Abstract
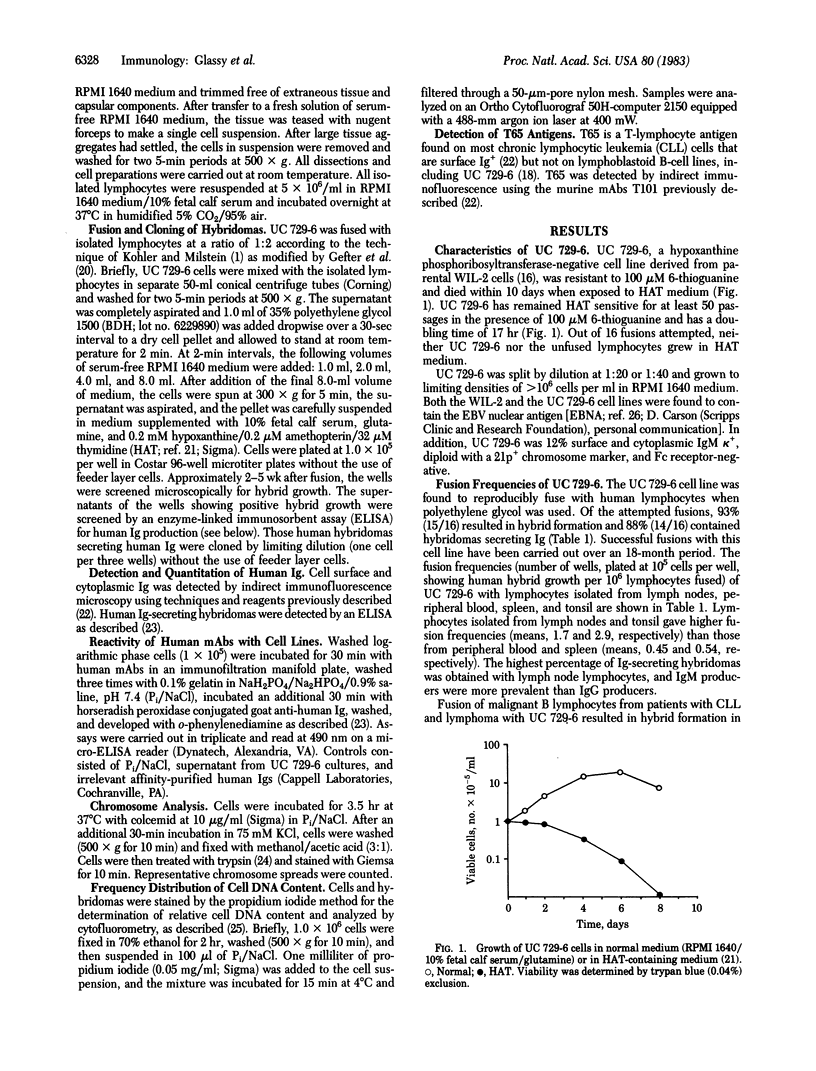
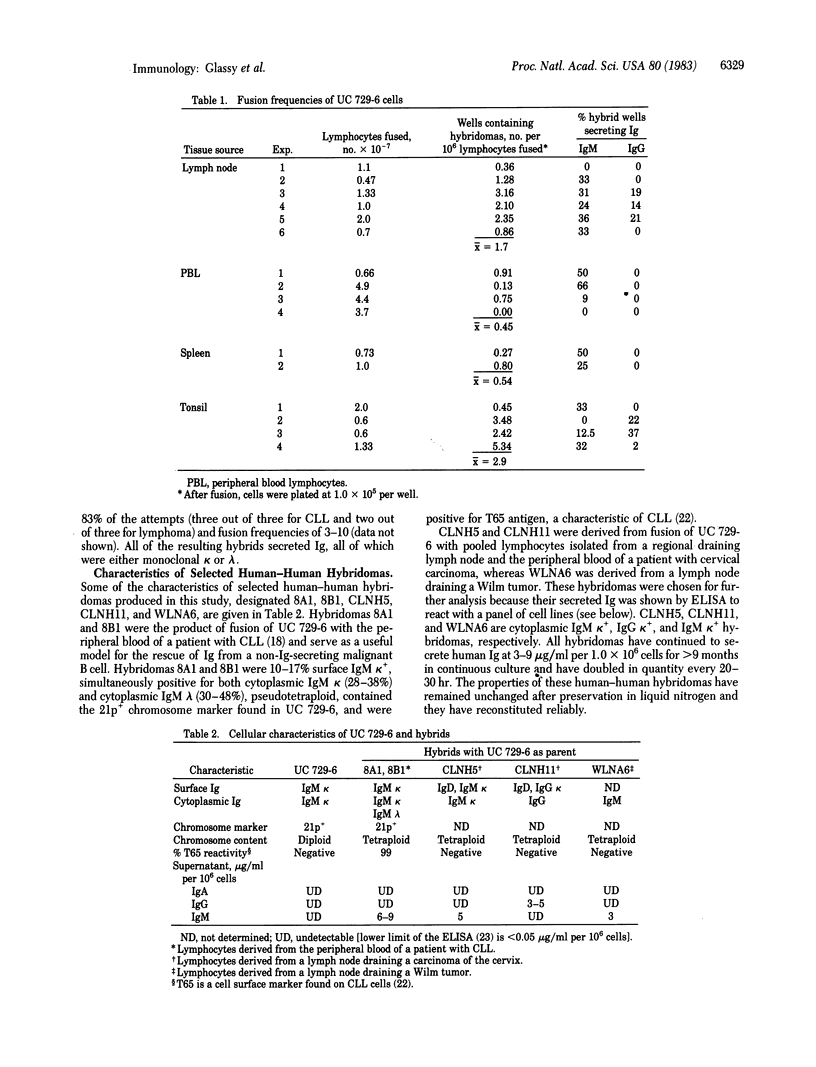
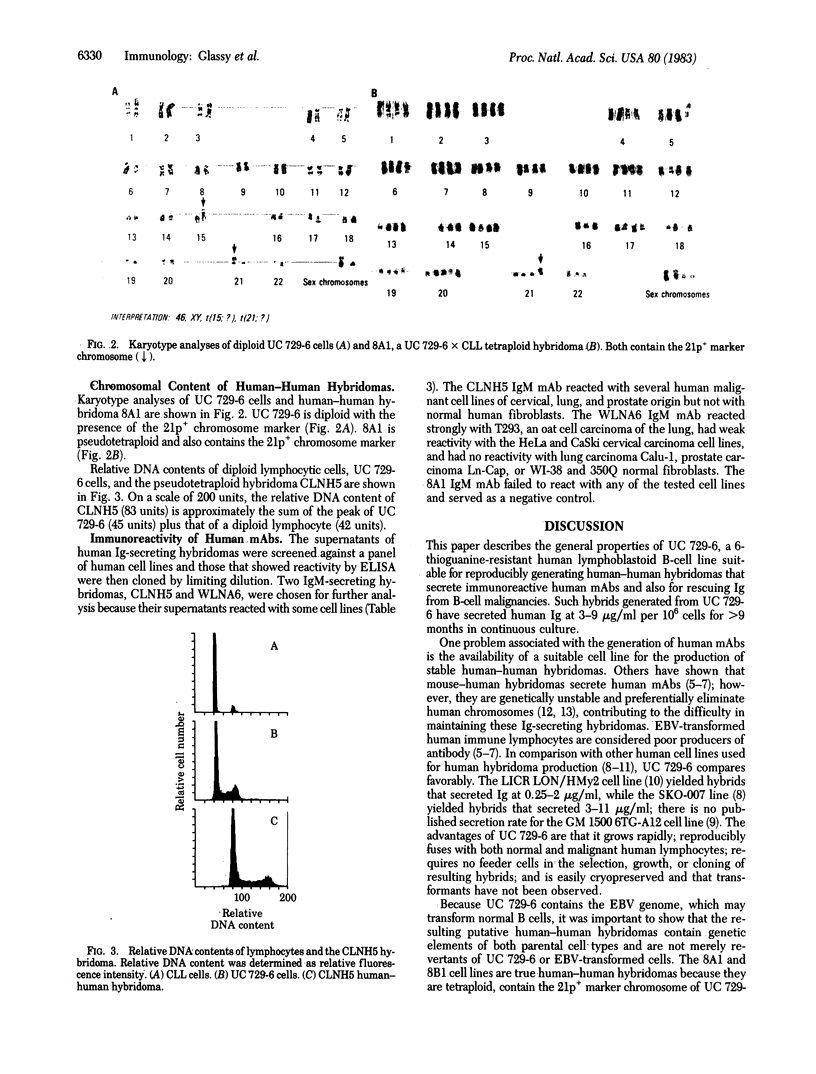
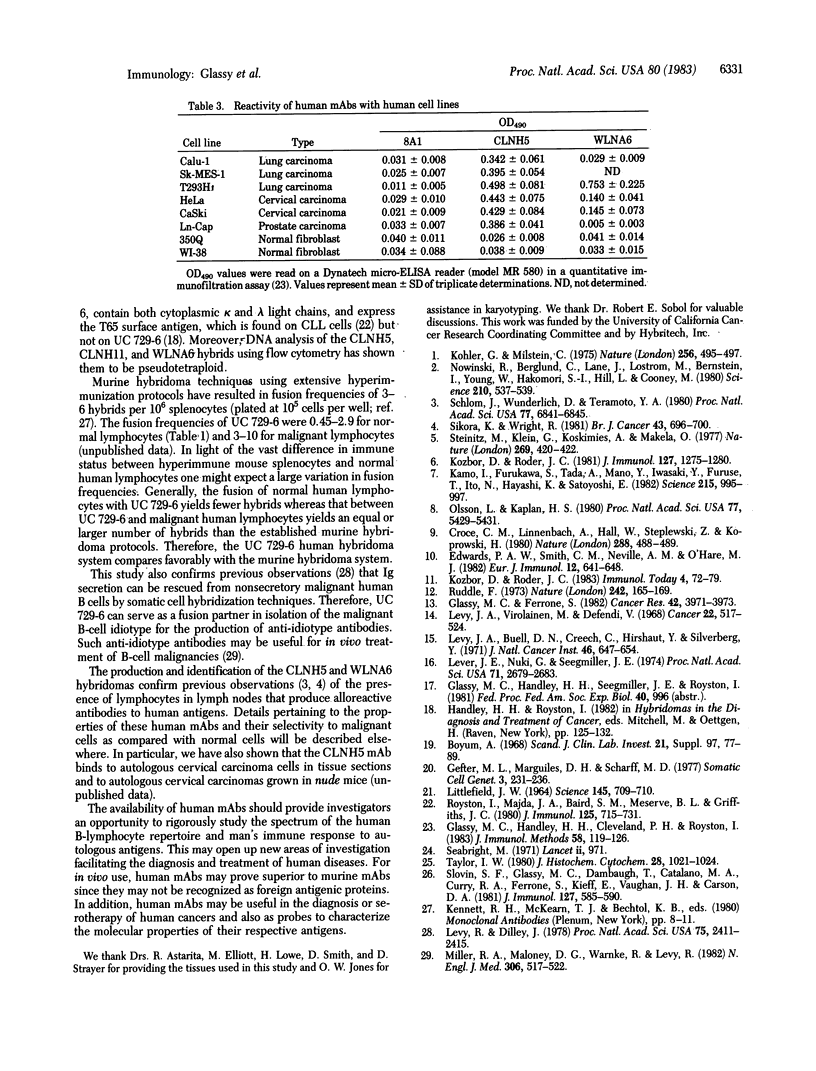
UC 729-6, a 6-thioguanine-resistant human lymphoblastoid B-cell line, was fused with normal and malignant human lymphocytes. Parent UC 729-6 cells were diploid with a 21p+ marker chromosome, expressed surface and cytoplasmic IgM kappa, and doubled every 17 hr. The resulting human-human hybridomas were pseudotetraploid containing the 21p+ marker, doubled every 20-30 hr, and secreted 3-9 micrograms of human Ig per ml per 10(6) hybrid cells for greater than 9 months in continuous culture. Human Ig-secreting hybridomas were generated in 88% (14/16) of the fusions carried out and were cloned by limiting dilution (one cell per three wells) without the use of feeder layers. The mean fusion frequencies (number of wells, plated at 10(5) cells per well, showing hybrid growth per 10(6) lymphocytes fused) of UC 729-6 with normal lymphocytes ranged from 0.45 to 2.9 and with malignant B lymphocytes, from 3 to 10. Analysis of the human-human hybridomas derived from lymphocytes isolated from regional draining lymph nodes of cancer patients revealed several that secreted human monoclonal antibody that reacted by an enzyme immunoassay with some carcinoma cell lines but not with normal fibroblast cell lines. These data suggest that (i) UC 729-6 can be fused with human lymphocytes to generate stable human-human hybridomas, some of which secrete antibody reactive to human cell surface antigens, and (ii) UC 729-6 can be used to rescue Ig from nonsecretory malignant B cells and thereby allow for the production of anti-idiotype antibodies.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Croce C. M., Linnenbach A., Hall W., Steplewski Z., Koprowski H. Production of human hybridomas secreting antibodies to measles virus. Nature. 1980 Dec 4;288(5790):488–489. doi: 10.1038/288488a0. [DOI] [PubMed] [Google Scholar]
