Abstract
Natural regulatory T cells constitutively express the IL-2Rα chain (CD25) on their surface. Consequently, administration of anti-CD25 antibodies is a commonly utilized technique to deplete regulatory T cell populations in vivo. However, activated effector T cells may also transiently express CD25, and are thus also potential targets for anti-CD25 antibodies. In this study utilizing Toxoplasma gondii as a model pro-inflammatory infection we have examined the capacity of anti-CD25 antibodies to target effector T cell populations during an inflammatory episode, to determine to what extent that this may modulate the outcome of disease. Anti-CD25 antibody treated C57BL/6 mice displayed significantly reduced CD4+ T cell IFN-γ production during acute T. gondii infection and exhibited reduced weight loss and liver pathology during early acute infection; aspects of infection previously associated with effector CD4+ T cell responses. In agreement, anti-CD25 antibody administration impaired parasite control and caused mice to succumb to infection during late acute/early chronic stages of infection with elevated tissue parasite burdens. In contrast, anti-CD25 antibody treatment of mice with established chronic infections did not markedly affect brain parasite burdens, suggesting that protective T cell populations do not express CD25 during chronic stages of T. gondii infection. In summary, we have demonstrated that anti-CD25 antibodies may directly abrogate effector T cell responses during an inflammatory episode, highlighting important limitations of the use of anti-CD25 antibody administration to examine regulatory T cell function during inflammatory settings.
Keywords: T cells, Parasitic-Protozoan, cytokine receptors, Regulatory T cells
Introduction
Natural regulatory T cells (nTreg) constitutively express high levels of the IL-2 receptor alpha chain (CD25) and until recently this was the most commonly utilized marker to identify these cells. Natural regulatory T cells, which comprise 5–10% of CD4+ T cells in naïve mice, have potent regulatory capacity and have been shown to have important roles in a number of auto-immune and infectious diseases (reviewed-1, 2). Importantly, however, CD25 is not a specific marker of nTreg and it may be expressed by a number of other cell populations, including activated T and B cells, monocytes and some dendritic cells (reviewed in 3). Moreover, the identification of the transcription factor Foxp3 as a lineage specific marker for nTregs has enabled more accurate differentiation of regulatory and effector CD4+ T cell populations, and has shown that populations of CD25 negative Foxp3+ regulatory T cells also exist (4). Nevertheless, administration of anti-CD25 antibodies remains a commonly utilized method to deplete nTreg in vivo. Some studies have obtained results consistent with the depletion of regulatory cell populations, as evidenced by augmented protective immune responses and enhanced pathogen control (for example 5–8), whereas others have observed no difference in immunity following anti-CD25 administration, possibly due to the disease model employed or the strain and immune status of mice utilized in the study (for example 9–13). In addition, anti-CD25 antibodies are also routinely utilized in vitro for the positive selection of nTreg from naïve and infected mice for the subsequent use in adoptive transfer systems or in vitro suppression assays. In general, these experiments have almost entirely focused on the effects of anti-CD25 antibodies on CD4+ natural regulatory T cells (nTregs), even though many cells other than nTreg, including effector T cells, could potentially be targeted by anti-CD25 treatment (3). Thus, anti-CD25 treatment in vivo may have effects beyond simply depleting CD25-expressing nTregs or affecting their regulatory capability, potentially leading to inaccurate conclusions on the role of nTreg in any particular disease setting.
Here we use oral Toxoplasma gondii infection as a model of inflammatory disease to directly investigate the effect of anti-CD25 antibody treatment on the effector arm of the immune response. The T. gondii infection model has several useful features for this purpose. During acute infection, which lasts one to two weeks, strong pro-inflammatory innate and adaptive responses develop as rapidly proliferating single cell tachyzoites develop from dormant encysted parasites and disseminate from the intestine to liver, lung, brain and other sites. Co-operation between neutrophils, natural killer cells, and macrophages is required for the production of IL-12 and IFN-γ, which then play critical protective roles during both acute and chronic infections (14–19). However, in susceptible mouse strains such as C57BL/6 (B6), type 1 cytokines cause immunopathology in small intestine and liver that is not evident in resistant mouse strains (e.g., BALB/c) (20). Notably, CD4+ cells, while not required for control of parasites in the first week or so of acute infection, are nonetheless important mediators of the immunopathology seen in B6 mice (20).
Following the development of sufficient adaptive T cell mediated immune responses, the acute stage of infection is controlled and tachyzoites are cleared in the intestine, liver and lungs, and parasites revert to a semidormant state within cysts in the brain. In this chronic infection phase, acquired immune mechanisms dependent on CD4+ and CD8+ T cells and B cells, along with IFN-γ and TNF-α are required to control tachyzoite dissemination and to prevent the development of toxoplasmic encephalitis (21–24). Thus, activated effector CD4+ T cells mediate damaging immunopathology early during acute infection, yet contribute to parasite control during late acute/chronic stages of infection and prevent the development of encephalitis during chronic infection.
Our results support the conclusion that anti-CD25 antibody administration directly targets CD25 expressing effector CD4+ T cells during acute T. gondii infection. We show that anti-CD25 antibody administration, whilst depleting approximately 40% of Foxp3+ nTreg, also significantly reduces effector CD4+ T cell numbers and diminishes IFN-γ production during acute T. gondii infection, ameliorating early immune mediated pathology but significantly impairing disease control and elevating mortality during late acute/early chronic stage T. gondii infection. Thus, our results strongly imply that anti-CD25 antibodies should be utilized with caution in vivo during highly pro-inflammatory disease models, and that the potential effects on all cells that can express CD25 should be considered when they are utilized to examine the function and importance of nTreg.
Materials and Methods
Mice
C57BL/6J (B6), Thy1.1 C57BL/6 (B6) and bicistronic IFN-gamma reporter mice (Yeti) (25) male mice were used between 6 to 12 weeks of age. Mice were bred in the Trudeau Institute Animal Breeding Facility or obtained from the Jackson Laboratory or Taconic Farms. All experiments were reviewed and approved by the Trudeau Institute Institutional Animal Care and Use Committee. The Trudeau Institute is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Parasites and infections
ME49 cysts were obtained from brains of chronically infected B6 mice. Infections were initiated by peroral administration of 10 cysts in 0.1 ml diluted brain suspension as previously described (26). Sham-infected mice received similarly diluted brain suspension containing no cysts. Weight loss was monitored as a sign of morbidity and is expressed as the percentage of baseline starting weight. Mice were monitored daily for survival.
Antibody treatments
Mice were given i.p. injections of 1 mg of anti-CD25 (clone PC61) or a control rat IgG (clone HRPN) obtained from BioXcell (Previously BioExpress, West Lebanon, NH). Antibodies were administered 5–7 days prior to infection or during infection on days stated in the text. PC61 administration promoted long lasting depletion/neutralisation of CD25 expression (>2 weeks) in naïve mice, consistent with previously published reports (27). Endotoxin levels of PC61 and HRPN were below 1EU/mg.
Flow cytometry
Single cell suspensions were prepared from spleens, liver, blood and mesenteric lymph nodes. Brains were gently mashed using a Teflon pestle, cell populations separated on a Percoll gradient, and lymphocytes recovered for analysis. Cells were counted using a hemacytometer. The surface antigen phenotype was determined by flow cytometry using the following fluorochrome-conjugated antibodies: anti-CD4 (clone GK1.5), anti-CD25 (clone PC61 or clone 7D4), anti-CD44 (clone IM7). Cells were preincubated with Fc block (clone 2.4G2) before staining. All antibodies were obtained from BD Biosciences (Franklin Lakes, USA). Foxp3 staining was performed using anti-Foxp3 (FJK-16s) antibody according to the manufacturers’ instructions (eBioscience, inc. San Diego, USA). It is important to note that fluorochrome labeled 7D4 was utilized for flow cytometric detection of CD25 expression in all experiments where PC61 was administered in vivo to deplete CD25 expressing cells; PC61 does not inhibit 7D4 detection of cell surface CD25 (27).
