Abstract
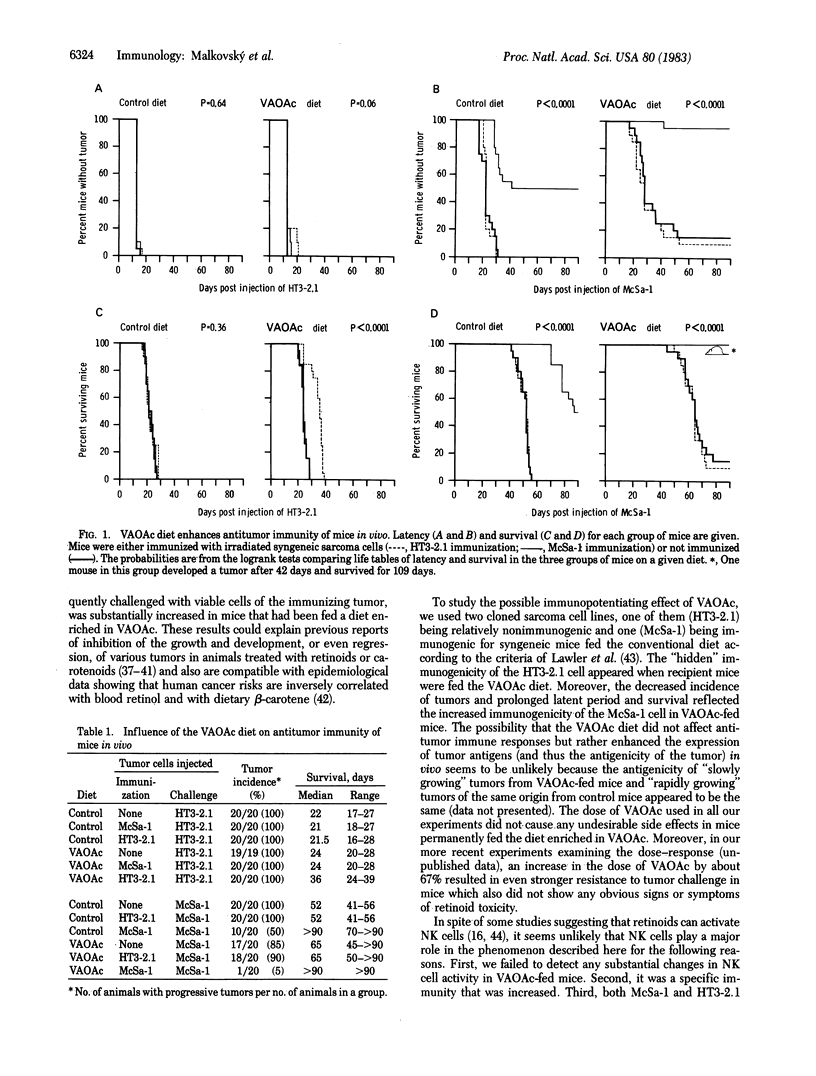
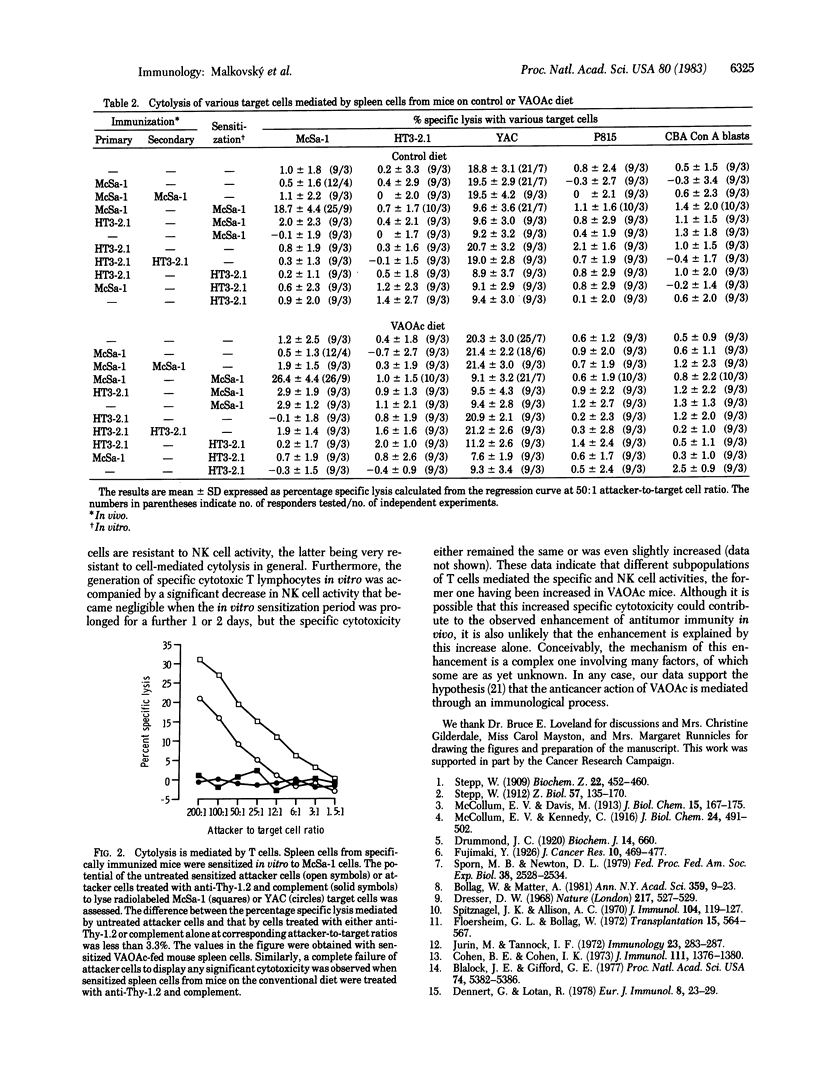
Age-matched male CBA mice on a conventional or a vitamin A acetate (VAOAc)-rich diet were immunized with irradiated cloned 3-methylcholanthrene- or Harvey sarcoma virus-induced (McSa-1 or HT3-2.1) sarcoma cells and then challenged with viable corresponding or unrelated (non-crossreacting) syngeneic sarcoma cells. The survival of the specifically immunized mice on the VAOAc diet was significantly prolonged in comparison with all control groups of mice as assessed by using logrank tests. Moreover, the specific immunization markedly decreased the incidence of tumors after the McSa-1 (but not HT3-2.1) challenge in a group of mice on the VAOAc diet (5% tumor incidence) compared with the equivalent group on the control diet (50% tumor incidence). Neither the VAOAc diet nor in vivo immunization alone or combined influenced natural killer cell activity. Specific T-cell-mediated cytotoxicity after in vivo priming and in vitro boosting with sarcoma cells was increased in VAOAc-fed mice. However, the marginal increase in cytotoxicity does not in itself explain the strikingly increased resistance to tumor transplants in preimmunized mice on the VAOAc diet in comparison with preimmunized mice on the control diet. The results indicate that a diet enriched in VAOAc can modify the ability of the immune system of a mouse to respond effectively to tumor antigens and can influence whether a tumor grows or regresses.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abb J., Abb H., Deinhardt F. Retinoic acid suppression of human leukocyte interferon production. Immunopharmacology. 1982 Aug;4(4):303–310. doi: 10.1016/0162-3109(82)90051-0. [DOI] [PubMed] [Google Scholar]
- Blalock J. E., Gifford G. E. Retinoic acid (vitamin A acid) induced transcriptional control of interferon production. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5382–5386. doi: 10.1073/pnas.74.12.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag W., Matter A. From vitamin A to retinoids in experimental and clinical oncology: achievements, failures, and outlook. Ann N Y Acad Sci. 1981 Feb 27;359:9–23. doi: 10.1111/j.1749-6632.1981.tb12733.x. [DOI] [PubMed] [Google Scholar]
