.3 ± 1.7 | 97.7 ± 1.6 | 13 |
| wild-type CaV2.1 | 166.3 ± 25.4 | 758.3 ± 78.7 | 84.2 ± 4.6 | 6 | 78.6 ± 3.2 | 94.6 ± 1.6 | 97.3 ± 1.9 | 11 |
| 280-bbs | 147.9 ± 15.9 | 857.4 ± 147.1 | 86.3 ± 2.6 | 9 | 82.4 ± 2.0b | 95.6 ± 1.3 | 98.2 ± 1.1 | 9 |
| 1722-bbs | 200.4 ± 49.7 | 867.8 ± 117.9 | 82.4 ± 6.7 | 5 | 67.8 ± 9.3b | 92.3 ± 2.8c | 96.9 ± 1.7 | 11 |
Inactivation after a 3-s depolarising pulse to Vmax was fit to a bi-exponential decay yielding time constants for both the fast (τfast) and the slow (τslow) component, as well as the contribution of the fast component (%τfast). Because of the high variability of measurements calculations did not reveal significance though obvious from graphical analysis, we calculated % inactivation after 3 predefined time points (350 ms, 1 s, 2 s) from the same data sets and analysed it statistically. Data are means ± SD for the indicated number of experiments. Statistical data were obtained by Student's t test and are indicated as
p < 0.001
p < 0.01
p < 0.05.
Wild-type data from experiments carried out with the same batches of injected oocytes are given as controls for each construct.
Next we investigated the suitability of 1722-HAGHA for staining CaV2.1 α1 subunits in cultured hippocampal neurons. To this end an additional N-terminal GFP tag was added to the 1722-HAGHA construct to allow a direct comparison of the GFP stain with the HA labelling pattern (eGFP-1722-HAGHA). In differentiated cultured hippocampal neurons eGFP-1722-HAGHA was expressed in the soma and dendrites as well as in presynaptic terminals (Fig. 4A). Presynaptic labelling in axonal processes appears as clusters along the soma and dendrites of non-transfected neighbouring neurons that colocalize with the presynaptic marker synapsin (Fig. 4A and D).17,18 Experimental conditions (fixation in pF and permeabilization with 0.2% Triton X-100) allowed access of antibodies to surface-expressed as well as intracellular CaV2.1 channels. However, a major advantage of extracellularly tagged α1 subunits is to distinguish the population of channels in the plasma membrane (i.e., putative current-conducting channels) from intracellular channels. In fact, HA-staining in pF fixed but unpermeabilized neurons (Fig. 4B) resulted in a distinct staining pattern showing fine calcium channel clusters along the neuronal surface of the soma and the dendrites and in presynaptic terminals. If this staining pattern indeed results from HA-tagged surface channels, then the ultimate proof for detection of surface channels would be the detection of a similar pattern obtained by a live cell staining protocol.7 In this protocol fixation before primary antibody application is omitted because it might already cause some permeabilization of cells. Surprisingly, no live cell staining of eGFP-1722-HAGHA by anti-HA antibodies was observed (Fig. 4C). However, the same staining protocol reproducibly permitted staining of an extracellularly HA-tagged CaV1.2 channel7 (data not shown).
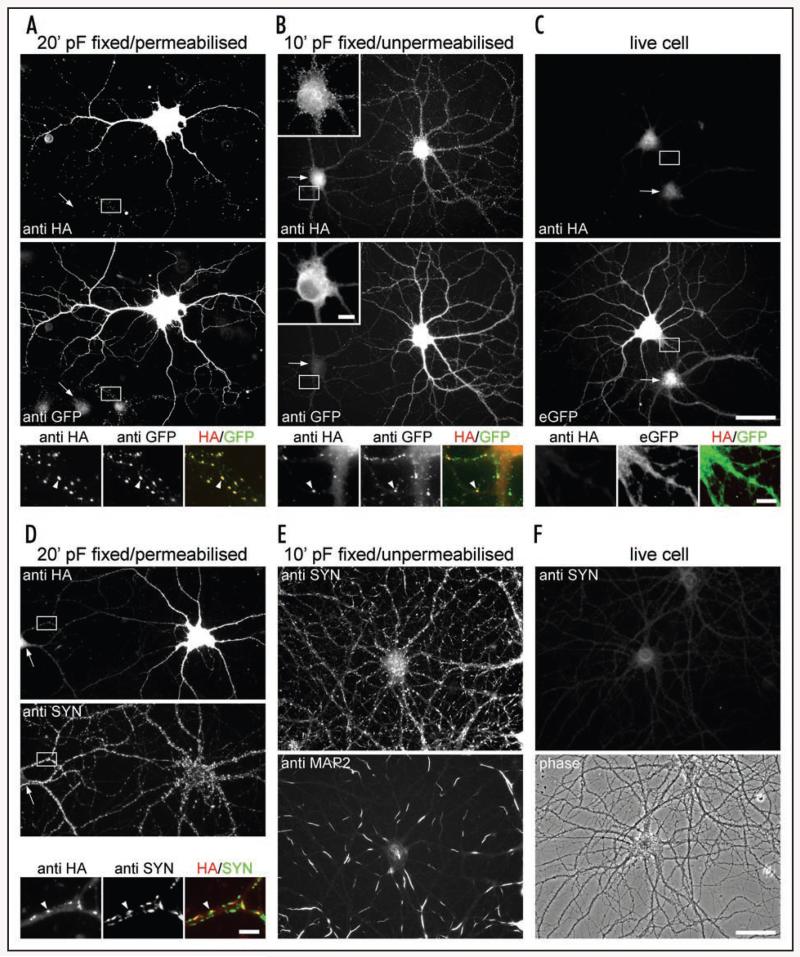
Figure 4. Characterization of the immunofluorescence labelling pattern of eGFP-1722-HAGHA heterologously expressed in cultured mouse hippocampal neurons.
Cultured mouse hippocampal neurons were immunostained after pF fixation and permeabilization with Triton X-100 (fixed/permeabilized) (A and D), after mild pF fixation without permeabilization (fixed/unpermeabilized) (B and E), and prior to fixation (live cell stain; C and E). (A) HA-labeling pattern (anti HA) of neurons transfected with eGFP-1722-HAGHA closely resembles that of the N-terminal eGFP tag (anti-GFP). Both antibodies stain channels in the soma and dendrites as well as in axonal clusters along the processes of neighbouring non-transfected cells (high resolution micrographs; arrowhead). These anti HA clusters colocalize with the presynaptic marker synapsin (anti SYN) in double labelling experiments (D), identifying them as presynaptic terminals (example indicated by an arrowhead in the high resolution micrographs in D). (B) Fixed and unpermeabilized neurons were HA-labeled and subsequently permeabilized for immunostaining of the N-terminal eGFP tag. Compared to the overall eGFP labelling pattern (anti-GFP), the HA antibody stains small clusters on the neuronal surface and in presynapitc terminals (high resolution micrographs, arrowhead). The insert in (B) is magnified and at reduced contrast to visualize clustering on the somatic surface. (C) HA labelling is absent from the surface of live cell stained transfected hippocampal neurons identified by the fluorescence of the N-terminal eGFP tag (eGFP). (E) Immunolabeling of fixed and unpermeabilized neurons with anti synapsin and anti MAP2 antibodies. Synaptic terminals are markedly stained with synapsin. MAP2 label displays a regular spacing pattern of permeable and impermeable segments as it was recently demonstrated by Taylor and Fallon.19 This demonstrates that internal synaptic and dendritic epitopes are accessible for immunolabelling in unpermeabilized neurons. (F) Live cell labelling with the synapsin antibody shows no staining of a hippocampal neuron identified by phase contrast. This indicates that the synapsin stain in (E) is caused by membrane permeabilization and not by staining of synapses along broken or disrupted axons. Regions selected for high resolution micrographs in (A–D) are indicated by white boxes and non-transfected neighbouring neurons are indicated by arrows. Arrowheads in the high resolution micrographs mark exemplary clusters. Number of experiments from independent culture preparations: (A–C): 3; (D): 2; (E and F): 1. Scale bars: Insert in (B), 10 μm; (C), 50 μm and 5 μm (high resolution micrograph); (D), 5 μm; (F), 50 μm.