Isolation of CD4+CD25− and CD4+CD25hi T cells
CD4+ T cells were purified from the spleens and brains of C57BL/6 mice using Miltenyi anti-CD4 midi Macs beads according to the manufacturer’s instructions (Miltenyi). Following separation CD4+ cells were stained with anti-CD4 (GK1.5) and anti-CD25 (PC61) and sorted into CD4+CD25− and CD4+CD25hi populations using a FACS vantage cell sorter. The sorted cell populations were routinely >99% pure.
In vitro re-stimulation experiments
CD4+CD25hi and CD4+CD25− cells, obtained as described, were cultured individually or at a ratio of 1:1. Cells were either stimulated with naïve antigen presenting cells (APC) and anti-CD3 antibody (1μg/ml) or with T. gondii pulsed APC (without anti-CD3 antibody co-stimulation). Antigen presenting cells were prepared by gamma irradiation (3000RAD) of spleen cells obtained from naïve B6 mice. Separately, T. gondii primed APC were prepared to measure parasite-specific T cell responses. RH strain tachyzoites, maintained through continuous in vitro culture in fibroblast monolayers, were irradiated at 15000RADS and added to APC generated from naïve mice as indicated above. Irradiated tachyzoites and APC were cultured for 12hrs at 5% CO2 and 37°C before being washed and added at required cell concentration to wells including purified CD4+ cells. The cells were then incubated at 5% CO2 and 37°C for 60 h. Supernatants were then harvested to assay cytokine production and proliferation was determined by pulsing cells with 0.2μCu/well 3H Thymidine (Amersham Biosciences, Piscataway, USA) for an extra 12hrs. 3H Thymidine incorporation and proliferation was measured using a beta counter.
ELISA and Real Time PCR
Serum IFN-γ levels were measured by ELISA as previously described (18). Tachyzoites were quantified by a real-time PCR-based method (28) based on the expression of the tachyzoite-limited Sag1 gene (29). RNA was isolated from tissues, cDNA prepared, amplified and quantitated using the following primer:probe sets: T. gondii Sag1. Forward TTTCCGAAGGCAGTGAGACG, Reverse GGATCCGATGCCATAGCG, Probe TTGCCGCGCCCACACTGATG. IFNγ. Forward CATTGAAAGCCTAGAAAGTCTGAATAAC, Reverse TGGCTCTGCAGGATTTTCATG, Probe TCACCATCCTTTTGCCAGTTCCTCCAG. Foxp3. Forward CCCAGGAAAGACAGCAACCTT Reverse TTCTCACAACCAGGCCACTTG Probe. ATCCTACCCACTGCTGGCAAATGGAGTC GAPDH Forward CTCGTCCCGTAGACAAAATGG, Reverse AATCTCCACTTTGCCACTGCA, Probe CGGATTTGGCCGTATTGGGCG. Values were normalized to GAPDH expression. IFN-γ message levels in brain are shown as fold change (log10) relative to control mAb-treated, infected mice. Tachyzoite numbers were calculated relative to a standard curve generated by inoculating known numbers of tachyzoites into uninfected liver samples.
Statistics
Unless otherwise noted, group means were compared by Student’s t-test. Intergroup survivals were compared by log-rank test. All statistical analyses were performed using GraphPad Prism software.
Results
Anti-CD25 antibody increases susceptibility of mice to late acute T. gondii infection
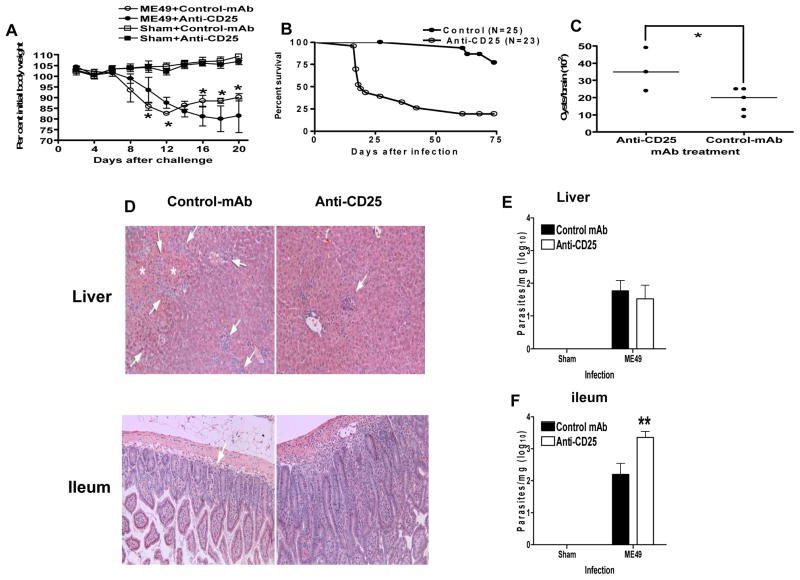
The administration of anti-CD25 antibody (PC61) significantly increased the susceptibility of mice to acute oral T. gondii infection (Fig. 1). The majority of C57BL/6 mice treated with PC61 succumbed to infection by day 20 of infection, and this correlated with a biphasic modulation of weight loss (Fig. 1 A, B); PC61 pre-treated mice exhibited reduced weight loss during early acute T. gondii infection, with significant differences compared with control antibody treated mice on days 6–8 of infection (P<0.01). However, in contrast to control antibody treated mice that survived acute infection and recovered weight, PC61 treated mice were unable to resolve the acute infection and exhibited significantly elevated weight loss during mid to late acute infection on days 15–18 (P<0.05)(Fig. 1A). This data is consistent with previous studies where the depletion of pro-inflammatory CD4+ T cells ameliorated weight loss and pathology during early acute infection but caused enhanced weight loss during late acute infection (30). Surviving PC61 pre-treated mice also harbored significantly increased brain cyst numbers compared with control mice on day 30 post-infection (P<0.033) (Fig. 1C).
Fig. 1. Anti-CD25 antibody administration significantly modulates the outcome of acute T. gondii infection.
Groups of 5 B6 males were given 1 mg control mAb (HRPN) or anti-CD25 (PC61) 5 or 7 days before oral infection with 10 ME49 cysts. (A) Changes in body weight were monitored and are presented as percentage change relative to starting weight. Symbols show mean ± std. dev. *, significant differences (p<0.05). (B) Compiled survival data from 3 experiments comparing anti-CD25-treated with control T. gondii-infected B6 male mice. Survivals differed significantly (p<0.01) by log-rank test. (C) Brain cysts of 3 anti-CD25-treated B6 male mice, and 5 controls 28 days after mice were sham-infected or infected with 10 ME49 cysts. Horizontal lines denote medians. (D) On day 8 post-infection livers and ileums were removed and stained with H & E for histological examination of tissue pathology with arrows marking areas of inflammation or necrosis (also marked by *). (E, F) On day 8 post-infection (E) livers and (F) ileums were removed for real-time PCR (taqman) quantification of tissue parasite burdens. The results shown are the mean ± std. dev. of the group (n = 5) and are representative of 2 independent experiments. ** (p<0.01).
