Abstract
The present study was designed to determine the effects of Ganoderma lucidum polysaccharides (GL-PS) on exhaustive exercise-induced oxidative stress in skeletal muscle tissues of mice. The mice were divided into four groups (three GL-PS administered groups and the control group). The control group was administered with distilled water and GL-PS administered groups were administered with GL-PS (50, 100 and 200 mg/kg body weight per day). After 28 days, the mice performed an exhaustive swimming exercise, along with the determination of superoxide dismutase (SOD), glutathione peroxidase (GPX), catalase (CAT) activities and malondialdehyde (MDA) levels in the skeletal muscle of mice. The results showed that GL-PS could increase antioxidant enzymes activities and decrease the MDA levels in the skeletal muscle of mice. This study provides strong evidence that GL-PS supplementation possessed protective effects against exhaustive exercise-induced oxidative stress.
Keywords: Ganoderma lucidum polysaccharides, Exhaustive exercise-induced oxidative, Mice, Swimming exercise
1. Introduction
It is well documented that regular physical exercise has many beneficial effects, and is an effective means for preventing chronic diseases (Goto et al., 2004; Warburton et al., 2006; Araújo et al., 2011). However, strenuous physical exercise is associated with a dramatic increase in oxygen uptake both by the whole body and particularly by the skeletal muscle (Bejma and Ji, 1999). Oxygen usage increases with increasing metabolic activity and the results in reactive oxygen species (ROS) production (Powers and Jackson, 2008). Production of ROS in quantities that overwhelm the endogenous antioxidant defense system has been referred to as oxidative stress, which can lead to the destruction of tissue and cell macromolecules such as lipids, proteins, and nucleic acids (Jówko et al., 2011). There is growing evidence that exercise-induced oxidative stress may be associated with muscle fatigue, muscle damage, and a decrease in physical performance (Kim et al., 2008; Wei and Jin, 2011; Shan et al., 2011).
Ganoderma lucidum (Leyss. ex Fr.) Karst), a popular medicinal mushroom, is a basidiomycete belonging to the polyporaceae. Its fruiting body is called “Lingzhi” in China and “Reishi” in Japan (Zhang et al., 2003). For hundreds of years, this mushroom has been regarded as a folk medicine used for the prevention and treatment of various human diseases, such as hepatitis, hypertension, chronic bronchitis, bronchial asthma, cancer and others in China (Berovic et al., 2003; Boh et al., 2007). In the past few years, many studies have demonstrated that the major bioactive components in G. lucidum are polysaccharides (Gl-PS), ganoderic acid (triterpene), and adenosine, while the Gl-PS are the major source of its biological activity and therapeutic use (Zhang et al., 2002; Sanodiya et al., 2009). Previous evidences showed that Gl-PS have anti-oxidant, hypoglycemic, anti-inflammatory, anti-tumor and immunomodulatory activities (Lin and Zhang, 2004; Li et al., 2011; Zhao et al., 2012). Our previous studies also have demonstrated that Gl-PS has anti-fatigue effects (Zhao et al., 2013), but the effects of Gl-PS on exercise-induced oxidative stress have not been investigated so far. Therefore, this study was designed to investigate the protective effects of Gl-PS against exhaustive exercise-induced oxidative stress in skeletal muscle tissues of mice.
2. Materials and methods
2.1. Plant materials
The dried fruiting bodies of G. lucidum were purchased from a local medicine shop (Hangzhou, China), and identified by Professor Zhang ML, Zhejiang Gongshang University. A voucher specimen (No. 1908024) has been deposited in the Herbarium of the Zhejiang Institute of Botany.
2.2. Chemicals and reagents
Analytical reagent grade chemicals and double distilled water were used to prepare all solutions. The reagent kits for the determination of superoxide dismutase (SOD), glutathione peroxidase (GPX), catalase (CAT) and malondialdehyde (MDA) were purchased from the Nanjing Jiancheng Bioengineering Institute, Nanjing, China.
2.3. Preparation of Ganoderma lucidum polysaccharides
G. lucidum polysaccharides (Gl-PS) were prepared according to the previously published method (Chen et al., 2009). In brief, the dried fruiting bodies of G. lucidum were ground to a fine powder, and powders were defatted with petroleum ether and extracted with double distilled water at 80 °C for 8–10 h in several batches. The extract was combined, filtered, and concentrated to about one third of the original volume and about five times the original volume of chilled ethanol was added and kept at 4 °C for 48 h. The precipitate was collected after centrifugation, redissolved in distilled water and treated with Sevag’s reagent several times to remove protein and then dialyzed against deionised water for 48 h at 4 °C. The GL-PS were again precipitated with ethanol and the precipitate thus obtained was lyophilized. GL-PS was dissolved in distilled water and stored at 4 °C before use.