To test whether this pattern of surface staining observed under mild pF fixation was the result of local membrane permeabilization, we labelled pF fixed/unpermeabilized neurons with antibodies to synapsin, an internal synaptic vesicle associated protein, and MAP2, an internal microtubule associated protein (Fig. 4E). Interestingly, synapsin staining revealed a pronounced synaptic staining pattern and staining of MAP2 resulted in a regularly spaced staining pattern of permeable and unpermeable segments, in accordance with a recent report.19 Live cell labeling with the synapsin antibody showed no staining (Fig. 4F) indicating that the synapsin stain in the pF fixed/unpermeabilized condition is indeed caused by membrane permeabilization and not by staining of synapses along broken or disrupted axons. These control experiments provided evidence that the proposed HA surface labelling pattern results from staining of intracellular channels available to the antibody after pF-induced fixation apparently causing limited membrane permeabilization.
Consequently, while the 1722-HAGHA tag allows staining of presynaptic CaV2.1 in cultured hippocampal neurons, we could not use it for selective detection of surface expressed channels. Interestingly, functional experiments (see below) indicate that the absence of surface staining is not due to a lack of accessibility of the epitope for the antibody.
In conclusion, 1722-HA and 1722-HAGHA both express at protein levels comparable to wild-type, show immunoreactivity to an anti-HA antibody and have biophysical properties that are indistinguishable from wild-type. Furthermore the 1722-HAGHA provides a novel tool for the specific staining of presynaptic CaV2.1 channels heterologously expressed in differentiated cultured hippocampal neurons.
Modulation of 1722-HA with anti-HA antibody
Due to our failure to exclusively detect surface channels by immunocytochemistry, we tested if antibody binding could be demonstrated in functional experiments. If such binding occurs then the position of the HA tag close to the extracellular channel mouth may allow modulation of 1722-HA currents by anti-HA antibodies. We therefore perfused oocytes while applying short pulses (100 ms) at low frequency (0.1 Hz) with 25 nM monoclonal rat anti-HA antibody (Roche, Fig. 3E). In order to avoid unspecific binding of the antibody to the tubes and measuring chamber we pre-incubated the oocytes with 0.1 mg/ml BSA in bath solution. BSA alone was not able to induce any statistically detectable changes in IBa of both wild-type and 1722-HA mediated currents (100.6 ± 2.5%, n = 5; 100.7 ± 3.4%, n = 5; respectively). 25 nM rat anti-HA antibody inhibited 1722-HA IBa to 83.0 ± 7.7% of control (n = 3, p = 0.0036 versus 0.1 mg/ml BSA, Fig. 3E). In control experiments 25 nM rabbit IgG neither inhibited wild-type nor 1722-HA currents (Fig. 3E). Our data clearly demonstrate that the HA tag is accessible for the antibody under native conditions and can serve as an artificial modulatory site in a VGCC α1 subunit.
Insertion of an oligo-histidine (His) tag at position 1722 in CaV2.1 α1
Having identified a site permissive for tag insertion that also allowed modulation of channel function we investigated if smaller ligands binding to other tags could also interfere with channel function. Oligo-His motifs are primary candidates because of their tight interaction with Ni-NTA. We therefore replaced the eight amino acids 1722–1729 in CaV2.1 α1 with 6 (1722-6His) or 9 (1722-9His) histidines (Fig. 2B). Expression of both full-length constructs at wild-type levels was confirmed by immunoblotting (Fig. 5A). 9His tagged CaV2.1 α1 (1722-9His) was chosen for further functional analysis because of the higher affinity of Ni-NTA for extended oligo-His stretches.20
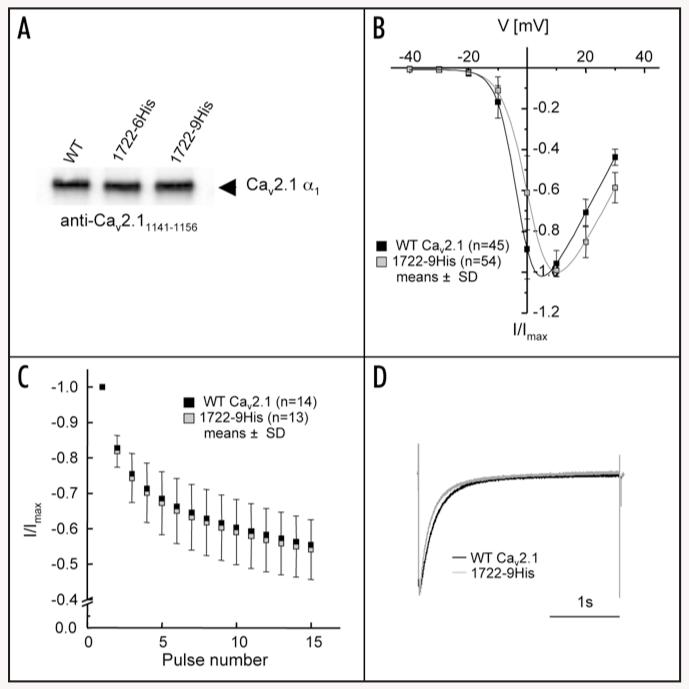
Figure 5. Oligo-histidine-tagged CaV2.1 α1 shows equal protein expression to wild-type but significant differences in functional analyses.
(A) Western blot analysis of 1722-6His and 1722-9His stained with rabbit antibody anti-CaV2.11141-1156.16 All subunits migrated at the expected molecular mass as indicated by an arrow. One representative experiment of four is shown. (B) Wild-type CaV2.1 α1 (black) and 1722-9His (grey) were injected into Xenopus oocytes and after 2-4 days IBa was measured (10 mM Ba2+ as charge carrier) in the indicated number of cells. IV-curves were determined by depolarising pulses to test potentials ranging from −40 to +30 mV from a holding potential of −80 mV and revealed a significant shift to more depolarised voltages (Table 1). (C) No changes could be detected in the use-dependence protocol but significant changes were obtained during a 3-s depolarising pulse to Vmax. (D) Representative traces for wild-type and 1722-9His all normalised to a peak IBa of 1 μA obtained during a 3-s depolarising pulse from holding potential −80 mV to Vmax. Data are presented as means ± SD.
1722-9His could be expressed with current amplitudes similar to wild-type. Its biophysical properties showed small but significant differences to wild-type. In particular, the voltage-dependence of activation was significantly shifted to more depolarised potentials by about 5 mV (Fig. 5B) which was due to a significant increase in the slope factor of the activation curve (Table 1). Acceleration of the inactivation time course during 3-s depolarising pulses to Vmax was also observed (Fig. 5D), but it was too small to significantly affect the parameters of bi-exponential current decay (Table 2). It was detected as a small but significant acceleration of percent current decay after three predefined time points (350 ms, 1 s, 2 s; Table 2). No statistically significant differences were detected for current inactivation during trains of short pulses (Fig. 5C, Table 1).
