Abstract
Chinese herbal medicine (中草藥) attracts much attention in the treatment of liver injuries. Numerous studies have revealed various biological activities of medicinal mushrooms such as Antrodia Cinnamomea (牛樟芝). Although A. cinnamomea is rare in the wild, recent developments in fermentation and cultivation technologies make the mycelia and fruiting bodies of this valuable medicinal mushroom readily available. Liver diseases such as fatty liver, hepatitis, hepatic fibrosis, and liver cancer are complicated processes of liver injuries that have tremendous impact on human society. In this article, we reviewed studies about the hepatoprotective effects of the fruiting bodies and mycelia of A. cinnamomea performed in different experimental models. The results of those studies suggest the potential application of A. cinnamomea in preventing and treating liver diseases and its potential to be developed into health foods or new drugs.
Keywords: Antrodia Cinnamomea, Fruiting bodies, Mycelia, Liver injury
Introduction
Several medicinal mushrooms are known to be valuable for their effectiveness in the treatment of diseases. Among the popular medicinal mushrooms such as Antrodia Cinnamomea (Figure 1), Ganoderma lucidum, Cordyceps sinensis, and Phellinus linteus, A. cinnamomea can be found only in Taiwan. Antrodia Cinnamomea is used to treat liver diseases, hypertension, abdominal pain, diarrhea, and other diseases (Ao et al., 2009). Historically, indigenous Taiwanese people have used A. cinnamomea to ameliorate liver disorders from excessive alcohol consumption. However, it is now becoming more difficult to collect wild A. cinnamomea fruiting bodies.
Figure 1.

Antrodia Cinnamomea fruiting bodies grown on wood.
With the development of fermentation technology, the mycelia of many medicinal mushrooms can now be obtained through submerged fermentation. This technology can effectively manage the identity and purity of medicinal mushrooms without contamination. The process of substrate bag culture for growing fruiting bodies also uses liquid fermentation to provide the seed medium (Sharma et al., 2005). However, the medicinal effectiveness and applicability of the different compounds contained in fruiting bodies and mycelia remain debatable.
In this review, we discuss the hepatoprotective activities of A. cinnamomea in animal models of carbon tetrachloride (CCl4)-and alcohol-induced liver injuries, as well as its in vitro anti-liver cancer activity.
The liver is the largest organ responsible for a spectrum of functions, including the uptake, metabolism, conjugation, and excretion of various endogenous and foreign substances (Hoekstra et al., 2012). Chronic hepatitis or toxification leads to severe liver injury. The damaged hepatocytes are initially denatured and then undergo fibrosis and necrosis. This process eventually leads to hepatoma (Friedman, 1997).
Antrodia Cinnamomea, a species of the genus Antrodia (Polyporaceae), is a parasitic fungus that lives in the inner cavity of Cinnamomum kanehirai, which is endemic to Taiwan. Antrodia Cinnamomea is believed to be one of the most potent liver-protecting herbs in Taiwan (Ao et al., 2009).
Numerous reports have been published on the chemical components of A. cinnamomea. Compounds isolated from this mushroom, such as benzenoids, diterpenes, triterpenoids, steroids, maleic/succinic acid derivatives, and polysaccharides, have been reported to exhibit some biological activities.