2.4. Animals and grouping
Male Kunming mice (18–20 g each) were obtained from the Zhejiang Animal Husbandry Center (Hangzhou, China). The animals were housed in a room maintained at 22 ± 1 °C with relative air humidity of 45–55% on a 12-h light/12-h dark cycle. Mice were provided a standard commercial diet and water ad libitum. All animal handling procedures were performed in strict accordance with the China legislation for the use and care of laboratory animals, with the guidelines established by the Institute for Experimental Animals of the Zhejiang Police College, and were approved by the College committee for animal experiments.
The mice were allowed to acclimate to the laboratory environment for a 7-day period before the experiments. After this period, the animals were randomly divided into four groups, ten in each. The first group designated as the control group was administered with distilled water by gavage each day for 28 days. The second group designated as the low dose group was administered with GL-PS of 50 mg/kg body weight by gavage each day for 28 days. The third group designated as the middle-dose group was administered with GL-PS of 100 mg/kg body weight by gavage each day for 28 days. The fourth group designated as the high-dose group was administered with GL-PS of 200 mg/kg body weight by gavage each day for 28 days. The mice were trained to swim for 20 min twice a week to adapt to swimming.
2.5. Exhaustive swimming exercise
After 28 days, the mice performed an exhaustive swimming exercise as described previously (Tang et al., 2008). Briefly, 30 min after the last administration, the mice were placed individually in a swimming pool (50 × 40 × 50 cm) with 30 cm depth of water maintained at 25 ± 0.5 °C. A tin wire (7% of body weight) was loaded onto the tail root of the mouse. The mice were determined to be exhausted when they sunk into the water and could not rise to the surface of water within a 10 s period. The mice were anesthetized with ethyl ether and sacrificed immediately after the exhaustive swimming exercise. Hind-limb skeletal muscle was quickly dissected out, frozen in liquid nitrogen, and kept at −70 °C until being analyzed.
2.6. Analytical oxidative stress-associated parameters
The skeletal muscle samples of mice were homogenized in ice-cold buffer (0.25 M sucrose, 10 mM Tris–HCl, and 0.25 Mm phenylmethylsulfonyl fluoride; pH 7.4), and a portion of the homogenate was measured immediately for malondialdehyde (MDA) levels. Another portion of the homogenate was centrifuged at 15,000g for 30 min at 4 °C, the supernatant was decanted and assayed for SOD, GPX and CAT activities. The SOD, GPX, CAT activities and MDA levels were tested following the recommended procedures provided by the reagent kits (Jiancheng Bioengineering Institute, Nanjing, China).
SOD activity was assayed spectrophotometrically at 550 nm by the use of a xanthine and xanthine oxide system. GPX was assayed spectrophotometrically by the use of glutathione as substrate by measuring the decrease of enzymatic reaction of GSH (except the effect of non-enzymatic reaction) at 412 nm. CAT activity was determined spectrophotometrically by monitoring the amount of complex compound at 405 nm due to H2O2 decomposition. MDA level was determined by the thiobarbituric acid (TBA) method. The MDA-TBA adduct formation was measured spectrophotometrically at 532 nm.
2.7. Statistical analysis
The data were expressed as means ± SD based on the indicated number in the experiment. All analyses of data were done with the Statistical Package for Social Sciences (version 11.0; SPSS, Chicago, IL, USA). The results were analyzed using 1-way analysis of variance followed by Student’s t test for comparison between different treatment groups. Statistical significance was set at P < 0.05.
3. Results
3.1. The effect of GL-PS on SOD activities in skeletal muscle of mice
Fig. 1 demonstrates the effect of GL-PS on SOD activities in the skeletal muscle of mice. Compared with the first (control) group, SOD activities of the second, third and fourth groups were significantly higher (P < 0.05). Compared with the second group, SOD activities of the third and fourth groups were significantly higher (P < 0.05). Compared with the third group, SOD activities of the fourth group were also higher but not significantly (P > 0.05).
Figure 1.
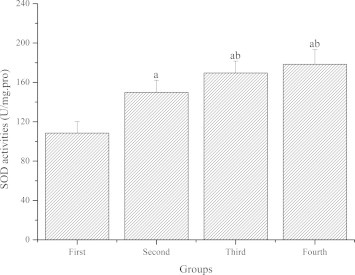
The effect of GL-PS on SOD activities in skeletal muscle. The data were expressed as means ± SD (n = 10 per group). ∗P < 0.05 when compared to the first group. ∗∗P < 0.05 when compared to the second group. ∗∗∗P < 0.05 when compared to the third group.