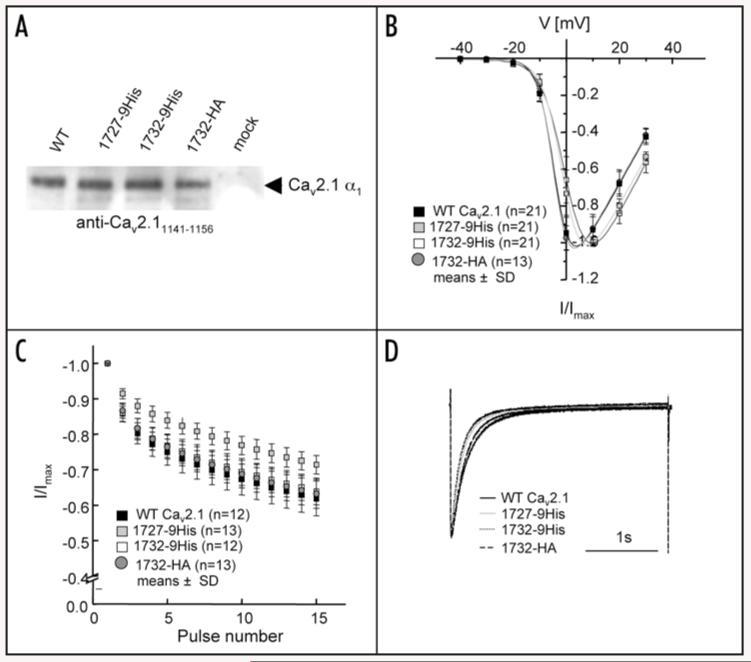
To test if the position of the His tag affects channel function we also generated 1727-9His and 1732-9His channels (Fig. 2B). A construct analogous to 1732-9His but harbouring an HA tag at position 1732 was also made (1732-HA; Fig. 2B). All these tagged α1 subunits expressed at wild-type levels as demonstrated by immunoblotting (Fig. 6A). Whereas all tested biophysical parameters of 1732-HA revealed wild-type properties (Fig. 6B-D, Tables 1 and 2), both 9His tagged constructs revealed slight (but significant) changes in gating properties very similar to 1722-9His (Fig. 6B-D, Tables 1 and 2). We also tested another tag of similar length, a 13-amino acid α-bungarotoxin-binding site (bbs) in position 1722 (1722-bbs; Fig. 2B) that can serve as a binding site for fluorescently labelled α-bungarotoxin. 1722-bbs expressed at wild-type levels (n = 3, not shown) and yielded robust IBa with properties nearly indistinguishable from wild-type (<1.5 mV shift in activation parameters; Tables 1 and 2). Taken together our data demonstrate that position 1722 is suitable for the introduction of different epitope tags resulting in no (HA tag) or only minor changes in CaV2.1 channel function.
Figure 6. Extended analysis of oligo-His-tagged CaV2.1 α1: positions 1727 and 1732.
(A) Western blot analysis of wild-type, 1727-9His, 1732-9His and 1732-HA after heterologous expression in tsA201 cells and staining of membranes with antibody anti-CaV2.11141-1156.16 All subunits migrated at the expected molecular weight (indicated by an arrow). One representative experiment of three is shown. (B–D) Electrophysiological analysis performed in Xenopus oocytes with measurements 1–4 days after cRNA injection of wild-type (black squares or black line), 1727-9His (grey squares or grey line), 1732-9His (white squares or dotted line), and 1732-HA (grey filled circles or dashed line). Data are presented as means ± SD.
As mentioned above, the transposon-mediated HA-tagging approach introduces a 27 amino acid insert which is slightly larger than the HA (9 amino acids), HAGHA (19 amino acids) and the bbs tag (13 amino acids). We therefore tested if the smaller bbs insert would be tolerated in positions 280 (280-bbs) and 336 (336-bbs) (Fig. 2B). Both bbs constructs expressed at wild-type levels (n = 3; data not shown). Like TP-336, 336-bbs also did not yield any detectable currents (n = 24, 3 independent cRNA injections; data not illustrated). In contrast to TP-280, the 280-bbs construct yielded robust currents which activated at about 5 mV more depolarising potentials compared to wild-type (Table 1).
Based on the observation that extracellular 9His-tagging of CaV2.1 α1 subunits in position 1722 results in functional channels with no or only minor differences in channel gating we asked the question if these tags located in a position adjacent to a pore forming region could also mediate channel modulation.
Pharmacological modulation of the His-tagged constructs by Ni-NTA and its derivatives
We first tested if the classical ligand Ni-NTA can specifically bind and modulate 1722-9His channels after expression in Xenopus oocytes. 660 μM Ni-NTA elicited a significant 71.9 ± 40.4% (n = 36, p = 0.0002) increase of IBa through 1722-9His channels (Fig. 7A). Two sets of control experiments were performed to demonstrate that this activation was a consequence of interaction with the 9His tag: First, NTA alone did elicit a significantly smaller increase in IBa (26.7 ± 17.4%, n = 6, p = 0.0107 versus Ni-NTA). This weaker modulation is explained by the formation of Ba-NTA due to the Ba2+ concentration (10 mM) in the bath solution which is well above the KD for Ba-NTA (KD for Ba2+: 15.1 μM, for Ni2+: 5.5 pM).21 Second, neither Ni-NTA nor NTA affected wild-type channel currents (Fig. 7A).
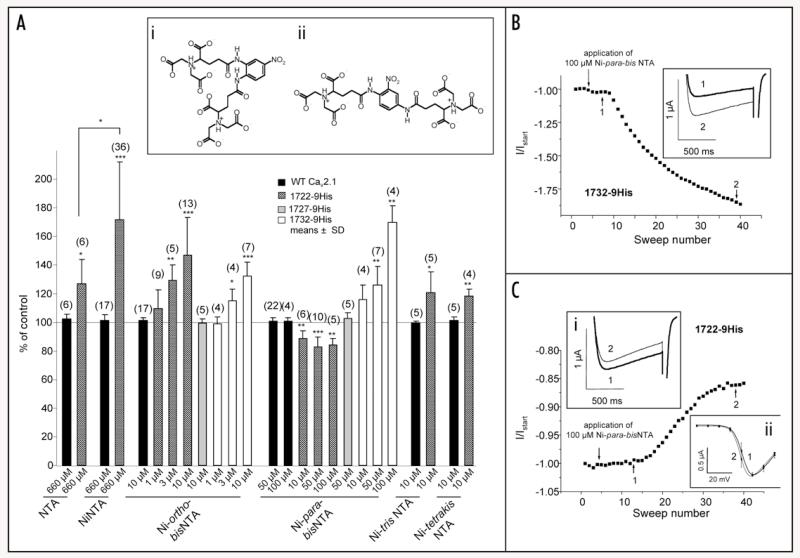
Figure 7. Pharmacological modulation of 1722-9His, 1727-9His and 1732-9His with Ni-NTA and its derivatives.