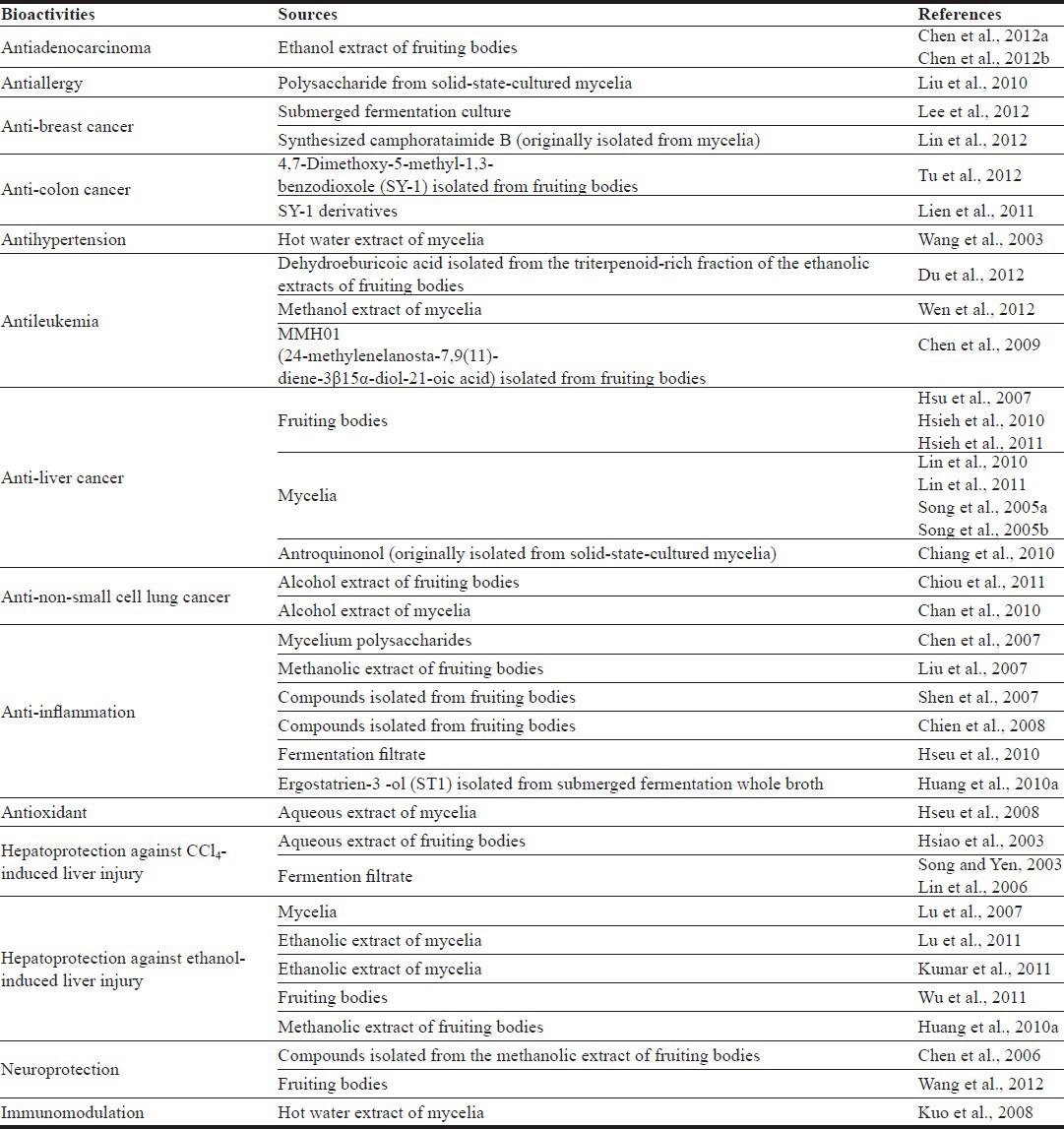
Table 1 summarizes the reported biological activities of A. cinnamomea, including antiallergy, anticancer, antihypertension, anti-inflammation, antioxidation, hepatoprotection, neuroprotection, and immunomodulation.
Table 1.
Reported bioactivities of Antrodia Cinnamomea.
Antrodia Cinnamomea and Alcohol-induced Liver Injury
Alcohol liver disease (ALD) is a kind of liver injury induced by alcohol drinking, which remains one of the most common causes of chronic liver disease worldwide. ALD includes steatosis (fatty liver), steatohepatitis (alcoholic hepatitis), and cirrhosis (Day and Yeaman, 1994). Steatosis is the earliest response of the liver to excessive alcohol use and is characterized by the accumulation of fat in hepatocytes. Steatohepatitis is characterized by infiltration of inflammatory cells into hepatocytes and hepatocellular injury (Gao and Bataller, 2011). Alcohol consumption suppresses the antifibrotic effects of natural killer cells and interferon-γ, and therefore enhances the progression of liver fibrosis (Jeong et al., 2008).
Alcohol promotes the accumulation of fat in the liver mainly by the substitution of ethanol for fatty acids as the major hepatic fuel (Baraona and Lieber, 1979). Ethanol increases the fatty acid synthesis by acetaldehyde through the upregulation of sterol regulatory element-binding protein 1c (SREBP-1c). AMP-activated protein kinase (AMPK) (You et al., 2004), sirtuin 1 (You et al., 2008), adiponectin (You, 2009), and signal transducer and activator of transcription 3 (STAT3) (Horiguchi et al., 2008) were reported to be downregulated by alcohol. The DNA-binding and transcriptional activation activities of peroxisome proliferator-activated receptor-α, a nuclear hormone receptor, in hepatocytes are directly inhibited by acetaldehyde (Galli et al., 2001).
Three main pathways are involved in the metabolism of ethanol: the alcohol dehydrogenase (ADH) pathway in the cytosol, the microsomal ethanol-oxidizing system (MEOS) in the endoplasmic reticulum, and the catalase pathway in peroxisomes (Jiménez-López et al., 2002). The ADH pathway is the major metabolic pathway during the early stage of chronic alcohol liver injury. During the ADH-mediated oxidation of ethanol, hydrogen is transferred from the substrate to the cofactor nicotinamide adenine dinucleotide (NAD), resulting in excess conversion to its reduced form (NADH) with the production of acetaldehyde (Cronholm et al., 1988). Excess NADH changes the redox state and leads to metabolic irregularity in the liver. The increase in α-glycerophosphate level and the suppression of the citric acid cycle are due to the elevated NADH-to-NAD ratio, thus favoring the accumulation of hepatic triglycerides in the liver (Ao et al., 2009). In the early 1960s, Lieber et al. (1963) formulated a liquid ethanol diet and used experimental models to study ethanol-induced hepatotoxicity. Therefore, the hepatoprotective effects of herbs, natural products, and chemicals have been widely investigated using these models.
In Taiwan, A. cinnamomea is believed to effectively ameliorate liver disorders induced by excessive alcohol consumption. However, the effects of A. cinnamomea, either its fruiting bodies or mycelia, on alcohol-induced liver injuries have only recently been reported (Huang et al., 2010a; Lu et al., 2007, 2011; Kumar et al., 2011; Wu et al. 2011).