Anti-CD25 antibodies modulate development of immune mediated pathology and significantly impair parasite control during acute T. gondii infection
As anti-CD25 antibody administration elevated mortality, modulated weight loss and increased brain cyst numbers, we next examined whether anti-CD25 antibody administration impeded tachyzoite control and/or limited acute stage immunopathology. In agreement with this hypothesis, we observed significantly reduced liver pathology in anti-CD25 antibody treated mice compared with control antibody treated mice on day 8 of infection, with ameliorated inflammatory cell infiltration (predominantly mononuclear cells and neutrophils) throughout the parenchamal and portal areas and significantly reduced levels of hepatic coagulation necrosis (Fig. 1D and Table 1). In addition, moderately severe perivasculitis, vasculitis and fibrinous thrombosis was observed in medium to large sized blood vessels of control antibody treated mice, which was relatively absent in anti-CD25 antibody treated mice (Fig. 1D). Nevertheless, parasite control in the liver was relatively unaffected by anti-CD25 antibody treatment, as comparable tachyzoite numbers were observed in control antibody and PC61 treated mice on day 8 post-infection (Fig. 1E), indicating that parasite control in the liver during early acute T. gondii infection is CD25-independent. In contrast, significantly elevated pathology was observed in the ileum of anti-CD25 antibody treated mice compared with control antibody treated mice on day 8 post-infection; extensive infiltration of moderate numbers of mononuclear cells was observed in the lamina propria of anti-CD25 antibody treated mice, which was relatively absent in control antibody treated mice (Fig. 1D and Table 1). Elevated pathology was directly correlated with increased parasite burdens in the ileum of anti-CD25 antibody treated mice on day 8 post-infection (Fig. 1F), suggesting that the failure of parasite control, rather than partial depletion or inactivation of nTreg following anti-CD25 antibody administration, was responsible for the increased pathology evident in the ileum. Combined, these data highlight the pathological role of CD25-expressing effector cells in mediating hepatic pathology during early acute T. gondii infection and demonstrate the importance of CD25-expressing effector cells for control of T. gondii parasites in the ileum during the acute stage of infection.
Table 1.
Quantification of tissue pathology during acute T. gondii infection following anti-CD25 antibody administration
| Days post-inoculation | Treatment | Liver | Ileum lesions | |
|---|---|---|---|---|
| Liver damage | Inflammatory responses | |||
| 8 | Controls | 3.0 (3.0–4.0) | 3.0 (2.0–3.0) | 1.0 (1.0–2.0) |
| PC61 | 1.0 (1.0–2.0) | 1.0 (1.0–1.0) | 3.0 (2.0–3.0) | |
Liver and ileal lesions were semi-quantitatively scored based on our previous scoring systems (28, 31).
Liver damage: 1, degeneration and necrosis of individual hepatocytes occasionally seen; 2, clusters or small aggregates of hepatocyte degeneration and necrosis; 3, medium to large aggregates of hepatocyte necrosis; 4, large areas of hepatocyte necrosis with loss of normal liver anatomic architecture.
Intensity of the inflammatory response: 1, small inflammatory infiltrates with a few inflammatory cells; 2, medium-size inflammatory infiltrates with small to moderate numbers of inflammatory cells; 3, large inflammatory infiltrates with moderate to large numbers of inflammatory cells; 4, extensive infiltration with large numbers of inflammatory cells.
The ileal lesions were scored for the degeneration and necrosis of villous epithelial cells, transmural inflammation, and lamina propria and crypt inflammation and an integer score between 0 (unremarkable) and 4 (severe) was assigned.
The values are medium with ranges in the parenthesis from five individual mice.
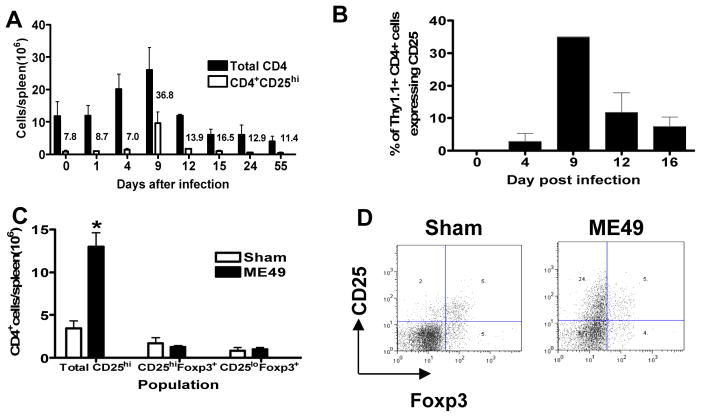
CD25 is transiently upregulated on CD4 T cells during acute T. gondii infection
To examine the kinetics of CD25 expression during T. gondii infection, to establish when it is upregulated and on which cells, we determined the expression level of CD25 on CD4+ and CD8+ splenic lymphocytes on various days during acute T. gondii infection. Fig. 2A shows the marked increase between days 4 and 12 of infection in both the absolute numbers of total splenic CD4+ T cells and the number of splenic CD4+ T cells expressing high levels of CD25. A similar transient upregulation of CD25 on splenic CD8+ T cells was also observed between day 4 and 12 of infection, before absolute numbers of splenic CD8+ T cells and CD8+CD25hi T cells returned to baseline pre-infection levels (results not shown).
Fig. 2. Transient upregulation of CD25 on effector T cells during acute T. gondii infection.
(A) Total numbers of splenic CD4+ T cell and CD4+CD25hi T cells were calculated on selected days following oral T. gondii infection. CD25 detection was performed using the PC61 clone anti-CD25 antibody. Bars show means +/− std. dev. Numbers above bars for CD4+CD25hi cells indicate the percentage of that population within the total CD4+ T cell population. (B) Cell sorted Thy1.1+CD4+CD25− cells were adoptively transferred into Thy1.2+ B6 mice prior to oral T. gondii infection. The frequency of adoptively transferred donor cells expressing CD25 in the spleen was determined on selected days post-infection. (C, D) In a separate experiment, groups of 5 B6 males were infected as in panel A, and spleens harvested on day 8 to analyse the percentage of CD4+CD25hi cells that co-expressed Foxp3. (C) The numbers of CD4+CD25hi cells and CD4+Foxp3+ cells and (D) representative dot plots gating on CD4+ T cells showing CD25 expression versus Foxp3 expression in naïve and T. gondii infected mice. Mean+ std. dev. numbers of indicated CD4+ populations are shown. *, p<0.01.
The expansion of CD4+ T cells expressing CD25 during acute T. gondii infection could potentially have been due to either the proliferation of CD4+CD25hi regulatory T cells (present in naïve mice) or due to the upregulation of CD25 on activated precursor CD25− naïve CD4+ T cells. Thus, to investigate the ability of CD4+CD25− T cells to upregulate CD25 during acute T. gondii infection we purified and adoptively transferred Thy1.1+CD4+CD25− cells into congenic Thy1.2+ mice prior to oral T. gondii infection. Importantly, significant upregulation of CD25 expression was observed on the CD4+CD25− donor T cell population (Fig. 2B). Moreover, the kinetics (and magnitude) of CD25 upregulation on the donor CD4+ T cells mirrored the upregulation of CD25 in the total CD4+ T cell population in WT mice (Fig. 2A), indicating that CD4+CD25− cells are the primary precursor cells of the CD4+CD25hi cells generated during acute T. gondii infection.
Although there were significantly more CD4+CD25hi spleen cells in infected mice than in sham-infected mice on day 8 post-infection, there was no significant difference in numbers of total CD4+Foxp3+ cells, or numbers of CD4+Foxp3+ cells expressing CD25 (Fig. 2C), suggesting that the majority of CD4+CD25hi T cells were not regulatory T cells, as shown in the representative dot plots from day 8 of infection (Fig. 2D). Moreover, a significant number of CD4+Foxp3+ cells were found within the CD25lo subset of CD4+ cells, revealing the presence of Foxp3+CD25-regulatory T cells during T. gondii infection and in naïve uninfected mice (Fig. 2C).