(A) Ni-NTA and its derivatives were applied to wild-type (black bars), 1722-9His (shaded bars), 1727-9His (grey bars), and 1732-9His (open bars) in the bath solution and effects were recorded by 0.1 Hz pulses from −80 mV to Vmax. The baseline IBa before application of drug was set to 100% (control) and % increase or decrease in IBa was calculated. Tested compound and concentration is indicated under the respective bar. Data are shown as means ± SD for the indicated number of experiments. Statistical data were obtained by Student’s t test and are indicated as ***p < 0.001; **p < 0.01; *p < 0.05. Statistics for modulation by Ni-ortho-bisNTA and Ni-para-bisNTA are given in the Results Section. Inset: Chemical structures of two novel Ni-NTA derivatives (i) Ni-nitro-o-phenyl-bisNTA (Ni-ortho-bisNTA) and (ii) Ni-nitro-p-phenyl-bisNTA (Ni-para-bisNTA). (B) Representative experiment showing increase of normalised current after application of 100 μM Ni-para-bisNTA on a 1732-9His expressing oocyte. Application time point is indicated by arrow. Inset: Corresponding trace before application of Ni-para-bisNTA (1, indicated by 1 and arrow in the experiment) after application of Ni-para-bisNTA (2, indicated by 2 and arrow in the experiment). (C) Representative experiment showing decrease of normalised current after application of 100 μM Ni-para-bisNTA on a 1722-9His expressing oocyte. Application time point is indicated by arrow. Inset i: Corresponding trace before application of Ni-para-bisNTA (1, indicated by 1 and arrow in the experiment) after application of Ni-para-bisNTA (2, indicated by 2 and arrow in the experiment). Inset ii: Current-voltage relationship for 1722-9His construct before (1, n = 9) and after addition (2, n = 7) of Ni-para-bisNTA.
Next we addressed the question if bulkier Ni-NTA derivatives are also active, perhaps also as channel blockers rather than activators and if they could modulate channel function at lower concentrations. We therefore tested the two recently developed Ni-NTA derivatives Ni-trisNTA and Ni-tetrakisNTA20 as well as two novel substances, Ni-nitro-o-phenyl-bisNTA (Ni-ortho-bisNTA) and Ni-nitro-p-phenyl-bisNTA (Ni-para-bisNTA) (Fig. 7A, inset). Ni-ortho-bisNTA and Ni-para-bisNTA (Fig. 7A) have two separate Ni-NTA moieties interconnected via a phenyl ring. Ni-ortho-bisNTA was tested on all three 9His-tagged calcium channel constructs as well as on wild-type. Like Ni-NTA it significantly stimulated 1722-9His—as well as 1732-9His—mediated currents which reached significance at concentrations ≥3 μM (Fig. 7A; 1722-9His: 1 μM: 109.7 ± 13.1% of control, n = 6; 3 μM: 129.3 ± 10.5%, n = 5, p = 0.0034; 10 μM: 146.9 ± 26.3%, n = 13, p < 0.0001; 1732-9His: 1 μM: 99.1 ± 4.9%, n = 4; 3 μM: 115.0 ± 8.4%, n = 4, p = 0.0379; 10 μM: 132.4 ± 9.7%, n = 7, p = 0.0001). No changes could be observed for wild-type (10 μM: 101.5 ± 2.0%, n = 17) and for 1727-9His (10 μM: 99.5 ± 3.1%, n = 5). Similar to Ni-ortho-bisNTA, Ni-para-bisNTA did not modulate wild-type (Fig. 7A; 50 μM: 101.0 ± 2.2%, n = 22; 100 μM: 101.1 ± 2.2%, n = 4) and 1727-9His (Fig. 7A; 50 μM: 103.0 ± 3.7%, n = 5) but it stimulated IBa through 1732-9His in concentrations of 50 and 100 μM (Fig. 7A; 10 μM: 115.9 ± 10.1%, n = 4; 50 μM: 125.8 ± 13.2%, n = 7, p = 0.0021; 100 μM: 169.7 ± 11.8%, n = 4, p = 0.0013; representative experiment shown in Fig. 7B). However, in contrast to the ortho isomer, it partially inhibited 1722-9His channels (Fig. 7A; 10 μM: 88.9 ± 5.1% of control, n = 6, p = 0.0031; 50 μM: 83.0 ± 6.6%, n = 10, p < 0.0001; 100 μM: 84.5 ± 4.3%, n = 5, p = 0.0013; representative experiment shown in Fig. 7C). Analysis of the current-voltage relationships before and after addition of either Ni-ortho-bisNTA or Ni-para-bisNTA revealed minimal shifts of the activation curves to more negative voltages. This shift was only significant for Ni-ortho-bisNTA action on 1722-9His (V0.5;act before addition of Ni-ortho-bisNTA: 1.5 ± 2.7 mV; V0.5;act after addition of Ni-ortho-bisNTA: −1.8 ± 2.0 mV, n = 14, p = 0.0012), in all other cases it was below 2 mV and did not reach statistical significance (Fig. 7C, inset ii).
Ni-tris-NTA and Ni-tetrakis-NTA, which show an even higher affinity for oligo-His sequences,20 were also able to enhance 1722-9His mediated IBa in the low micromolar concentration range (10 μM Ni-tris-NTA: 121.0 ± 14.3% of control, n = 5, p = 0.0304; for 10 μM Ni-tetrakis-NTA: 118.7 ± 4.5%, n = 4, p = 0.0038; Fig. 7A).
Taken together our experiments demonstrate that activation and partial inhibition of CaV2.1 channels is feasible by introducing an NTA-binding site into the extracellular channel surface close to the channel pore. Modulation is both dependent on the structure of Ni-NTA-containing molecules and on the position of the 9His tag.
Discussion
Here we describe the successful identification of a position within the voltage-gated Ca2+ channel CaV2.1 α1 subunit that permits introduction of different extracellularly accessible epitope tags with no or only minimal consequences for channel expression and function. We also demonstrate that permissive positions in the vicinity of the pore cannot only be exploited for analysing the subcellular targeting of these channels in transfected hippocampal neurons or labelling in heterologously transfected cells22-24 but also for the introduction of artificial modulatory sites for either antibodies or small synthetic molecules.
Our detailed characterisation of several insertion sites revealed position 1722 in the E3 region13 of the pore loop9 of domain IV as a privileged region for the introduction of a variety of different tags varying in length from 6 (6-His) to 19 amino acids (double-HA). The bbs tag was also tolerated in position 280 which is located at about the same distance (37 amino acids) upstream from the selectivity filter glutamate in domain I as is position 1722 in domain IV (Fig. 2). The suitability of this position for insertion of different tags has to be tested in future experiments. However, our results already show that it may be more susceptible to tag-induced gating changes (5 mV shift of the activation curve observed for 280-bbs but not for 1722-bbs) and does not tolerate the 27 amino acid peptide resulting from transposon-mediated HA-tagging. In contrast to 280-bbs, insertion of this tag into position 336 did not yield functional channels. Although we have not performed a systematic analysis yet, our data indicate that insertion upstream the selectivity filter glutamate (E3 region) may be tolerated better than insertion between the selectivity filter and transmembrane segment S6 (Fig. 2). This is also supported by our findings with TP-685. Functional channels with tags inserted into the E3 region were also obtained for HA-tagged CaV1.2 α1,5 CaV1.3 α1,8 and CaV3.2 α1,4 although a systematic analysis of expression densities and biophysical properties in comparison to wild-type channels has not been published for these Ca2+ channel constructs. In position 1722 the bbs tag and single (1722-HA) or double HA-tagging (1722-HAGHA) did not result in detectable changes of channel gating under our experimental conditions. This was also true for 1732-HA. In contrast, all 9His- and bbs-tagged constructs displayed slight but significant changes in the activation and inactivation parameters. From this we conclude that a single or double HA tag (9 or 19 amino acids, respectively) is more easily tolerated than 9 histidines or a 13 amino acid long bungarotoxin-binding site. A closer analysis of these tags revealed an overall negative charge for both HA and bbs and a positive charge for the 9His tag. Charge and tag size therefore do not seem to account for the observed differences.