Hepatoprotective Effect of A. cinnamomea Fruiting Bodies Against Alcohol-induced Liver Injury
The effects of A. cinnamomea fruiting bodies on alcohol-induced liver damage have been reported. Huang et al. (2010a) investigated the effects of A. cinnamomea fruiting bodies in a chronic alcohol consumption model. Alcoholic fatty liver disease was induced by adding 20% (w/w) alcohol to the drinking water of experimental rats. Antrodia Cinnamomea fruiting body (0.1 g per kilogram body weight per day [g•kg BW-1•day-1]) orally administered to the chronic alcohol consumption group for 4 weeks increased the levels of fecal cholesterol and bile acid. The gene expression of 3-hydroxy-3-methoxyglutaryl-CoA reductase, SREBP-1c, acetyl-CoA carboxylase, fatty acid synthase, and malic enzyme was downregulated by A. cinnamomea fruiting body. The results of histological examination revealed that the alcohol-induced liver injuries, i.e., hepatocyte necrosis and inflammatory cell infiltration, were prevented by A. cinnamomea fruiting body (0.1 g•kg BW-1•day-1) and that its effect was even better than that of silymarin (0.25 g•kg BW-1•day-1).
Wu et al. (2011) investigated the beneficial effects of A. cinnamomea fruiting bodies on alcohol-induced liver fibrosis. The expression of hepatic mRNAs, i.e., matrix metalloproteinase (MMP)-9, tumor necrosis factor (TNF)-α, Kruppel-like factor (KLF)-6, and transforming growth factor (TGF)-β1, were downregulated by orally administered A. cinnamomea fruiting bodies (0.025 g/kg BW, once a day). The hepatoprotective effect of A. cinnamomea fruiting bodies (0.025 g/kg BW, once a day) was comparable to that of silymarin (0.25 g/kg BW, once a day). This protective effect might account for the acceleration of alcohol clearance and the suppression of ethanol-induced elevation of MMP-9, TNF-α, KLF-6, and TGF-β1 levels.
Hepatoprotective Effect of A. cinnamomea Mycelia Against Alcohol-induced Liver Injury
Lu et al. (2007) reported the effects of A. cinnamomea mycelia against ethanol-induced liver injury. Male Sprague-Dawley (SD) rats were orally administered with 0.5 and 1.0 g/kg BW of A. cinnamomea mycelia for 10 days. At the end of 10th day, rats were administered with ethanol (5.0 g/kg BW) by gavage to induce acute hepatic injury. The levels of serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and bilirubin in rats given A. cinnamomea were comparable with the silymarin positive group (0.1 g/kg BW). Thus, A. cinnamomea showed hepatoprotective activity in this acute ethanol-induced liver injury rat model. Lu et al. (2011) further reported that a triterpenoid-enriched fraction of the ethanolic extract of A. cinnamomea mycelia showed the greatest effectiveness in preventing ethanol-induced acute liver injury and free radical generation in rats.
Kumar et al. (2011) evaluated the effect of the ethanolic extract of A. cinnamomea in ethanol-induced acute hepatotoxicity. Ethanolic extracts of A. cinnamomea mycelia (0.25, 0.5, and 1 g/kg BW, once a day) were orally administered to rats by gavage for 10 days. Silymarin (0.2 g/kg BW) was used as a positive control. After the final administration, hepatotoxicity was induced by administering ethanol (5 g/kg BW) through oral gavage. The results showed that the serum levels of ALT and AST in the group given the ethanolic extract of A. cinnamomea (1 g/kg BW) were comparable to those in the silymarin positive group (0.2 g kg BW). The levels of protein expression of hemeoxygenase-1 (HO-1) and NF-E2 related factor-2 (Nrf-2) were increased in groups orally administered with A. cinnamomea ethanolic extract and silymarin. The transcription factor Nrf-2 can induce the expression of a variety of cytoprotective and detoxifying genes (e.g., HO-1). This study suggested that the hepatoprotective effects of A. cinnamomea ethanolic extract might be through a mechanism that involves Nrf-2 activation and upregulation of the expression of the downstream antioxidant gene.
Figure 2 shows the hepatoprotective mechanisms of A. cinnamomea against ethanol-induced liver injuries according to the above results.
Figure 2.
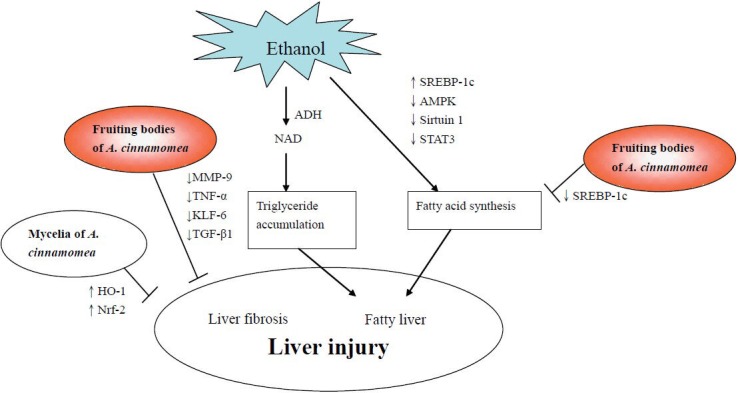
Mechanisms of fruiting bodies and mycelia of Antrodia Cinnamomea against ethanol-induced liver injuries.
