Abstract
Andrographis paniculata is a traditional Chinese herb and displays diverse biological activities including antioxidation, anti-tumorigenesis, anti-virus, and anti-atherogenesis. In this study, we investigated the up-regulation of ethanolic extract of A. paniculata (APE) on the antioxidant defense in rat livers and whether this enhancement protected against carbon tetrachloride (CCl4)-induced liver damage. Male Sprague-Dawley rats were orally administered (i.g.) 0, 0.75, or 2 g/kg/d APE for 5 d. At d 6, rats were sacrificed and liver tissues were removed. Some animals (n=8) were intraperitoneally injected CCl4 (1 mL/kg, 50% in olive oil) and blood was drawn 24 h after CCl4 treatment. The results showed that APE increased hepatic glutathione (GSH) content and superoxide dismutase, GSH peroxidase, and GSH S-transferase activities in a dose-dependent manner (p < 0.05). Results of immunoblotting and RT-PCR revealed that rats treated with APE had higher glutamate cysteine ligase catalytic and modifier subunits, heme oxygenase 1, superoxide dismutase 1, and GSH S-transferase Ya and Yb protein and mRNA expression than those of control rats. Moreover, APE increased Nrf2 nuclear translocation and Nrf2 binding to DNA in rat liver. In the presence of CCl4, APE decreased hepatic thiobarbituric acid-reactive substances production and plasma aspartate aminotransferase and alanine aminotransferase activities. These results suggest that APE protection against CCl4 insult is attributed, at least in part, to its up-regulation of antioxidant defense in rat liver.
Keywords: Andrographis paniculata, Antioxidation, Nrf2, Carbon tetrachloride
Introduction
Andrograph is Herba (穿 心 蓮 chuān xīn lián; Andrographis paniculata (Burm. f)) is widely used as a traditional medicine in Southeast Asia including Taiwan, China, India, and Thailand. It is commonly applied for treating infections, cold, fever, inflammation, and diarrhea (Chao and Lin, 2010). The leaves and stems of A. paniculata are rich in numerous diterpene lactones and andrographolide is the most abundant one. It is estimated that andrographolide accounts for 1.7 and 0.8% of the dried leaf and stem weight, respectively (Pholphana et al., 2004). Recently, several lines of evidence indicate that A. paniculata and andrographolide own diverse physiological functions and therapeutic potentials including antioxidation, anti-inflammation, anti-apoptosis, anti-atherosclerosis, anti-cancer, anti-virus, anti-hyperglycemia, and hepatoprotection (Chao and Lin, 2010). Aqueous and ethanolic extracts of A. paniculata (APE) and andrographolide lower blood glucose level and induce glucose transporter 4 activity in streptozotocin-induced diabetes rats (Husen et al., 2004; Yu et al., 2003; Zhang and Tan, 2000). Glutathione (GSH) peroxidase, GSH reductase, superoxide dismutase (SOD), and catalase activities are increased in mice dosed with 50 or 100 mg/kg APE for 14 days (Singh et al., 2001). In cardiomyocytes, andrographolide up-regulates both glutamate cysteine ligase (GCL) catalytic (GCLC) and modifier subunit (GCLM) and increases cellular GSH contents, which lead to protection of cells against hypoxia/reoxygenation-induced oxidative damage (Woo et al., 2008). Andrographolide ameliorates tumor necrosis factor -induced monocyte adhesion to the endothelial cells by suppressing intracellular adhesion molecule 1 expression in vascular endothelial cells (Chen et al., 2011).
In aerobic organisms, antioxidant defense is vital for protecting cells against oxidative insult. This defense is composed of a number of antioxidants and enzymes, such as GSH, ascorbic acid, n-tocopherol, GSH peroxidase, GSH reductase, SOD, catalase, GCLC, GCLM, and heme oxygenase (Vassort and Turan, 2010). GSH peroxidase is responsible for reducing hydrogen peroxide and organic hydroperoxides and is accompanied with GSH oxidation to GSH disulfide (GSSG). GSSG then can be reduced to GSH via the action of GSH reductase. GCL catalyzes the rate-limiting step in GSH synthesis, and it is a heterodimeric protein composed of GCLC and GCLM subunits that are expressed by distinct genes (Franklin et al., 2009). Heme oxygenase 1 (HO-1) is an inducible enzyme and catalyzes the rate-limiting step of free heme degradation into Fe2+, carbon monoxide, and biliverdin (Ryter et al., 2000). HO-1 is known for its cytoprotective effect against oxidative stress (Abraham and Kappas, 2005) and plays an important role in the resolution of inflammation (Pae et al., 2008).
Nuclear factor erythroid 2-related 2 (Nrf2) is a transcription factor and plays a key role in the induction of numerous antioxidant enzymes and phase II drug metabolizing enzymes (Itoh et al., 1997; Surh et al., 2008). Under unstimulated conditions, Nrf2 is retained in the cytosol by binding to Klech-like ECH-associated protein 1 (Keap1). The Keap1-Nrf2 complex is disrupted in response to several electrophilic antioxidants, and free Nrf2 translocates into the nucleus where it conjugates with small Maf proteins and then binds to the antioxidant response element (ARE) of the target gene promoter (Itoh et al., 2004; Owuor and Kong, 2002). ARE has been shown to be located in the promoters of many antioxidant and detoxifying enzyme genes including GCLC, GCLM, GSH peroxidase 1/2, GSH reductase, HO-1, GSH S-transferase (GST), and UDP-glucuronyl transferase (Surh et al., 2008).