- Bubeník J., Indrová M., Nemecková S., Malkovský M., Von Broen B., Pálek V., Anderlíková J. Solubilized tumour-associated antigens of methyl-cholanthrene-induced mouse sarcomas. Comparative studies by in vitro sensitization of lymph-node cells, macrophage electrophoretic mobility assay and transplantation tests. Int J Cancer. 1978 Mar 15;21(3):348–355. doi: 10.1002/ijc.2910210316. [DOI] [PubMed] [Google Scholar]
- Cohen B. E., Cohen I. K. Vitamin A: adjuvant and steroid antagonist in the immune response. J Immunol. 1973 Nov;111(5):1376–1380. [PubMed] [Google Scholar]
- DUNN T. B., POTTER M. A transplantable mast-cell neoplasm in the mouse. J Natl Cancer Inst. 1957 Apr;18(4):587–601. [PubMed] [Google Scholar]
- Dennert G., Crowley C., Kouba J., Lotan R. Retinoic acid stimulation of the induction of mouse killer T-cells in allogeneic and syngeneic systems. J Natl Cancer Inst. 1979 Jan;62(1):89–94. [PubMed] [Google Scholar]
- Dennert G., Lotan R. Effects of retinoic acid on the immune system: stimulation of T killer cell induction. Eur J Immunol. 1978 Jan;8(1):23–29. doi: 10.1002/eji.1830080106. [DOI] [PubMed] [Google Scholar]
- Dresser D. W. Adjuvanticity of vitamin A. Nature. 1968 Feb 10;217(5128):527–529. doi: 10.1038/217527a0. [DOI] [PubMed] [Google Scholar]
- Drummond J. C. The Nomenclature of the so-called Accessory Food Factors (Vitamins). Biochem J. 1920 Oct;14(5):660–660. doi: 10.1042/bj0140660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix E. L., Loyd B., Cohen M. H. Inhibition of the growth and development of a transplantable murine melanoma by vitamin A. Science. 1975 Sep 12;189(4206):886–888. doi: 10.1126/science.1154026. [DOI] [PubMed] [Google Scholar]
- Floersheim G. L., Bollag W. Accelerated rejection of skin homografts by vitamin A acid. Transplantation. 1972 Nov;14(5):564–567. doi: 10.1097/00007890-197211000-00006. [DOI] [PubMed] [Google Scholar]
- Glaser M., Lotan R. Augmentation of specific tumor immunity against a syngeneic SV40-induced sarcoma in mice by retinoic acid. Cell Immunol. 1979 Jun;45(1):175–181. doi: 10.1016/0008-8749(79)90373-3. [DOI] [PubMed] [Google Scholar]