Antrodia Cinnamomea and CCl4-induced Liver Injury
Chronic CCl4 treatment causes liver injury, oxidative stress, and nitrosative stress. Collagen accumulation, which causes hepatic fibrosis, was also found to be a result of chronic CCl4 treatment (Tipoe et al., 2010). The hepatotoxicity of CCl4 consists of 2 steps. The first step involves the production of free radicals (CCl3• and CCl3OO•) through hepatic metabolism mediated by the cytochrome P450 system. The second phase involves the activation of Kupffer cells, which is accompanied by the production of inflammatory and profibrogenic mediators. Cytochrome P450 2E1 (CYP2E1) knockout mice (cyp2e1-/-) were used to investigate the involvement of CYP2E1 in the development of CCl4-induced hepatotoxicity, and CYP2E1 was found to be a major factor in CCl4-induced hepatotoxicity in mice (Wong et al., 1998).
The enzymatic activation of CCl4, leading to the generation of CCl3 radicals, also disrupts the structure and function of lipids and proteins in the membrane and cell organelles (Xiao et al., 2012). The injured hepatic tissue is thus degraded by MMPs, while tissue inhibitor of MMPs (TIMPs) act as regulators of MMPs, preventing further degradation and damage to the newly synthesized collagen and unaffected tissue (Hemmann et al., 2007). CCl4 intoxication increased the expression of mRNAs, including MMP-2, MMP-9, TIMP-1, TIMP-2, TGF-β1, α-smooth muscle actin (SMA), and procollagen (Knittel et al., 2000). The synthesis of collagen leads to liver fibrosis. CCl4-induced liver injury involves the increased expression of TGF-β1 mRNA and hydroxyproline (the unique component of collagen) content, as observed in liver fibrosis.
Heptaprotective Effect of A. cinnamomea Fruiting Bodies Against CCl4-induced Liver Injury
Hsiao et al. (2003) investigated the hepatoprotective activity of the water extract of A. cinnamomea fruiting bodies against CCl4-induced liver injury in ICR mice. Mice were subcutaneously injected with CCl4 (40% CCl4 diluted with olive oil, 0.1 mL/10 g BW) twice a week for 8 weeks to induce chronic chemical liver injury. Water extracts of A. cinnamomea fruiting bodies (0.25, 0.75, 1.25 mg/kg BW, day, 4 days a week) were orally administered for 8 weeks. The levels of plasma transaminases, i.e., AST and ALT, were significantly lower in the group of mice orally administered with the water extract of A. cinnamomea (1250 mg/mL) than in the corn oil control group, and were comparable to the silymarin positive group (100 mg/kg BW). The activities of hepatic superoxide dismutase and catalase were dose-dependently increased by treatment with the water extract of A. cinnamomea. The water extract of A. cinnamomea fruiting bodies showed an ability to scavenge 1,1-diphenyl-2-picrylhydrazyl radicals in an in vitro study (Hseu et al., 2008). Thus, the water extract of A. cinnamomea fruiting bodies could effectively prevent CCl4-induced hepatotoxicity by scavenging the free radical formation or by inhibiting the inflammatory mediators in CCl4-mediated lipid peroxidation. The protective mechanisms of the fruiting bodies of A. cinnamomea against CCl4-induced liver injuries, liver fibrosis, and lipid peroxidation are shown in Figure 3.
Figure 3.
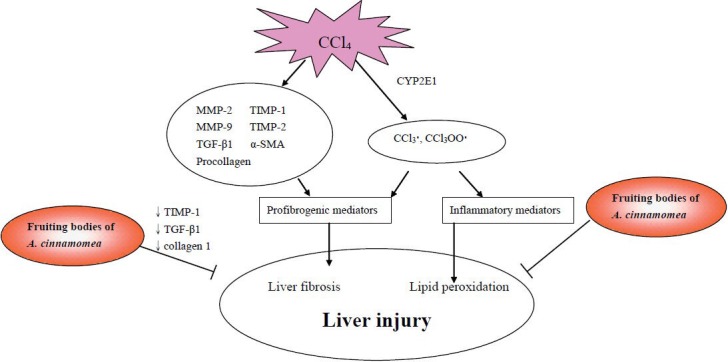
Mechanisms of fruiting bodies of Antrodia Cinnamomea against CCl4-induced liver injuries.
Antrodia Cinnamomea and Liver Cancer
Hepatocellular carcinoma (HCC) is one of the most lethal malignancies worldwide, especially in Taiwan, China, Korea, and Sub-Saharan Africa (Marrero, 2006). When cancer cells become invasive and metastatic, the excess breakdown of the extracellular matrix (ECM) occurs (Christofori et al., 2006). MMP-2 and MMP-9, members of the MMP family that degrade the ECM during tissue remodeling, are believed to play important roles in the invasion and metastasis of liver cancers (Hofmann et al., 2005).