- Edwards P. A., Smith C. M., Neville A. M., O'Hare M. J. A human-hybridoma system based on a fast-growing mutant of the ARH-77 plasma cell leukemia-derived line. Eur J Immunol. 1982 Aug;12(8):641–648. doi: 10.1002/eji.1830120804. [DOI] [PubMed] [Google Scholar]
- Gefter M. L., Margulies D. H., Scharff M. D. A simple method for polyethylene glycol-promoted hybridization of mouse myeloma cells. Somatic Cell Genet. 1977 Mar;3(2):231–236. doi: 10.1007/BF01551818. [DOI] [PubMed] [Google Scholar]
- Glassy M. C., Ferrone S. Differential segregation patterns of human chromosomes in somatic cell hybrids constructed with human B-lymphocytes and human melanoma cells. Cancer Res. 1982 Oct;42(10):3971–3973. [PubMed] [Google Scholar]
- Glassy M. C., Handley H. H., Cleveland P. H., Royston I. An enzyme immunofiltration assay useful for detecting human monoclonal antibody. J Immunol Methods. 1983 Mar 11;58(1-2):119–126. doi: 10.1016/0022-1759(83)90268-5. [DOI] [PubMed] [Google Scholar]
- Kamo I., Furukawa S., Tada A., Mano Y., Iwasaki Y., Furuse T., Ito N., Hayashi K., Satoyoshi E. Monoclonal antibody to acetylcholine receptor: cell line established from thymus of patient with Myasthenia gravis. Science. 1982 Feb 19;215(4535):995–997. doi: 10.1126/science.6297000. [DOI] [PubMed] [Google Scholar]
- Kozbor D., Roder J. C. Requirements for the establishment of high-titered human monoclonal antibodies against tetanus toxoid using the Epstein-Barr virus technique. J Immunol. 1981 Oct;127(4):1275–1280. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Lever J. E., Nuki G., Seegmiller J. E. Expression of purine overproduction in a series of 8-azaguanine-resistant diploid human lymphoblast lines. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2679–2683. doi: 10.1073/pnas.71.7.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A., Buell D. N., Creech C., Hirshaut Y., Silverberg H. Further characterization of the WI-L1 and WI-L2 lymphoblastoid lines. J Natl Cancer Inst. 1971 Mar;46(3):647–654. [PubMed] [Google Scholar]
- Levy J. A., Virolainen M., Defendi V. Human lymphoblastoid lines from lymph node and spleen. Cancer. 1968 Sep;22(3):517–524. doi: 10.1002/1097-0142(196809)22:3<517::aid-cncr2820220305>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Levy R., Dilley J. Rescue of immunoglobulin secretion from human neoplastic lymphoid cells by somatic cell hybridization. Proc Natl Acad Sci U S A. 1978 May;75(5):2411–2415. doi: 10.1073/pnas.75.5.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. A., Maloney D. G., Warnke R., Levy R. Treatment of B-cell lymphoma with monoclonal anti-idiotype antibody. N Engl J Med. 1982 Mar 4;306(9):517–522. doi: 10.1056/NEJM198203043060906. [DOI] [PubMed] [Google Scholar]
- Nowinski R., Berglund C., Lane J., Lostrom M., Bernstein I., Young W., Hakomori S. I., Hill L., Cooney M. Human monoclonal antibody against Forssman antigen. Science. 1980 Oct 31;210(4469):537–539. doi: 10.1126/science.7423202. [DOI] [PubMed] [Google Scholar]
- Olsson L., Kaplan H. S. Human-human hybridomas producing monoclonal antibodies of predefined antigenic specificity. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5429–5431. doi: 10.1073/pnas.77.9.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston I., Majda J. A., Baird S. M., Meserve B. L., Griffiths J. C. Human T cell antigens defined by monoclonal antibodies: the 65,000-dalton antigen of T cells (T65) is also found on chronic lymphocytic leukemia cells bearing surface immunoglobulin. J Immunol. 1980 Aug;125(2):725–731. [PubMed] [Google Scholar]
- Ruddle F. H. Linkage analysis in man by somatic cell genetics. Nature. 1973 Mar 16;242(5394):165–169. doi: 10.1038/242165a0. [DOI] [PubMed] [Google Scholar]
- Schlom J., Wunderlich D., Teramoto Y. A. Generation of human monoclonal antibodies reactive with human mammary carcinoma cells. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6841–6845. doi: 10.1073/pnas.77.11.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971 Oct 30;2(7731):971–972. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- Sikora K., Wright R. Human monoclonal antibodies to lung-cancer antigens. Br J Cancer. 1981 May;43(5):696–700. doi: 10.1038/bjc.1981.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slovin S. F., Glassy M. C., Dambaugh T., Catalano M. A., Curry R. A., Ferrone S., Kieff E., Vaughan J. H., Carson D. A. Discordant expression of 2 Epstein-Barr virus-associated antigens, EBNA and RANA, in man-rodent somatic cell hybrids. J Immunol. 1981 Aug;127(2):585–590. [PubMed] [Google Scholar]
- Steinitz M., Klein G., Koskimies S., Makel O. EB virus-induced B lymphocyte cell lines producing specific antibody. Nature. 1977 Sep 29;269(5627):420–422. doi: 10.1038/269420a0. [DOI] [PubMed] [Google Scholar]
- Taylor I. W. A rapid single step staining technique for DNA analysis by flow microfluorimetry. J Histochem Cytochem. 1980 Sep;28(9):1021–1024. doi: 10.1177/28.9.6157714. [DOI] [PubMed] [Google Scholar]