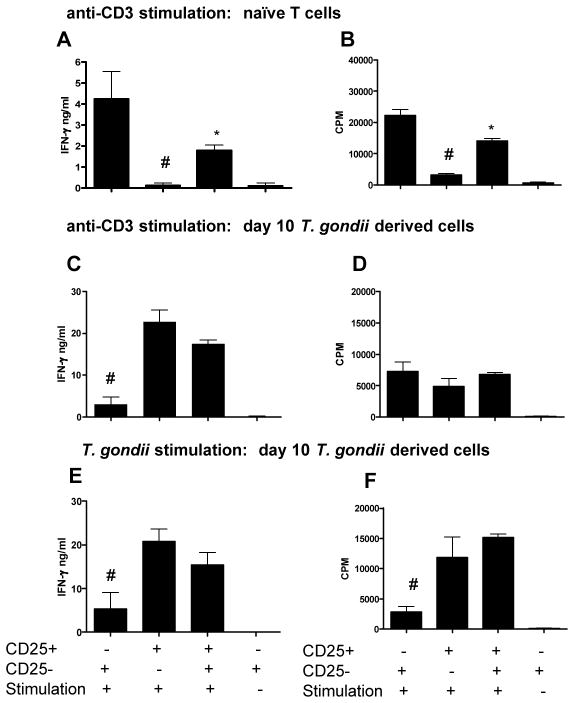
CD4+CD25hi T cells are primarily effector T cells during acute T. gondii infection
It is well established that CD4+CD25hi T cells derived from naïve mice express high levels of Foxp3 and exhibit potent regulatory capabilities. In contrast, extremely strong systemic pro-inflammatory immune responses develop during T. gondii infection and the majority of CD4+CD25hi cells do not express Foxp3 (Fig. 2), indicating that these cells may exert effector rather than regulatory functions. To address this likelihood, we purified CD4+CD25− and CD4+CD25hi cells from uninfected mice and mice acutely infected with T. gondii and assessed the ability of these cells to respond to mitogenic (anti-CD3) and T. gondii antigen specific stimulation. As expected, CD4+CD25hi cells derived from uninfected mice closely matched the functional characteristics of natural regulatory T cells; they failed to produce IFN-γ or proliferate following mitogenic stimulation and suppressed proliferation and IFN-γ production by CD4+CD25− cells (P<0.05) (Fig. 3A, B). Conversely, CD4+CD25hi cells derived from acutely infected mice failed to suppress CD4+CD25− cell responses, produced significant quantities of IFN-γ and proliferated rapidly following mitogenic and T. gondii antigenic challenge (Fig. 3 C–F). Importantly, CD4+CD25hi T cells derived from acutely infected mice produced more IFN-γ and proliferated more rapidly than infection derived CD4+CD25− cells following specific T. gondii stimulation (Fig. 3E, F), suggesting that during acute T. gondii infection, the majority of parasite-specific effector CD4+ T cells are found within the CD4+CD25hi T cell population.
Fig. 3. CD4+ T cells that upregulate CD25 expression during acute T. gondii infection are Th1 effector cells that produce high levels of IFN-γ.
CD4+CD25− and CD4+CD25hi splenic T cells were sort purified from (A, B) naïve mice or (B–F) T. gondii infected mice (day 10 of infection). CD25 detection was performed using the PC61 clone anti-CD25 antibody. CD4+CD25− and CD4+CD25hi cells were cultured with (A–D) naïve APC and anti-CD3 antibody or (E–F) T. gondii pulsed APC. (A, C, E) IFN-γ production was assayed in supernatant by ELISA. (B, D, F) Proliferation was determined by 3H Thymidine incorporation. The results shown are the mean ± std. dev. of the group (n = 3) and are representative of 2 independent experiments. # denotes significant difference between CD4+CD25− and CD4+CD25hi cultures (p<0.05). * denotes significant difference between CD4+CD25− and CD4+CD25− and CD4+CD25hi co-cultures (P<0.05).
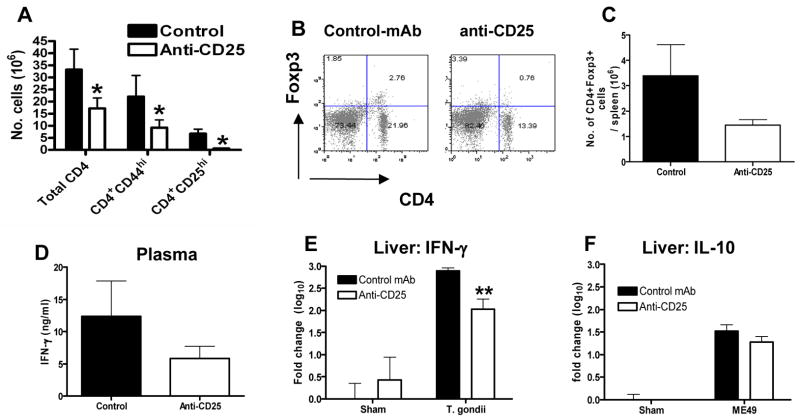
Reduced numbers of effector T cells and suppressed IFN-γ production in anti-CD25 antibody treated T. gondii-infected mice
Numerous studies have shown that anti-CD25 antibody (PC61 clone) treatment leaves mice essentially devoid of cells expressing CD25 for an extended period (27, 32), an observation we confirm in this study during T. gondii infection (Fig. 4); CD25 expressing splenic CD4+ T cells were almost completely undetectable following anti-CD25 antibody administration (detection day 9 post-infection with antibody injected i.p. 4 days prior to infection) (Fig. 4A). These results were largely reproduced when examining mesenteric lymph node cells (data not shown). Importantly, and consistent with previous reports (27, 32), PC61 administration failed to induce complete depletion of Foxp3+ regulatory T cells, instead promoting a maximal 40% reduction in Foxp3-expressing cell numbers (Fig. 4B, C). As we have demonstrated that during acute stage T. gondii infection the overwhelming majority of CD4CD25hi cells display effector rather than regulatory characteristics, these results directly indicate that anti-CD25 antibodies primarily deplete effector T cells during T. gondii infection. In agreement, anti-CD25 antibodies principally targeted activated T cell populations, as shown by the significant decrease in the numbers of CD4+ T cells expressing high levels of the activation marker CD44 following PC61 administration (28.9±11.6×106 in control mAb-treated vs. 13.3±4.5×106 in anti-CD25-treated) (Fig. 4A).
Fig. 4. Anti-CD25 administration depletes activated CD25+ T cells and significantly reduces IFN-γ production.
Groups of 5 B6 males were given 1 mg anti-CD25 (PC61) or control mAb (HRPN) i.p 5 days before oral infection with 10 ME49 cysts. (A) The numbers of splenic activated CD4+ T cells were calculated on day 9 post-infection by measuring CD25 and CD44 expression. CD25 detection was performed using the 7D4 clone anti-CD25 antibody. (B) Representative dot plots showing Foxp3 expression on splenic CD4+ T cells on day 8 post-infection. (C) The numbers of splenic CD4+Foxp3+ cells on day 8 post-infection in control antibody and anti-CD25 antibody treated mice. (D, E) The level of IFN-γ and IL-10 in PC61 and HRPN treated mice was determined on day 9 post-infection in (D) plasma by ELISA and in (E, F) liver by real time PCR (taqman). The results shown are the mean ± std. dev. of the group (n = 5) and are representative of 2 independent experiments. * (p<0.05), ** (p<0.01).
Consequently, to examine whether the depressed effector T cell responses observed during T. gondii infection following anti-CD25 antibody administration correlated with a functional defect in pro-inflammatory immune responses, we next determined the levels of the prototypic type 1 cytokine IFN-γ in T. gondii infected mice treated with anti-CD25 antibodies. Our results show that PC61 administration reduced systemic plasma IFN-γ levels (detected on day 8 post-infection) compared with control antibody treated infected mice (Fig. 4D). Although the reduction in IFN-γ production failed to reach statistical significance in any individual experiments, a reproducible reduction in IFN-γ production was observed in 4 separate experiments, with percent reductions relative to controls of 11, 20, 27, and 52%. Furthermore the reduced serum IFN-γ levels were paralleled by significantly reduced levels of IFN-γ mRNA in the liver of anti-CD25 antibody treated mice on day 8 post-infection (Fig. 4E).
CD4+ T cells, specifically Th1 cells that express T-bet and co-produce IFN-γ, have also been shown to be the primary source of host-protective IL-10 during T. gondii infection (33). Importantly, however, the expression level of IL-10 mRNA in the liver was comparable in anti-CD25 antibody and control-treated mice (Fig. 4F), indicating that the regulatory IL-10 response was largely intact in anti-CD25 antibody treated mice. Combined these data definitively demonstrate that in vivo administration of anti-CD25 antibodies can negatively effect pro-inflammatory immune responses during an inflammatory disorder, and that CD25 expression is upregulated on T. gondii specific activated effector T cells, which are required for effective pro-inflammatory type-1 responses.