Protective Effect of A. cinnamomea Fruiting Bodies Against Liver Cancer
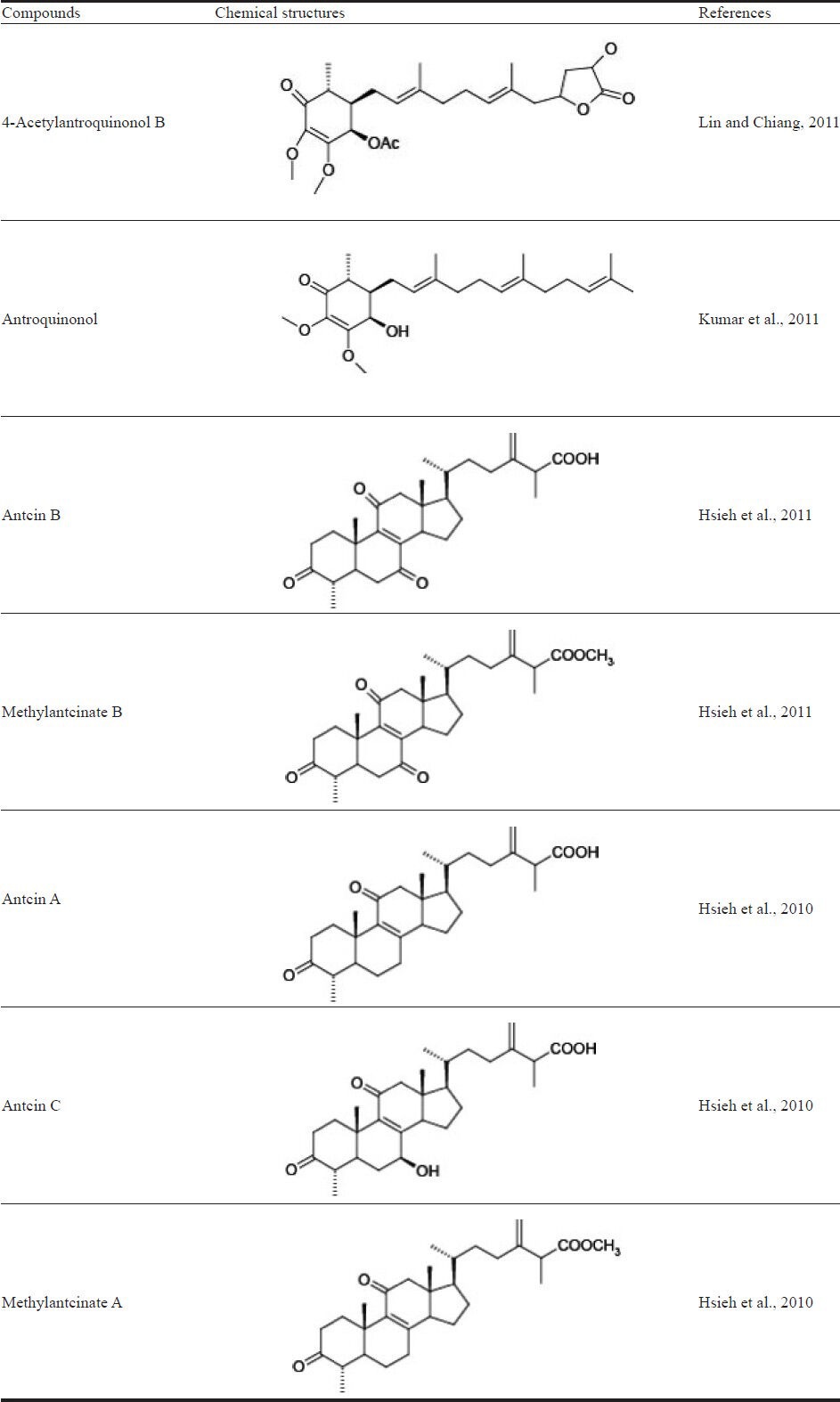
Although the hepatoprotective effects of A. cinnamomea fruiting bodies and mycelia have been reported, studies on the effect of A. cinnamomea fruiting bodies in liver cancer are few. Hsu et al. (2005) reported the apoptotic effects of the ethyl acetate extract of A. cinnamomea fruiting bodies in human HCC cell lines (i.e., HepG2 and PLC/PRF/5). They found that the extract inhibited cell survival signaling by increasing the expression of IκBα in the cytosol and decreasing the amount of NF-κB in the nucleus, leading to the increased expression of Bcl-XL in both HepG2 and PLC/PRF/5 cells. Hsu et al. (2007) further reported the anti-invasion potential of the ethyl acetate extract of A. cinnamomea fruiting bodies in PLC/PRF/5 cells. This extract decreased the expression of MMP-2, MMP-9, membrane type 1-MMP, and vascular endothelial growth factor, which resulted in the anti-invasion effects on PLC/PRF/5 cells. Hsieh et al. (2010) isolated 3 triterpenoid compounds, antcin A, antcin C, and methyl antcinate A, from the fruiting bodies and investigated the growth-inhibitory effects of these 3 compounds in human liver cancer cells (i.e., HepG2, Hep3B, and Huh7). Methyl antcinate A showed more inhibitory activity than the other 2 compounds and was most potent in Huh7 cells. Methyl antcinate A decreased the levels of antiapoptotic proteins (i.e., Bcl-2 and Bcl-XL), increased the cytochrome c release from mitochondria to the cytosol, increased the mitochondrial translocation of cofilin and Bax, and enhanced NADPH activity in cells, suggesting that the apoptotic effects of methyl antcinate A were partially through a mitochondrial signaling pathway. These results indicated that methyl antcinate A isolated from A. cinnamomea fruiting bodies is a potential candidate in the treatment of HCC. Hsieh et al. (2011) further reported the apoptotic activities of antcin B and methylantcinate B, 2 triterpenoids isolated from A. cinnamomea fruiting bodies. Antcin B and methylantcinate B both showed cytotoxicity in 3 HCC cell lines, i.e., HepG2, Hep3B, and Huh7. HepG2 showed more sensitivity to antcin B and methylantcinate B than the other cell lines and was further used for a mechanism study. Internucleosomal DNA fragmentation and subdiploid peak formation were observed in both antcin B and methylantcinate B-treated HepG2 cells, which suggested that both compounds triggered apoptotic cell death. The caspase activation and mitochondria-dependent pathways were both involved in the apoptosis induced by antcin B and methylantcinate B. The results indicated that antcin B and methylantcinate B induced NADPH oxidase-provoked oxidative stress and extrinsic and intrinsic apoptosis, as a critical mechanism of causing cell death in HCC cells.