3.2. The effect of GL-PS on GPX activities in skeletal muscle of mice
Fig. 2 demonstrates the effect of GL-PS on GPX activities in the skeletal muscle of mice. Compared with the first group, GPX activities of the second, third and fourth groups were significantly higher (P < 0.05). Compared with the second group, GPX activities of the third and fourth groups were significantly higher (P < 0.05). Compared with the third group, GPX activities of the fourth groups were significantly higher (P < 0.05).
Figure 2.
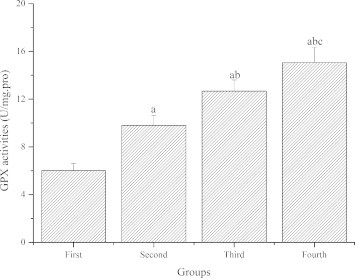
The effect of GL-PS on GPX activities in skeletal muscle. The data were expressed as means ± SD (n = 10 per group). ∗P < 0.05 when compared to the first group. ∗∗P < 0.05 when compared to the second group. ∗∗∗P < 0.05 when compared to the third group.
3.3. The effect of GL-PS on CAT activities in skeletal muscle of mice
Fig. 3 demonstrates the effect of GL-PS on CAT activities in the skeletal muscle of mice. Compared with the first group, CAT activities of the third and fourth groups were significantly higher (P < 0.05), CAT activities of the second group were also higher but not significantly (P > 0.05). Compared with the second group, CAT activities of the fourth group were significantly higher (P < 0.05), CAT activities of the third group were also higher but not significantly (P > 0.05). Compared with the third group, CAT activities of the fourth group were also higher but not significantly (P > 0.05).
Figure 3.
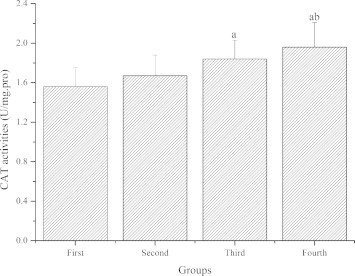
The effect of GL-PS on CAT activities in skeletal muscle. The data were expressed as means ± SD (n = 10 per group). ∗P < 0.05 when compared to the first group. ∗∗P < 0.05 when compared to the second group. ∗∗∗P < 0.05 when compared to the third group.
3.4. The effect of GL-PS on MDA levels in skeletal muscle of mice
Fig. 4 demonstrates the effect of GL-PS on MDA levels in the skeletal muscle of mice. Compared with the first group, MDA levels of the second, third and fourth groups were significantly lower (P < 0.05). Compared with the second group, MDA levels of the third and fourth groups were significantly lower (P < 0.05). Compared with the third group, MDA levels of the fourth groups were significantly lower (P < 0.05).
Figure 4.
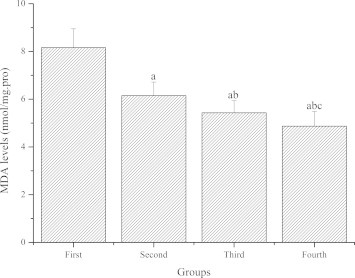
The effect of GL-PS on MDA levels in skeletal muscle. The data were expressed as means ± SD (n = 10 per group). ∗P < 0.05 when compared to the first group. ∗∗P < 0.05 when compared to the second group. ∗∗∗P < 0.05 when compared to the third group.
4. Discussion
The present work has been designed to determine the effects of G. lucidum polysaccharides on exhaustive swimming exercise-induced Oxidative stress by noting the SOD, GPX and CAT activities as well as by the MDA levels in skeletal muscle tissues of mice. The antioxidant defense systems of the living body consist of antioxidant enzymes and antioxidant nutrients, which may be involved in reducing oxidative stress (Lee et al., 2009). Principal antioxidant enzymes include SOD, GPX and CAT. SOD catalyzes the dismutation of superoxide into oxygen and hydrogen peroxide. GPX is a selenoenzyme which catalyzes the reduction of hydroperoxides at the expense of reduced glutathione. CAT is a primary antioxidant defense component that works to catalyze the decomposition of hydrogen peroxide to water, sharing this function with GPX (Apel and Hirt, 2004). The significant decrease in the activities of SOD, GPX and CAT in the muscle, liver tissues and blood after exhaustive exercise may be an indication of oxidative stress (Powers and Jackson, 2008). In the present study, the data showed that GL-PS promote increases in the activities of SOD, GPX and CAT in skeletal muscle of mice, and the high-dose GL-PS (200 mg/kg body weight) presented the best effect. These results indicated that GL-PS was able to up-regulate antioxidant enzyme activities in skeletal muscle to protect against oxidative stress induced by exhaustive exercise.
