Abstract
The cytokine “macrophage migration inhibitory factor (MIF)” is generally recognized as a proinflammatory cytokine, and MIF is involved in broad range of acute and chronic inflammatory states. With regard to glucose metabolism and insulin secretion, MIF is produced by pancreatic β cells and acts as a positive regulator of insulin secretion. In contrast, it is evident that MIF expressed in adipose tissues causes insulin resistance. Concerning MIF gene analysis, we found four alleles: 5-, 6-, 7-and 8-CATT at position −794 of MIF gene in a Japanese population. Genotypes without the 5-CATT allele were more common in the obese subjects than in the lean or overweight groups. It is conceivable that promoter polymorphism in the MIF gene is profoundly linked with obesity relevant to lifestyle diseases, such as diabetes. Obesity has become a serious social issue due to the inappropriate nutritional balance, and the consumption of functional foods (including functional foods to reduce fat mass) is expected to overcome this issue. In this context, MIF would be a reliable quantitative biomarker to evaluate the effects of functional foods on adiposity.
Keywords: Adipokine, Diabetes, Gene polymorphism, Macrophage migration inhibitory factor, Obesity
Introduction
A number of cytokines called adipokines that are expressed in adipocytes are involved in diabetes, hypertension, and other lifestyle-linked diseases. A cytokine known as macrophage migration inhibitory factor (MIF) was originally identified as a cytokine produced by activated T cells (Bloom & Bennett, 1966; David, 1966). MIF was found to be secreted from monocytes and macrophages as major sources (Calandra et al., 1994), and has the potential to exacerbate endotoxin shock (Bernhagen et al., 1993; Bucala, 1996). A number of pathophysiological roles of MIF have been described. MIF functions as an essential mediator in T-cell activation (Bacher, 1996) and delayed-type hypersensitivity (Bernhagen et al., 1998). We cloned rat MIF cDNA and elucidated its tertiary protein structure by X-ray crystallography, and we reported a number of physicochemical properties of this cytokine (Nishihira, 1998; Nishihira, 2000).
By immunohistochemical analysis, MIF was observed in the cytosol of immune cells such as macrophages, and also in adipocytes (Hirokawa, 1997) and pancreatic cells (Waeber et al., 1997). It is generally accepted that adipocytes are not only an energy deposit but also secrete a variety of bioactive molecules such as leptin, plasminogen activator inhibitor-1, and tumor necrosis factor (TNF)-α (Weisberg et al., 2003). In particular, the TNF-α expression of adipocytes was found to be increased in obese patients, and TNF-α exerts an inhibitory effect on the insulin signal transduction system, resulting in insulin resistance (Hotamisligil et al., 1993; Hotamisligil et al., 1995). Since obesity is associated with a chronic inflammatory response, MIF may have an impact on the pathophysiology of adiposity. The findings regarding adipose tissue and functional molecules prompted us to investigate the role of MIF in diabetes and obesity.
Among the various functions of MIF, we first focused on the issue of MIF mRNA in adipose tissues in response to glucose and insulin. We confirmed that MIF plays an important role in glucose metabolism and its related diseases. In this review, we discuss the biological characteristics of MIF, focusing on diabetes and obesity and the functionality of the MIF gene with respect to promoter polymorphisms in association with adiposity. We also discuss the potential role of MIF as a biomarker for the evaluation of pharmaceuticals and functional foods applicable for the amelioration of a pandemic lifestyle disease, e.g., obesity.
The pathophysiological role of MIF in adipocytes
Expression and Regulation of MIF in Adipocytes
First, we investigated whether glucose and insulin could stimulate MIF mRNA expression in 3T3-L1 adipocytes in vitro. In brief, MIF mRNA was markedly enhanced, reaching the maximum level at the dose of 1 μg/ml insulin with a 2.8-fold increase in the presence of 400 mg/dL glucose (Sakaue et al., 1999). In contrast, no significant increase was observed in the presence of 100 mg/dL glucose. These findings show that concomitant stimuli of high glucose level and insulin are essential for the up-regulation of MIF mRNA.
Following this experiment, we quantitated the intracellular MIF protein content in response to insulin. After a 24-hr incubation of mouse adipocytes in the presence of various concentrations of glucose and insulin, aliquots of cell lysates were collected, and the MIF concentration was measured with an ELISA (enzyme-linked immunosorbent assay). The results revealed that the intracellular MIF protein level increased in parallel with the up-regulation of MIF mRNA. In contrast, the MIF concentration in the cell culture medium decreased when the adipocytes were stimulated with both glucose and insulin. Thus, we concluded that the intracellular content of MIF in adipocytes increases in response to insulin and glucose, whereas the extracellular release of MIF is taken up by the cells and stored within them. The precise mechanism is not well understood, but MIF could be used as a recycling bioactive molecule between intracellular and extracellular spaces.