Our single and tandem HA-tagged constructs have been successfully used by others22-24 in immunocytochemical studies in non-permeabilized HEK293 and COS7 cells. Here we show for the first time a staining of the double HA-tagged construct in permeabilized cultured hippocampal neurons. CaV2.1 showed an overall expression in the soma and dendrites and was well visible in presynaptic terminals (Fig. 4A and D). This staining pattern is a known feature for heterologously expressed presynaptic Ca2+ channels7,25 and likely represents membrane expressed channels together with channels in the biosynthetic pathway (Golgi, ER) and possible intracellular pools. HA-staining of CaV2.1 in unpermeabilized neurons showed a surface expression pattern while, surprisingly, live cell staining did not. Two possible explanations exist: First, in living neurons the HA epitope might not be properly accessible for stable antibody binding. Second, the density of CaV2.1 channels in the presynaptic membrane may be too low for reliable detection by immunocytochemistry. This is not a general limit of the method because our live cell staining protocol is sensitive enough to reliably identify clusters consisting of only a few CaV1.2-HA molecules in the same neuronal cultures.7 A critical finding reported here is that the surface labelling pattern in fixed/unpermeabilized neurons is evidently the consequence of membrane permeabilization caused by the pF fixation. This was proven by the staining of the intracellular proteins synapsin and MAP2 under these experimental conditions. This is a critical observation because similar surface staining protocols have been applied to study the surface expression of Ca2+ channels in cultured hippocampal neurons.26 Therefore, when studying the surface expression of Ca2+ channels and other surface transmembrane proteins, we suggest live cell staining of endogenous proteins located in the same neuronal compartment as a proper control.
Ni-tetrakisNTA-His tag interaction has recently been used to specifically and reversibly control the proteolytic activity of the 20S proteasome complex.27 Here we demonstrate for the first time that such interaction can also be exploited to introduce artificial modulatory sites into VGCCs. Integration of engineered binding sites for known ligands could be exploited to generate channel constructs with unique pharmacological properties. This concept would allow to selectively activate or block such constructs e.g., after expression in cultured cells. Moreover, appropriate mouse strains expressing such mutant channels would allow selectively inhibiting or stimulating these channels in vivo. This could help to address the question if selective modulation of a particular channel would lead to therapeutically relevant effects. Given the structural similarities of the pore regions within the cation channel family this concept may be applicable not only to VGCCs but also to other members for which selective modulators are absent. Here we provide a proof-of-concept for this approach. HA-tagged CaV2.1 calcium channels could be inhibited by an anti-HA antibody. This inhibition was incomplete at the highest antibody concentration available for our experiments (25 nM). His-tagged channels were also modulated by multivalent Ni-NTA compounds. Surprisingly, most compounds increased IBa through different His constructs. Although we have not investigated the biophysical basis of this phenomenon in single channel recordings, our data indicate that binding of these compounds near the ion permeation pathway can somehow facilitate permeation through the pore. Our data do not allow distinction between effects on gating (e.g., promotion of open channel states and enhanced open probability) or increased single channel conductance. We consider it unlikely that Ni-NTA and its derivatives only reversed a decreased permeability of the 9His constructs because 1722-9His was stimulated by the Ni-ortho-bisNTA but inhibited by the corresponding para derivative. The position of the 9His tag was also critical because insertion of the 9His tag 10 amino acids downstream (1732-9His) resulted in activation by both compounds. We expected that moving the 9His tag closer to the selectivity filter and, hence, closer to the ion conducting pathway, would favour inhibitory effects. This was not the case. Likewise, using bulkier Ni-NTA derivatives did (with the exception of 1722-HA inhibition by Ni-para-bisNTA) also not result in inhibitory effects. Previous attempts to develop inhibitory antibodies for different cation channels (including TRPC5, NaV1.5)13 have focused on antibodies binding to the so-called E3 region, the extracellular region between S5 and the selectivity filter (“pore”). These antibodies also caused only partial inhibition of the ion currents suggesting that their binding domain is not close enough to the cation permeation pathway to induce full obstruction of the pore. Future experiments should therefore test if 9His tags even closer to the selectivity filter can favour, if functionally tolerated, more pronounced inhibition or even complete block.
In conclusion we suggest that aside position 1722, the population of individually tagged CaV2.1 α1 subunits reported in our study could also contain useful tools for future experiments, serving studies that rang from calcium channel activity in neuronal physiology and pathophysiology to tertiary structure of α1 subunits.
Materials and Methods
Accession numbers
Human CaV2.1 α1 (GenBank™ accession number FJ040507) was used for this study. For alignments between the different α1 subunits the following accession numbers were used: CaV1.1: NM_000069, CaV1.2: AF_465484; CaV1.3: NM_000720; CaV1.4: NM_005183; CaV2.2: NM_000718; CaV2.3: NM_000721.
Synthesis of Ni-NTAs
Nickel N-nitrilotriacetic acid (Ni-NTA) was synthesised by dissolving nitrilotriacetic acid (NTA, Aldrich) in water at pH 7.0. An equimolar amount of NiCl2 * 6H2O (Riedel-de-Haën) was added while holding the pH constant at 7.0 with 1 M NaOH under constant stirring. The solution turned from a light green colour of the Ni2+ into deep blue-green. Synthesis of Ni-nitro-o-phenyl-bisNTA (Ni-ortho-bisNTA) and Ni-nitro-p-phenyl-bisNTA (Ni-para-bisNTA) will be published elsewhere. The purity and structural integrity of the two substances were confirmed by 1H- and 13C-NMR as well as mass spectrometry.
Epitope-tagging of CaV2.1 α1
For random insertion of HA-tags into CaV2.1 α1 a Tn5 transposon-based approach (EZ::TN™, Epicentre® technologies)28 was used. Transposon Tn5 was redesigned in our lab to encode a kanamycin resistance gene (kanr) under the control of a bacterial promoter, an eGFP (enhanced Green Fluorescent Protein) gene under the control of the promoter of the target DNA and the HA-epitope tag (9 amino acids), flanked by the recognition sequences for the transposase (mosaic ends, MEs, 9 amino acids in translation). The sequences encoding the selection markers (kanr, eGFP expression) were removed from positive clones by restriction enzyme digest resulting in the HA-epitope flanked by the MEs (total 27 amino acids in translation).
Equimolar amounts (0.03 pmol each) of transposon and the target DNA CaV2.1 α1 in the mammalian expression vector pCMV15 were incubated following the manufacturer’s recommendations. The transposition mix was electroporated into electrically competent E. coli DH5-α cells and plated on agar containing ampicillin (100 mg/l) and kanamycin (30 mg/l) to recover transposed clones harbouring both resistance genes. Clones were amplified to mini cultures and extracted DNA was transfected into mammalian cells in 96 well plates. eGFP fluorescence representing in-frame insertion was determined 48 hours after transfection and fluorescing clones were sequenced (MWG-Biotech, Germany) to reveal the insertion positions of the transposon sequence. As the transposase opens the target DNA in a 9 base pair staggered cut, the three amino acids preceding the transposon are repeated immediately 3′ of insertion. HA-tagged α1 subunits were named after the codon number 5′ of insertion.
