Abstract
Adlay (薏苡 yì yĭ “soft-shelled job's tears”, the seeds of Coix lachryma-jobi L. var. ma-yuen Stapf) is a grass crop that has long been used in traditional Chinese medicine (TCM) and as a nourishing food in China for the treatment of warts, chapped skin, rheumatism, neuralgia, inflammatory, and neoplastic diseases. In addition, adlay also has been said to have stomachic, diuretic, antipholgistic, anodynic, and antispasmodic effects. Carcinogenesis is a multistage process that begins with exposure of viruses or chemicals that are found in the environment. Chemoprevention refers to the use of natural or synthetic, non-toxic chemical substances to reverse, repress, or prevent carcinogenesis. In this review, we summarize recent research attempting to study the chemopreventive blocking and suppressing potential of adlay and its active components in scavenging electrophiles and reactive oxygen species, antimutagenicity, enhancing Nrf2-mediated detoxification and antioxidant effect, altering carcinogen metabolism, suppressing proliferation, decreasing inflammation, and enhancing antitumor immunity. In addition, several active components with diverse chemopreventive properties have been also mentioned in this review article.
Keywords: Adlay, Traditional Chinese medicine, Cancer chemoprevention, Blocking agent, Suppressing agent
Adlay (Coix lachryma-jobi L. var. ma-yuen Stapf.)
Adlay (薏苡 yì yǐ; the seeds of Coix lachryma-jobi L. var. ma-yuen Stapf.), also named coix seeds, Chinese pearl barley, pearl barley, semen coicis, yokuinin, 薏 苡仁 (yì yǐ rén), and 薏米 (yì mǐ), belongs to the family Gramineae. It is an annual or perennial herb, 100 ˜ 180 cm high, flowering from July to September and fruiting from September to October. The adlay seed consists of four parts from outside to inside including the hull, testa, bran, and endosperm (polished adlay) (Figure 1). Adlay is widely planted in Taiwan, China, and Japan, where it is considered a healthy food supplement.
Figure 1.
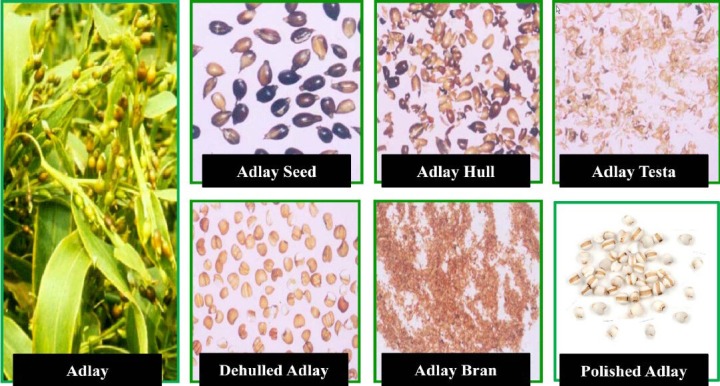
The photograph of adlay plant, adlay seed and its fractions, including adlay hull, adlay testa, dehulled adlay, adlay bran, and polished adlay.
According to the first pharmaceutical monograph in ancient China, The Divine Husbandman's Herbal Foundation Canon (神農本草經 shén nóng běn cǎo jīng), adlay is considered as a top grade (上品 shàng pǐn) of Traditional Chinese Medicine (TCM). People received adlay for a long period can nourish life (養命 yǎng mìng), boost qi (益氣 yì qì), rejuvenate the body (輕身 qīng shēn), and extend life (增年 zēng nái). Since adlay can make body healthy and prolong life with non-toxic properties, it has been thought to be the longevity of monarch herb (不老長生之君藥), and generally form the basis of dietary functional foods. Adlay traditionally has long been used in China for the treatment of warts, chapped skin, rheumatism, neuralgia, and inflammatory diseases. In addition, adlay also has been said to have stomachic, diuretic, antipholgistic, anodynic, antispasmodic, and antitumor effects.
Although medicinal functionality of adlay has been around for thousands of years, recently, it is interesting to note that integration exists between traditional medicine and modern medicine because a number of biological activities of adlay have been elucidated by scientific investigation, including antioxidant/free radical scavenging (Kuo et al., 2001; Kuo et al., 2002; Yao et al., 2011; Yu et al., 2011; Chen et al., 2012a; Wang et al., 2012), anti-inflammatory (Otsuka et al., 1988; Huang et al., 2009a; Huang et al., 2009b), anti-mutagenic (Huang and Chiang, 1999; Chen et al., 2011), anti-tumor (Tanimura, 1961; Numata et al., 1994; Kuo et al., 2001; Chang et al., 2003; Hung and Chang, 2003; Shih et al., 2004; Lee et al., 2008; Chung et al., 2010; Chung et al., 2011b; Li et al., 2011), anti-allergic (Hsu et al., 2003; Chen et al., 2010; Chen et al., 2012b; Chen et al., 2012c), hypolipidemic (Kim et al., 2004; Yu et al., 2004; Huang et al., 2005; Yeh et al., 2006; Son et al., 2008; Yu et al., 2011), hypocholesterolemic (Wang et al., 2012), hypoglycemic (Takahashi et al., 1986; Huang et al., 2005; Yeh et al., 2006; Lin et al., 2010), anti-obesity (Kim et al., 2004; Huang et al., 2005; Kim et al., 2007), anti-ulcer (Chung et al., 2011a), prebiotic activity (Chiang et al., 2000), abortifacient (Tzeng et al., 2005), hormonal modulating (Kondo et al., 1988; Chang et al., 2006; Hsia et al., 2006; Hsia et al., 2007; Hsia et al., 2008; Hsia et al., 2009), osteoporosis preventing (Yang et al., 2008), and antimicrobial (Ishiguro et al., 1993) effect. Since cancer mortality and incidence worldwide has increased rapidly in recent decades, thus, this review article focused on chemopreventive role of adlay in blocking carcinogenesis progress, preventing cancer formation and progression.