Protective Effect of A. cinnamomea Mycelia Against Liver Cancer
Not only the fruiting bodies but also the fermented mycelia of A. cinnamomea were investigated for their anticancer activities. Song et al. (2005a) reported the effect of the methanolic extract of mycelia (MEM) from a submerged culture of A. cinnamomea on the inhibition of cell viability, as well as the mechanism of this effect. Antrodia Cinnamomea MEM inhibited the viability of liver cancer cells, i.e., HepG2 and Hep3B, in a dose-dependent manner. HepG2 cells showed more susceptibility to MEM than Hep3B cells. Furthermore, MEM had no significant inhibitory effect on normal primary rat hepatocytes. MEM induced DNA fragmentation in both HepG2 and Hep3B cells. However, G0/G1 cell cycle arrest was only observed in MEM-treated HepG2 cells. MEM resulted in the increase of caspase-3 and caspase-8 proteolytic activities and induced the proteolysis of pro-caspase-8 and procaspase-3. The above results indicated that MEM induced HepG2 apoptosis through the activation of caspase-3 and caspase-8 cascades and the regulation of cell cycle progression. Furthermore, Song et al. (2005b) reported the effects of MEM on the Fas and Bcl-2 pathways. Since Fas belongs to the TNF receptor (TNFR) superfamily, Fas ligands are known to induce apoptosis through Fas-induced caspase cascade activation, first caspase-8 followed by caspase-3 (Yoon et al., 2002). The expression of Fas was significantly increased in MEM-treated HepG2 cells (Song et al., 2005b). The expression of death receptors (DR), including DR3, DR4, TNFRI, and TNFRII, were decreased by MEM in both a dose-and time-dependent manner. When HepG2 was treated with MEM, the expression of the antiapoptotic protein Bcl-2 was decreased but that of the proapoptotic protein Bcl-XL increased. The MEM-induced cell apoptosis possibly involves the upregulation of Fas expression, which promotes Fas and FasL ligation and then passes the death message to cytosolic messengers. Therefore, procaspase-8 is activated to caspase-8, thus triggering the caspase activation cascade. The studies of Song et al. (2005a,b) did not identify the active compounds that contribute to the anti-HCC effects of A. cinnamomea mycelia extracts. Lin et al. (2010) reported 4-acetylantroquinonol B isolated from the ethanolic extract of A. cinnamomea mycelia to be the major antihepatoma constituent. The authors used antiproliferative activity-guided isolation to trace the active compound and identify its structure. 4-Acetylantroquinonol B was purified and identified as the most potent compound to inhibit the proliferation of HepG2 cells. The authors also reported that the antiproliferation mechanism of 4-acetylantroquinonol B (Lin and Chiang, 2011) in HepG2 cells was through a dose-dependent cell cycle arrest at the G phase. In the G1 phase, cyclin D, cyclin E, CDK2, and CDK4/6 work together to promote cell cycle progression. In 4-acetylantroquinonol B-treated HepG2 cells, the protein expression of CDK2 and CDK4 was slightly decreased. p27, a cell-cycle regulator, was reported to be inactivated in HCC and considered a suppressor of liver cancer cells. The expression level of p27 in HepG2 cells was increased by 4-acetylantroquinonol B. Thus, the growth-inhibitory effect of 4-acetylantroquinonol B was mostly mediated by cell cycle arrest through the decrease in CDK2 and CDK4 and increase in p27. Figure 4 shows the protective mechanisms of A. cinnamomea against liver cancer cells, including grow inhibition, apoptosis, and cell cycle arrest.
Figure 4.
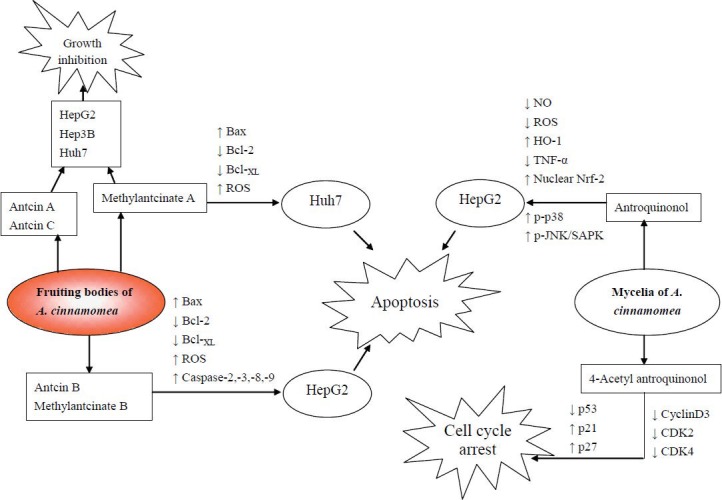
Mechanisms of fruiting bodies, mycelia, and active compounds of Antrodia Cinnamomea against liver cancer cells.