All other constructs were generated by SOE PCR. Constructs and corresponding forward and reverse primer sequences:
1722-HA_CaV2.1: 5′-TAT CCA TAT GAC GTA CCT GAC TAT GCC GAT GAG TTC CAA ATC ACT GAG-3′ and 5′-GGC ATA GTC AGG TAC GTC ATA TGG ATA GTC GAT GCC AAT GTT ACC AAA CAC-3′;
1722-HAGHA_CaV2.1: 5′-TAT GCC GGG TAC CCA TAT GAC GTA CCT GAC TAT GCC GAT GAG TTC CAA ATC ACT GAGC-3′ and 5′-GGT ACG TCA TAT GGG TAC CCG GCA TAG TCA GGT ACG TCA TAT GGA TAG TCG ATG CCA ATG-3′;
1722-6His_CaV2.1: 5′-ATT GGC ATC GAC CAC CAC CAC CAC CAC CAC GAT GAG TTC CAA ATC ACT GAG CAC AAT AAC-3′ and 5′-TTG GAA CTC ATC GTG GTG GTG GTG GTG GTG GTC GAT GCC AAT GTT ACC AAA CAC CTG-3′;
1722-9His_CaV2.1: 5′-CAC CAC CAC CAC CAC CAC CAC CAC CAC GAT GAG TTC CAA ATC ACTG-3′ and 5′-GTG GTG GTG GTG GTG GTG GTG GTG GTG GTC GAT GCC AAT GTT ACC-3′;
1727-9His_CaV2.1: 5′-GAC CAC CAC CAC CAC CAC CAC CAC CAC CAC ACT GAG CAC AAT AAC TTC CG-3′ and 5′-AGT GTG GTG GTG GTG GTG GTG GTG GTG GTG GTC CTC GTC CTC CAC GTC G-3′;
1732-9His_CaV2.1: 5′-CAC CAC CAC CAC CAC CAC CAC CAC CAC TTC CAA ATC ACT GAG CAC AAT AAC TTC C-3′ and 5′-GTG GTG GTG GTG GTG GTG GTG GTG GTG CTC ATC TTC ATC ACT GTC CTC GTC CTC C-3′;
1732-HA_CaV2.1: 5′-TAT CCA TAT GAC GTA CCT GAC TAT GCC TTC CAA ATC ACT GAG CAC AAT AAC TTC C-3′ and 5′-GGC ATA GTC AGG TAC GTC ATA TGG ATA CTC ATC TTC ATC ACT GTC CTC GTC CTC C-3′;
280-bbs_CaV2.1: 5′-TAC GAG AGC TCC CTG GAG CCC TAC CCT GAC TGC CCC AAT GGG ACC AAA TGT CAG-3′ and 5′-GTA GGG CTC CAG GGA GCT CTC GTA GTA TCT CCA GGT GCG GGC GGG CTC TTC TGT C-3′;
336-bbs_CaV2.1: 5′-TAC GAG AGC TCC CTG GAG CCC TAC CCT GAC TGG TTG TAC TTC ATC CCC CTC ATC-3′ and 5′-GTA GGG CTC CAG GGA GCT CTC GTA GTA TCT CCA GTT CCA AGT GTT CCC TGA GGC ATC-3′;
1722-bbs_CaV2.1: 5′-TAC GAG AGC TCC CTG GAG CCC TAC CCT GAC GAT GAG TTC CAA ATC ACT GAG CAC-3′ and 5′-GTA GGG CTC CAG GGA GCT CTC GTA GTA TCT CCA GTC GAT GCC AAT GTT ACC AAA CAC-3′.
All constructs were verified by sequencing (MWG-Biotech, Germany).
Transient expression of CaV2.1 in mammalian cells
tsA201 cells were maintained in Dulbecco’s modified Eagle’s medium/Coon’s F-12 medium (Invitrogen) supplemented with 2 mM L-glutamine, 10% (v/v) foetal calf serum (Invitrogen), and 100 units/ml penicillin/streptomycin at 37°C at 5% CO2. To check for correct transposon insertion, cells were transfected one day after plating in 96 well plates with a total amount of 0.40 μg DNA per well (0.12 μg of transposed CaV2.1 α1, 0.09 μg rabbit α2δ-1,29 and 0.08 μg rat β3 subunit30 plus 0.11 μg of pUC18 carrier DNA). Cells were analysed 48 hours after transfection for eGFP fluorescence.
For Western blot experiments cells were transfected one day after plating in dishes (d = 10 cm) with a DNA mix containing 3.0 μg α1, 2.0 μg rat β3, 2.5 μg rabbit α2δ-1, and 2.5 μg pUC18 carrier DNA following standard protocols for calcium phosphate precipitation. Cells were incubated at 37°C for 48 hours and harvested with PBS for membrane preparation.
Membrane preparation and western blotting
For each construct two dishes (d = 10 cm) were transfected. Cells were precipitated by slow centrifugation and shock frozen in liquid nitrogen. After resuspension in ice-cold homogenisation buffer,31 cells were mechanically disrupted by suction through a cannula (d = 0.4 mm) for four times. After centrifugation at 585 × g microsomal membranes were collected from the supernatant by centrifugation at 81000 × g. Protein concentration was assessed following the Lowry protocol using bovine serum albumin as a standard. Western blot experiments were carried out as described31 by separation of 30 μg of membrane protein on a 7% SDS-polyacrylamide gel and immunostaining of α1 subunits using a sequence-directed antibody (anti-CaV2.11141-1156)16 or a mouse anti-HA antibody (clone 12CA5, Roche).
Cell culture of hippocampal neurons and immunocytochemistry
Low-density cultures of hippocampal neurons were prepared from 16.5-day-old embryonic BALB/c mice as previously described.7,17 Briefly, pregnant mice were decapitated and hippocampi were dissociated by trypsin treatment and trituration. Neurons were plated on poly-L-lysine-coated glass coverslips in 60-mm culture dishes at a density of 3500 cells/cm2. After plating, cells were allowed to attach for 3–4 h before transferring the coverslips neuron-side-down into a 60-mm culture dish with a glial feeder layer. For maintenance, the neurons and glial feeder layer were cultured in serum-free neurobasal medium (Invitrogen GmbH, Karlsruhe, Germany) supplemented with GlutaMax and B27 supplements (Invitrogen GmbH). Ara-C (5 μm) was added three days after plating to stop proliferation of non-neuronal cells. Cultures were transfected with 1.5 μg plasmid DNA on day 6 using lipofectamine 2000 as previously described.7,25 18 and 24 days after plating neurons were fixed and processed for immunocytochemistry according to three different protocols (see below). For neuronal expression and N-terminal eGFP-tagging the CaV2.1 construct bearing an extracellular double HA was cloned into the pβA-eGFP7 plasmid yielding eGFP-1722-HAGHA.
Twenty min paraformaldehyde fixation and permeabilization
Neurons were fixed for 20 min in 4% paraformaldehyde/4% sucrose in PBS (pF) at room temperature and subsequently rinsed in PBS. Fixed neurons were incubated in 5% normal goat serum (NGS) in PBS containing 0.2% bovine serum albumin (BSA) and 0.2% Triton X-100 (PBS/BSA/Triton) for 30 min. Primary antibodies were applied in PBS/BSA/Triton for 4 h at room temperature. Subsequently, cells were washed and incubated for 1 h in fluorochrome-conjugated secondary antibodies.
10 min pF fixation without permeabilization
For unpermeabilized staining of CaV2.1-HA neurons were fixed with pF for 10 min. Nonspecific binding was blocked in 5% NGS in PBS/BSA (without Triton) for 30 min and then neurons were incubated overnight at 4°C with the rat anti-HA antibody in PBS/BSA. After rinsing in PBS/BSA, neurons were incubated for 1 h in the fluorochrome-conjugated secondary antibody goat anti-rat Alexa 594. Next, cells were postfixed in pF for 5 min and rinsed again in PBS. Neurons were then permeabilized and blocked with 5% NGS in PBS/BSA/Triton. The rabbit polyclonal anti-GFP antibody was applied in PBS/BSA/Triton for 4 h at room temperature, cells were washed and incubated for 1 h with the Alexa 488-conjugated secondary antibody. For control experiments involving the anti-synapsin and anti-MAP2 antibodies the entire immunocytochemsitry was performed in PBS/BSA without subsequent permeabilization.