Multidrug Carcinogenesis
Carcinogenesis is a multistage process that begins with exposure of viruses or chemicals that are found in the environment. This process involves various molecular and cellular events that transform a normal cell to a malignant neoplastic cell. It can be conceptually identified as tumor initiation, tumor promotion, and tumor progression (Figure 2) (Panayiotidis,2008). Tumor initiation is a step where the normal cell undergoes irreversible genetic and epigenetic alterations to generate an initiated cell (Lengauer et al., 1998). Oxidative stress plays a major role in the initiation of carcinogenesis (Khansari et al., 2009). Oxidative stress can be induced through a number of endogenous sources, such as the production of free radical by mitochondrial oxidative phosphorylation, and exogenous sources, including ionizing radiations, ultraviolet exposure and environmental carcinogens. In fact, chemical carcinogens cannot damage DNA until they are metabolized by cytochrome P-450 in cells and converted to reactive eletrophiles. Reactive oxygen species (ROS) can reacts with DNA as well as chromatin proteins, resulting in several types of DNA damage, including depurination, depyrimidination, single-and double-strand breaks, base modifications, and DNA-protein cross linkages (Barzilai and Yamamoto, 2004; Fruehauf and Meyskens, 2007). In addition, carcinogen-DNA adduct formation is an important molecular mechanism that contributes to chemical carcinogenesis (Windmill et al., 1997). ROS-mediated DNA damage contributes to the initiation of carcinogenesis through increasing a cell's mutation rate, influencing the activation of protooncogene and tumor-suppressor gene, and promoting the oncogenic phenotype (Campos et al., 2007). Tumor promotion involves the clonal expansion of initiated cells to form an actively premalignant, proliferating lesion. Direct DNA interaction and damage is not involved in this process. Tumor promoters generally alter gene expression resulting in decrease of apoptosis cell death and increase of cell proliferation (Klaunig and Kamendulis, 2004). Tumor progression involved in the expression of the malignant, aggressive, invasive, and metastatic phenotype. During this process, further genetic and epigenetic alterations may occur resulting in the formation of neoplasms.
Figure 2.
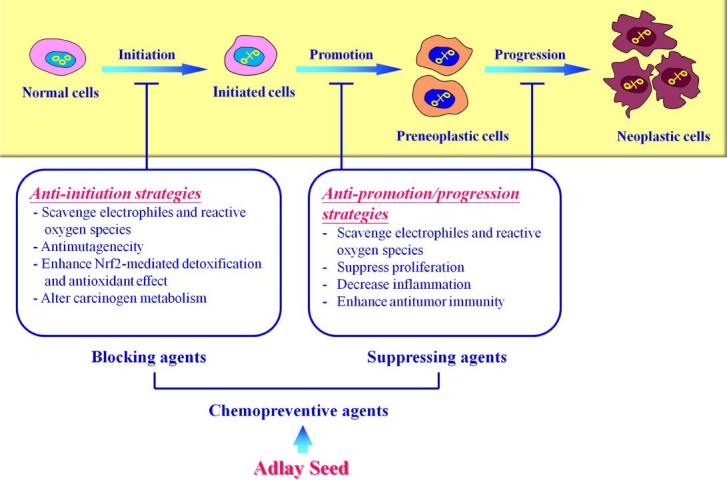
Multistage carcinogenesis and strategies for cancer chemoprevention.
Cancer Chemoprevention
The prominent and effective strategy to inhibit cancer formation is elimination, reverse or delay of genetic and epigenetic alterations induced by chemical carcinogens (Hail et al., 2008). Chemoprevention refers to the use of natural or synthetic, non-toxic chemical substances to reverse, repress, or prevent carcinogenesis. These chemopreventive agents can be broadly categorized into two types: blocking agents and suppressing agents (Figure 2) (Wattenberg, 1985; Manson et al., 2000). Blocking agents generally inhibit the initiation of carcinogenesis. Blocking agents alter carcinogen metabolism and enhance carcinogen detoxification, resulting in elimination of carcinogens reaching their target site to induce DNA damage (Surh, 2003). In addition, blocking agents effectively scavenge eletrophiles and ROS to prevent DNA alterations. Furthermore, blocking agents induce DNA repair machinery to reduce DNA damage as well as genetic mutations. Suppressing agents inhibit pre-malignant or malignant conversion of initiated cells during the stages of tumor promotion and progression. They efficiently modulate gene expression, including oncogenes and tumor-suppressor genes, resulting in suppressing cell proliferation, encouraging apoptosis, and inhibiting angiogenesis (Hayes and McMahon, 2001).
Adlay Acts as a Blocking Agent for Cancer Chemoprevention
Scavenge Electrophiles and Reactive Oxygen Species
Reactive oxygen species (ROS) are induced through a variety of endogenous and exogenous sources. Overwhelming of antioxidant and DNA repair mechanisms in a cell by ROS may result in oxidative stress and oxidative damage to a cell. Oxidative damage resulting from ROS generation can participate in all stages of the carcinogenesis process (Klaunig et al., 2011). Therefore, elimination of ROS or electrophiles by chemopreventive blocking agents is an important strategy to prevent the malignant development. Kuo et al. have demonstrated that the methanol extract of adlay hull (AHM) is a highly effective ROS scavenger that inhibits free radical-generating enzymes, blocks tumor promoter-generated oxidative processes in neutrophile-like leukocytes, and exhibits a cytoprotective effect on cultured cells exposed to tert-butyl hydroperoxide (Kuo et al., 2001). Furthermore, active component that represent strong antioxidant activity were isolated from the methanol extract of adlay hull to be coniferyl alcohol, syringic acid, ferulic acid, syringaresinol, 4-ketopinoresinol, and mayuenolide (Kuo et al., 2002).