- Goldfarb R. H., Herberman R. B. Natural killer cell reactivity: regulatory interactions and among phorbol ester, interferon, cholera toxin, and retinoic acid. J Immunol. 1981 Jun;126(6):2129–2135. [PubMed] [Google Scholar]
- Herberman R. B., Ortaldo J. R. Natural killer cells: their roles in defenses against disease. Science. 1981 Oct 2;214(4516):24–30. doi: 10.1126/science.7025208. [DOI] [PubMed] [Google Scholar]
- Jurin M., Tannock I. F. Influence of vitamin A on immunological response. Immunology. 1972 Sep;23(3):283–287. [PMC free article] [PubMed] [Google Scholar]
- KLEIN E., KLEIN G. ANTIGENIC PROPERTIES OF LYMPHOMAS INDUCED BY THE MOLONEY AGENT. J Natl Cancer Inst. 1964 Mar;32:547–568. [PubMed] [Google Scholar]
- Malkovsky M., Asherson G. L., Stockinger B., Watkins M. C. Nonspecific inhibitor released by T acceptor cells reduces the production of interleukin-2. Nature. 1982 Dec 16;300(5893):652–655. doi: 10.1038/300652a0. [DOI] [PubMed] [Google Scholar]
- Malkovský M., Asherson G. L., Chandler P., Colizzi V., Watkins M. C., Zembala M. Nonspecific inhibitor of DNA synthesis elaborated by T acceptor cells. I. Specific hapten- and I-J-driven liberation of an inhibitor of cell proliferation by Lyt-1-2+ cyclophosphamide-sensitive T acceptor cells armed with a product of Lyt-1+2+-specific suppressor cells. J Immunol. 1983 Feb;130(2):785–790. [PubMed] [Google Scholar]
- Malkovský M., Edwards A. J., Hunt R., Palmer L., Medawar P. B. T-cell-mediated enhancement of host-versus-graft reactivity in mice fed a diet enriched in vitamin A acetate. Nature. 1983 Mar 24;302(5906):338–340. doi: 10.1038/302338a0. [DOI] [PubMed] [Google Scholar]
- Marx J. L. Tumors; a mixed bag of cells. Science. 1982 Jan 15;215(4530):275–277. doi: 10.1126/science.7053575. [DOI] [PubMed] [Google Scholar]
- Medawar P. B., Hunt R. Anti-cancer action of retinoids. Immunology. 1981 Feb;42(2):349–353. [PMC free article] [PubMed] [Google Scholar]
- Medawar P., Hunt R., Mertin J. An influence of diet on transplantation immunity. Proc R Soc Lond B Biol Sci. 1979 Dec 31;206(1164):265–280. doi: 10.1098/rspb.1979.0104. [DOI] [PubMed] [Google Scholar]
- Meltzer M. S., Cohen B. E. Tumor suppression by Mycobacterium bovis (strain BCG) enhanced by vitamin A. J Natl Cancer Inst. 1974 Aug;53(2):585–587. doi: 10.1093/jnci/53.2.585. [DOI] [PubMed] [Google Scholar]
- Patek P. Q., Collins J. L., Yogeeswaran G., Dennert G. Anti-tumor potential of retinoic acid: stimulation of immune mediated effectors. Int J Cancer. 1979 Nov 15;24(5):624–628. doi: 10.1002/ijc.2910240516. [DOI] [PubMed] [Google Scholar]
- Peto R., Doll R., Buckley J. D., Sporn M. B. Can dietary beta-carotene materially reduce human cancer rates? Nature. 1981 Mar 19;290(5803):201–208. doi: 10.1038/290201a0. [DOI] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettura G., Schittek A., Hardy M., Levenson S. M., Demetriou A., Seifter E. Antitumor action of vitamin A in mice inoculated with adenocarcinoma cells. J Natl Cancer Inst. 1975 Jun;54(6):1489–1491. doi: 10.1093/jnci/54.6.1489. [DOI] [PubMed] [Google Scholar]
- Seifter E., Rettura G., Padawer J., Levenson S. M. Moloney murine sarcoma virus tumors in CBA/J mice: chemopreventive and chemotherapeutic actions of supplemental beta-carotene. J Natl Cancer Inst. 1982 May;68(5):835–840. [PubMed] [Google Scholar]
- Selfter E., Rettura G., Levenson S. M. Decreased resistance of C3H/HeHa mice to C3HBA tumor transplants; increased resistance due to supplemental vitamin A. J Natl Cancer Inst. 1981 Aug;67(2):467–472. [PubMed] [Google Scholar]
- Simpson E., Gordon R., Taylor M., Mertin J., Chandler P. Micromethods for induction and assay of mouse mixed lymphocyte reactions and cytotoxicity. Eur J Immunol. 1976 Jul;5(7):451–455. doi: 10.1002/eji.1830050705. [DOI] [PubMed] [Google Scholar]
- Spitznagel J. K., Allison A. C. Mode of action of adjuvants: retinol and other lysosome-labilizing agents as adjuvants. J Immunol. 1970 Jan;104(1):119–127. [PubMed] [Google Scholar]
- Sporn M. B., Newton D. L. Chemoprevention of cancer with retinoids. Fed Proc. 1979 Oct;38(11):2528–2534. [PubMed] [Google Scholar]
- Tannock I. F., Suit H. D., Marshall N. Vitamin A and the radiation response of experimental tumors: an immune-mediated effect. J Natl Cancer Inst. 1972 Mar;48(3):731–741. [PubMed] [Google Scholar]
- Trown P. W., Buck M. J., Hansen R. Inhibition of growth and regression of a transplantable rat chondrosarcoma by three retinoids. Cancer Treat Rep. 1976 Nov;60(11):1647–1653. [PubMed] [Google Scholar]