MDA is the breakdown product of the major chain reactions leading to the oxidation of polyunsaturated fatty acids and thus serves as a reliable marker of oxidative stress (Finaud et al., 2006). Significant increases in the MDA levels in muscle after exhaustive exercise have been noted in several studies (Alessio et al., 1988; Liu et al., 2000; Cabral et al., 2001; Niu et al., 2008). When MDA exceeds the regulatory level of the body, surplus free radicals will induce unsaturated fatty acid to form lipid peroxide and overwhelm the protective mechanisms, resulting in oxidative damage (Yu et al., 2006). In the present study, the data showed that GL-PS could effectively decrease MDA levels in the skeletal muscle of mice, and the high-dose GL-PS (200 mg/kg body weight) presented the best effect. These results indicated that GL-PS could reduce lipid peroxidation and prevent exercise-induced oxidative damage.
5. Conclusion
In conclusion, the present study clearly indicates that GL-PS could increase antioxidant enzymes activities and decrease the MDA levels in the skeletal muscle of mice. This study provides strong evidence that GL-PS supplementation attenuates exhaustive exercise-induced oxidative stress.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alessio H.M., Goldfarb A.H., Cutler R.G. MDA content increases in fast- and slow-twitch skeletal muscle with intensity of exercise in a rat. Am. J. Physiol. 1988;255(6 Pt 1):C874–C877. doi: 10.1152/ajpcell.1988.255.6.C874. [DOI] [PubMed] [Google Scholar]
- Apel K., Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Araújo M.B., Moura L.P., Ribeiro C., Dalia R., Voltarelli F.A., Mello M.A. Oxidative stress in the liver of exercised rats supplemented with creatine. Int. J. Nutr. Metabol. 2011;3(5):58–64. [Google Scholar]
- Bejma J., Ji L.L. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J. Appl. Physiol. 1999;87(1):465–470. doi: 10.1152/jappl.1999.87.1.465. [DOI] [PubMed] [Google Scholar]
- Berovic M., Habijanic J., Zore I., Wraber B., Hodzar D., Boh B., Pohleven F. Submerged cultivation of Ganoderma lucidum biomass and immunostimulatory effects of fungal polysaccharides. J. Biotechnol. 2003;103(1):77–86. doi: 10.1016/s0168-1656(03)00069-5. [DOI] [PubMed] [Google Scholar]
- Boh B., Berovic M., Zhang J., Zhi-Bin L. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol. Annu. Rev. 2007;13:265–301. doi: 10.1016/S1387-2656(07)13010-6. [DOI] [PubMed] [Google Scholar]
- Cabral de Oliveira A.C., Perez A.C., Merino G., Prieto J.G., Alvarez A.I. Protective effects of Panax ginseng on muscle injury and inflammation after eccentric exercise. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001;130(3):369–377. doi: 10.1016/s1532-0456(01)00262-9. [DOI] [PubMed] [Google Scholar]
- Chen X.P., Chen Y., Li S.B., Chen Y.G., Lan J.Y., Liu L.P. Free radical scavenging of Ganoderma lucidum polysaccharides and its effect on antioxidant enzymes and immunity activities in cervical carcinoma rats. Carbohydr. Polym. 2009;77:389–393. [Google Scholar]
- Finaud J., Lac G., Filaire E. Oxidative stress: relationship with exercise and training. Sports Med. 2006;36(4):327–358. doi: 10.2165/00007256-200636040-00004. [DOI] [PubMed] [Google Scholar]
- Goto S., Radák Z., Nyakas C., Chung H.Y., Naito H., Takahashi R., Nakamoto H., Abea R. Regular exercise: an effective means to reduce oxidative stress in old rats. Ann. NY Acad. Sci. 2004;1019:471–474. doi: 10.1196/annals.1297.085. [DOI] [PubMed] [Google Scholar]
- Jówko E., Sacharuk J., Balasińska B., Ostaszewski P., Charmas M., Charmas R. Green tea extract supplementation gives protection against exercise-induced oxidative damage in healthy men. Nutr. Res. 2011;31(11):813–821. doi: 10.1016/j.nutres.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Kim S., Park S.H., Lee H.N., Park T. Prunus mume extract ameliorates exercise-induced fatigue in trained rats. J. Med. Food. 2008;11(3):460–468. doi: 10.1089/jmf.2007.0097. [DOI] [PubMed] [Google Scholar]