Live cell staining
For live cell staining cultures were incubated with the rat anti-HA (1:100) antibody or, for control experiments, the rabbit anti-synapsin (1:1000) antibody for 30 min at 37°C. The cells were quickly rinsed with Hank’s buffered salt solution and fixed with pF for 10 min. Neurons were incubated in 5% NGS in PBS/BSA (without Triton) for 30 min followed by 1 h in the Alexa 594-conjugated secondary antibody (Molecular Probes).
Antibodies
Primary antibodies: rat monoclonal anti-HA (clone 3F10, Roche Diagnostics GmbH, Vienna, Austria) at 1:1,000 or 1:100 for live cell labeling; rabbit polyclonal anti-GFP (Molecular Probes, Eugene, OR, USA) at 1:20000; rabbit polyclonal anti-synapsin 1 and 2 (Synaptic Systems) at 1:20000 or 1:2000 for live cell labeling; mouse monoclonal anti-MAP2 antibody (clone AP-20; Sigma-Aldrich) at 1:1000. Secondary antibodies and fluorochromes: goat anti-rat Alexa 594, goat anti-rabbit Alexa 488 and Alexa 594, and goat anti-mouse Alexa 488 (Invitrogen, all at 1:4000).
Imaging
After staining cover slips were washed and mounted in p-phenylene-diamine-glycerol to retard photo-bleaching. Preparations were analysed on an Axio Imager microscope (Carl Zeiss, Inc.) using a 63 ×, 1.4 NA and a 25 ×, 0.8 NA objective and images were recorded with a cooled CCD camera (SPOT; Diagnostic Instruments, Stirling Heights, MI, USA) and Metaview image processing software (Universal Imaging, Corp., West Chester, PA, USA). Image composite was arranged in Adobe Photoshop 9 (Adobe Systems Inc.) and linear adjustments were performed to correct black level and contrast.
Electrophysiological recordings in Xenopus oocytes
Stage V-VI oocytes harvested from Xenopus laevis by transverse laparotomy were incubated for 70–90 min in collagenase (type I, Sigma). After extensive washes, the follicle layer was removed mechanically. After an overnight rest, oocytes were injected with cRNA mixes (23–63 nl) containing equimolar amounts of the α1 subunit of interest, β3, and α2δ-1. Measurement of IBa was performed 1–4 days after injection using the two-electrode voltage clamp technique as described earlier.16 To determine endogenous barium currents (IBa), oocytes injected only with β3 and α2δ-1 were analysed in parallel. Only oocytes with peak IBa at least 10-fold higher than the highest endogenous current were included into analysis. Data analysis and acquisition were performed by using the pClamp software package (9.0 and 9.2, Axon Instruments). Leak current correction was performed by adjusting the current traces by a factor calculated from the difference between leak at −80 and −90 mV, respectively. Both voltage-recording and current-injecting microelectrodes were filled with (in M) 2.8 CsCl, 0.2 CsOH, 0.01 HEPES and 0.01 EGTA (adjusted to pH 7.4 with HCl). Resistances were between 0.1 and 1.5 MΩ. Bath solution contained (in M): 0.01 Ba(OH)2; 0.095 NaOH, 0.002 CsOH and 0.005 HEPES (229 mOsM; adjusted to pH 7.4 with methanesulfonic acid). 1722-HA blocking experiments were performed with rat anti-HA antibody (clone 3F10, Roche). Drugs were diluted to the required end concentrations in bath solution (containing 0.1 mg/ml BSA where indicated) and were applied to the recording chamber through a gravity-driven perfusion system with a flow rate of 150 μl/min. The voltage-dependence of activation was determined from IV curves obtained by step-depolarisations from a holding potential −80 mV to various test potentials ranging from −40 and +30 mV. IV curves were fitted according to the following equation:
where V is the membrane potential, I is the peak current, Vrev corresponds to the extrapolated reversal potential of IBa, Gmax is the maximal conductivity of the cell, V0.5;act is the voltage of half-maximal activation, k0.5;act is the slope factor of the Boltzmann term, and C represents a constant term.
Inactivation during pulse trains was determined by applying 1 Hz trains of 15 100 ms pulses to Vmax from a holding potential of −60 mV. Voltage-dependent inactivation during depolarisation was estimated during 3-s pulses from a holding potential of −80 mV to a test potential corresponding to the peak potential (Vmax) of the IV relations of the respective cell. Percent inactivation after three different time points (i.e., 350 ms, 1 s and 2 s after peak inward current) was analysed. Short pulse protocol was performed by applying 100 ms pulses to Vmax with a frequency of 0.1 Hz from a holding potential of −80 mV.
Statistics
All data are given as mean ± standard deviation (SD). Statistical significance was determined by two-tailed Student’s t test. Data were analysed using Clampfit (9.0 and 9.2, Axon Instruments), Origin® 6.1 (OriginLab Corporation), Microsoft® Office Excel 2003 (Microsoft Corporation) and GraphPad Prism® 4.03 (GraphPad Software Inc).
Acknowledgements
We thank Douglas L. Sheridan for kindly providing us with the transposon construct, Hermann Gruber for advice on the Ni-NTA synthesis protocol, Jasmin Aldrian, Gilda Pelster, and Sabine Baumgartner for excellent technical help. This work was supported by the Austrian Science Fund (P-17109, P-17159, P-17807-B05) and the Tyrolean Science Fund to Alexandra Koschak and Gerald J. Obermair.