Carbon tetrachloride (CCl4) is an organic solvent and a hepatotoxicant. When CCl4 enters liver, reactive metabolites trichloromethyl free radical (CCL3) and proxy trichloromethyl radical (OOCCl3) are generated by the mixed function cytochrome p450 system (Recknagel et al., 1966). These free radicals lead to lipid peroxidation and damage cell membrane and enzyme activity. Acute liver damage or chronic hepatotoxicity such as fatty liver and cirrhosis are observed. In this study, the effect of A. paniculata ethanolic extract (APE) on hepatic antioxidant defense capacity and its protection against CCl4-induced liver damage were investigated.
Materials and Methods
Materials
GSH, GSSG, NADPH, leupeptin, aprotinin, PMSF, pyrogallol, 2-thiobarbituric acid, Ellman's reagent, and 1-chloro-2,4-dinitrobenzene were obtained from Sigma (St. Louis, MO). Trizol reagent was from Invitrogen (Carlsbad, CA). The antibodies against GCLM and Nrf2 were from Santa Cruz Biotechnology (Santa Cruz, CA). The antibodies against GCLC and HO-1 were from Abcam (Cambridge, UK) and Calbiochem (Darmstadt, Germany), respectively. The antibodies against GST Ya and Yb were from Oxford Biomedical Research (Cambridge, UK). The antibody against SOD1 was from Gene Tex (San Antonio, TX). LightShiftTM Chemiluminescent EMSA kit was from Pierce Chemical (Rockford, IL). All other chemicals and reagents were of analytical grade and were obtained commercially. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) kits were obtained from DiaSYS (Holzheim, Germany).
Preparation of the ethanolic extract of A. paniculata (APE)
Dried whole plants of A. paniculata were procured from Hualien, Taiwan. Powdered plant material (10 g) was extracted with 95% ethanol (250 mL) by stirring overnight at room temperature. The ethanol extract portion was collected by centrifugation at 1,350 xg for 10 min at 4°C. The extract was concentrated by using a rotary evaporator and was then dried by freeze dry, and stored at -20°C.
Animals and treatments
Male Sprague-Dawley rats (6-week-old) were purchased from the Bio LASCO Experimental Animal Center (Taipei, Taiwan). The animals were housed in plastic cages in a room kept at 23 ± 1°C and 60 ± 5% relative humidity with a 12-h light and dark cycle (lighting from 6:00 a.m. to 6:00 p.m.). Rats were fed a standard chow diet and drinking water ad libitum. After one week of acclimation, rats were randomly assigned to control, 0.75 g/kg APE, or 2 g/kg APE groups (n=6-7). APE was suspended in 0.5% methyl cellulose solution (w/v) and was intragastrically (i.g.) given (10 mL/kg) for 5 consecutive days. Animals were fasted overnight and were sacrificed by exsanguinations via the abdominal aorta under carbon dioxide (70%/30%, CO2/O2) anesthesia. Liver and kidneys were removed immediately, weighed, freeze-clamped in liquid nitrogen, and stored at -80°C. Animals in this study were treated based on animal ethics guidelines of Institutional Animal Ethics Committee.
For CCl4-induced liver damage, rats were intraperitoneally injected of 1 mL/kg CCl4 (50% in olive oil, v/v) after a 5-d i.g. dosed with 0, 0.75 or 2 g/kg/d APE (n=8). Blood was drawn 24 h after CCl4 treatment with heparin as an anticoagulant and plasma was prepared for transaminase activity assay. Rats were sacrificed and liver was removed for lipid peroxides determination.
Determination of antioxidant enzyme activities
Livers were homogenized (1:4, w/v) in a buffer (pH 7.4) containing 10 mM potassium phosphate and 1.5 % KCl, and centrifuged at 10,000 xg for 30 min at 4°C. The supernatant was further ultracentrifuged at 105,000 xg for 1 h and the final cytosol and microsome fractions were used for enzyme activity and protein expression measurement. The protein content of the cytosolic and microsomal fraction was measured by using the Coomassie plus protein assay kit (Pierce, Rockford, IL). Hepatic GSH peroxidase activity was determined spectrophotometrically with the coupled method using hydrogen peroxide (H2O2) as a substrate (Lawrence and Burk, 1976). GSH reductase activity was measured as described by Bellomo et al. (1987). GST activity was determined according to the method of Habig and Jakoby (1981) using 2,4-chloro-dinitrobenzene (CDNB) as substrate. Superoxide dismutase (SOD) activity was determined by measuring the degree of inhibition of pyrogallol oxidation as described by Marklund and Marklund (1974). One unit SOD activity is defined as the amount of enzyme required to inhibit the rate of pyrogallol oxidation by 50%. Catalase activity was determined according to the method of Abei et al. (1984) using H2O2 as substrate.
SDS-PAGE and Western blotting
Equal amounts of cytosol and microsome proteins were electrophoresed in a SDS-polyacrylamide gel and transferred to a polyvinylidene fluoride membrane. After blocking the nonspecific binding sites with 5% nonfat dry milk in 15 mM Tris-150 mM NaCl buffer (pH 7.4) at 4°C overnight, membranes were hybridized with anti-GCLC, GCLM, HO-1, SOD1, and GST Ya and Yb antibodies. The immunoreactive bands were detected by using an enhanced chemiluminescence plus Western blotting detection reagent (Amersham Biosciences, Boston, MA).
RNA isolation and RT-PCR
Frozen livers were homogenized in Trizol reagent (1:20, w/v) and total RNA was extracted. RNA extracts were suspended in nuclease-free water and were frozen at -20°C until the RT-PCR analysis was performed (Yang et al., 2011). Total RNA (0.4 μg) was reversely transcribed by Moloney murine leukemia virus reverse transcriptase (Promega) in the presence of 150 μM of each dNTP, 40 units RNase inhibitor, and 250 nmol oligo(dT) in a final volume of 20 μL. cDNA was amplified in a thermocycler in a reaction volume of 50 μL containing 20 μL of cDNA, BioTaq PCR buffer, 50 μmol of each deoxyribonucleotide triphosphate, 1.25 mM MgCl2, and 1 unit of BioTaq DNA polymerase (BioLine). The sequences for the PCR primers were as follows: for GCLC (forward: 5’-CCTTCTGGCACAGCACGTTG-3’; reverse: 5’-TAAGACGGCATCTCGCTCCT-3’), GCLM (forward: 5’-CTGACATTGAAGCCCAGGAG-3’; reverse: 5’-ACATTGCCAAACCACCACA-3’), HO-1 (forward: 5’-AGCATGTCCCAGGATTTGTC-3’; reverse: 5’-AAGGCGGTCTTAGCCTCTTC-3’), SOD1 (forward: 5’-GCAGGGCGTCATTCACTT-3’; reverse: 5’-TTCTCGTGGACCACCATA-3’), GST Ya (forward: 5’-CCATCACCATCTTCCAGGAG-3’; reverse: 5’-CCT GCTTCACCACCTTCTTG-3’), and GST Yb (forward: 5’-TGGCACTCACAGGGAGGACC-3’; reverse: 5’-TTAAAGATGAGACAGGCCTGGG-3’). The PCR for GCLC and GCLM was performed as follows: 25 cycles for 60 s at 94°C, 60 s at 60°C, and 90 s at 72°C. For HO-1 amplification, the PCR was 25 cycles for 30 s at 94°C, 45 s at 55°C, and 45 s at 72°C. For SOD1, the PCR was 30 cycles for 60 s at 95°C, 60 s at 48°C, and 60 s at 72°C. For GST Ya, the PCR was 29 cycles for 60 s at 95°C, 60 s at 45°C, and 60 s at 72°C. For GST Yb, the PCR was 30 cycles for 45 s at 95°C, 60 s at 52°C, and 80 s at 72°C. The PCR amplicons were then electrophoresed in 1%-agarose gels containing 1x TAE buffer (40 mM Tris/20 mM glacial acetic acid/2 mM EDTA).
Nuclear extraction and electrophoretic mobility shift assay (EMSA)
Nuclear proteins of liver tissues were extracted as described by Tian et al. (2004) with some modifications. Briefly, 50 mg liver were homogenized (1:18, w/v) in ice-cold hypotonic buffer containing 10 mM HEPES, 10 mM KCl, 1 mM MgCl2, 0.1 mM EDTA, 0.5 mM dithiothreitol, 0.5 mM PMSF, 4 μg/mL leupeptin, 20 μg/mL aprotinin, pH 7.9. The homogenates were sat at ice bath for 15 min and then were centrifuged at 600 xg for 10 min. The supernatant was mixed with 100 μL 10% Nonidet P-40 and was allowed to sit at ice bath for 10 min. After centrifugation at 5,000 xg for 5 min, pellets containing crude nuclei were resuspended in 100 μL of hypertonic buffer containing 10 mM HEPES, 400 mM KCl, 1 mM MgCl2, 0.1 mM EDTA, 0.5 mM dithiothreitol, 0.5 mM PMSF, 4 μg/mL leupeptin, 20 μg/mL aprotinin, and 25% glycerol at 4°C for 45 min. Nuclear proteins were then collected by centrifugation at 12,000 xg for 15 min.
EMSA was performed according to our previous study (Yang et al., 2011). The LightShift Chemiluminescent EMSA Kit (Pierce Chemical Company, Rockford, IL) and synthetic biotin-labeled double-stranded rHO-1 ARE consensus oligonucleotides (5′-AACCATGACACAGCATAAAA-3′) were used to measure Nrf2 nuclear protein-DNA binding activity. Unlabeled double-stranded ARE oligonucleotide and a mutant double-stranded oligonucleotide were used to confirm the protein-binding specificity.
Measurement of intracellular GSH content
Hepatic GSH and GSSG were determined by HPLC-MS as described by Yao et al. (2011). Briefly, 100 μL of liver cytosolic fraction was reacted with 200 μL of 10 mM Ellman's reagent by gentle mixing, and followed by adding 60 μL of 20% 5-sulfosalicylic acid to cause acid precipitation. After centrifugation at 10,000 xg for 10 min at 4°C, supernatant was used to analyze reduced and oxidized GSH content.
Biochemical assays
Plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured by commercial kits (Randox Laboratories, Antrim, UK). The lipid peroxidation was determined by measuring the thiobarbituric acid-reactive substances (TBARS) in a fluorescence spectrophotometer (Hitachi F4500, Tokyo, Japan) as described by Fraga et al. (1988).
Statistical Analysis
Statistical analysis was performed with SAS statistical software (Cary, NC). The significance of the difference among group means was determined by one-way ANOVA followed by Duncan's test. P values <0.05 were taken to be statistically significant.
Results
APE on rat growth characteristics
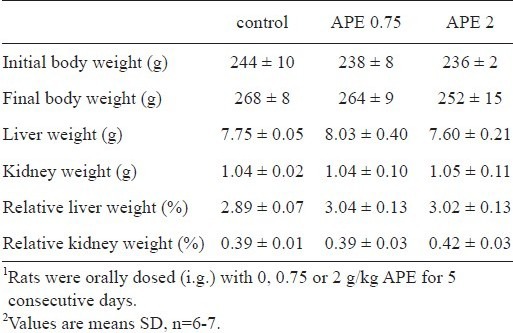
With up to 2 g/kg/d APE over this 5-d experiment, the body weight, liver weight, kidney weight, and the percentage of liver weight and kidney weight to body weight were not different from those of the control rats (Table 1).
Table 1.
Effect of ethanolic extract of A. paniculata (APE) on rat growth characteristics1,2.
APE on rat liver antioxidant enzyme activities and GSH content
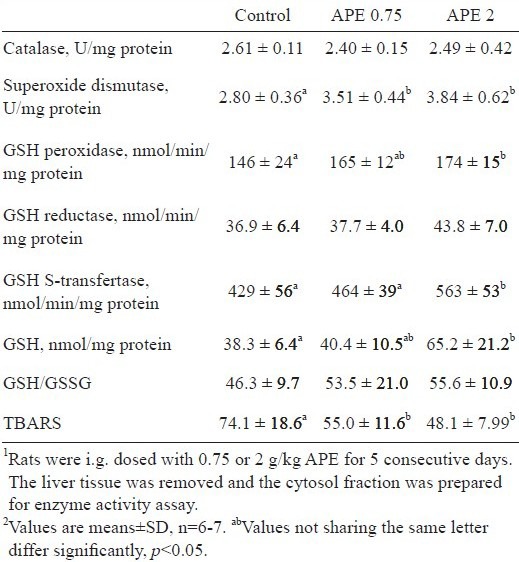
Whether A. paniculata changed hepatic antioxidation capacity, activities of antioxidant enzymes and content of cellular GSH and GSSG were determined. As shown in Table 2, APE dose-dependently increased SOD activity. As compared to controls, a 25% and 37% increase of SOD activity was noted in rats dosed with 0.75 and 2 g/kg/d APE, respectively (p <0.05). Similarly, a dose-dependent increase of GSH peroxidase and GST activities was noted in APE-treated rats. Rats dosed with 2 g/kg APE had 19% higher GSH peroxidase and 31% higher GST activity than control rats did (p <0.05). Activities of catalase and GSH reductase in rat livers, however, were not changed by APE. In addition to the changes of antioxidant enzyme activities, APE in a dose of 2 g/kg increased GSH content in rat liver as compared to that in control rats (p <0.05). TBARS production in rat livers was dose-dependently decreased by APE (p <0.05).
Table 2.
Changes of rat liver antioxidant enzyme activity and glutathione (GSH) content by the ethanolic extract of A. paniculata (APE)1,2.
APE on GCLC, GCLM, SOD1, HO-1, GST Ya, and GST Yb protein and mRNA levels
Next, we investigated the effect of APE on the expression of antioxidant enzymes in rat livers. Compared to the control rats, higher levels of GCLC, GCLM, SOD1, HO-1, and GST Ya and Yb protein were noted in rats treated with either 0.75 or 2 g/kg/d APE (Figure 1A). Similar to the changes of protein expression, mRNA expression of GCLC, GCLM, SOD1, HO-1, and GST Ya and Yb were increased in APE-treated rats (Figure 1B).
Figure 1.
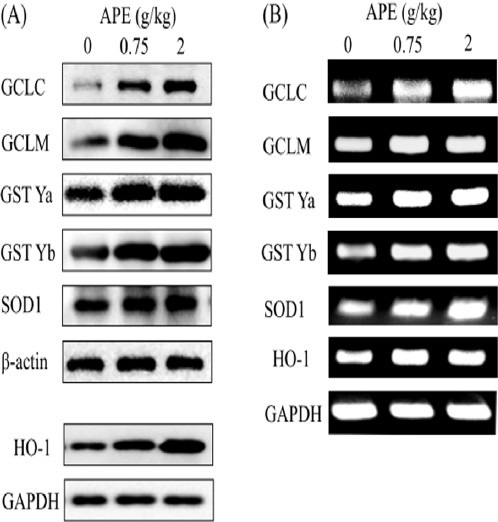
Induction of hepatic antioxidant enzyme protein (A) and mRNA (B) expression in rats treated with ethanolic extract of A. paniculata (APE). Rats were i.g. administered with 0, 0.75, or 2 g/kg APE for 5 consecutive days. Protein and mRNA levels were determined by immunoblotting and RT-PCR, respectively, as described in the Materials and Methods.
APE increases DNA binding activity of nuclear Nrf2
Transcription of GCLC, GCLM, HO-1, SOD1, and GST Ya and Yb genes are known to be regulated by transcription factor Nrf2. Activation of Nrf2 nuclear translocation and nuclear Nrf2 binding to DNA were examined. As shown in Figure 2A, rats treated with APE had a higher level of Nrf2 accumulation in the nuclear fraction. Moreover, EMSA showed that APE increased the binding activity of nuclear Nrf2 to the ARE consensus sequences (Figure 2B).
Figure 2.
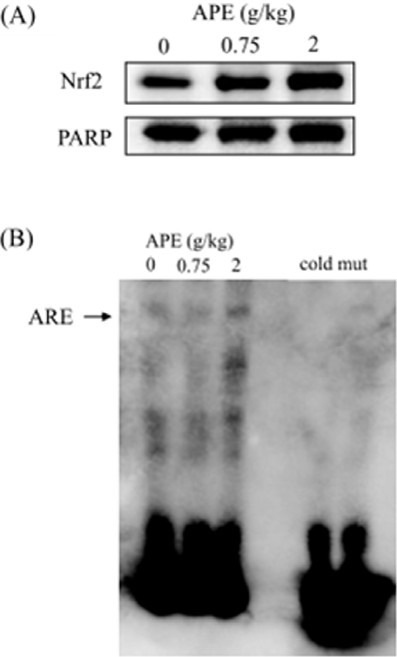
Effect of ethanolic extract of A. paniculata (APE) on Nrf2 nuclear translocation (A) and Nrf2-DNA binding activity (B). After 5 days of APE (0, 0.75, or 2 g/kg/d) treatment, nuclear extracts of rat liver were prepared and used for Western blotting assay and EMSA. To confirm the specificity of the nucleotide, 200-fold cold probe (biotin-unlabeled ARE binding site) and biotin-unlabeled double-stranded mutant ARE oligonucleotide (2 ng) were included in the EMSA. PARP, poly (ADP-ribose) polymerase.
Hepatoprotection of APE on CCl4-induced liver damage
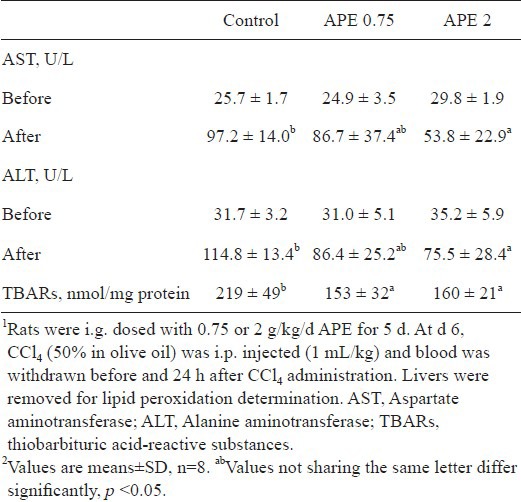
Because APE up-regulated antioxidant enzyme expression and activity and increased reduced GSH level in rat liver, we determined whether APE decreases CCl4-induced hepatotoxicity. As shown in Table 3, plasma AST and ALT activities were significantly increased after intraperitoneal injection of CCl4 (p <0.05). With APE pretreatment, plasma AST and ALT activities were dose-dependently decreased. Compared to the controls, rats receiving 2 g/kg APE for 5 consecutive days had significantly less AST and ALT activities (p <0.05). In addition, CCl4 induced TBARs production in rat liver was attenuated by APE (p <0.05).
Table 3.
Protection of ethanolic extract of A. paniculata (APE) against CCl4-induced liver damage1,2.
Discussion
Oxidative damage is known to be one of the major determining factors in the pathogenesis of many chronic diseases including atherosclerosis, cancer, and neurodegenerative diseases (Cooke et al., 2003; Halliwell and Gutteridge, 2007). The chemoprevention of many phytochemicals on these chronic diseases is partly attributed to their antioxidation potency. Akowuah and colleagues (2009) reported that rats received 1 g/kg methanolic extract of A. paniculata for 14 consecutive days increases SOD and catalase activity and decreases plasma ALT and AST activity induced by CCl4. Andrographolide in a dose of 100 mg/kg for 8 days was shown to attenuate CCl4-induced mouse liver damage and this effect is related to its induction of hepatic GSH content and HO-1 expression (Ye et al., 2011). In this study, we further demonstrated that, in addition to HO-1, A. paniculata is effective on increasing antioxidant defense by up-regulating Nrf2-dependent GCLC, GCLM, SOD1, and GST isozymes gene expression and increases GSH content in rat liver, and these are associated with attenuation of CCl4-induced liver damage.
Under oxidative stress, cellular macromolecules such as DNA, protein, and membrane unsaturated fatty acids are susceptible to oxidation, which leads to cell damage. To prevent the impact of oxidative stress, antioxidant defense is evolved in all aerobic organisms. Cellular antioxidant defense system is composed of many antioxidant enzymes and radical scavengers that convert reactive oxygen species to harmless products. Superoxide anion production is accompanied with the respiratory chain in mitochondria and also the phase I drug metabolism in microsomes (Halliwell and Gutteridge, 2007). To diminish its potent reactivity, superoxide anion is dismutated to H2O2 by SOD. Then, H2O2 is reduced to water by catalase and by GSH peroxidase that consumes GSH. To restore cellular GSH content, GSH regeneration and de novo synthesis occur simultaneously. GSH reductase is responsible for regeneration of GSH from GSSG. GCL catalyzes the rate limiting step in GSH synthesis (Franklin et al., 2009). In this study, dose-dependent increases of hepatic GSH peroxidase and SOD activity and GSH levels were noted in rats dosed with APE for 5 days (Table 2). These results indicate that APE enhances antioxidation capacity in rat liver and, thus, lowers plasma aminotransferase activity caused by CCl4 (Table 3).
In mammalian cells, GSH concentrations range 0.5-10 mM (Kosower and Kosower, 1978). GSH acts as a radical scavenging antioxidant and also is the preferred substrate for several enzymes in drug metabolism and antioxidant defense (Lu, 2009). Alterations in GSH content are associated to many pathological conditions including diabetes, cancer, AIDS, neurodegenerative, and liver diseases (Franco et al., 2007). This explains high exogenous GSH attenuated LPS-induced systemic inflammatory response and reduced mortality in rats (Sun et al., 2006). The GSH biosynthesis is completed in two steps that are catalyzed by GCL and GSH synthetase. GCL is a heterodimeric holoenzyme composed of GCLC and GCLM subunits and is the rate-limiting enzyme in GSH biosynthesis (Gipps et al., 1992, 1995). Up-regulation of GCLC expression and the resultant increase of GSH level are responsible for the protection of chalcon flavonoids butein and phloretin against CCl4-induced rat liver damage (Yang et al., 2011). In this study, APE induced both GCLC and GCLM protein and mRNA expression in rat liver (Figure 1A and B). Accompanied by the increase of GCLC and GCLM expression, more GSH production was noted in rats treated with APE (Table 2).
GST is classified as a phase II detoxification enzyme which catalyzes the conjugation of GSH with a variety of electrophilic xenobiotics and facilitates their excretion rate. Because of its reduction of organic hydroperoxides to alcohols, GST also acts as an antioxidant enzyme and is named as non-selenium-dependent GSH peroxidase. HO-1 is a highly inducible enzyme that catalyzes the first and rate-limiting step of free heme degradation into biliverdin, carbon monoxide, and free ferrous iron (Ryter et al., 2000). Accumulating evidence indicates that increase of HO-1 expression is involved in resistance to H2O2-, tumor necrosis factor ,-, and ischemia/reperfusion-mediated cytotoxicity in vascular smooth muscle cells and endothelial cells (Chao et al., 2011; Clark et al., 2000; Jansen et al., 2010; Yet et al., 2001). Resveratrol induction of HO-1 expression attenuates D-galactosamine/lipopolysaccharide-and tert-butyl hydroperoxide-induced toxicity in rat liver and in isolated hepatocytes (Cerný et al., 2009; Farghali et al., 2009). Similar to GCLC and GCLM induction, APE induced HO-1 and GST Ya and Yb mRNA and protein expression (Fig. 1A and B) and activity of GST (Table 2) in rat liver. This implies that APE protection against CCl4 insult can also be associated with its induction of HO-1 and GST expression and increased activity of GST.
Diterpene lactones are recognized to be the most bioactive and abundant components of A. paniculata (Chao and Lin, 2010). Among those, andrographolide, 14-deoxy-11,12-didehydroandrographolide, and neoandrographolide make up about 1.75, 1.74, and 0.61% (w/w) of dried leaves and 0.84, 0.26, and 0.37% of dried stem, respectively (Pholphana et al., 2004). By HPLC, Burgos and colleagues reported that andrographolide accounts for 6.1% dry weight of whole plant (Burgos et al., 1997). In this study, diterpenoid content of APE was determined by HPLC/MS and results showed that andrographolide makes up about 5.32% (w/w) of APE (data not shown). This means 0.75 and 2 g APE with andrographolide equivalent of 40 and 106 mg, respectively. Recently, andrographolide in a dose of 100 mg/kg for 8 days has been shown to attenuate mouse liver injury induced by CCl4 (Ye et al., 2011). This indicates that hepatoprotection observed in this study can be partly explained by the andrographolide in APE.
The Nrf2 is a member of basic leucine zipper (bZip) transcription factor family with a Cap ‘n’ Collar structure. In response to electrophilic compounds and antioxidants, Nrf2 is activated and, then, dissociates from Keap1, translocates into the nuclei, and thereby up-regulates Nrf2-driven gene transcription. It is well known that Nrf2 plays an important role in modulating antioxidant and phase II detoxification enzyme gene transcription (Surh et al., 2008). For instance, ERK-Nrf2 signaling pathway is responsible for andrographolide, flavonoids butein and phloretin, and organosulfur compounds sulforaphane, allyl isothiocynate, and diallyl trisulfide up-regulation of GCLC, HO-1, NAD(P)H: quinone oxidoreductase, and GST mRNA and protein expression in liver and stomach and in vascular endothelial cells (Lii et al., 2010; McWalter et al., 2004; Yang et al., 2011; Yu et al., 2010). We found that nuclear Nrf2 contents and DNA binding activity were increased in rat administered with APE (Figure 2), suggesting that APE up-regulation of GCLC, GCLM, HO-1, SOD1, and GST Ya and Yb mRNA and protein expression is associated with Nrf2 activation.
In conclusion, we have shown that A. paniculata up-regulates the expression and activity of antioxidant enzymes, including GCLC, GCLM, HO-1, SOD1, and GST Ya and Yb, in rat liver through the Nrf2 activation. The antioxidant property of A. paniculata confers it with protective activity against CCl4-induced liver damage.
Acknowledgement
This work was supported by CMU98-CT-21.
References
- 1.Abraham N.G., Kappas A. Heme oxygenase and the cardiovascular-renal system. Free Radic Biol Med. 2005;39:1–25. doi: 10.1016/j.freeradbiomed.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Aebi H. Catalase in vitro Methods. Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 3.Akowuah G.A., Zhari I., Mariam A., Yam M.F. Absorption of andrographolides from Andrographis paniculata and its effect on CCl(4)-induced oxidative stress in rats. Food Chem Toxicol. 2009;47:2321–2326. doi: 10.1016/j.fct.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 4.Bellomo G., Mirabelli F., DiMonte D., Richelmi P., Thor H., Orrenius C., Orrenius S. Formation and reduction of glutathione-protein mixed disulfides during oxidative stress. Biochem Pharmacol. 1987;36:1313–1320. doi: 10.1016/0006-2952(87)90087-6. [DOI] [PubMed] [Google Scholar]
- 5.Burgos R.A., Caballero E.E., Sánchez N.S., Schroeder R.A., Wikman G.K., Hancke J.L. Testicular toxicity assessment of Andrographis paniculata dried extract in rats. J Ethnopharmacol. 1997;58:219–224. doi: 10.1016/s0378-8741(97)00099-8. [DOI] [PubMed] [Google Scholar]
- 6.Cerný D., Canová N.K., Martínek J., Horínek A., Kmonícková E., Zídek Z., Farghali H. Effects of resveratrol pretreatment on tert-butylhydroperoxide induced hepatocyte toxicity in immobilized perifused hepatocytes: involvement of inducible nitric oxide synthase and hemoxygenase-1. Nitric Oxide. 2009;20:1–8. doi: 10.1016/j.niox.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Chao C.Y., Lii C.K., Tsai I.T., Li C.C., Liu K.L., Tsai C.W., Chen H.W. Andrographolide inhibits ICAM-1 expression and NF-κB activation in TNF-α-treated EA.hy926. Cells J Agric Food Chem. 2011;59:5263–5271. doi: 10.1021/jf104003y. [DOI] [PubMed] [Google Scholar]
- 8.Chao W.W., Lin B.F. Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian) Chin Med. 2010;5:17. doi: 10.1186/1749-8546-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H.W., Lin A.H., Chu H.C., Li C.C., Tsai C.W., Chao C.Y., Wang C.J., Lii C.K., Liu K.L. Inhibition of TNF-α-induced inflammation by andrographolide via down-regulation of the PI3K/Akt signaling pathway. J Nat Prod. 2011;74:2408–2413. doi: 10.1021/np200631v. [DOI] [PubMed] [Google Scholar]
- 10.Clark J.E., Foresti R., Green C.J., Motterlini R. Dynamics of haem oxygenase-1 expression and bilirubin production in cellular protection against oxidative stress. Biochem J. 2000;348:615–619. [PMC free article] [PubMed] [Google Scholar]
- 11.Cooke M.S., Evans M.D., Dizdaroglu M., Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17:1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 12.Fraga C.G., Leibovitz B.E., Tappel A.L. Lipid peroxidation measured as thiobarbituric acid-reactive substance in tissue slices: characterization and comparison between homogenates and microsomes. Free Radic Biol Med. 1988;4:155–161. doi: 10.1016/0891-5849(88)90023-8. [DOI] [PubMed] [Google Scholar]
- 13.Farghali H., Cerný D., Kameníková L., Martínek J., Horínek A., Kmonícková E., Zídek Z. Resveratrol attenuates lipopolysaccharide-induced hepatitis in D-galactosamine sensitized rats: role of nitric oxide synthase 2 and heme oxygenase-1. Nitric Oxide. 2009;21:216–225. doi: 10.1016/j.niox.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Franco R., Schoneveld O.J., Pappa A., Panayiotidis M.I. The central role of glutathione in the pathophysiology of human diseases. Arch Physiol Biochem. 2007;113:234–258. doi: 10.1080/13813450701661198. [DOI] [PubMed] [Google Scholar]
- 15.Franklin C.C., Backos D.S., Mohar I., White C.C., Forman H.J., Kavanagh T.J. Structure function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol Aspects Med. 2009;30:86–98. doi: 10.1016/j.mam.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gipp J.J, Chang C., Mulcahy R.T. Cloning and nucleotide se quence of a full-length c DNA for human liver gamma - glutamylcysteine synthetase. Biochem Biophys Res Commun. 1992;185:29–35. doi: 10.1016/s0006-291x(05)80950-7. [DOI] [PubMed] [Google Scholar]
- 17.Gipp J.J., Bailey H.H., Mulcahy R.T. Cloning and sequencing of the cDNA for the light subunit of human liver gamma - glutamylcysteine synthetase and relative mRNA levels for heavy and light subunits in human normal tissues. Biochem Biophys Res Commun. 1995;206:584–589. doi: 10.1006/bbrc.1995.1083. [DOI] [PubMed] [Google Scholar]
- 18.Habig W.H., Jakoby W.B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- 19.Halliwell B., Gutteridge JMC. 4th ed. Oxford University Press; 2007. Free Radicals in Biology and Medicine. [Google Scholar]
- 20.Husen R., Pihie A.H., Nallappan M. Screening for antihyperglycaemic activity in several local herbs of Malaysia. J Ethnopharmacol. 2004;95:205–208. doi: 10.1016/j.jep.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I., Yamamoto M., Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 22.Itoh K., Tong K.I., Yamamoto M. Molecular mechanism activating Nrf-2-keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med. 2004;36:1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- 23.Jansen T., Hortmann M., Oelze M., Opitz B., Steven S., Schell R., Knorr M., Karbach S., Schuhmacher S., Wenzel P., Münzel T., Daiber A. Conversion of biliverdin to bilirubin by biliverdin reductase contributes to endothelial cell protection by heme oxygenase-1-evidence for direct and indirect antioxidant actions of bilirubin. J Mol Cell Cardiol. 2010;49:186–195. doi: 10.1016/j.yjmcc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Kosower N.S., Kosower E.M. The glutathione status of cells. Intl Rev Cytol. 1978;54:109–160. doi: 10.1016/s0074-7696(08)60166-7. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence R., Burk R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976;71:952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- 26.Lii C.K., Liu K.L., Cheng Y.P., Lin A.H., Chen H.W., Tsai C.W. Sulforaphane and α-lipoic acid up-regulate the expression of the pi class of glutathione S-transferase through c-Jun and Nrf2 activation. J Nutr. 2010;140:885–892. doi: 10.3945/jn.110.121418. [DOI] [PubMed] [Google Scholar]
- 27.Lu S.C. Regulation of glutathione synthesis. Mol Aspects Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 29.McWalter G.K., Higgins L.G., Mc Lellan L.I., Henderson C.J., Song L., Thornalley P.J., Itoh K., Yamamoto M., Hayes J.D. Transcription factor Nrf2 is essential for induction of NAD(P)H: quinone oxidoreductase 1, glutathione S - transferases, and glutamate cysteine ligase by broccoli seeds and isothiocyanates. J Nutr. 2004;134(12 Suppl):3499S–3506S. doi: 10.1093/jn/134.12.3499S. [DOI] [PubMed] [Google Scholar]
- 30.Owuor E.D., Kong A.N. Antioxidants and oxidants regulated signal transduction pathways. Biochem Pharmacol. 2002;64:765–770. doi: 10.1016/s0006-2952(02)01137-1. [DOI] [PubMed] [Google Scholar]
- 31.Pae H.O., Lee Y.C., Chung H.T. Heme oxygenase-1 and carbon monoxide: emerging therapeutic targets in inflammation and allergy. Recent Pat Inflamm Allergy Drug Discov. 2008;2:159–165. doi: 10.2174/187221308786241929. [DOI] [PubMed] [Google Scholar]
- 32.Pholphana N., Rangkadilok N., Thongnest S., Ruchirawat S., Ruchirawat M., Satayavivad J. Determination and variation of three active diterpenoids in Andrographis paniculata (Burmf) Nees Phytochem Anal. 2004;15:365–371. doi: 10.1002/pca.789. [DOI] [PubMed] [Google Scholar]
- 33.Recknagel R.O., Ghoshal A.K. Quantitative estimation of peroxidative degeneration of rat liver microsomal and mitochondrial lipids after carbon tetrachloride poisoning. Exp Mol Pathol. 1966;5:413–426. doi: 10.1016/0014-4800(66)90023-2. [DOI] [PubMed] [Google Scholar]
- 34.Ryter S.W., Tyrrell R.M. The heme synthesis and degradation pathways: role in oxidant sensitivity Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med. 2000;28:289–309. doi: 10.1016/s0891-5849(99)00223-3. [DOI] [PubMed] [Google Scholar]
- 35.Singh R.P., Banerjee S., Rao A.R. Modulatory influence of Andrographis paniculata on mouse hepatic and extrahepatic carcinogen metabolizing enzymes and antioxidant status. Phytother Res. 2001;15:382–390. doi: 10.1002/ptr.730. [DOI] [PubMed] [Google Scholar]
- 36.Sun S., Zhang H., Xue B., Wu Y., Wang J., Yin Z., Luo L. Protective effect of glutathione against lipopolysaccharide-induced inflammation and mortality in rats. Inflamm Res. 2006;55:504–510. doi: 10.1007/s00011-006-6037-7. [DOI] [PubMed] [Google Scholar]
- 37.Surh Y.J., Kundu J.K., Na H.K. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008;74:1526–1539. doi: 10.1055/s-0028-1088302. [DOI] [PubMed] [Google Scholar]
- 38.Tian J., Lin X., Guan R., Xu J.G. The effects of hydroxyethyl starch on lung capillary permeability in endotoxic rats and possible mechanisms. Anesth Analg. 2004;98:768–774. doi: 10.1213/01.ane.0000099720.25581.86. [DOI] [PubMed] [Google Scholar]
- 39.Vassort G., Turan B. Protective role of antioxidants in diabetes-induced cardiac dysfunction. Cardiovasc Toxicol. 2010;10:73–86. doi: 10.1007/s12012-010-9064-0. [DOI] [PubMed] [Google Scholar]
- 40.Woo A.Y., Waye M.M., Tsui S.K., Yeung S.T., Cheng C.H. Andrographolide up-regulates cellular-reduced glutathione level and protects cardiomyocytes against hypoxia/reoxygenation injury. J Pharmacol Exp Ther. 2008;325:226–235. doi: 10.1124/jpet.107.133918. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y.C., Lii C.K., Lin A.H., Yeh Y.W., Yao H.T., Li C.C., Liu K.L., Chen H.W. Induction of glutathione synthesis and heme oxygenase 1 by the flavonoids butein and phloretin is mediated through the ERK/Nrf2 pathway and protects against oxidative stress. Free Radic Biol Med. 2011;51:2073–2081. doi: 10.1016/j.freeradbiomed.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 42.Yao H.T., Lin J.H., Chiang M.T., Chiang W., Luo M.N., Lii C.K. Suppressive effect of the ethanolic extract of adlay bran on cytochrome P-450 enzymes in rat liver and lungs. J Agric Food Chem. 2011;59:4306–4314. doi: 10.1021/jf200117m. [DOI] [PubMed] [Google Scholar]
- 43.Ye J.F., Zhu H., Zhou Z.F., Xiong R.B., Wang X.W., Su L.X., Luo B.D. Protective mechanism of andrographolide against carbon tetrachloride-induced acute liver injury in mice. Biol Pharm Bull. 2011;34:1666–1670. doi: 10.1248/bpb.34.1666. [DOI] [PubMed] [Google Scholar]
- 44.Yet S.F., Tian R., Layne M.D., Wang Z.Y., Maemura K., Solovyeva M., Ith B., Melo L.G., Zhang L., Ingwall J.S., Dzau V.J., Lee M.E., Perrella M.A. Cardiac-specific expression of heme oxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ Res. 2001;89:168–173. doi: 10.1161/hh1401.093314. [DOI] [PubMed] [Google Scholar]
- 45.Yu B.C., Hung C.R., Chen W.C., Cheng J.T. Antihyperglycemic effect of andrographolide in streptozotocin-induced diabetic rats. Planta Med. 2003;69:1075–1079. doi: 10.1055/s-2003-45185. [DOI] [PubMed] [Google Scholar]
- 46.Yu A.L., Lu C.Y., Wang T.S., Tsai C.W., Liu K.L., Cheng Y.P., Chang H.C., Lii C.K., Chen H.W. Induction of heme oxygenase 1 and inhibition of TNF-α-induced ICAM-1 expression by andrographolide in EA.hy926 cells. J Agric Food Chem. 2010;58:7641–7648. doi: 10.1021/jf101353c. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X.F., Tan B.K. Antihyperglycaemic and anti-oxidant properties of Andrographis paniculata in normal and diabetic rats. Clin Exp Pharmacol Physiol. 2000;27:358–363. doi: 10.1046/j.1440-1681.2000.03253.x. [DOI] [PubMed] [Google Scholar]