As for insulin secretion from pancreatic β cells, it was demonstrated that anti-MIF antibody administration suppressed insulin secretion from these cells following the glucose challenge (Waeber et al., 1997). We hypothesized that MIF produced by pancreatic β cells acts as a positive regulator of insulin secretion in both an autocrine and paracrine manner. Elevated insulin may stimulate the up-take of glucose by adipocytes. However, some of pro-inflammatory cytokines, e.g., interleukin-6 can cause insulin resistance in concert with TNF-α. In our understanding, MIF appears to play dual roles; that is, positive and negative functions in the regulation of the serum glucose level. In this context, it is critical to elucidate the mechanisms of how MIF is secreted in the extracellular space. When such mechanisms are identified, the pathophysiological roles of MIF in glucose intolerance will be more clearly understood.
The abnormal production of proinflammatory cytokines (such as TNF-α) in adipose tissue has been reported in obese rodents (Xu et al., 2003) and humans (Norman et al., 1995). The dysregulation of cytokines could contribute to the insulin resistance often seen in obesity. MIF is known as a pro-inflammatory cytokine, and it exacerbates the inflammatory reaction in concert with TNF-α. Regarding the pathophysiological actions of MIF, further insight into the local and systemic actions of MIF is necessary for a complete understanding of the functions of this molecule in diabetes and obesity.
We hypothesize that MIF functions to stimulate insulin secretion from pancreatic β cells in a local tissue-specific manner that controls the serum insulin level, whereas MIF secreted from macrophages or other cells circulating through the entire body would enhance insulin resistance together with TNF-α. We hypothesized that MIF bi-functionally regulates insulin availability and functionality in a positive manner for insulin secretion in pancreas, and in a negative manner for insulin resistance in adipose tissues.
MIF mRNA Expression in a Adipose Tissue in vivo
We also elucidated the characteristics of MIF in adipose tissues obtained from rats. In contrast to the findings with cultured adipocytes, MIF mRNA was significantly down-regulated in the adipose tissues (epididymal fat pads) of two different animal models, noninsulin-dependent diabetes mellitus (NIDDM)-
Otsuka Long-Evans Tokushima fatty (OLETF) rats and Wistar fatty rats (Sakaue et al., 1999). Although the reasons for the decreased MIF mRNA levels in fat tissues are unclear, this result suggests a possible biological link between the expression level of MIF and the severity of obesity and diabetes. To investigate this in detail, we examined the profile of MIF mRNA expression of primary adipocytes obtained from rats.
Expression of MIF mRNA in Primary Culture Cells
To examine the relationship between MIF expression levels and the severity of adiposity, we measured MIF mRNA expression in cultured rat primary cells at regular intervals through day 9 after the initiation of cell culture. The MIF mRNA expression increased depending on the time of incubation and reached a maximum at a certain time interval (day 6), but the expression gradually decreased when the cells became overgrown (Figure 1). This in vitro experiment may explain the down-regulation of MIF mRNA expression observed in the excessive obese state of OLETF rats. We also observed that the profile of MIF mRNA expression was similar to that of adiponectin mRNA. The profile of leptin mRNA increased as the cells grew. These data indicate that MIF plays an important role in the regulation of the cytokine network system of adipocytes in concert with adiponectin in a positive or negative manner, but this needs further investigation.
Figure 1.
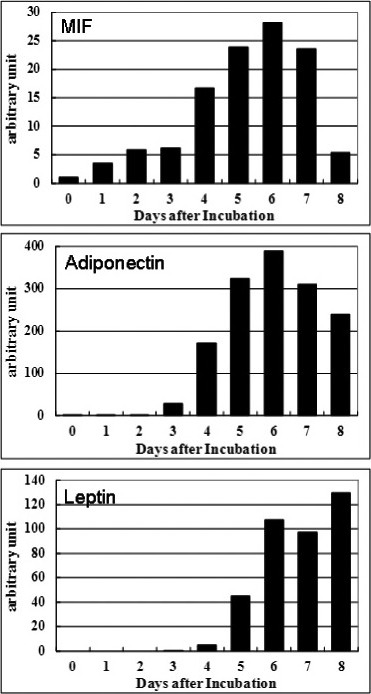
MIF mRNA expression of rat primary adipocytes. Pre-adipocytes collected from abdominal fat tissue of rats were harvested and cultured for 9 days, and the mRNA expressions of MIF, adiponectin and leptin were measured by real-time PCR at daily intervals.