Antimutagenicity
It is well-documented that cancer begins after a mutational episode in a single cell and then it progressively transforms to malignancy in multiple stages through sequential acquisition of additional mutations (Khan and Pelengaris, 2006). The Ames Salmonella typhimurium mutagenicity assay (Ames test) is a short-term bacterial reverse mutation assay specifically designed to detect a wide range of chemical substances that can produce genetic damage leading to gene mutations (Mortelmans and Zeiger, 2000). Several lines of evidence showed that the compound prevents mutagenesis caused by known carcinogens in the Ames test indicates a possible role in chemoprevention (Weisburger, 2001). Notably, Huang and Chiang previously investigated the antimutagenic activity of different portions of adlay, including adlay hull, adlay testa, adlay bran, dehulled adlay, and polished adlay. Among them, the acetone extract of adlay hull and adlay bran possesses most potent antimutagenic activity against Benzo(a)pyrene (B[a]P), 2-amino-3-methylimidazo[4,5-f]quinoline (IQ), and 4-nitroquinoline-N-oxide (NQNO) induced mutagenesis in Salmonella typhimurium TA98 (Huang and Chiang, 1999). Recently, Chen et al. further identified the antimutagenic constituents from adlay hull by used Ames antimutagenic activity-guide isolation procedures, and found that p-hydroxybenzaldehyde, vanillin, syringaldehyde, trans-coniferylaldehyde, sinapaldehyde, and coixol exert great antimutagenic activity in suppressing IQ-induced mutagenecity in Salmonella typhimurium TA98 (Chen et al., 2011). In addition to antimutagenic activity, trans-coniferylaldehyde and sinapaldehyde also exhibit potent effect in scavenging of 2,2’-diphenyl-1-picrylhydrazyl (DPPH) radicals and inhibiting 12-O-tetradecanoylphorbol-13-acetate (TPA) stimulated superoxide anion generation in neutrophil-like leukocytes (Chen et al., 2011).
Enhance Nrf2-mediated Detoxification and Antioxidant Effect
Recently, a potential chemopreventive strategy involving the induction of a battery of cytoprotective genes has represented a recent focus of attention in cancer research. NF-E2-related factor 2 (Nrf2), a member of the Cap ‘n’ Collar (CNC) family of transcription factors that share a highly conserved basic region-leucine zipper (bZIP) structure mainly regulates transcriptional activation through antioxidant responsive element (ARE) (Huang et al., 2002). Nrf2-mediated cytoprotective genes are thought to protect cells against carcinogensis and attenuate cancer development via neutralization of ROS or carcinogens (Klaunig et al., 2010; Kwak and Kensler, 2010). The gene families regulated by the Nrf2/ARE pathway include phase II metabolizing/detoxifying enzymes [e.g. aldo keto reductase (AKR), glutathione S-transferases (GST), UDP-glucuronoyltransferase (UGT), NADP(H) quinone oxidoreductase 1 (NQO1), heme oxygenase-1(HO-1)], and antioxidants [e.g. superoxide dismutase (SOD), catalase, ferritin, s-glutamyl cysteine synthetase modifier subunit (GCLM),)-glutamyl cysteine synthetase catalytic subunit (GCLC), glutathione peroxidase (GPx), glutathione reductase (GR), thioredoxin, thioredoxin reductase (TR), peroxiredoxins, metallothionein, etc.] (Zhang and Gordon, 2004; Giudice and Montella, 2006). Therefore, one of the most prominent strategies of cancer chemoprevention is to protect cells or tissues against various carcinogens and carcinogenic metabolites, both exogenous and endogenous, through the induction of metabolizing/ detoxifying and antioxidant enzymes. We recently found that 4-ketopinoresinol, trans-coniferylaldehyde and sinapaldehyde, the active component isolated by free radical scavenge or antimutagenecity, significantly induced Nrf2/ARE-driven luciferase activity (Chen et al., 2011; Chen et al., 2012a). Further study demonstrated that 4-ketopinoresinol strongly induces Nrf2-mediated detoxifying/antioxidant proteins, including HO-1, AKR1C1, AKR1C2, AKR1C3, GR, TR, GCLC, GCLM, etc., via the PI3K/AKT pathway, and at least in part, 4-ketopinoresinol -induced HO-1 expression contributes to a cellular defense mechanism against hydrogen peroxide-induced cell injury (Chen et al., 2012a). These results suggested that the cytoprotective effects induced by 4-ketopinoresinol may come from direct scavenging ROS directly (Kuo et al., 2002) and activating of PI3K/AKT/Nrf2/HO-1 axis (Chen et al., 2012a). Future studies evaluating the efficacy of 4-ketopinoresinol as a potential chemopreventive agent, using different carcinogenesis animal models, are warranted.
Alter Carcinogen Metabolism
Mammalian cytochrome P-450 (CYP) enzymes are phase I metabolizing enzymes, generally catalyze the oxidative metabolism of various xenobiotics, including drug, chemical carcinogens, etc. (Rendic, 2002). Despite the phase I oxidative metabolic reactions of chemicals result in the formation of more-water-soluble and less-toxic metabolites, however, some CYP enzymes, such as CYP1A1, CYP3A, and CYP2E1, have been indicated to involve in the metabolic activation of carcinogens, such as benzo[a]pyrene, N-nitrosodimethylamine, or aflatoxin B1 (Gonzalez and Gelboin, 1994). In addition, CYP1A2 have been addressed to activate a tobacco pre-carcinogen to a carcinogen [i.e. 4-methylnitrosamino-1-(3-pyridyl)-1-butanone; NNK] (Smith et al., 1996). Yao et al. demonstrated that the ethanolic extract of adlay bran (ABE) significantly suppresses CYP1A1 and CYP1A2 activities in the liver and CYP1A1 activity in the lungs of rats after 4 weeks of feeding (Yao et al., 2011). This change may have resulted in a decrease in the metabolism of chemical carcinogens. Indeed, a recent study reported that rats fed the ethyl acetate fraction of ABE showed reduced 1,2-dimethylhydrazine (DMH)-induced colon carcinogenesis (Chung et al., 2010). Because DMH is a pre-carcinogen that requires activation by liver CYP to form DNA-reactive metabolites, therefore, ABE may have chemopreventive effect against colon carcinogenesis in the initiation stage.
Adlay Acts as a Suppressing Agents for Cancer Chemoprevention
Suppress Proliferation
Dysregulated proliferation is a well-established hallmark of carcinogenesis (Hanahan and Weinberg,2000). The earliest antitumor study of adlay was demonstrated by Tanimura's group at 1961. They reported that the growth of Ehrlich ascites sarcoma was inhibited by adlay (Tanimura, 1961; Ukita and Tanimura, 1961). Adlay is also applied as an adjuvant to treat lung, stomach, breast, colon, and cervical cancers (Chang et al., 2003; Hung and Chang, 2003; Woo et al., 2007; Hu et al., 2009). In addition to tumor transplantation experiment, Chang et al. used NNK-induced lung tumorigenesis model to investigate the chemopreventive potential of adlay seed. As the result, mice received the powder of adlay seed significantly reduced the number of surface lung tumors (Chang et al., 2003).
The active compound with anticancer property was first identified by Tanimura's group to be a oil component, coixenolide (Tanimura, 1961; Ukita and Tanimura, 1961). Therefore, it is important to know the distribution of this compound in adlay seed. Huang and Chiang demonstrated that among different part of adlay seed, adlay bran contained the highest amount of coixenolide (473 ppm) (Huang and Chiang, 1999). In addition to coixenolide, other oil components, including palmitic acid, stearic acid, oleic acid, and linoleic acid, also showed potent growth inhibitory activity on a transplantable mouse tumor (Numata et al., 1994). These results suggested that oil components of adlay play a critical role of anticancer efficacy. Recently, Kanglaite injection (KLT), an aqueous microemulsion of an oil extracted from adlay seed, have been developed using the latest and most complex modern technologies (Lu et al., 2008). KLT is a new biphase extended-spectrum anticancer medicine, the food and drug administration (FDA) of United States also approved a phase II trial of KLT to test its efficacy in treating patients with stage IV of non-small-cell lung cancer or combination of KLT with gemicitabine to investigate the therapeutic efficacy in pancreatic cancer patients (http://www.clinicaltrial.gov). The mechanisms contributed to antitumor effect of neutral lipid components, at least in part, through cell cycle arrest and apoptosis induction involving upregulation of cyclin-dependent kinase inhibitor p21 and inhibition of antiapoptotic protein Bcl-2 expression (Bao et al., 2005).
In addition to oil extract, Chiang's group recently identified several active components from adlay bran with anticancer growth inhibitory ability, including: (i) caffeic acid and chlorogenic acid toward gastric cancer cell growth (Chung et al., 2011a); (ii) coixspirolactam D, coixspirolactam E, coixspiroenone, coixspirolactam A, coixspirolactam C, and coixlactam toward breast cancer cells growth (Chung et al., 2011b); and (iii) coixspirolactam A, coixspirolactam B, coixspirolactam C, coixlactam, and methyl dioxindole-3-acetate toward non-small cell lung and colorectal cancer cell growth (Lee et al., 2008). The mechanism underlying anticancer effect of these component merit for further investigation.
Decrease Inflammation
A causal association between inflammation and cancer has long been suspected. Multiple lines of compelling evidence from clinical, epidemiologic and laboratory studies support that inflammation plays a critical role in the promotion and progression stages of carcinogenesis (Fitzpatrick, 2001).
1. The Nuclear Factor kappa-B (NF-κB)
NF-κB comprises a family of transcription factors involved in the regulation of a wide variety of biological responses. NF-κB plays a well-known function in the regulation of immune responses and inflammation, but growing evidences support a major role in oncogenesis. NF-κB regulates the expression of genes involved in many processes that play a key role in the development and progression of cancer such as proliferation, migration and apoptosis (Wang and Cho, 2010). Thus, clinical trials with drugs that block NF-κB are currently in progress with promising result (http://www.clinicaltrial.gov). Woo et al. showed that anti-neoplastic activity of an adlay extract in breast MDA-MB-231 xenografts (Woo et al., 2007). Using gene array technology, adlay extract significantly inhibits NF-κB-dependent gene transcription, including downregulation of COX-2 and matrixmetalloproteinases (MMP), in these cells. In addition, adlay extract also inhibits activity of protein kinase C, a major mediator of signal transduction and activator of NF-κB (Woo et al., 2007). Likewise, treatment with KLT decreased the level of NF-κB in the nucleus in a dose-dependent manner, and KLT markedly decreased the expression of IκBα, IKK and EGFR in the cytoplasm of lung tumor cells (Pan et al., 2012).
2. Cyclooxygenase-2 (COX-2)
Aberrant upregulation of COX-2, a key player in inflammatory signaling, is frequently observed in various precancerous and malignant tissues. Therefore, the normalization of inappropriately overamplified signaling cascades implicated in chronic inflammation-associated carcinogenesis by use of COX-2 specific inhibitors has been recognized as a rational and pragmatic strategy in molecular target-based cancer prevention (Surh and Kundu, 2007). Huang et al. demonstrated that the ethanolic extract of adlay hull (AHE) and adlay testa (ATE) possessed potent anti-inflammatory activity through inhibiting lipopolysaccharide (LPS)-induced COX-2 expression in RAW264.7 macrophage (Huang et al., 2009a; Huang et al., 2009b). The active COX-2 inhibiting components were identified to be ceramide and naringenin in AHE (Huang et al., 2009a), and gallic acid, caffeic acid, and ferulic acid in ATE (Huang et al., 2009b), respectively. In the in vivo investigation, rats received dehulled adlay significantly reduced the expression level of COX-2 and the number of preneoplastic aberrant crypt foci (ACF) in azoxymethane (AOM)-induced colon carcinogenesis animal model (Shih et al., 2004). Li et al. further showed that adlay bran and its ethanolic extract and residue play an important role in suppressing ACF formation in an early stage of colon carcinogenesis (Li et al., 2011). In addition, Chung et al. demonstrated that the ethyl acetate fraction of ABE significantly suppresses DMH-induced preneoplastic lesion of the colon in F344 rats through an anti-inflammatory pathway, involved COX-2 inhibition (Chung et al., 2010). Moreover, Hung and Chang also suggested that the methanolic extract of adlay seed also inhibits cancer growth and prevents lung tumorigenesis through COX-2 suppression (Hung and Chang, 2003).
Enhance Antitumor Immunity
Cytotoxic T lymphocytes and natural killer cells are essential effectors of anti-tumor immune responses in vivo (Seino et al., 2006). Miyai's group have investigated the changes in lymphocyte subsets in seven volunteers before, during (four weeks) and after taking six adlay seed tablets. As the results, the level of CD3+CD56+ (MHC-non restricted cytotoxic T cells) markedly increased at four weeks. The level of CD16+CD57- (the mature, most active natural killer cells) also increased at three weeks (Hidaka et al., 1992; Kaneda et al., 1992). These results indicate that adlay seeds increase peripheral cytotoxic lymphocytes and may be effective not only to viral infection through the enhancement of cytotoxic activity but also to boost antitumor immunity. In addition, it is interesting to note that KLT boosted anticancer immunity in Lewis lung carcinoma-bearing C57BL/6 mice through stimulating T cell proliferation and IL-2 secretion (Pan et al., 2012).
CONCLUSION REMARKS
In summary, adlay is a potential chemopreventive TCM to block multistage carcinogenesis with anti-initiation, -promotion and -progression properties. The chemopreventive functionality of adlay involving in scavenging electrophiles and reactive oxygen species, antimutagenicity, enhancing Nrf2-mediated detoxification and antioxidant effect, altering carcinogen metabolism, suppressing proliferation, decreasing inflammation, and enhancing antitumor immunity. Oil extract, especially KLT, have been proved to test its therapeutic efficacy in clinical setting.
Otherwise, several active components with diverse chemopreventive properties have been identified, including coniferyl alcohol, syringic acid, ferulic acid, syringaresinol, 4-ketopinoresinol, and mayuenolide (scavenge electrophiles and reactive oxygen species); p-hydroxybenzaldehyde, vanillin, syringaldehyde, trans-coniferylaldehyde, sinapaldehyde, and coixol (antimutagenicity); 4-ketopinoresinol, trans-coniferylaldehyde and sinapaldehyde (enhance Nrf2-mediated detoxification and antioxidant effect); coixenolide, Kanglaite injection (KLT; neutral lipid components), caffeic acid, chlorogenic acid, coixspirolactam D, coixspirolactam E, coixspiroenone, coixspirolactam A, coixspirolactam C, coixlactam, coixspirolactam A, coixspirolactam B, coixspirolactam C, coixlactam, and methyl dioxindole-3-acetate (suppressing proliferation); ceramide and naringenin, gallic acid, caffeic acid, and ferulic acid (decrease inflammation); and KLT (enhance antitumor immunity). The underlying mechanism of these active components is merit for further investigation.
ABBREVIATIONS
ACKNOWLEDGEMENTS
The study was supported by grants from the National Health Research Institutes (CA-101-PP-05), the National Science Council (NSC98-2320-B-400-003-MY3), and the Department of Health (DOH101-TD-C-111-004), Taiwan, R.O.C.
REFERENCES
- 1.Bao Y, Yuan Y, Xia L, Jiang H, Wu W, Zhang X. Neutral lipid isolated from endosperm of Job's tears inhibits the growth of pancreatic cancer cells via apoptosis, G2/M arrest, and regulation of gene expression. J Gastroenterol Hepatol. 2005;20:1046–1053. doi: 10.1111/j.1440-1746.2005.03864.x. [DOI] [PubMed] [Google Scholar]
- 2.Barzilai A, Yamamoto K. DNA damage responses to oxidative stress. DNA repair. 2004;3:1109–1115. doi: 10.1016/j.dnarep.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Campos A.C, Molognoni F, Melo F.H, Galdieri L.C, Carneiro C.R, D’Almeida V, Correa M, Jasiulionis M.G. Oxidative stress modulates DNA methylation during melanocyte anchorage blockade associated with malignant transformation. Neoplasia. 2007;9:1111–1121. doi: 10.1593/neo.07712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang H.C, Huang Y.C, Hung W.C. Antiproliferative and chemopreventive effects of adlay seed on lung cancer in vitro and in vivo. J Agric Food Chem. 2003;51:3656–3660. doi: 10.1021/jf021142a. [DOI] [PubMed] [Google Scholar]
- 5.Chang L.L, Wun A.W, Hung C.T, Hsia S.M, Chiang W, Wang P.S. Effects of crude adlay hull acetone extract on corticosterone release from rat zona fasciculata-reticularis cells. Naunyn Schmiedebergs Arch Pharmacol. 2006;374:141–152. doi: 10.1007/s00210-006-0094-x. [DOI] [PubMed] [Google Scholar]
- 6.Chen H.H, Chen Y.T, Huang Y.W, Tsai H.J, Kuo C.C. 4-Ketopinoresinol a novel naturally occurring ARE activator, induces the Nrf2/HO-1 axis and protects against oxidative stress- induced cell injury via activation of PI3K/AKT signaling. Free Radic Biol Med. 2012a;52:1054–1066. doi: 10.1016/j.freeradbiomed.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Chen H.H, Chiang W, Chang J.Y, Chien Y.L, Lee C.K, Liu K.J, Cheng Y.T, Chen T.F, Kuo Y.H, Kuo C.C. Antimutagenic constituents of adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) with potential cancer chemopreventive activity. J Agric Food Chem. 2011;59:6444–6452. doi: 10.1021/jf200539r. [DOI] [PubMed] [Google Scholar]
- 8.Chen H.J, Hsu H.Y, Chiang W. Allergic immune-regulatory effects of Adlay Bran on an OVA-immunized mice allergic model. Food Chem Toxicol. 2012b doi: 10.1016/j.fct.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Chen H.J, Lo Y.C, Chiang W. Inhibitory effects of adlay bran (Coix lachryma-jobi L. var. ma-yuen Stapf) on chemical mediator release and cytokine production in rat basophilic leukemia cells. J Ethnopharmacol. 2012c;141:119–127. doi: 10.1016/j.jep.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Chen H.J, Shih C.K, Hsu H.Y, Chiang W. Mast cell- dependent allergic responses are inhibited by ethanolic extract of adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) testa. J Agric Food Chem. 2010;58:2596–2601. doi: 10.1021/jf904356q. [DOI] [PubMed] [Google Scholar]
- 11.Chiang W, Cheng C, Chiang M, Chung K.T. Effects of dehulled adlay on the culture count of some microbiota and their metabolism in the gastrointestinal tract of rats. J Agric Food Chem. 2000;48:829–832. doi: 10.1021/jf990473t. [DOI] [PubMed] [Google Scholar]
- 12.Chung C.P, Hsia S.M, Lee M.Y, Chen H.J, Cheng F, Chan L.C, Kuo Y.H, Lin Y.L, Chiang W. Gastroprotective activities of adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) on the growth of the stomach cancer AGS cell line and indomethacin-induced gastric ulcers. J Agric Food Chem. 2011a;59:6025–6033. doi: 10.1021/jf2009556. [DOI] [PubMed] [Google Scholar]
- 13.Chung C.P, Hsu C.Y, Lin J.H, Kuo Y.H, Chiang W, Lin Y.L. Antiproliferative lactams and spiroenone from adlay bran in human breast cancer cell lines. J Agric Food Chem. 2011b;59:1185–1194. doi: 10.1021/jf104088x. [DOI] [PubMed] [Google Scholar]
- 14.Chung C.P, Hsu H.Y, Huang D.W, Hsu H.H, Lin J.T, Shih C.K, Chiang W. Ethyl acetate fraction of adlay bran ethanolic extract inhibits oncogene expression and suppresses DMH-induced preneoplastic lesions of the colon in F344 rats through an anti- inflammatory pathway. J Agric Food Chem. 2010;58:7616–7623. doi: 10.1021/jf101084e. [DOI] [PubMed] [Google Scholar]
- 15.Fitzpatrick F.A. Inflammation carcinogenesis and cancer. Int Immunopharmacol. 2001;1:1651–1667. doi: 10.1016/s1567-5769(01)00102-3. [DOI] [PubMed] [Google Scholar]
- 16.Fruehauf J.P, Meyskens F.L., Jr Reactive oxygen species: a breath of life or death? Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13:789–794. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 17.Giudice A, Montella M. Activation of the Nrf 2-AR signaling pathway a promising strategy in cancer prevention. Bioessays. 2006;28:169–181. doi: 10.1002/bies.20359. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez F.J, Gelboin H.V. Role of human cytochromes P450 in the metabolic activation of chemical carcinogens and toxins. Drug Metab Rev. 1994;26:165–183. doi: 10.3109/03602539409029789. [DOI] [PubMed] [Google Scholar]
- 19.Hail N, Jr, Cortes M, Drake E.N, Spallholz J.E. Cancer chemoprevention: a radical perspective. Free Radic Biol Med. 2008;45:97–110. doi: 10.1016/j.freeradbiomed.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan D, Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 21.Hayes J.D, Mc Mahon M. Molecular basis for the contribution of the antioxidant responsive element to cancer chemoprevention. Cancer letters. 2001;174:103–113. doi: 10.1016/s0304-3835(01)00695-4. [DOI] [PubMed] [Google Scholar]
- 22.Hidaka Y, Kaneda T, Amino N, Miyai K. Chinese medicine, Coix seeds increase peripheral cytotoxic T and NK cells. Biotherapy. 1992;5:201–203. doi: 10.1007/BF02171052. [DOI] [PubMed] [Google Scholar]
- 23.Hsia S.M, Chiang W, Kuo Y.H, Wang P.S. Downregulation of progesterone biosynthesis in rat granulosa cells by adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) bran extracts. Int J Impot Res. 2006;18:264–274. doi: 10.1038/sj.ijir.3901405. [DOI] [PubMed] [Google Scholar]
- 24.Hsia S.M, Kuo Y.H, Chiang W, Wang P.S. Effects of adlay hull extracts on uterine contraction and Ca2+ mobilization in the rat. Am J Physiol Endocrinol Metab. 2008;295:E719–726. doi: 10.1152/ajpendo.90367.2008. [DOI] [PubMed] [Google Scholar]
- 25.Hsia S.M, Tseng Y.W, Wang S.W, Kuo Y.H, Huang D.W, Wang P.S, Chiang W. Effect of adlay (Coix lachryma-jobi L. var. ma-yuen Stapf.) hull extracts on testosterone release from rat Leydig cells. Phytother Res. 2009;23:687–695. doi: 10.1002/ptr.2706. [DOI] [PubMed] [Google Scholar]
- 26.Hsia S.M, Yeh C.L, Kuo Y.H, Wang P.S, Chiang W. Effects of adlay (Coix lachryma-jobi L. var. ma-yuen Stapf.) hull extracts on the secretion of progesterone and estradiol in vivo and in vitro. Exp Biol Med (Maywood) 2007;232:1181–1194. doi: 10.3181/0612-RM-306. [DOI] [PubMed] [Google Scholar]
- 27.Hsu H.Y, Lin B.F, Lin J.Y, Kuo C.C, Chiang W. Suppression of allergic reactions by dehulled adlay in association with the balance of TH1/TH2 cell responses. J Agric Food Chem. 2003;51:3763–3769. doi: 10.1021/jf021154w. [DOI] [PubMed] [Google Scholar]
- 28.Hu S.H, Xiao X.N, Yi X, Zhang J.P. Study progress of coix seed. Shizhen Guoyi Guoyao. 2009;20:1059–1060. [Google Scholar]
- 29.Huang B.W, Chiang M.T, Yao H.T, Chiang W. The effect of adlay oil on plasma lipids, insulin and leptin in rat. Phytomedicine. 2005;12:433–439. doi: 10.1016/j.phymed.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Huang D.W, Chung C.P, Kuo Y.H, Lin Y.L, Chiang W. Identification of compounds in adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) seed hull extracts that inhibit lipopolysaccharide- induced inflammation in RAW 2647 macrophages. J Agric Food Chem. 2009a;57:10651–10657. doi: 10.1021/jf9028514. [DOI] [PubMed] [Google Scholar]
- 31.Huang D.W, Kuo Y.H, Lin F.Y, Lin Y.L, Chiang W. Effect of Adlay ( Coix lachryma-jobi L. var. ma-yuen Stapf) Testa and its phenolic components on Cu2+-treated low-density lipoprotein (LDL) oxidation and lipopolysaccharide (LPS)-induced inflammation in RAW 2647 macrophages. J Agric Food Chem. 2009b;57:2259–2266. doi: 10.1021/jf803255p. [DOI] [PubMed] [Google Scholar]
- 32.Huang H.C, Nguyen T, Pickett C.B. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J Biol Chem. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- 33.Huang S-L, Chiang W. Composition of the different fractions of adlay seed and the demutagenic effect of their acetone extract. Food Sci (Chinese) 1999;26:121–130. [Google Scholar]
- 34.Hung W.C, Chang H.C. Methanolic extract of adlay seed suppresses COX-2 expression of human lung cancer cells via inhibition of gene transcription. J Agric Food Chem. 2003;51:7333–7337. doi: 10.1021/jf0340512. [DOI] [PubMed] [Google Scholar]
- 35.Ishiguro Y, Okamoto K, Sakamoto H, Sonoda Y. Antimicrobial substances coixindens A and B in etiolated seedlings of adlay. Nippon Nogei Kagaku Kaishi. 1993;67:1405–1410. [Google Scholar]
- 36.Kaneda T, Hidaka Y, Kashiwai T, Tada H, Takano T, Nishiyama S, Amino N, Miyai K. [Effect of coix seed on the changes in peripheral lymphocyte subsets] Rinsho Byori. 1992;40:179–181. [PubMed] [Google Scholar]
- 37.Khan M, Pelengaris S. Black-well Publishing Massachusetts; 2006. The molecular biology of cancer. [Google Scholar]
- 38.Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent patents on inflammation & allergy drug discovery. 2009;3:73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- 39.Kim S.O, Yun S.J, Jung B, Lee E.H, Hahm D.H, Shim I, Lee H.J. Hypolipidemic effects of crude extract of adlay seed (Coix lachrymajobi var. mayuen) in obesity rat fed high fat diet: relations of TNF-alpha and leptin mRNA expressions and serum lipid levels. Life Sci. 2004;75:1391–1404. doi: 10.1016/j.lfs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 40.Kim S.O, Yun S.J, Lee E.H. The water extract of adlay seed (Coix lachrymajobi var. mayuen) exhibits anti-obesity effects through neuroendocrine modulation. Am J Chin Med. 2007;35:297–308. doi: 10.1142/S0192415X07004825. [DOI] [PubMed] [Google Scholar]
- 41.Klaunig J.E, Kamendulis L.M. The role of oxidative stress in carcinogenesis. Annual review of pharmacology and toxicology. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 42.Klaunig J.E, Kamendulis L.M, Hocevar B.A. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol. 2010;38:96–109. doi: 10.1177/0192623309356453. [DOI] [PubMed] [Google Scholar]
- 43.Klaunig J.E, Wang Z, Pu X, Zhou S. Oxidative stress and oxidative damage in chemical carcinogenesis. Toxicol Appl Pharmacol. 2011;254:86–99. doi: 10.1016/j.taap.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 44.Kondo Y, Nakajima K, Nozoe S, Suzuki S. Isolation of ovulatory-active substances from crops of Job's tears (Coix lacryma-jobi L. var. ma-yuen STAPF.) Chem Pharm Bull (Tokyo) 1988;36:3147–3152. doi: 10.1248/cpb.36.3147. [DOI] [PubMed] [Google Scholar]
- 45.Kuo C.C, Chiang W, Liu G.P, Chien Y.L, Chang J.Y, Lee C.K, Lo J.M, Huang S.L, Shih M.C, Kuo Y.H. 2,2’-Diphenyl-1-picrylhydrazyl radical-scavenging active components from adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) hulls. J Agric Food Chem. 2002;50:5850–5855. doi: 10.1021/jf020391w. [DOI] [PubMed] [Google Scholar]
- 46.Kuo C.C, Shih M.C, Kuo Y.H, Chiang W. Antagonism of free-radical-induced damage of adlay seed and its antiproliferative effect in human histolytic lymphoma U937 monocytic cells. J Agric Food Chem. 2001;49:1564–1570. doi: 10.1021/jf001215v. [DOI] [PubMed] [Google Scholar]
- 47.Kwak M.K, Kensler T.W. Targeting NRF2 signaling for cancer chemoprevention. Toxicol Appl Pharmacol. 2010;244:66–76. doi: 10.1016/j.taap.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee M.Y, Lin H.Y, Cheng F, Chiang W, Kuo Y.H. Isolation and characterization of new lactam compounds that inhibit lung and colon cancer cells from adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) bran. Food Chem Toxicol. 2008;46:1933–1939. doi: 10.1016/j.fct.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 49.Lengauer C, Kinzler K.W, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 50.Li S.C, Chen C.M, Lin S.H, Chiang W, Shih C.K. Effects of adlay bran and its ethanolic extract and residue on preneoplastic lesions of the colon in rats. J Sci Food Agric. 2011;91:547–552. doi: 10.1002/jsfa.4219. [DOI] [PubMed] [Google Scholar]
- 51.Lin M.H, Wu M.C, Lu S, Lin J. Glycemic index, glycemic load and insulinemic index of Chinese starchy foods. World J Gastroenterol. 2010;16:4973–4979. doi: 10.3748/wjg.v16.i39.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu Y, Li C.S, Dong Q. Chinese herb related molecules of cancer-cell-apoptosis: a minireview of progress between Kanglaite injection and related genes. J Exp Clin Cancer Res. 2008;27:31. doi: 10.1186/1756-9966-27-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manson M.M, Gescher A, Hudson E.A, Plummer S.M, Squires M.S, Prigent S.A. Blocking and suppressing mechanisms of chemoprevention by dietary constituents. Toxicology letters. 2000;112-113:499–505. doi: 10.1016/s0378-4274(99)00211-8. [DOI] [PubMed] [Google Scholar]
- 54.Mortelmans K, Zeiger E. The Ames Salmonella/microsome mutagenicity assay. Mutat Res. 2000;455:29–60. doi: 10.1016/s0027-5107(00)00064-6. [DOI] [PubMed] [Google Scholar]
- 55.Numata M, Yamamoto A, Moribayashi A, Yamada H. Antitumor components isolated from the Chinese herbal medicine. Coix lachryma-jobi Planta Med. 1994;60:356–359. doi: 10.1055/s-2006-959500. [DOI] [PubMed] [Google Scholar]
- 56.Otsuka H, Hirai Y, Nagao T, Yamasaki K. Anti-inflammatory activity of benzoxazinoids from roots of Coix lachryma-jobi var. ma-yuen. J Nat Prod. 1988;51:74–79. doi: 10.1021/np50055a009. [DOI] [PubMed] [Google Scholar]
- 57.Pan P, Wu Y, Guo Z.Y, Wang R, Wang Y.J, Yuan Y.F. Antitumor activity and immunomodulatory effects of the intraperitoneal administration of Kanglaite in vivo in Lewis lung carcinoma. J Ethnopharmacol. 2012;143:680–685. doi: 10.1016/j.jep.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 58.Panayiotidis M. Reactive oxygen species (ROS) in multistage carcinogenesis. Cancer letters. 2008;266:3–5. doi: 10.1016/j.canlet.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 59.Rendic S. Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002;34:83–448. doi: 10.1081/dmr-120001392. [DOI] [PubMed] [Google Scholar]