Anti-CD25 antibody administration suppresses IFN-γ production by CD4+ T cells
To examine the T cell populations affected by anti-CD25 antibody administration leading to impaired IFN-γ expression, we utilized IFN-γ bicistronic reporter mice (YETI; 25), enabling the detection and quantification of cell specific IFN-γ production directly ex vivo, negating the requirement for subsequent re-stimulation in vitro. Importantly, yFP expression in YETI mice not only marks cells actively producing IFN-γ, but also marks cells that have expressed IFN-γ, providing an accurate representation of both current and historical cellular function (25). As expected (25), the majority of CD4+ T cells in naïve mice were found to be yFP negative, indicating that few CD4+ T cells were polarized type 1 effector/memory cells (Fig. 5A). The CD4+ yFP+ cells found in liver, spleen, blood and mesenteric lymph nodes in naïve mice displayed heterogeneity in CD25 expression, suggesting the existence of a number of different populations of IFN-γ producing CD4+ T cells at various stages of cellular activation. All yFP+ cells irrespective of CD25 expression or tissue/organ location were, however, uniformly CD44hi (results not shown).
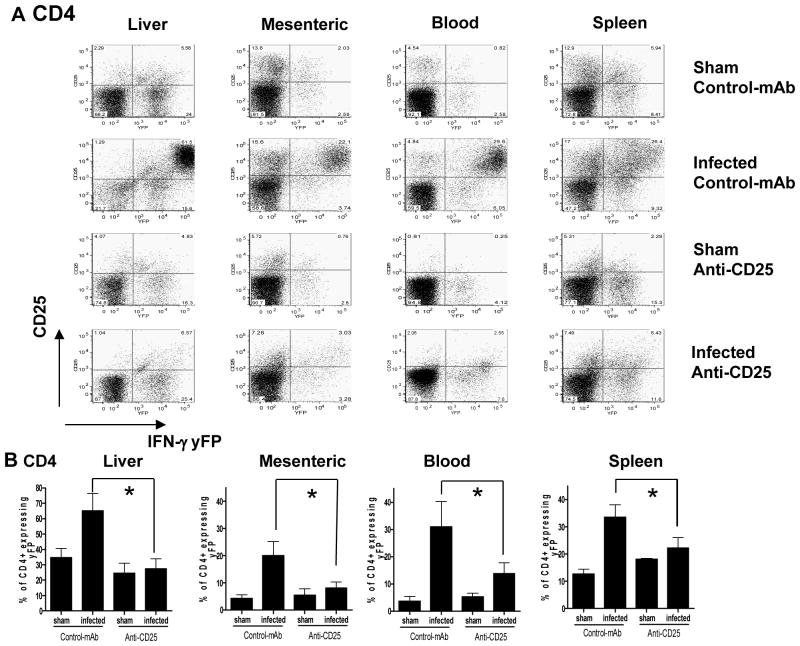
Figure 5. Anti CD25 antibody administration suppresses IFN-γ production by CD4+ T cells.
Groups of 3 bicistronic IFN-gamma-enhanced yellow fluorescent protein (IFN-gamma-YFP) reporter mice (Yeti) male mice were given 1 mg anti-CD25 (PC61) or control mAb (HRPN) i.p 5 days before oral infection with brain homogenate containing10 ME49 cysts or sham uninfected brain homogenate. (A) Representative dot plots showing yFP expression relative to CD25 expression (utilizing 7D4 to detect) on CD4+ T cells from spleen, mesenteric lymph, liver and blood on day 8 post-infection. (B) Relative frequencies of CD4+ T cells expressing yFP in anti-CD25 antibody treated and control antibody treated T. gondii infected and sham infected mice on day 8 post-infection. The results shown are the mean ± std. dev. of the group (n = 3) and are representative of 2 independent experiments. * (p<0.05).
In agreement with figure 2, upregulation of CD25 on CD4+ T cells was observed on day 8 post-infection in all tissues examined and this correlated with significant upregulation of yFP expression; importantly in all the tissues examined the majority of yFP expressing CD4+ T cells co-expressed CD25 (Fig. 5A). Thus, virtually all CD4+CD25hi cells derived from liver and blood were yFP+, marking CD4CD25hi cells as predominantly effector rather than regulatory T cells in these tissues during acute T. gondii infection. In contrast, a number of CD4+CD25hi cells derived from spleen and mesenteric lymph nodes of T. gondii infected mice did not co-express yFP, suggesting that these cells are either regulatory T cells or activated T cells that have failed to produce IFN-γ (Fig. 5A). Irrespective of this, our results in figure 3 clearly show that splenic CD4+CD25hi T cells predominantly exert effector rather than regulatory functionality during acute stage T. gondii infection.
In support of our hypothesis that anti-CD25 antibody administration directly suppresses pro-inflammatory protective immune responses during acute T. gondii infection, we observed significantly reduced frequencies of yFP+ CD4+ T cells in the liver, mesenteric lymph node, blood and spleen of T. gondii infected mice treated with anti-CD25 antibodies as compared with control antibody treated infected mice (Fig. 5B). Similarly, significantly reduced frequencies of yFP+ CD8+ T cells were observed in the liver of T. gondii infected mice treated with anti-CD25 antibodies as compared with control antibody treated infected mice (results not shown). In contrast anti-CD25 antibody administration did not significantly reduce yFP expression by CD8+ T cells in the spleen, mesenteric lymph node or blood, suggesting that effector CD8+ T cells are less affected by anti-CD25 antibody than CD4+ T cells (results not shown).
Heterogeneous expression of yFP by CD4+ T cells occurs during T. gondii infection (as shown in infected, isotype control group: Fig. 5) and as published in Mayer et al (34). Importantly, the expression level of yFP is directly correlated to the capacity to produce IFN-γ following in vitro re-stimulation, with cells expressing highest levels of yFP capable of producing most IFN-γ protein (34). Interestingly, the highest level of yFP expression was routinely observed in CD4+ T cells expressing highest levels of CD25, marking these potent IFN-γ producing cells as principal targets of anti-CD25 antibodies. Thus, anti-CD25 antibody administration significantly reduced the YFPhi CD4+ T cell subsets in the liver and blood (a reduction in the frequency of yFPhi CD4+ T cells was also observed in the mesenteric lymph node and spleen, but the reduction failed to reach statistical significance (results not shown). Combined these results demonstrate that anti-CD25 antibodies can directly target effector IFN-γ producing CD4+ T cells during acute T. gondii infection leading to systemic reductions in IFN-γ production and impaired protective T cell immunity.