Effect of Pioglitazone on the MIF Secretion of Adipocytes
Pioglitazone is known as an antidiabetic agent and has a pharmacological action to improve insulin resistance. We clarified the effect of pioglitazone on MIF mRNA expression and MIF protein production by adipocytes (Sakaue et al., 1999). When the adipocytes were incubated with pioglitazone (5 µM) for 24 hr, the MIF protein content in the culture medium was increased to about two fold that of the untreated cells, whereas the MIF mRNA expression level was minimally changed. This result shows that pioglitazone has the potential to enhance MIF secretion from adipocytes. We hypothesize that pioglitazone improves insulin resistance in part by stimulating the release of MIF into the extracellular space. Taken together, the relevant findings indicate that MIF may have a key role as a positive regulator of insulin secretion from pancreatic β cells, which would ameliorate, at least in part, insulin resistance together with pioglitazone. In this context, as mentioned above, the functions of MIF in terms of glucose metabolism should be determined at the local level, e.g., pancreatic cells regarding insulin secretion and at the systemic level, e.g., insulin resistance.
Promoter Polymorphism in the MIF Gene in Association with Adiposity
Analysis of the MIF Gene in Association with Obesity
Obesity is an independent risk factor for a wide variety of diseases, especially lifestyle diseases, diabetes, hypertension, and hyperlipidemia. As the morbidity of these diseases is increasing, obesity has become a serious health problem not only in western countries but also in Asia, especially Japan, China, and Korea. A high-calorie and high-fat diet and a sedentary lifestyle contribute to the development of obesity. It is evident that genetic factors also play an important role in adiposity. Several studies of the relationship between body mass index (BMI) and gene polymorphisms have been reported, and the gene polymorphisms have been widely used as biomarkers for the severity of adiposity. It has also become clear that any promoter polymorphisms of the MIF gene relevant to obesity would be useful to account for the entire genetic contribution to obesity (Sakaue et al., 2006).
The MIF gene is located on chromosome 22q11.23, which was found to be associated with abdominal obesity in Caucasians in a genome-wide linkage scan (Rice et al., 2002). This finding suggested that this chromosomal region is a susceptibility locus for abdominal adiposity in a particular population, such as Japanese. Polymorphism has been identified within the promoter region of the MIF gene, a tetranucleotide CATT repeat located at position −794 (−794[CATT]5–8) (Figure 2) (Baugh et al., 2002). In addition, a specific single-nucleotide polymorphism (SNP) was identified at −173 (Donn et al., 2001), which affects the MIF mRNA expression level. From several lines of molecular-basis studies, it became evident that the polymorphism affects MIF promoter activity and regulates the expression of MIF mRNA (Hizawa et al., 2004).
Figure 2.
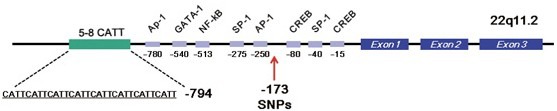
Polymorphism of the promoter region of MIF gene. A tetranucleotide CATT repeat located at position -794 (-794[CATT]5-8) affects MIF mRNA expression, and is considered to be associated with adiposity. In addition, a specific single nucleotide polymorphism is identified at -173, which affects MIF mRNA expression level, but not adiposity.
Promoter Polymorphisms of the MIF Gene in a Japanese Population
We explored the relationship between promoter polymorphisms of the MIF gene and obesity in a Japanese population. In parallel with the available gene-related information from other laboratories, we found a significant relationship between MIF gene polymorphism and obesity in Japanese (Sakaue et al., 2006). Briefly, 223 unrelated individuals were divided into three groups according to WHO definitions: lean (BMI<25 kg/m2), overweight (25>BMI>30 kg/m2) and obese (BMI>30 kg/m2). Polymorphism at −794 was analyzed by PCR, in which the PCR products and the GS-500 TAMRA size standard were analyzed. The sizes of the PCR products were 340, 344, 348 and 352 base pairs in length; these corresponded to 5-, 6-, 7-, and 8-CATT repeats, respectively.
We clarified that the frequencies of the 5-, 6-, 7- and 8-CATT alleles located at −794 of the MIF gene were 40.6%, 41.3%, 17.8% and 0.2%, respectively. The transcriptional activity of the MIF promoter with the 5-CATT repeat was lower than that with the other alleles at −794[CATT]5–8. We classified the subjects into two groups for further analysis; subjects with the 5-CATT allele (5/5 and 5/X) and those without the 5-CATT allele (X/X) (X indicates 6-, 7- or 8-CATT allele).