Active Compounds Against Liver Injury in the Fruiting Bodies and Mycelia of A. cinnamomea
Antrodia Cinnamomea fruiting bodies were reported to be rich in flavonoids, terpenoids, polyphenols, and polysaccharides (Chiang et al., 2010). In the study of Huang et al. (2010a), the authors used high-performance liquid chromatography to analyze the components of the methanolic extract of A. cinnamomea fruiting bodies. Ten compounds were run under the same condition as standards to identify the chemical components in the sample, including ascorbic acid, 2,3-dimethoxy-5-methyl[1,4] benzoquinone, 2,4,5-trimethoxybenzaldehyde (COX-2 inhibition, Chen et al., 2007), 4,7-dimethoxy-5-methyl-1,3-benzodioxide (antitumor, Tu et al., 2012), R,S-zhankuic acid C, dehydrosulphurenic acid (anti-inflammatory activity, Shen et al., 2007), R,S-zhankuic acid A, zhankuic acid B (anti-inflammatory activity, Shen et al., 2004), dehydroeburicoic acid (antileukemia, Du et al., 2012), and ergostatrien-3β-ol (anti-inflammation, Huang et al., 2010b). All 10 compounds were detected in the methanolic extract of the fruiting bodies. Wu et al. (2011) also analyzed the chemical contents of the A. cinnamomea fruiting body. According to their data, the fruiting bodies contain 0.24% polysaccharides, 12.95% triterpenoids, 0.3% total polyphenols, 0.05% flavonoids, and 0.015% condensed tannins.
Submerged fermentation or solid-state culture is a process extensively used for obtaining mycelia. Mycelial components such as maleic and succinic acid derivatives and triterpenoids were also investigated (Lu et al., 2011; Nakamura et al., 2004; Shao et al., 2008). Nakamura et al. (2004) reported 5 new maleic and succinic acid derivatives together with ergosterol peroxide from A. cinnamomea mycelia. Shao et al. (2008) identified 12 compounds from the methanolic extract of A. cinnamomea mycelia. Among them are 4 new compounds: 10-hydroxy-γ-dodecalactone, 11-hydroxy-γ-dodecalactone, 2-(2-hydroxyethyl)phenol, and 12-hydroxydodecanoic acid methyl ester; the other 8 compounds were ergostatrien-3 β-ol (ST-1), ergosterol peroxide, methyl (4-hydroxyphenyl) acetate, vanillin, 4-hydroxybenzaldehyde, hexadecanoic acid, 5-methoxymethylfuran-2-carbaldehyde, and 5-hydroxymethylfuran-2-carbaldehyde.
Lu et al. (2011) investigated the hepatoprotective effect of the fractions from ethanolic extracts of A. cinnamomea mycelia and analyzed the composition of fractions (Fr-) I to III. Fr-I is a triterpenoid-enriched fraction making up 359.8 mg per gram of mycelia. Fr-II is the polysaccharide content (483.1mg/g), which consists of rhamnose, arabinose, xylose, mannose, glucose, and galactose in a ratio of 0.11:0.23:0.06:0.77:0.29:1.00. Finally, Fr-III is a polyphenol-enriched fraction. The triterpenoid-enriched fraction, Fr-I, showed the best hepatoprotective activity against ethanol-induced acute liver injury in SD rats. However, the active compounds contributing to these hepatoprotective effects have not been reported up to now.
The active compounds isolated from A. cinnamomea shown to exhibit anti-liver cancer activities are listed in Table 2. Anticin A, anticin B, anticin C, and their derivatives methylantcinate A and methylantcinate B belong to the ergostane-type triterpenoids. Antroquinonol and 4-acetylantroquinonol B are ubiquinones. These compounds were reported to have anti-liver cancer activities in human hepatoma cell lines such as HepG2, Hep3B, and Huh7. As shown in Figure 4, anticin B, methylantcinate A, methylantcinate B, and antroquinonol exerted apoptosis-inducing effects through the mitochondrial-or ROS-mediated pathways, whereas 4-acetylantroquinonol inhibited cell growth by activating cell cycle arrest.
Table 2.
Compounds isolated from Antrodia Cinnamomea exhibiting anti-liver cancer activity.
Conclusion
Antrodia Cinnamomea attracts much attention for its effectiveness in treating liver injuries, as shown in numerous published articles. The hepatoprotective effects of the fruiting bodies and mycelia of this mushroom have been investigated in animal models of ethanol-and CCl4-induced liver injury. The extracts of A. cinnamomea fruiting bodies and mycelia have also been investigated for their anti-liver cancer activities. Several active compounds were identified to have anti-cancer activity in vitro.