Anti-CD25 antibody administration does not impair resistance to chronic stage T. gondii infection
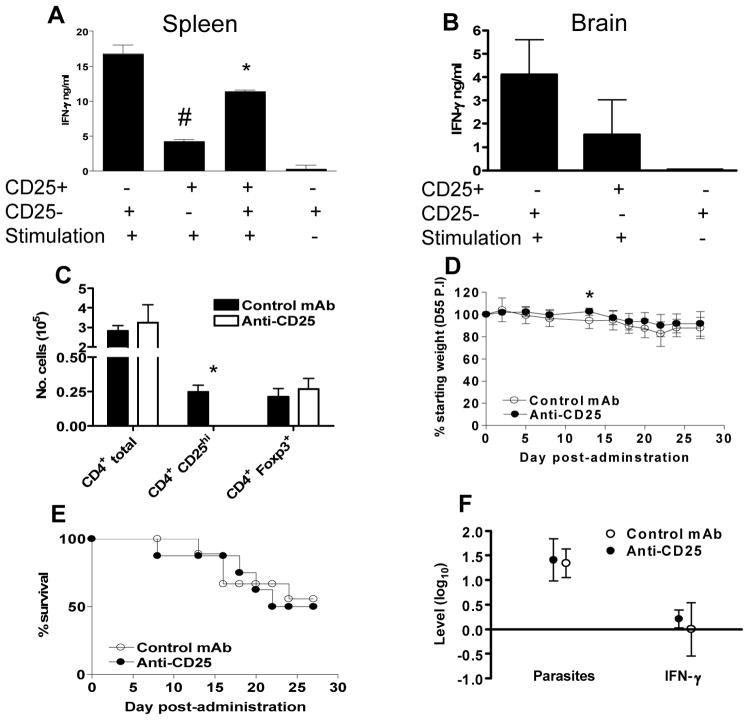
It is well established that anti-parasitic CD4+ T cells and CD8+ T cells are required for the control of parasite re-activation in the brain and prevention of Toxoplasma encephalitis during chronic T. gondii infection (22). However, the activation status of these CD4+ and CD8+ T cells is somewhat unknown. Consequently it is unclear if protective CD4+ and CD8+ T cells in the spleen or brain express CD25 during chronic infection, and if these cells may also be affected by anti-CD25 antibody administration. Additionally, it has previously been shown that nTreg cells that co-express Foxp3 and CD25 can suppress protective immunity during a number of infections, including Leishmania major infection, enabling parasite persistence and the establishment of chronic infections (35, 36). Thus, CD25 expressing CD4+ T cells may also play an important regulatory role during chronic T. gondii infection, lowering pro-inflammatory responses, moderating immune-mediated pathology but impairing parasite control. As a result, anti-CD25 antibody administration during chronic T. gondii infection could potentially augment anti-parasitic immune responses and enhance parasite control. In support of this hypothesis Foxp3 mRNA levels in the brain were increased during chronic infection compared with uninfected control levels (results not shown). Moreover, we have shown that CD25 upregulation on effector T cells is transient during T. gondii infection and occurs primarily during the acute phase of infection, suggesting that CD4+CD25hi cells are functionally disparate during acute and chronic stages of T. gondii infection (Fig. 2). In agreement with a regulatory rather than effector role of CD25 expressing cells during chronic infection, CD4+CD25hi cells isolated from both brain and spleen during chronic infection failed to produce significant quantities of IFN-γ following T. gondii antigenic re-stimulation (as compared with CD4+CD25− cells) and splenic CD4+CD25hi cells moderately suppressed IFN-γ production by CD4+CD25− cells (Fig. 6A, B). To directly determine the relative importance of CD25 expressing nTreg during chronic T. gondii infection, we administered anti-CD25 antibodies to chronically infected mice (Day 56 post-infection) and determined whether antibody administration significantly modulated disease control. Anti-CD25 antibody administration effectively neutralized CD25 expression in the spleen (results not shown) and brain (Fig. 6C) 5 and 10 days post-administration (days 5 and 10 post-administration were examined in separate experiments with results from day 5 post-administration shown). However, Foxp3 levels were not significantly reduced in anti-CD25 antibody treated mice (Fig. 6C). Importantly, it has previously been shown that Foxp3+ cells that survive anti-CD25 antibody treatment are functionally inactive (32), indicating that anti-CD25 antibody administration should still ablate regulatory T cell functionality during chronic T. gondii infection even if all Foxp3+ T cells are not eliminated. Nonetheless, antibody administration did not significantly alter the level of weight loss (relative to pre-antibody administration baseline levels) or mortality over a 30 day period post-treatment (Fig. 6D, E) and this correlated with unaltered parasite control (measuring invasive tachyzoite forms of parasite) and IFN-γ production in the brain of anti-CD25 antibody treated mice compared with control treated groups (Fig. 6F). Combined these results demonstrate that CD25 expressing nTreg cells do not suppress anti-parasitic immune responses during chronic T. gondii infection and are not required to limit excessive inflammation. In support of this conclusion, the frequency of CD4+Foxp3+ cells in the brain (of total CD4+ T cells) was not elevated in chronically infected mice compared with naïve mice. Thus, the increase in brain Foxp3 mRNA expression during chronic infection is due to the increase in total CD4+ T cells that migrate to and reside in brains of chronically infected mice, rather than specific localization and migration of regulatory T cells to the site of infection, as is observed during L. major infection (35).
Fig. 6. Anti CD25 antibody administration does not affect control of chronic T. gondii infection.
(A–B) CD4+CD25-ve and CD4+CD25hi splenic T cells were sort purified from (A) spleen or (B) brain of mice chronically infected with T. gondii (day 50 post-infection). CD4+CD25− and CD4+CD25hi cells were cultured with T. gondii pulsed APC for 60h and IFN-γ production was assayed in supernatant by ELISA. The results shown are the mean ± st. dev. of the group (n = 3) and are representative of 2 independent experiments. # denotes significant difference between CD4+CD25− and CD4+CD25hi cultures (p<0.05). * denotes significant difference between CD4+CD25− and CD4+CD25− and CD4+CD25hi co-cultures (P<0.05). (C–E) 1mg of anti-CD25 antibody (PC61) or control antibody was administered to mice chronically infected with T. gondii (day 56 pi). (c) 5 days post-antibody administration the numbers of total brain CD4+ T cells, CD4CD25hi (using 7D4 to detect) and CD4+Foxp3+ cells were enumerated to determine the effectiveness of anti-CD25 antibody mediated depletion. * denotes significant difference between control antibody and anti-CD25 antibody treated groups (p<0.05) (D) Weight loss and (E) survival were followed following antibody administration. Weights are expressed as the percentage weight relative to pre-antibody administration (day 56 weights). * denotes significant difference between control antibody and anti-CD25 antibody treated groups (p<0.05). (F) The effect of anti-CD25 antibody administration on brain parasite control and immune responses were determined 30 days post antibody administration by quantifying parasite (tachyzoite) and IFN-γ levels by real time PCR. Results are shown as fold change relative to control antibody treated mice. None of the differences were statistically significant in this or a replicate experiment
Discussion
In this study we demonstrate that during acute T. gondii infection transient upregulation of CD25 expression occurs on CD4+ T cells during the expansion phase of the T cell response. Importantly, the vast majority of CD4+CD25hi cells generated during acute T. gondii infection do not co-express Foxp3, clearly defining the cells as effector cells rather than regulatory T cells. Anti-CD25 antibodies directly target these CD25 expressing effector CD4+ T cell populations leading to impaired pro-inflammatory immune responses, exemplified by significantly reduced T cell derived IFN-γ production. Thus anti-CD25 antibody administration initially reduced the severity of T cell dependent immune-mediated pathology during early acute T. gondii infection, but led to the failure of parasite control and ultimately death in a large number of anti-CD25 antibody treated mice. In contrast, anti-CD25 antibody administration did not affect parasite control during chronic T. gondii infection, suggesting that CD25 expressing T cells do not significantly regulate anti-parasitic immunity during the later chronic stage of T. gondii infection.
The results observed in this study are entirely consistent with a direct effect of anti-CD25 antibody administration on pro-inflammatory effector T cell populations. Thus, whilst (as expected) anti-CD25 antibody administration reduced the numbers of Foxp3+ nTreg, it also reduced circulating IFN-γ levels (in all 4 experiments examined) and resulted in significantly reduced IFN-γ mRNA levels in the liver. Utilizing IFN-γ-yFP reporter (YETI) mice we subsequently showed that anti-CD25 antibody administration leads to reduced IFN-γ production by CD4+ T cells in the liver, spleen, blood and mesenteric lymph node. Consequently our results differ from those obtained by Lund et al (37), where specific nTreg depletion reduced localized pro-inflammatory immune responses at the mucosal site of herpes simplex virus infection, but enhanced pro-inflammatory immune responses in tissue draining lymph nodes. Therefore, whereas in their model, the specific depletion of nTreg (performed in Foxp3 diptheria toxin receptor transgenic mice) inhibited effector T cell migration to non-lympoid tissue sites of infection, we show that anti-CD25 antibody administration promoted systemic suppression of effector T cell responses. Combined, these data demonstrate the lack of specificity of anti-CD25 antibodies to target nTreg compared with the more recently generated Foxp3-dtr system (38).