The X/X genotype was significantly more common in the obese subjects than in the lean or overweight groups. The subjects with the X/X genotype were at increased risk for obesity compared to those with the 5-CATT allele, adjusted by age and sex. The Japan Society for the Study of Obesity (JASSO) has defined BMIs of 25 kg/m2 or higher as ‘obese’. When the JASSO criterion was applied, the risk of obesity (BMI >25 kg/m2) in the subjects with the X/X genotype was also increased, with the odds ratio of 2.05. This finding indicates that the X-CATT allele is more frequent in obese subjects than in lean or overweight subjects. In contrast, SNPs at −173 involved in the MIF mRNA expression level did not affect the adiposity of this population.
Perspective on MIF Studies with Regard to Functional Foods
In this review, we presented various physiologic and pathogenic characteristics of MIF, focusing on the expression and regulation of MIF mRNA in adipocytes and adipose tissues in relation to adiposity. From the available data, MIF expressed in muscle has a catabolic effect via the induction of 6-phosphofructo-2-kinase/fructose-2, 6-bisphosphatase (Benigni et al., 2000). This report strongly supports the idea that MIF is profoundly linked to energy metabolism. Although it remains unclear whether this catabolic effect is physiologically associated with the regulation of whole body weight, we speculate that MIF expression in adipose tissues is responsible for obesity through the catabolic effect of the MIF molecule. We observed a marked reduction of MIF contents in adipose tissues of obese rats (OLETF), as mentioned previously. It is of interest to investigate whether MIF is responsible for the adiposity of the obese rats in terms of energy consumption.
In conclusion, we are currently investigating the efficacy of foods such as onion, asparagus and broccoli on the amelioration of lifestyle diseases caused by visceral obesity. In the past few years, a number of studies have demonstrated that functional foods can ameliorate adiposity, resulting in the improvement of diabetes and hypertension. Based on our continued studies of MIF, we suspect that the measurement of MIF serum levels will be useful for the evaluation of the functions of foods, particularly in regard to diabetes and obesity. For an example, we measured the serum contents of MIF in prediabetic patients (HbA1c 6.0% −6.5%), and found a significant increase compared to normal controls (Yabunaka, 2000). Likewise, elevated serum MIF has been found in American Pima Indians, who generally show a high incidence of type 2 diabetes. However, the serum MIF level would be affected by multiple factors, such as inflammatory disorders. Thus, any causal relationship between MIF and obesity should be carefully determined for each individual. Moreover, the polymorphism of MIF promoter gene analysis is considered to be useful for the prevention of lifestyle diseases through evidence-based nutrition education. MIF-based investigations of food, exercise, and prevention of disease are underway, and they may produce a novel scientific approach for food science.
Acknowledgement
The authors thank all of their collaborators in their MIF studies, particularly the investigators in the First Department of Medicine, Hokkaido University School of Medicine. This work was supported in part by the Regional Innovation of Research program of the Hokkaido municipal government.
References
- 1.Bacher M., Metz C.N., Calandra T., Mayer K., Chesney J., Lohoff M., Gemsa D., Donnelly T., Bucala R. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc Natl Acad Sci USA. 1996;93:7849–7854. doi: 10.1073/pnas.93.15.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baugh J.A., Chitnis S., Donnelly S.C., Monteiro J., Lin X., Plant B.J., Wolfe F., Gregersen P.K., Bucala R. A functional promoter polymorphism in the macrophage migration inhibitory factor (MIF) gene associated with disease severity in rheumatoid arthritis. Genes Immun. 2002;3:170–176. doi: 10.1038/sj.gene.6363867. [DOI] [PubMed] [Google Scholar]
- 3.Benigni F., Atsumi T., Calandra T., Metz C., Echtenacher B., Peng T., Bucala R. The proinflammatory mediator macrophage migration inhibitory factor induces glucose catabolism in muscle. J Clin Invest. 2000;106:1291–1300. doi: 10.1172/JCI9900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernhagen J., Calandra T., Bucala R. Regulation of the immune response by macrophage migration inhibitory factor: biological and structural features. J Mol Med. 1998;76:151–161. doi: 10.1007/s001090050204. [DOI] [PubMed] [Google Scholar]
- 5.Bernhagen J., Calandra T., Mitchell R.A., Martin S.B., Tracey K.J., Voelter W., Manogue K.R., Cerami A., Bucala R. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- 6.Bloom B.R., Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966;153:80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- 7.Bucala R. MIF re-discovered: pituitary hormone and glucocorticoid-induced regulator of cytokine production. Cytokine Growth Factor Rev. 1996;7:19–24. doi: 10.1016/1359-6101(96)00008-1. [DOI] [PubMed] [Google Scholar]
- 8.Calandra T., Bernhagen J., Mitchell R.A., Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994;179:1985–1992. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.David J.R. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci USA. 1966;56:72–77. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donn R.P., Shelley E., Ollier W.E., Thomson W. A novel 5’-flanking region polymorphism of macrophage migration inhibitory factor is associated with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2001;44:1782–1785. doi: 10.1002/1529-0131(200108)44:8<1782::AID-ART314>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 11.Hirokawa J., Sakaue S., Tagami S., Kawakami Y., Sakai M., Nishi S., Nishihira J. Identification of macrophage migration inhibitory factor in adipose tissue and its induction by tumor necrosis factor-alpha. Biochem Biophys Res Commun. 1997;235:94–98. doi: 10.1006/bbrc.1997.6745. [DOI] [PubMed] [Google Scholar]
- 12.Hizawa N., Yamaguchi E., Takahashi D., Nishihira J., Nishimura M. Functional polymorphisms in the promoter region of macrophage migration inhibitory factor and atopy. Am J Respir Crit Care Med. 2004;169:1014–1018. doi: 10.1164/rccm.200307-933OC. [DOI] [PubMed] [Google Scholar]
- 13.Hotamisligil G.S., Arner P., Caro J.F., Atkinson R.L., Spiegelman B.M. Increased adipose tissue expression of tumor necrosis factor-α in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity- linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 15.Nishihira J. Novel pathophysiological aspects of macrophage migration inhibitory factor. Int J Mol Med. 1998;2:17–28. doi: 10.3892/ijmm.2.1.17. [DOI] [PubMed] [Google Scholar]
- 16.Nishihira J. Macrophage migration inhibitory factor (MIF): its essential role in the immune system and cell growth. J Interferon Cytokine Res. 2000;20:751–762. doi: 10.1089/10799900050151012. [DOI] [PubMed] [Google Scholar]
- 17.Norman R.A., Bogardus C., Ravussin E. Linkage between obesity and a marker near the tumor necrosis factor- locus in Pima Indians. J Clin Invest. 1995;96:158–162. doi: 10.1172/JCI118016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rice T., Chagnon Y.C., Pérusse L., Borecki I.B., Ukkola O., Rankinen T., Gagnon J., Leon A.S., Skinner J.S., Wilmore J.H., Bouchard C., Rao D.C. A genomewide linkage scan for abdominal subcutaneous and visceral fat in black and white families: The HERITAGE Family Study. Diabetes. 2002;51:848–855. doi: 10.2337/diabetes.51.3.848. [DOI] [PubMed] [Google Scholar]
- 19.Sakaue S., Ishimaru S., Hizawa N., Ohtsuka Y., Tsujino I., Honda T., Suzuki J., Kawakami Y., Nishihira J., Nishimura M. Promoter polymorphism in the macrophage migration inhibitory factor gene is associated with obesity. Int J Obes. 2006;30:238–242. doi: 10.1038/sj.ijo.0803148. [DOI] [PubMed] [Google Scholar]
- 20.Sakaue S., Nishihira J., Hirokawa J., Yoshimura H., Honda T., Aoki K., Tagami S., Kawakami Y. Regulation of macrophage migration inhibitory factor (MIF) expression by glucose and insulin in adipocytes in vitro. Mol Med. 1999;5:361–371. [PMC free article] [PubMed] [Google Scholar]
- 21.Waeber G., Calandra T., Roduit R., Haefliger J.A., Bonny C., Thompson N., Thorens B., Temler E., Meinhardt A., Bacher M., Metz C.N., Nicod P., Bucala R. Insulin secretion is regulated by the glucose-dependent production of islet beta cell macrophage migration inhibitory factor. Proc Natl Acad Sci USA. 1997;94:4782–4787. doi: 10.1073/pnas.94.9.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisberg S.P., Mc Cann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu H., Barnes G.T., Yang Q., Tan G., Yang D., Chou C.J., Sole J., Nichols A., Ross J.S., Tartaglia L.A., Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yabunaka N., Nishihira J., Mizue Y., Tsuji M., Kumagai M., Ohtsuka Y., Imamura M., Asaka M. Elevated serum content of macrophage migration inhibitory factor in patients with type 2 diabetes. Diabetes Care. 2000;23:256–258. doi: 10.2337/diacare.23.2.256. [DOI] [PubMed] [Google Scholar]