The protective mechanisms of A. cinnamomea against ethanol-induced liver injuries were found to be through the inhibition of fatty acid synthesis and liver fibrosis (Figure 2). In addition, A. cinnamomea inhibited lipid peroxidation and liver fibrosis in CCl4-intoxicated animals (Figure 3). As for the anti-liver cancer effects, 7 compounds from A. cinnamomea were found to inhibit cell growth by the activating apoptosis or cell cycle arrest in human hepatoma cells (Figure 4).
The present report provides scientific evidences for the use of A. cinnamomea in ameliorating liver diseases. However, human clinical trials for studying the effectiveness of A. cinnamomea are still ongoing (http://clinicaltrials.gov/ct2/show/results/NCT01287286). The results of the studies described here suggest the potential of A. cinnamomea to prevent and treat liver diseases, as well as its potential to be developed into health foods or new drugs.
References
- 1.Ao Z.H, Xu Z.H, Lu Z.M, Xu H.Y, Zhang X.M, Dou W.F. Niuchangchih (Antrodia camphorata) and its potential in treating liver diseases. J Ethnopharmacol. 2009;121:194–212. doi: 10.1016/j.jep.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 2.Achliya G.S, Wadodkar S.G, Dorle A.K. Evaluation of hepatoprotective effect of Amalkadi Ghrita against carbon tetrachloride induced hepatic damage in rats. J Ethnopharmacol. 2004;90:229–232. doi: 10.1016/j.jep.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 3.Baraona E, Lieber C.S. Effects of ethanol on lipid metabolism. J Lipid Res. 1979;20:289–315. [PubMed] [Google Scholar]
- 4.Chen C.C, Liu Y.W, Ker Y.B, Wu Y.Y, Lai E.Y, Chyau C.C, Hseu T.H, Peng R.Y. Chemical characterization and anti-inflammatory effect of polysaccharides fractionated from submerge-cultured Antrodia camphorata mycelia. J Agric Food Chem. 2007;55:5007–5012. doi: 10.1021/jf063484c. [DOI] [PubMed] [Google Scholar]
- 5.Chiang P.C, Lin S.C, Pan S.L, Kuo C.H, Tsai I.L, Kuo M.T, Wen W.C, Chen P, Guh J.H. Antroquinonol displays anticancer potential against human hepatocellualr carcinoma cells: a crucial role of AMPK and mTOR pathway. Biochem Pharmacol. 2010;79:162–171. doi: 10.1016/j.bcp.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Christofori G. New signals from the invasive front. Nature. 2006;441:444–450. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- 7.Cronholm T, Jones A.W, Skagerberg S. Mechanism and regulation of ethanol elimination in humans: intermolecular hydrogen transfer and oxidoreduction in vivo. Alcohol Clin Exp Res. 1988;12:683–686. doi: 10.1111/j.1530-0277.1988.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 8.Day C.P, Yeaman S.J. The biochemistry of alcohol-induced fatty liver. Biochim Biophys Acta. 1994;1215:33–48. doi: 10.1016/0005-2760(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 9.Du Y.C, Chang F.R, Wu T.Y, Hsu Y.M, El-Shazly M, Chen C.F, Sung P.J, Lin Y.Y, Lin Y.H, Wu Y.C, Lu M.C. Antileukemia component, dehydroeburicoic acid from Antrodia camphorata induces DNA damage and apoptosis in vitro and in vivo models. Phytomedicine. 2012;19:788–796. doi: 10.1016/j.phymed.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Friedman S.L. Molecular mechanisms of hepatic fibrosis and principles of therapy. J Gastroenterol. 1997;32:424–430. doi: 10.1007/BF02934504. [DOI] [PubMed] [Google Scholar]
- 11.Galli A, Pinaire J, Fischer M, Dorris R, Crabb D.W. The transcriptional and DNA binding activity of peroxisome proliferator-activated receptor alpha is inhibited by ethanol metabolism A novel mechanism for the development of ethanol- induced fatty liver. J Biol Chem. 2001;276:68–75. doi: 10.1074/jbc.M008791200. [DOI] [PubMed] [Google Scholar]
- 12.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemmann S, Graf J, Roderfeld M, Roeb E. Expression of MMPs and TIMPs in liver fibrosis - a systematic review with special emphasis on anti-fibrotic strategies. J Hepatol. 2007;46:955–975. doi: 10.1016/j.jhep.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann U.B, Houben R, Brocker E.B, Becker J.C. Role of matrix metalloproteinases in melanoma cell invasion. Biochimie. 2005;87:307–314. doi: 10.1016/j.biochi.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Horiguchi N, Wang L, Mukhopadhyay P, Park O, Jeong W.I, Lafdil F, Osei-Hyiaman D, Moh A, Fu X.Y, Pacher P, Kunos G, Gao B. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury. Gastroenterology. 2008;134:1148–1158. doi: 10.1053/j.gastro.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoekstra L.T, de Graaf W, Nibourg G.A, Heger M, Bennink R.J, Stieger B, van Gulik T.M. Physiological and biochemical basis of clinical liver function tests: A Review. Ann Surg. 2012 doi: 10.1097/SLA.0b013e31825d5d47. [Google Scholar]
- 17.Hseu Y.C, Chen S.C, Yech Y.J, Wang L, Yang H.L. Antioxidant activity of Antrodia camphorata on free radical-induced endothelial cell damage. J Ethnopharmacol. 2008;118:237–245. doi: 10.1016/j.jep.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Hsiao G, Shen M.Y, