Abstract
This case-control study examines whether chronic diarrhea at initiation of antiretroviral therapy (ART) affects survival of human immunodefiency virus–infected patients. Cases (288) were treatment-naive, non-pregnant, adults with self report of frequent loose stool for > 3 weeks at the time ART was initiated. One-third of patients had an enteric pathogen identified including Cryptosporidium spp., Giardia spp., Isospora belli, Cyclospora cayetanensis, and Entamoeba histolytica. Control patients (400) did not have diarrhea when initiating ART. At six weeks, mortality was 10% in the patients with diarrhea and 5% in the patients without diarrhea (P = 0.009). Chronic diarrhea in patients requesting ART in Haiti is associated with increased early mortality.
INTRODUCTION
Chronic diarrhea is one of the most common symptoms of acquired immunodeficiency syndrome (AIDS) in resourcelimited countries. 1–11 Patients with AIDS and diarrhea have increased mortality rates. 12,13 Studies of patients in resourcerich countries suggest that highly active antiretroviral therapy (ART) sharply reduces or eliminates chronic diarrhea and improves survival. 14,15 However, no studies have evaluated patients with chronic diarrhea treated with ART in resource-limited settings where tropical enteric infections, high parasite burden, and severe malnutrition may adversely affect the outcome of ART.
Patients with AIDS in resource-poor countries who are treated with ART have higher rates of mortality in the first few months than patients in wealthier settings receiving comparable regimens. 16–22 Some studies suggest that chronic tropical diarrhea may decrease the absorption of antiretroviral drugs and therefore could contribute to the excess mortality documented in the first months after initiating ART. 23–28 Other studies suggest that the wasting associated with chronic diarrhea leads to early mortality. 29–31 Therefore, we conducted a study in Port au Prince, Haiti to determine whether the presence of chronic diarrhea at the initiation of ART affected clinical outcomes of patients with AIDS.
METHODS
We conducted a nested case-control study within a cohort of patients with AIDS receiving antiretroviral therapy at the clinic of the Haitian Group for the Study of Kaposi’s Sarcoma and Opportunistic Infections (GHESKIO). The GHESKIO Centers in Port au Prince, Haiti provides voluntary counseling, testing, and care for patients with HIV/AIDS, other sexually transmitted diseases, and tuberculosis. The study was reviewed and approved by the institutional review boards at GHESKIO, Weill Cornell Medical College, and the University of Virginia.
All adult patients coming to the GHESKIO clinic with HIV symptoms provide a medical history, are given a physical examination, and have a complete blood count and a CD4 T cell count measured (Becton-Dickinson, Franklin Lakes, NJ). Patients with an AIDS-defining illness or a CD4 T cell count < 200 cells/mm3 are given ART according to World Health Organization (WHO) guidelines. The CD4 counts are measured every six months. GHESKIO maintains an electronic medical record (EMR) that includes clinical, laboratory, and pharmacy data on all patients receiving ART.
All patients coming to GHESKIO with chronic diarrhea are asked to provide a stool specimen. Guaiac testing is performed to evaluate for occult blood. Methylene blue staining is performed on a wet mount for leukocytes. Trained technologists perform a microscopic examination for ova and parasites. Coccidial oocysts are identified with modified Kinyoun acid-fast staining. Studies for viral or bacterial pathogens are not routinely performed. Chronic diarrhea is treated empirically with metronidazole (500 mg three times a day) and/or trimethoprim/sulfamethoxazole (800 mg/160 mg twice a day) at the discretion of the treating physician. Pathogen-specific therapy is provided in the event that a treatable enteric pathogen is identified.
Clinical definitions
Cases were defined as adult (≥ 18 years of age), non-pregnant patients with no prior exposure to antiretroviral drugs who initiated ART from March 1, 2003, through April 30, 2004, and had chronic diarrhea at the initiation of ART (self report of frequent loose stool for > 3 weeks). Controls included all adult, non-pregnant patients without prior exposure to antiretroviral drugs who initiated ART during the same time period, and had no history of diarrhea in the three-week period prior to starting ART.
Early mortality was defined as death in the first six weeks after initiation of ART. 22 An AIDS-defining illness is an opportunistic infection or malignancy that satisfied the WHO defnition of stage IV HIV disease. We define low body weight as a weight below the lowest quartile for all patients in the study stratified by sex. We define weight loss at month 3 as a weight that is > 3% lower than the weight at ART initiation. 31
Data collection
The EMR was used to generate a list of all patients who had initiated ART at GHESKIO from March 2003 through April 2004. The list included 1,069 patients. Demographics, clinical history, physical examination findings, pregnancy history, antiretroviral drug history, CD4 T cell counts, weights, mortality, and follow-up data through January 2007 were obtained from the EMR for all patients.
All patients at GHESKIO are specifically asked about diarrhea at each clinic visit, and this information is entered into the EMR. The EMR was queried to identify all patients who reported diarrhea as a symptom at the time of ART initiation. The charts of all patients with diarrhea were reviewed by a single author (RAD) to evaluate them for inclusion as case patients and to collect information from the medical chart using a standardized form about the diagnosis, treatment, and course of the diarrheal illness.
Statistical analysis
Baseline characteristics and clinical outcomes between cases and controls were compared using a chisquare test for categorical variables and an analysis of variance test for continuous variables. Significance was pre-assigned as P < 0.05. Survival estimates were calculated using the Kaplan-Meier method with significant differences reported using the log rank probability test. The day of initiation of ART was considered baseline, and patients were censored at death or on the date of last clinic visit up to January 1, 2007. A multivariable Cox regression model was used to evaluate significant univariate Kaplan-Meier analyses. Statistical analyses were performed using SPSS software version 15.0 (SPSS Inc., Chicago, IL).
RESULTS
Patient population
From March 1, 2003, through April 30, 2004 GHESKIO initiated ART in 1,069 treatment-naive patients with either an AIDS-defining illness or a CD4 T cell count < 200 cells/mL. Of these patients, 288 (27%) ART-naive, non-pregnant adults with chronic diarrhea were included in the analysis as cases, and 400 (37%) ART-naive, non-pregnant adults without diarrhea were included as controls. Not included in this analysis were 139 (13%) children, 73 (7%) pregnant women, 80 (7%) adults with prior ART, 41 (4%) adult patients with acute diarrhea for less than three weeks, and 53 (5%) patients with incomplete electronic medical records. The median follow-up of the 288 cases and 400 controls was 36 months.
Baseline characteristics of cases and controls are detailed in Table 1 . Seventy percent of patients with chronic diarrhea had an AIDS-defining illness by WHO criteria compared with 22% of patients without diarrhea. Patients with diarrhea weighed approximately 3.5 kg less than controls and were more likely to fall into the lowest quartile for weight by sex than controls. The median CD4 T cell count of patients with diarrhea was 25 CD4 T cells/mm3 lower than that of control patients.
Table 1.
Baseline characteristics of AIDS patients with and without chronic diarrhea, Haiti*
| Characteristic | Chronic diarrhea (n = 288) |
No diarrhea (n = 400) |
P |
|---|---|---|---|
| Age, years, no. (%) | |||
| 18–29 | 44 (15) | 46 (12) | |
| 30–39 | 114 (40) | 148 (37) | |
| 40–49 | 88 (31) | 148 (37) | 0.251 |
| 50–59 | 30 (10) | 47 (12) | |
| > 60 | 11 (4) | 10 (3) | |
| Female, no. (%) | 144 (50) | 223 (56) | 0.136 |
| Earn < $1.00/day, no. (%) | 155 (44) | 223 (56) | 0.616 |
| Educational level, no. (%) | |||
| None | 53 (18) | 60 (15) | |
| Primary | 87 (30) | 125 (31) | 0.716 |
| Secondary | 131 (46) | 180 (45) | |
| University | 15 (5) | 24 (6) | |
| AIDS-defining illness,† no. (%) | 202 (70) | 90 (22) | < 0.001 |
| Tuberculosis at ART start, no. (%) | 22 (8) | 31 (8) | 0.748 |
| Tuberculosis after ART start, no. (%) | 16 (6) | 19 (5) | 0.796 |
| Body weight, kg | |||
| Men | |||
| Median | 55.2 | 58.2 | 0.004 |
| Interquartile range | 47.7–61.3 | 51.9–64.8 | |
| Women | |||
| Median | 48.6 | 52.9 | < 0.001 |
| Interquartile range | 41.8–53.2 | 45.5–58.9 | |
| Low body weight,‡ no. (%) | 89 (31) | 77 (19) | < 0.001 |
| CD4 T cells/mm3 | |||
| Median | 106 | 131 | 0.005 |
| Interquartile range | 21–161 | 38–204 | |
| Hemoglobin level, g/dL | |||
| Median | 10.5 | 10.5 | 0.737 |
| Interquartile range | 9.2–11.6 | 9.3–11.6 | |
| Initial ART regimen, no. (%) | |||
| Zidovudine, lamivudine, efavirenz | 145 (51) | 195 (49) | |
| Zidovudine, lamivudine, nevirapine | 118 (41) | 170 (43) | 0.939 |
| Other | 24 (8) | 35 (8) |
AIDS = acquired immunodeficiency syndrome; ART = antiretroviral therapy.
Defined by the World Health Organization.
Defined as a weight below the 25% quartile for all patients in the study stratified by sex.
Of the 288 patients with chronic diarrhea, 243 (85%) submitted a stool sample for analysis. Of the 243 patients with a stool examination, 14(6%) had occult fecal blood, 15(6%) had fecal leukocytes, and 80 (33%) had an enteric pathogen identified. The patients with pathogens identified in the 243 patients included 39 patients with Cryptosporidium spp. (16%), 14 with Giardia spp. (6%), 11 with Isospora belli (5%), 8 with Cyclospora cayetanensis (3%), and 1 with Entamoeba histolytica (0.4%).
Treatment and outcomes
All patients in this study were initiated on three-drug ART according to WHO guidelines. The regimens are described in Table 1 . Of note, 109 (38%) of the 288 patients with chronic diarrhea received trimethoprim/sulfamethoxazole prophylaxis prior to initiating ART.
Physicians also provided antimicrobial therapy to 239 (83%) of 288 patients with chronic diarrhea. In addition to antibiotics, patients with diarrhea received a variety of additional therapies on the basis of the clinicians’ judgment. These therapies included oral rehydration solution (56%), multivitamins (44%), and food supplements (15%). Diarrhea persisted after the initiation of ART in the case-patients for a mean of 27 days (range = 7–140 days).
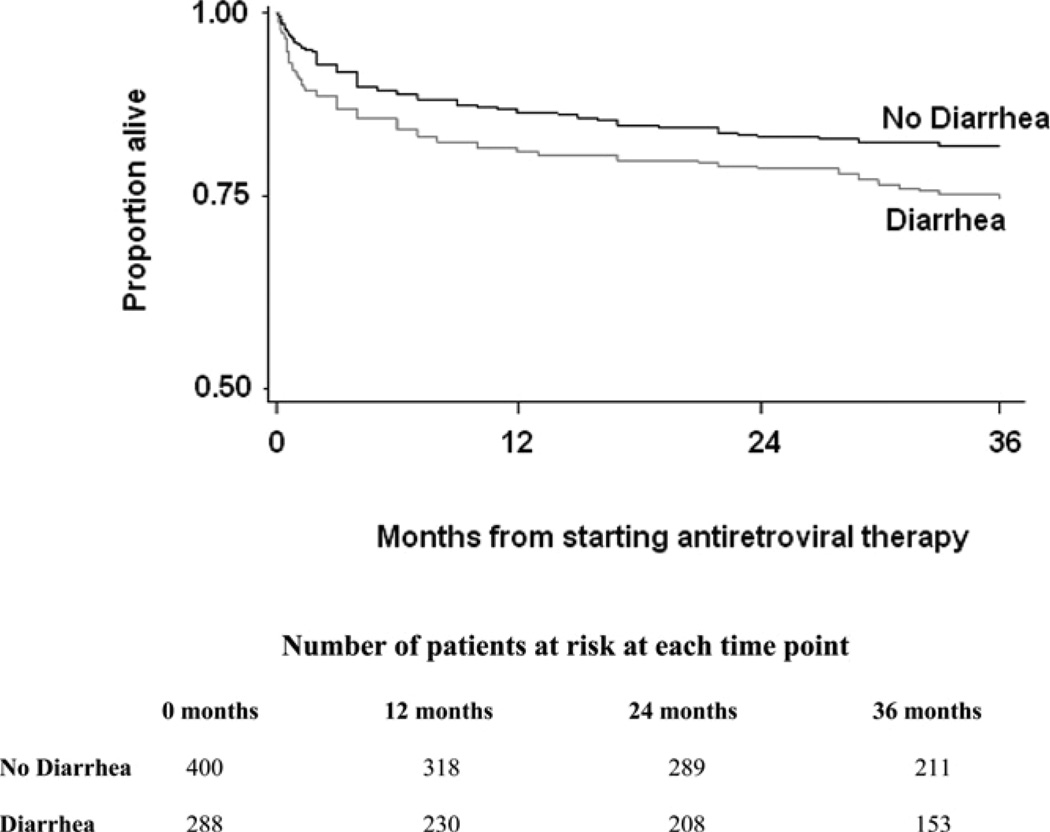
The mortality in the first six weeks in patients with diarrhea was twice the mortality in the controls without diarrhea. Kaplan-Meier estimation showed that mortality six weeks after the initiation of ART was 10% in patients with diarrhea and 5% in control patients, (P = 0.0092). After six weeks, the survival curves for the cases and controls are nearly parallel. As shown in Figure 1, the Kaplan-Meier estimate of mortality at 36 months was 25% in the patients with chronic diarrhea and 18% in the control patients (P = 0.0288).
Figure 1.
Kaplan-Meier curves demonstrating the difference in survival between cases (patients with diarrhea) and controls (patients without diarrhea) over 36 months of follow-up (P = 0.03, by log-rank test).
Multivariable analysis of the 688 patients in this study (288 cases and 400 controls) showed that baseline CD4 T cell counts, low body weight, and the presence of an AIDS-defining illness were predictive of mortality (Table 2 ). When controlling for these three variables, diarrhea was not an independent risk factor for death. Interaction terms (CD4 cell count and diarrhea, and low body weight and diarrhea) were entered into the model but were not significant.
T able 2.
Predictors of death among patients receiving antiretroviral therapy in Haiti*
| Variable | Hazards ratio† | 95% confidence interval | P |
|---|---|---|---|
| Low body weight‡ | 1.6 | (1.3–2.0) | < 0.0001 |
| CD4 count < 50 cells/mL | 1.6 | (1.1–2.4) | 0.0196 |
| AIDS-defining illness§ | 1.5 | (1.0–2.3) | 0 0.0189 |
AIDS = acquired immunodeficiency syndrome.
Hazards ratios are adjusted for other variables in the table.
Defined as a weight below the 25% quartile for all patients in the study stratified by sex.
As defined by the World Health Organization and included wasting syndrome, candida esophagitis, cryptosporidiosis, and extrapulmonary tuberculosis.
Among the 243 patients with diarrhea who survived to 3 months, the median weight gain at month 3 was 8.0 kg (interquartile range = 1.2–15 kg). The 323 patients without diarrhea gained a median of 4.0 kilograms at month 3 (interquartile range = 0.5–11.0 kg) (P = 0.001). At 3 months, 217 (89%) of 243 patients with diarrhea gained weight and 26 (11%) lost weight. In the controls, 274 (85%) of 323 patients gained weight and 49 (15%) lost weight, (P = 0.110). Patients who lost weight at 3 months were significantly more likely to die during the remainder of the follow-up period (hazards ratio = 2.1, 95% confidence interval = 1.2–3.8, P = 0.02). Case-patients who lost weight at 3 months had a significantly longer duration of diarrhea (6.2 versus 3.3 weeks; P < 0.0001).
Among the 182 patients with diarrhea who survived to 6 months and had baseline and 6 month CD4 counts measured, the median increase in CD4 T cell count was 125 cells/mm3 (interquartile range = 60–199 cells/mm3). Among 259 control patients with diarrhea who survived to 6 months and had baseline and 6 month CD4 count measured, the median increase in CD4 T cell count was 126 cells/mm3 (interquartile range = 62–189 cells/mm3) (P = 0.7688).
DISCUSSION
Ten percent of AIDS patients with chronic diarrhea died within six weeks despite the provision of ART, antibiotics for enteric pathogens, and supportive therapy. This mortality rate is twice that of HIV-infected control patients without diarrhea starting ART at the same in the first six weeks of ART.
Several recent reports demonstrate that HIV-infected patients receiving ART in resource-limited settings have higher mortality in the first six weeks compared with patients in wealthier settings. 19–21 The authors of these studies rightly emphasize that this high early mortality indicates a need to expand HIV testing and to help patients to access therapy earlier. Furthermore, simple clinical markers are needed to identify patients most at risk of early mortality. Our data suggest that self-report of chronic diarrhea is one such indicator.
Chronic diarrhea in our cohort was strongly associated with low body weight. This study and others have identified low body weight as a predictor of mortality. 22,29 In addition, patients in our study who lost weight in the first three months of therapy were more likely to die, as has also been demonstrated in a U.S. cohort. 31 Therefore, patients with chronic diarrhea may need closer follow-up to ensure that they are receiving adequate medical therapy and nutritional support to address low body weight and to prevent weight loss.
Less than 15% of patients had an enteric pathogen identified that was susceptible to the two drugs, metronidazole and trimethoprim/sulfamethoxazole, that are used as empiric therapy for chronic AIDS diarrhea in Haiti and many other resource-poor settings. In past studies conducted in Haiti, approximately 25% of HIV-infected patients with chronic diarrhea had Cyclospora spp. or Isospora spp. identified in stool samples. 32–34 In the current study, we found these two pathogens in less than 10% of patients with chronic diarrhea. This decrease is likely due to increasing use of trimethoprim/ sulfamethoxazole as prophylaxis for patients with HIV in Haiti. In the current study, nearly 40% of case-patients had received trimethoprim/sulfamethoxazole prior to presentation. Eighty-five percent of patients in our study had either Cryptospridia spp., which are not susceptible to the antibiotics used, or no pathogen identified. Given this finding and the strong association between chronic diarrhea and early mortality, we recommend that clinicians in resource-poor settings start ART immediately for HIV-infected patients with chronic AIDS diarrhea.
As noted in the introduction, some authors have suggested that HIV-infected patients with diarrhea may not absorb their ART because of enteropathy. They have then hypothesized that low ART levels may lead to virologic failure and ultimately death. Our data do not support this view. Although we did not measure viral load, the immunologic recovery of case-patients was statistically equivalent to that of control patients. Patients with diarrhea who survived the first few months of antiretroviral therapy did nearly as well as other patients during three years of follow-up. If diarrhea resulted in low drug levels and virologic failure, one would expect diarrhea to be an independent risk for mortality with persistent treatment failures and deaths throughout the follow- up period.
In conclusion, chronic diarrhea in HIV-infected patients presenting for ART in Haiti is associated with increased early mortality. In resource-poor settings, self-report of chronic diarrhea can serve as a simple clinical marker of increased risk for mortality and should prompt aggressive therapy and close monitoring.
Acknowledgments
Financial support: This study was supported by University of Virginia infectious disease and biodefense training grant, 5T32AI007046-31; by Fogarty International Center grants TW 00018, TW006896, and TW006901; and National Institute of Allergy and Infectious Diseases grants AI058257 and A1077339-01A1. Clinical care of the patients in this cohort was supported by grants from the Global Fund Against AIDS, Malaria, and Tuberculosis and the President’s Emergency Plan for AIDS Relief. The funders had no role in the design, conduct, or reporting of this study or in the decision to publish its results.
REFERENCES
- 1.Boncy M, Laroche AC, Liautaud B, Mathurin JR, Pape JW, Pamphile M, Péan V, St-Amand MM, Thomas F, Arnoux E, Elie R, Guérin JM, Laroche AC, Maelbranche R, Pierre G. Acquired immunodeficiency in Haitians. N Engl J Med. 1983;308:1419–1420. doi: 10.1056/nejm198306093082314. [DOI] [PubMed] [Google Scholar]
- 2.Brink A-K, Mahe C, Watera C, Lugada E, Gilks C, Whitworth J, French N. Diarrhoea, CD4 counts and enteric infections in a community-based cohort of HIV-infected adults in Uganda. J Infect. 2002;45:99–106. doi: 10.1053/jinf.2002.1002. [DOI] [PubMed] [Google Scholar]
- 3.Carcamo C, Hooton T, Wener MH, Weiss NS, Gilman R, Arevalo J, Carrasco J, Seas, Caballero M, Holmes KK. Etiologies and manifestations of persistent diarrhea in adults with HIV-1 infection: a case-control study in Lima, Peru. J Infect Dis. 2005;191:11–19. doi: 10.1086/426508. [DOI] [PubMed] [Google Scholar]
- 4.Dallabetta GA, Miotti PG. Chronic diarrhoea in AIDS patients in the tropics: a review. Trop Doct. 1992;22:3–9. doi: 10.1177/004947559202200102. [DOI] [PubMed] [Google Scholar]
- 5.Mwachari CW, Meier AS, Muyodi J, Gatei W, Waiyaki P, Cohen CR. Chronic diarrhoea in HIV-1-infected adults in Nairobi, Kenya: evaluation of risk factors and the WHO treatment algorithm. AIDS. 2003;17:2124–2126. doi: 10.1097/01.aids.0000088182.01779.09. [DOI] [PubMed] [Google Scholar]
- 6.Serwadda D, Mugerwa RD, Sewankambo NK, Lwegaba A, Carswell JW, Kirya GB, Bayley AC, Downing RG, Tedder RS, Clayden SA. Slim disease: a new disease in Uganda and its association with HTLV-III infection. Lancet. 1985;2:849–852. doi: 10.1016/S0140-6736(85)90122-9. [DOI] [PubMed] [Google Scholar]
- 7.Chakraborty N, Mukherjee A, Santra S, Sarkar RN, Banerjee D, Guha SK, Chakraborty S, Bhattacharyya SK. Current trends of opportunistic infections among HIV-seropositive patients from Eastern India. Jpn J Infect Dis. 2008;61:49–53. [PubMed] [Google Scholar]
- 8.Gupta S, Narang S, Nunavath V, Singh S. Chronic diarrhoea in HIV patients: prevalence of coccidian parasites. Indian J Med Microbiol. 2008;26:172–175. doi: 10.4103/0255-0857.40536. [DOI] [PubMed] [Google Scholar]
- 9.Akolo C, Ukoli CO, Ladep GN, Idoko JA. The clinical features of HIV/AIDS at presentation at the Jos University Teaching Hospital. Niger J Med. 2008;17:83–87. doi: 10.4314/njm.v17i1.37363. [DOI] [PubMed] [Google Scholar]
- 10.Lavy V. Presenting symptoms and signs in children referred for palliative care in Malawi. Palliat Med. 2007;21:333–339. doi: 10.1177/0269216307077689. [DOI] [PubMed] [Google Scholar]
- 11.Ferrand RA, Luethy R, Bwakura F, Mujuru H, Miller RF, Corbett EL. HIV infection presenting in older children and adolescents: a case series from Harare, Zimbabwe. Clin Infect Dis. 2007;44:874–878. doi: 10.1086/511873. [DOI] [PubMed] [Google Scholar]
- 12.Weber R, Ledergerber B, Zbinden R, Altwegg M, Pfyffer GE, Spycher MA, Briner J, Kaiser L, Opravil M, Meyenberger C, Flepp M. Enteric infections and diarrhea in human immunodeficiency virus-infected persons: prospective communitybased cohort study. Swiss HIV Cohort Study. Arch Intern Med. 1999;159:1473–1480. doi: 10.1001/archinte.159.13.1473. [DOI] [PubMed] [Google Scholar]
- 13.Lindan CP, Allen S, Serufilira A, Lifson AR, Van de Perre P, Chen- Rundle A, Batungwanayo J, Nsengumuremyi F, Bogaerts J, Hulley S. Predictors of mortality among HIV-infected women in Kigali, Rwanda. Ann Intern Med. 1992;116:320–328. doi: 10.7326/0003-4819-116-4-320. [DOI] [PubMed] [Google Scholar]
- 14.Maggi P, Larocca AM, Quarto M, Serio G, Brandonisio O, Angarano G, Pastore G. Effect of antiretroviral therapy on cryptosporidiosis and microsporidiosis in patients infected with human immunodeficiency virus type 1. Eur J Clin Microbiol Infect Dis. 2000;19:213–217. doi: 10.1007/s100960050461. [DOI] [PubMed] [Google Scholar]
- 15.Carr A, Marriott D, Field A, Vasak E, Cooper DA. Treatment of HIV-1-associated microsporidiosis and cryptosporidiosis with combination antiretroviral therapy. Lancet. 1998;351:256–261. doi: 10.1016/S0140-6736(97)07529-6. [DOI] [PubMed] [Google Scholar]
- 16.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, Wood R, Laurent C, Sprinz E, Seyler C, Bangsberg DR, Balestre E, Sterne JA, May M, Egger M Antiretriviral Therapy in Lower Incomes Countries (ART-LINC) Collaboration; ART Cohort Collaboration (ART-CC) groups. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 17.Ferradini L, Jeannin A, Pinoges L, Izopet J, Odhiambo D, Mankhambo L, Karungi G, Szumilin E, Balandine S, Fedida G, Carrieri MP, Spire B, Ford N, Tassie JM, Guerin PJ, Brasher C. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367:1335–1342. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- 18.Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19:2141–2148. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 19.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coetzee D, Hildebrand K, Boulle A, Maartens G, Louis F, Labatala V, Reuter H, Ntwana N, Goemaere E. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;18:887–895. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 21.Etard JF, Ndiaye I, Thierry-Mieg M, Gueye NF, Gueye PM, Laniece I, Dieng AB, Diouf A, Laurent C, Mboup S, Sow PS, Delaporte E. Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: a 7-year cohort study. AIDS. 2006;20:1181–1189. doi: 10.1097/01.aids.0000226959.87471.01. [DOI] [PubMed] [Google Scholar]
- 22.Jerene D, Endale A, Hailu Y, Lindtjorn B. Predictors of early death in a cohort of Ethiopian patients treated with HAART. BMC Infect Dis. 2006;6:136. doi: 10.1186/1471-2334-6-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brantley RK, Williams KR, Silva TM, Sistrom M, Thielman NM, Ward H, Lima AA, Guerrant RL. AIDS-associated diarrhea and wasting in northeast Brazil is associated with subtherapeutic plasma levels of antiretroviral medications and with both bovine and human subtypes of Cryptosporidium parvum . Braz J Infect Dis. 2003;7:16–22. doi: 10.1590/s1413-86702003000100003. [DOI] [PubMed] [Google Scholar]
- 24.Bushen OY, Davenport JA, Lima AB, Piscitelli SC, Uzgiris AJ, Silva TM, Leite R, Kosek M, Dillingham RA, Girao A, Lima AA, Guerrant RL. Diarrhea and reduced levels of antiretroviral drugs: improvement with glutamine or alanyl-glutamine in a randomized controlled trial in northeast Brazil. Clin Infect Dis. 2004;38:1764–1770. doi: 10.1086/421394. [DOI] [PubMed] [Google Scholar]
- 25.Pinheiro VG, Ramos LM, Monteiro HS, Barroso EC, Bushen OY, Facanha MC, Peloquin CA, Guerrant RL, Lima AA. Intestinal permeability and malabsorption of rifampin and isoniazid in active pulmonary tuberculosis. Braz J Infect Dis. 2006;10:374–379. doi: 10.1590/s1413-86702006000600003. [DOI] [PubMed] [Google Scholar]
- 26.Macnab KA, Gill MJ, Sutherland LR, De Boer Visser N, Church D. Erratic zidovudine bioavailability in HIV seropositive patients. J Antimicrob Chemother. 1993;31:421–428. doi: 10.1093/jac/31.3.421. [DOI] [PubMed] [Google Scholar]
- 27.Murphy B, Taylor C, Crane R, Okong P, Bjarnason I. Comparison of intestinal function in human immunodeficiency virus-seropositive patients in Kampala and London. Scand J Gastroenterol. 1999;34:491–495. doi: 10.1080/003655299750026227. [DOI] [PubMed] [Google Scholar]
- 28.Gurumurthy P, Ramachandran G, Kumar A, Rajasekaran S, Padmapriyaarsini C, Swaminathan S, Venkatesan P, Sekar L, Kumar S, Krishnarajasekhar OR, Paramesh P. Malabsorption of rifampin and isoniazid in HIV-infected patients with and without tuberculosis. Clin Infect Dis. 2004;38:280–283. doi: 10.1086/380795. [DOI] [PubMed] [Google Scholar]
- 29.Paton NI, Sangeetha S, Earnest A, Bellamy R. The impact of malnutrition on survival and the CD4 count response in HIVinfected patients starting antiretroviral therapy. HIV Med. 2006;7:323–330. doi: 10.1111/j.1468-1293.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 30.Severe P, Leger P, Charles M, Noel F, Bonhomme G, Bois G, George E, Kenel-Pierre S, Wright PF, Gulick R, Johnson WD, Jr, Pane JW, Fitzgerald DW. Antiretroviral therapy in a 1064 DILLINGHAM AND OTHERS thousand patients with AIDS in Haiti. N Engl J Med. 2005;353:2325–2334. doi: 10.1056/NEJMoa051908. [DOI] [PubMed] [Google Scholar]
- 31.Tang AM, Forrester J, Spiegelman D, Knox TA, Tchetgen E, Gorbach SL. Weight loss and survival in HIV-positive patients in the era of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31:230–236. doi: 10.1097/00126334-200210010-00014. [DOI] [PubMed] [Google Scholar]
- 32.DeHovitz JA, Pape JW, Boncy M, Johnson WD., Jr Clinical manifestations and therapy of Isospora belli infection in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1986;315:87–90. doi: 10.1056/NEJM198607103150203. [DOI] [PubMed] [Google Scholar]
- 33.Verdier RI, Fitzgerald DW, Johnson WD, Jr, Pape JW. Trimethoprim-sulfamethoxazole compared with ciprofloxacin for treatment and prophylaxis of Isospora belli and Cyclospora cayetanensis infection in HIV-infected patients. A randomized, controlled trial. Ann Intern Med. 2000;132:885–888. doi: 10.7326/0003-4819-132-11-200006060-00006. [DOI] [PubMed] [Google Scholar]
- 34.Pape JW, Liautaud B, Thomas F, Mathurin JR, St Amand MM, Boncy M, Pean V, Pamphile M, Larouche AC, Johnson WD., Jr Characteristics of the acquired immunodeficiency syndrome (AIDS) in Haiti. N Engl J Med. 1983;309:945–950. doi: 10.1056/NEJM198310203091603. [DOI] [PubMed] [Google Scholar]