Abbreviations
- HA
hemagglutinine
- His
histidine
- IBa
inward Ba2+ currents
- NTA
N-nitrilo triacetic acid
- kanr
kanamycin resistance gene
- eGFP
enhanced green fluorescent protein
- ME
mosaic ends
- pF
paraformaldehyde
- SOE PCR
splice overlap extension polymerase chain reaction
- Vmax
voltage of maximum inward current
- SD
standard deviation
- WT
wild-type
- V0.5;act
half-maximal voltage of activation
- k0.5;act
steepness of the curve at V0.5;act
- τfast
fast component of inactivation
- τslow
slow component of inactivation
- %τfast
the contribution of the fast component
- VGCCs
voltage-gated calcium channels
References
- 1.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–25. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 2.Striessnig J, Koschak A. Exploring the function and pharmacotherapeutic potential of voltage-gated calcium channels with gene knockout models. Channels. 2008;2(4):233–251. doi: 10.4161/chan.2.4.5847. [DOI] [PubMed] [Google Scholar]
- 3.Davies JN, Zamponi GW. Old proteins, developing roles. Channels. 2008;2:130–8. doi: 10.4161/chan.2.2.6214. [DOI] [PubMed] [Google Scholar]
- 4.Dubel SJ, Altier C, Chaumont S, Lory P, Bourinet E, Nargeot J. Plasma membrane expression of T-type calcium channel α1 subunits is modulated by high voltage-activated auxiliary subunits. J Biol Chem. 2004;279:29263–9. doi: 10.1074/jbc.M313450200. [DOI] [PubMed] [Google Scholar]
- 5.Altier C, Dubel SJ, Barrere C, Jarvis SE, Stotz SC, Spaetgens RL, Scott JD, Cornet V, De Waard M, Zamponi GW, Nargeot J, Bourinet E. Trafficking of L-type calcium channels mediated by the postsynaptic scaffolding protein AKAP79. J Biol Chem. 2002;277:33598–603. doi: 10.1074/jbc.M202476200. [DOI] [PubMed] [Google Scholar]
- 6.Altier C, Khosravani H, Evans RM, Hameed S, Peloquin JB, Vartian BA, Chen L, Beedle AM, Ferguson SS, Mezghrani A, Dubel SJ, Bourinet E, McRory JE, Zamponi GW. ORL1 receptor-mediated internalization of N-type calcium channels. Nat Neurosci. 2006;9:31–40. doi: 10.1038/nn1605. [DOI] [PubMed] [Google Scholar]
- 7.Obermair GJ, Szabo Z, Bourinet E, Flucher BE. Differential targeting of the L-type calcium channel α1C (CaV1.2) to synaptic and extrasynaptic compartments in hippocampal neurons. Eur J Neurosci. 2004;19:2109–22. doi: 10.1111/j.0953-816X.2004.03272.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, Fu Y, Altier C, Platzer J, Surmeier DJ, Bezprozvanny I. CaV1.2 and CaV1.3 neuronal L-type calcium channels: differential targeting and signaling to pCREB. Eur J Neurosci. 2006;23:2297–310. doi: 10.1111/j.1460-9568.2006.04734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stary A, Shafrir Y, Hering S, Wolschmann P, Guy R. Structural model of the CaV1.2 pore. Channels. 2008;2(3):210–215. doi: 10.4161/chan.2.3.6158. [DOI] [PubMed] [Google Scholar]
- 10.Pietrobon D. Calcium channels and channelopathies of the central nervous system. Mol Neurobiol. 2002;25:31–50. doi: 10.1385/MN:25:1:031. [DOI] [PubMed] [Google Scholar]
- 11.Pietrobon D, Striessnig J. Neurobiology of migraine. Nat Rev Neurosci. 2003;4:386–98. doi: 10.1038/nrn1102. [DOI] [PubMed] [Google Scholar]
- 12.Lorenzon N, Beam KG. Disease causing mutaitons of calcium channels. Channels. 2008;2(3):163–179. doi: 10.4161/chan.2.3.5950. [DOI] [PubMed] [Google Scholar]
- 13.Xu SZ, Zeng F, Lei M, Li J, Gao B, Xiong C, Sivaprasadarao A, Beech DJ. Generation of functional ion-channel tools by E3 targeting. Nat Biotechnol. 2005;23:1289–93. doi: 10.1038/nbt1148. [DOI] [PubMed] [Google Scholar]
- 14.Goryshin IY, Miller JA, Kil YV, Lanzov VA, Reznikoff WS. Tn5/IS50 target recognition. Proc Natl Acad Sci USA. 1998;95:10716–21. doi: 10.1073/pnas.95.18.10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grabner M, Dirksen RT, Beam KG. Tagging with green fluorescent protein reveals a distinct subcellular distribution of L-type and non-L-type calcium channels expressed in dysgenic myotubes. Proc Natl Acad Sci USA. 1998;95:1903–8. doi: 10.1073/pnas.95.4.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wappl E, Koschak A, Poteser M, Sinnegger MJ, Walter D, Eberhart A, Groschner K, Glossmann H, Kraus RL, Grabner M, Striessnig J. Functional consequences of P/Q-type calcium channel CaV2.1 missense mutations associated with episodic ataxia type 2 and progressive ataxia. J Biol Chem. 2002;277:6960–6. doi: 10.1074/jbc.M110948200. [DOI] [PubMed] [Google Scholar]
- 17.Obermair GJ, Kaufmann WA, Knaus HG, Flucher BE. The small conductance Ca2+-activated K+ channel SK3 is localized in nerve terminals of excitatory synapses of cultured mouse hippocampal neurons. Eur J Neurosci. 2003;17:721–31. doi: 10.1046/j.1460-9568.2003.02488.x. [DOI] [PubMed] [Google Scholar]
- 18.Di Biase V, Flucher BE, Obermair GJ. Resolving sub-synaptic compartments with double immunofluorescence labeling in hippocampal neurons. J Neurosci Methods. 2008 doi: 10.1016/j.jneumeth.2008.08.025. DOI: 10.1016/j.jneumeth.2008.08.025. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Taylor AB, Fallon JR. Dendrites contain a spacing pattern. J Neurosci. 2006;26:1154–63. doi: 10.1523/JNEUROSCI.4424-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lata S, Reichel A, Brock R, Tampe R, Piehler J. High-affinity adaptors for switchable recognition of histidine-tagged proteins. J Am Chem Soc. 2005;127:10205–15. doi: 10.1021/ja050690c. [DOI] [PubMed] [Google Scholar]
- 21.Furia TE. Sequestrants in food. In: Furia TE, editor. CRC Handbook of Food Additives. CRC Press; 1972. pp. 271–94. [Google Scholar]
- 22.Heblich F, Tran Van Minh A, Hendrich J, Watschinger K, Dolphin AC. Time course and specificity of the pharmacological disruption of the trafficking of voltage-gated calcium channels by gabapentin. Channels. 2008;2:4–9. doi: 10.4161/chan.2.1.6045. [DOI] [PubMed] [Google Scholar]
- 23.Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, Wratten J, Davies A, Dolphin AC. Pharmacological disruption of calcium channel trafficking by the α2δ ligand gabapentin. Proc Natl Acad Sci USA. 2008;105:3628–33. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mezghrani A, Monteil A, Watschinger K, Sinnegger-Brauns MJ, Barrere C, Bourinet E, Nargeot J, Striessnig J, Lory P. A destructive interaction mechanism accounts for dominant-negative effects of misfolded mutants of voltage-gated calcium channels. J Neurosci. 2008;28:4501–11. doi: 10.1523/JNEUROSCI.2844-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szabo Z, Obermair GJ, Cooper CB, Zamponi GW, Flucher BE. Role of the synprint site in presynaptic targeting of the calcium channel CaV2.2 in hippocampal neurons. Eur J Neurosci. 2006;24:709–18. doi: 10.1111/j.1460-9568.2006.04947.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Maximov A, Fu Y, Xu F, Tang TS, Tkatch T, Surmeier DJ, Bezprozvanny I. Association of CaV1.3 L-type calcium channels with Shank. J Neurosci. 2005;25:1037–49. doi: 10.1523/JNEUROSCI.4554-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulze K, Mulder A, Tinazli A, Tampe R. Controlling the activity of the 20S proteasome complex by synthetic gatekeepers. Angew Chem Int Ed Engl. 2006;45:5702–5. doi: 10.1002/anie.200600644. [DOI] [PubMed] [Google Scholar]
- 28.Sheridan DL, Berlot CH, Robert A, Inglis FM, Jakobsdottir KB, Howe JR, Hughes TE. A new way to rapidly create functional, fluorescent fusion proteins: random insertion of GFP with an in vitro transposition reaction. BMC Neurosci. 2002;3:7. doi: 10.1186/1471-2202-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellis SB, Williams ME, Ways NR, Brenner R, Sharp AH, Leung AT, Campbell KP, McKenna E, Koch WJ, Hui A, Schwartz A, Harpold MM. Sequence and expression of mRNAs encoding the α1 and α2 subunits of a DHP-sensitive calcium channel. Science. 1988;241:1661–4. doi: 10.1126/science.2458626. [DOI] [PubMed] [Google Scholar]
- 30.Castellano A, Wei X, Birnbaumer L, Perez-Reyes E. Cloning and expression of a third calcium channel β subunit. J Biol Chem. 1993;268:3450–5. [PubMed] [Google Scholar]
- 31.Pichler M, Cassidy TN, Reimer D, Haase H, Kraus R, Ostler D, Striessnig J. Beta subunit heterogeneity in neuronal L-type calcium channels. J Biol Chem. 1997;272:13877–82. doi: 10.1074/jbc.272.21.13877. [DOI] [PubMed] [Google Scholar]