- Lee S.P., Mar G.Y., Ng L.T. Effects of tocotrienol-rich fraction on exercise endurance capacity and oxidative stress in forced swimming rats. Eur. J. Appl. Physiol. 2009;107(5):587–595. doi: 10.1007/s00421-009-1159-6. [DOI] [PubMed] [Google Scholar]
- Li F., Zhang Y., Zhong Z. Antihyperglycemic effect of Ganoderma lucidum polysaccharides on streptozotocin-induced diabetic mice. Int. J. Mol. Sci. 2011;12(9):6135–6145. doi: 10.3390/ijms12096135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z.B., Zhang H.N. Anti-tumor and immunoregulatory activities of Ganoderma lucidum and its possible mechanisms. Acta. Pharmacol. Sin. 2004;25(11):1387–1395. [PubMed] [Google Scholar]
- Liu J., Yeo H.C., Overvik-Douki E., Hagen T., Doniger S.J., Chyu D.W., Brooks G.A., Ames B.N. Chronically and acutely exercised rats: biomarkers of oxidative stress and endogenous antioxidants. J. Appl. Physiol. 2000;89(1):21–28. doi: 10.1152/jappl.2000.89.1.21. [DOI] [PubMed] [Google Scholar]
- Niu A.J., Wu J.M., Yu D.H., Wang R. Protective effect of Lycium barbarum polysaccharides on oxidative damage in skeletal muscle of exhaustive exercise rats. Int. J. Biol. Macromol. 2008;42(5):447–449. doi: 10.1016/j.ijbiomac.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Powers S.K., Jackson M.J. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008;88(4):1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanodiya B.S., Thakur G.S., Baghel R.K., Prasad G.B., Bisen P.S. Ganoderma lucidum: a potent pharmacological macrofungus. Curr. Pharm. Biotechnol. 2009;10(8):717–742. doi: 10.2174/138920109789978757. [DOI] [PubMed] [Google Scholar]
- Shan X., Zhou J., Ma T., Chai Q. Lycium barbarum polysaccharides reduce exercise-induced oxidative stress. Int. J. Mol. Sci. 2011;12(2):1081–1088. doi: 10.3390/ijms12021081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W., Zhang Y., Gao J., Ding X., Gao S. The anti-fatigue effect of 20(R)-ginsenoside Rg3 in mice by intranasally administration. Biol. Pharm. Bull. 2008;31(11):2024–2027. doi: 10.1248/bpb.31.2024. [DOI] [PubMed] [Google Scholar]
- Warburton D.E., Nicol C.W., Bredin S.S. Health benefits of physical activity: the evidence. Can. Med. Assoc. J. 2006;174(6):801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei P., Jin H.M. Blueberries extract supplementation improves physical performance and decreases oxidative stress in mice. Afr. J. Biotech. 2011;10(60):12999–13003. [Google Scholar]
- Yu F., Lu S., Yu F., Feng S., McGuire P.M., Li R., Wang R. Protective effects of polysaccharide from Euphorbia kansui (Euphorbiaceae) on the swimming exercise-induced oxidative stress in mice. Can. J. Physiol. Pharmacol. 2006;84(10):1071–1079. doi: 10.1139/y06-052. [DOI] [PubMed] [Google Scholar]
- Zhang G.L., Wang Y.H., Ni W., Teng H.L., Lin Z. Hepatoprotective role of Ganoderma lucidum polysaccharide against BCG-induced immune liver injury in mice. World J. Gastroenterol. 2002;8(4):728–733. doi: 10.3748/wjg.v8.i4.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.N., He J.H., Yuan L., Lin Z.B. In vitro and in vivo protective effect of Ganoderma lucidum polysaccharides on alloxan-induced pancreatic islets damage. Life Sci. 2003;73(18):2307–2319. doi: 10.1016/s0024-3205(03)00594-0. [DOI] [PubMed] [Google Scholar]
- Zhao W., Jiang X., Deng W., Lai Y., Wu M., Zhang Z. Antioxidant activities of Ganoderma lucidum polysaccharides and their role on DNA damage in mice induced by cobalt-60 gamma-irradiation. Food Checol. 2012;50(2):303–309. doi: 10.1016/j.fct.2011.10.071. [DOI] [PubMed] [Google Scholar]
- Zhao Z.H., Zheng X.W., Fang F. Effects of Ganoderma lucidum polysaccharides on exercise-induced fatigue in mice. Asian J. Anim. Vet. Adv. 2013;8(3):511–518. [Google Scholar]