It has previously been demonstrated that effector CD4+ T cells are responsible for the onset of immune-mediated pathology and severe weight loss in B6 mice following oral T. gondii infection. {20, 30, 39, 40). Consistent with this, anti-CD25 antibody administration modulated body weight loss during acute T. gondii infection and significantly reduced the severity of liver pathology on day 8 of infection, indicating a direct effect on effector T cell populations. Nevertheless, it has also previously been reported that Th1 CD4+ T cells that co-produce IFN-γ are the primary source of host-protective IL-10 during T. gondii infection (33). Consequently, given the effect of anti-CD25 antibody administration on CD4+ T cell derived IFN-γ production, it was foreseeable that antibody administration could also have impaired IL-10 production, and that this secondary antibody effect may have contributed to the enhanced susceptibility of anti-CD25 antibody treated mice during acute T. gondii infection. Importantly, however, our results clearly show that IL-10 mRNA levels were not significantly reduced in the liver following anti-CD25 antibody administration, suggesting that CD25 expression is not critical for the production of host-protective IL-10 during acute T. gondii infection. This further underlines the effects of anti-CD25 antibody administration on effector pro-inflammatory responses, rather than regulatory based responses during acute T. gondii infection. Interestingly, although we highlight the importance of CD25 expression on effector T cells during the acute stage of primary T. gondii infection, administration of anti-CD25 antibodies did not affect vaccine-induced protection during secondary challenge infection. Thus, anti-CD25 antibody administration to mice previously infected with the non-cyst forming attenuated TS-4 strain of parasites did not inhibit protective immune responses and anti-CD25 antibody and control treated vaccinated mice displayed equivalent ability to prevent brain cyst development (results not shown). These results indicate that CD25 expressing effector T cell populations are not critically required for vaccine-induced protection to T. gondii infection.
Despite the pathological role of CD4+ T cells during early acute T. gondii infection, it is well established that effector CD4+ T cells and CD8+ T cells are required for protection and parasite control during late acute and chronic stages of infection (41–43). Consequently, as anti-CD25 antibody treatment reduced the number of activated (CD44hi) CD4+ spleen cells by more than 50% on day 9 of infection, it is not surprising that anti-CD25 antibody treated mice displayed elevated parasite levels in the ileum on days 8 and 15 post-infection, exhibited more brain cysts on day 30 post infection and succumbed more rapidly to late/acute and chronic stage infection. Nonetheless, we have also demonstrated that anti-CD25 antibody administration during established chronic stage of T. gondii infection did not affect brain-localized parasite control or anti-T. gondii immunity, suggesting that anti-CD25 antibodies do not negatively affect protective T cell responses at this stage. In agreement, transient expression of CD25 on effector T cells was observed only during acute T. gondii infection and CD25 expression on splenic CD4+ and CD8+ T cells during chronic stages of infection was comparable to that observed in naïve mice. Moreover, brain infiltrating CD4+ T cell populations did not express higher levels of CD25 than splenic CD4+ T cells during chronic T. gondii infection, indicating that CD25 expressing lymphocytes do not preferentially migrate to and reside at the local site of parasite persistence during chronic infection (results not shown). In addition, CD4+CD25hi cells isolated from the spleen and brain of chronically infected mice displayed regulatory rather than effector phenotypes following in vitro antigen-specific stimulation. Thus, CD25 expressing CD4+ T cells are primarily regulatory rather than effector cells during chronic T. gondii infection and effector CD4+ and CD8+ T cells do not require CD25 expression to mediate parasite control during the later stages of infection. Importantly, these results also demonstrate that natural regulatory T cells do not control anti-parasitic immune responses during chronic T. gondii infection, as administration of anti-CD25 antibodies did not augment parasite control. Consequently, it is unlikely that nTreg are the source of IL-10 that is required for the limitation of immune-mediated pathology during chronic T. gondii infection (44)
Most studies that utilize anti-CD25 antibody administration to investigate Tregs assume that the antibody affects only that subset and, for the most part, these studies have disregarded effects on cells other than Tregs that can upregulate CD25 in response to infection. This is surprising since antigen-stimulated effector T cells (and others) upregulate CD25 and IL-2 drives T cell proliferation in response to antigen stimulation. Notably, anti-CD25 antibodies have been used clinically to dampen autoimmunity and reduce rejection of transplants (45), uses that run counter to the notion that anti-CD25 augments immunity. The reason for the pronounced effect of anti-CD25 antibody on effector T cell populations during acute T. gondii infection, but not in other models is unclear, but is likely correlated with the extreme polarized systemic pro-inflammatory immune response that develops during T. gondii infection. As CD25 is upregulated on a significant percentage of the CD4+ and CD8+ T cell population during acute T. gondii infection, it is foreseeable that any effect of anti-CD25 antibody treatment on effector T cell populations is more pronounced during acute T. gondii infection, and might not be as apparent in other models.
In conclusion, we have shown that during acute T. gondii infection, anti-CD25 antibodies directly target effector CD4+ T cell populations, significantly modulating the pro-inflammatory immune response to T. gondii infection. Although initially this limits immune-mediated pathology, it also leads to the failure to control parasite dissemination and encystment and finally death. Thus, whilst highlighting the role of CD25 expressing T cells for the control of acute T. gondii infection, we importantly also demonstrate the significant limitations of using anti-CD25 antibodies in vivo during inflammatory diseases to investigate the importance of regulatory T cells. On the basis of our findings, we propose that studies that utilize anti-CD25 antibodies during inflammatory settings should take note of the effects on the entire T cell population, not solely those expressing markers associated with regulatory T cells.
Acknowledgments
Grant Support: This work was support by a Public Health Service Grant AI061587 from the National Institute of Allergy and Infectious Diseases and by funds provided by the Trudeau Institute.
We thank Katja Mohrs for technical assistance. We also thank Dr Julius Hafalla and Dr Brian deSouza for critical reading of the manuscript.