- 60.Seino K, Motohashi S, Fujisawa T, Nakayama T, Taniguchi M. Natural killer T cell-mediated antitumor immune responses and their clinical applications. Cancer Sci. 2006;97:807–812. doi: 10.1111/j.1349-7006.2006.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shih C.K, Chiang W, Kuo M.L. Effects of adlay on azoxymethane-induced colon carcinogenesis in rats. Food Chem Toxicol. 2004;42:1339–1347. doi: 10.1016/j.fct.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 62.Smith T.J, Guo Z, Guengerich F.P, Yang C.S. Metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) by human cytochrome P450 1A2 and its inhibition by phenethyl isothiocyanate. Carcinogenesis. 1996;17:809–813. doi: 10.1093/carcin/17.4.809. [DOI] [PubMed] [Google Scholar]
- 63.Son B.K, Kim J.Y, Lee S.S. Effect of adlay, buckwheat and barley on lipid metabolism and aorta histopathology in rats fed an obesogenic diet. Ann Nutr Metab. 2008;52:181–187. doi: 10.1159/000138121. [DOI] [PubMed] [Google Scholar]
- 64.Surh Y.J. Cancer chemoprevention with dietary phytochemicals. Nature reviews Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 65.Surh Y.J, Kundu J.K. Cancer preventive phytochemicals as speed breakers in inflammatory signaling involved in aberrant COX-2 expression. Curr Cancer Drug Targets. 2007;7:447–458. doi: 10.2174/156800907781386551. [DOI] [PubMed] [Google Scholar]
- 66.Takahashi M, Konno C, Hikino H. Isolation a n d hypoglycemic activity of coixans A, B and C glycans of Coix lachryma-jobi var. ma-yuen seeds. Planta Med. 1986:64–65. doi: 10.1055/s-2007-969074. [DOI] [PubMed] [Google Scholar]
- 67.Tanimura A. Studies on the antitumor components in the seeds of Coix lachryma-jobi L var. ma-yuen (Roman) Stapf II The structure of coixenolide. Chem Pharm Bull (Tokyo) 1961;9:47–53. [Google Scholar]
- 68.Tzeng H.P, Chiang W, Ueng T.H, Liu S.H. The abortifacient effects from the seeds of Coix lachryma-jobi L var. ma-yuen. Stapf J Toxicol Environ Health A. 2005;68:1557–1565. doi: 10.1080/15287390590967504. [DOI] [PubMed] [Google Scholar]
- 69.Ukita T, Tanimura A. Studies on the antitumor components in the seeds of Coix lachryma-jobi L. var. ma-yuen (Roman) Stapf I Isolation and antitumor activity of coixenolide. Chem Pharm Bull (Tokyo) 1961;9:43–46. [Google Scholar]
- 70.Wang H, Cho C.H. Effect of NF-kappaB signaling on apoptosis in chronic inflammation-associated carcinogenesis. Curr Cancer Drug Targets. 2010;10:593–599. doi: 10.2174/156800910791859425. [DOI] [PubMed] [Google Scholar]
- 71.Wang L, Sun J, Yi Q, Wang X, Ju X. Protective Effect of Polyphenols Extract of Adlay (<em>Coix lachryma-jobi </em>L. var<em>. ma-yuen Stapf</em>) on Hypercholesterolemia-Induced Oxidative Stress in Rats. Molecules. 2012;17:8886–8897. doi: 10.3390/molecules17088886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wattenberg L.W. Chemoprevention of cancer. Cancer research. 1985;45:1–8. [PubMed] [Google Scholar]
- 73.Weisburger J.H. Antimutagenesis and anticarcinogenesis, from the past to the future. Mutat Res. 2001;480-481:23–35. doi: 10.1016/s0027-5107(01)00166-x. [DOI] [PubMed] [Google Scholar]
- 74.Windmill K.F, Mc Kinnon R.A, Zhu X, Gaedigk A, Grant D.M, Mc Manus M.E. The role of xenobiotic metabolizing enzymes in arylamine toxicity and carcinogenesis: functional and localization studies. Mutat Res. 1997;376:153–160. doi: 10.1016/s0027-5107(97)00038-9. [DOI] [PubMed] [Google Scholar]
- 75.Woo J.H, Li D, Wilsbach K, Orita H, Coulter J, Tully E, Kwon T.K, Xu S, Gabrielson E. Coix seed extract, a commonly used treatment for cancer in China, inhibits NFkappaB and protein kinase C signaling. Cancer Biol Ther. 2007;6:2005–2011. doi: 10.4161/cbt.6.12.5168. [DOI] [PubMed] [Google Scholar]
- 76.Yang R.S, Chiang W, Lu Y.H, Liu S.H. Evaluation of osteoporosis prevention by adlay using a tissue culture model. Asia Pac J Clin Nutr 17 Suppl. 2008;1:143–146. [PubMed] [Google Scholar]
- 77.Yao H.T, Lin J.H, Chiang M.T, Chiang W, Luo M.N, Lii C.K. Suppressive effect of the ethanolic extract of adlay bran on cytochrome P-450 enzymes in rat liver and lungs. J Agric Food Chem. 2011;59:4306–4314. doi: 10.1021/jf200117m. [DOI] [PubMed] [Google Scholar]
- 78.Yeh P.H, Chiang W, Chiang M.T. Effects of dehulled adlay on plasma glucose and lipid concentrations in streptozotocin- induced diabetic rats fed a diet enriched in cholesterol. Int J Vitam Nutr Res. 2006;76:299–305. doi: 10.1024/0300-9831.76.5.299. [DOI] [PubMed] [Google Scholar]
- 79.Yu F, Gao J, Zeng Y, Liu C.X. Effects of adlay seed oil on blood lipids and antioxidant capacity in hyperlipidemic rats. J Sci Food Agric. 2011;91:1843–1848. doi: 10.1002/jsfa.4393. [DOI] [PubMed] [Google Scholar]
- 80.Yu Y.M, Chang W.C, Liu C.S, Tsai C.M. Effect of young barley leaf extract and adlay on plasma lipids and LDL oxidation in hyperlipidemic smokers. Biol Pharm Bull. 2004;27:802–805. doi: 10.1248/bpb.27.802. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Y, Gordon G.B. A strategy for cancer prevention: stimulation of the Nrf2-ARE signaling pathway. Mol Cancer Ther. 2004;3:885–893. [PubMed] [Google Scholar]