References
- 1.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol. 2007;7:875–888. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 3.Waldmann TA. The multichain interleukin-2 receptor: from the gene to the bedside. Harvey Lect. 1986;82:1–17. [PubMed] [Google Scholar]
- 4.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aseffa A, Gumy A, Launois P, MacDonald HR, Louis JA, Tacchini-Cottier F. The early IL-4 response to Leishmania major and the resulting Th2 cell maturation steering progressive disease in BALB/c mice are subject to the control of regulatory CD4+CD25+ T cells. J Immunol. 2002;169:3232–3241. doi: 10.4049/jimmunol.169.6.3232. [DOI] [PubMed] [Google Scholar]
- 7.Mendez S, Reckling SK, Piccirillo CA, Sacks D, Belkaid Y. Role for CD4(+) CD25(+) regulatory T cells in reactivation of persistent leishmaniasis and control of concomitant immunity. J Exp Med. 2004;200:201–210. doi: 10.1084/jem.20040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hisaeda H, Maekawa Y, Iwakawa D, Okada H, Himeno K, Kishihara K, Tsukumo S, Yasutomo K. Escape of malaria parasites from host immunity requires CD4+ CD25+ regulatory T cells. Nat Med. 2004;10:29–30. doi: 10.1038/nm975. [DOI] [PubMed] [Google Scholar]
- 9.Taylor MD, LeGoff L, Harris A, Malone E, Allen JE, Maizels RM. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J Immunol. 2005;174:4924–4933. doi: 10.4049/jimmunol.174.8.4924. [DOI] [PubMed] [Google Scholar]
- 10.Nardelli DT, Warner TF, Callister SM, Schell RF. Anti-CD25 antibody treatment of mice vaccinated and challenged with Borrelia spp. does not exacerbate arthritis but inhibits borreliacidal antibody production. Clin Vaccine Immunol. 2006;13:884–891. doi: 10.1128/CVI.00137-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dittmer U, He H, Messer RJ, Schimmer S, Olbrich AR, Ohlen C, Greenberg PD, Stromnes IM, Iwashiro M, Sakaguchi S, Evans LH, Peterson KE, Yang G, Hasenkrug KJ. Functional impairment of CD8(+) T cells by regulatory T cells during persistent retroviral infection. Immunity. 2004;20:293–303. doi: 10.1016/s1074-7613(04)00054-8. [DOI] [PubMed] [Google Scholar]
- 12.Kotner J, Tarleton R. Endogenous CD4(+) CD25(+) regulatory T cells have a limited role in the control of Trypanosoma cruzi infection in mice. Infect Immun. 2007;75:861–869. doi: 10.1128/IAI.01500-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Couper KN, Blount DG, Wilson MS, Hafalla JC, Belkaid Y, Kamanaka M, Flavell RA, de Souza JB, Riley EM. IL-10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS Pathog. 2008;4:e1000004. doi: 10.1371/journal.ppat.1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci U S A. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennouna S, Bliss SK, Curiel TJ, Denkers EY. Cross-talk in the innate immune system: neutrophils instruct recruitment and activation of dendritic cells during microbial infection. J Immunol. 2003;171:6052–6058. doi: 10.4049/jimmunol.171.11.6052. [DOI] [PubMed] [Google Scholar]
- 16.Bliss SK, Gavrilescu LC, Alcaraz A, Denkers EY. Neutrophil depletion during Toxoplasma gondii infection leads to impaired immunity and lethal systemic pathology. Infect Immun. 2001;69:4898–4905. doi: 10.1128/IAI.69.8.4898-4905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter CA, Subauste CS, Van Cleave VH, Remington JS. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect Immun. 1994;62:2818–2824. doi: 10.1128/iai.62.7.2818-2824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson LL, VanderVegt FP, Havell EA. Gamma interferon-dependent temporary resistance to acute Toxoplasma gondii infection independent of CD4+ or CD8+ lymphocytes. Infect Immun. 1993;61:5174–5180. doi: 10.1128/iai.61.12.5174-5180.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mordue DG, Sibley LD. A novel population of Gr-1+-activated macrophages induced during acute toxoplasmosis. J Leukoc Biol. 2003;74:1015–1025. doi: 10.1189/jlb.0403164. [DOI] [PubMed] [Google Scholar]
- 20.Liesenfeld O, Kosek J, Remington JS, Suzuki Y. Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J Exp Med. 1996;184:597–607. doi: 10.1084/jem.184.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denkers EY, Gazzinelli RT. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev. 1998;11:569–588. doi: 10.1128/cmr.11.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol. 1992;149:175–180. [PubMed] [Google Scholar]
- 23.Johnson LL. SCID mouse models of acute and relapsing chronic Toxoplasma gondii infections. Infect Immun. 1992;60:3719–3724. doi: 10.1128/iai.60.9.3719-3724.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson LL. A protective role for endogenous tumor necrosis factor in Toxoplasma gondii infection. Infect Immun. 1992;60:1979–1983. doi: 10.1128/iai.60.5.1979-1983.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, Kronenberg M, Locksley RM. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson LL, Berggren KN, Szaba FM, Chen W, Smiley ST. Fibrin-mediated protection against infection-stimulated immunopathology. J Exp Med. 2003;197:801–806. doi: 10.1084/jem.20021493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Couper KN, Blount DG, de Souza JB, Suffia I, Belkaid Y, Riley EM. Incomplete depletion and rapid regeneration of Foxp3+ regulatory T cells following anti-CD25 treatment in malaria-infected mice. J Immunol. 2007;178:4136–4146. doi: 10.4049/jimmunol.178.7.4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson LL, Lanthier P, Hoffman J, Chen W. Vaccination protects B cell-deficient mice against an oral challenge with mildly virulent Toxoplasma gondii. Vaccine. 2004;22:4054–4061. doi: 10.1016/j.vaccine.2004.03.056. [DOI] [PubMed] [Google Scholar]
- 29.Kasper LH, Bradley MS, Pfefferkorn ER. Identification of stage-specific sporozoite antigens of Toxoplasma gondii by monoclonal antibodies. J Immunol. 1984;132:443–449. [PubMed] [Google Scholar]
- 30.Smiley ST, Lanthier PA, Couper KN, Szaba FM, Boyson JE, Chen W, Johnson LL. Exacerbated susceptibility to infection-stimulated immunopathology in CD1d-deficient mice. J Immunol. 2005;174:7904–7911. doi: 10.4049/jimmunol.174.12.7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindgren H, Stenmark S, Chen W, Tarnvik A, Sjostedt A. Distinct roles of reactive nitrogen and oxygen species to control infection with the facultative intracellular bacterium Francisella tularensis. Infect Immun. 2004;72:7172–7182. doi: 10.1128/IAI.72.12.7172-7182.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohm AP, McMahon JS, Podojil JR, Begolka WS, DeGutes M, Kasprowicz DJ, Ziegler SF, Miller SD. Cutting Edge: Anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J Immunol. 2006;176:3301–3305. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- 33.Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, Wynn TA, Kamanaka M, Flavell RA, Sher A. Conventional T-bet(+)Foxp3(−) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 2007;204:273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayer KD, Mohrs K, Crowe SR, Johnson LL, Rhyne P, Woodland DL, Mohrs M. The functional heterogeneity of type 1 effector T cells in response to infection is related to the potential for IFN-gamma production. J Immunol. 2005;174:7732–7739. doi: 10.4049/jimmunol.174.12.7732. [DOI] [PubMed] [Google Scholar]
- 35.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 36.Belkaid Y, Blank RB, Suffia I. Natural regulatory T cells and parasites: a common quest for host homeostasis. Immunol Rev. 2006;212:287–300. doi: 10.1111/j.0105-2896.2006.00409.x. [DOI] [PubMed] [Google Scholar]
- 37.Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320:1220–1224. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 39.Liesenfeld O, Kang H, Park D, Nguyen TA, Parkhe CV, Watanabe H, Abo T, Sher A, Remington JS, Suzuki Y. TNF-alpha, nitric oxide and IFN-gamma are all critical for development of necrosis in the small intestine and early mortality in genetically susceptible mice infected perorally with Toxoplasma gondii. Parasite Immunol. 1999;21:365–376. doi: 10.1046/j.1365-3024.1999.00237.x. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki Y, Sher A, Yap G, Park D, Neyer LE, Liesenfeld O, Fort M, Kang H, Gufwoli E. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J Immunol. 2000;164:5375–5382. doi: 10.4049/jimmunol.164.10.5375. [DOI] [PubMed] [Google Scholar]
- 41.Araujo FG. Depletion of L3T4+ (CD4+) T lymphocytes prevents development of resistance to Toxoplasma gondii in mice. Infect Immun. 1991;59:1614–1619. doi: 10.1128/iai.59.5.1614-1619.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Araujo FG. Depletion of CD4+ T cells but not inhibition of the protective activity of IFN-gamma prevents cure of toxoplasmosis mediated by drug therapy in mice. J Immunol. 1992;149:3003–3007. [PubMed] [Google Scholar]
- 43.Johnson LL, Sayles PC. Deficient humoral responses underlie susceptibility to Toxoplasma gondii in CD4-deficient mice. Infect Immun. 2002;70:185–191. doi: 10.1128/IAI.70.1.185-191.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson EH, Wille-Reece U, Dzierszinski F, Hunter CA. A critical role for IL-10 in limiting inflammation during toxoplasmic encephalitis. J Neuroimmunol. 2005;165:63–74. doi: 10.1016/j.jneuroim.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 45.Morris JC, Waldmann TA. Advances in interleukin 2 receptor targeted treatment. Ann Rheum Dis. 2000;59(Suppl 1):i109–114. doi: 10.1136/ard.59.suppl_1.i109. [DOI] [PMC free article] [PubMed] [Google Scholar]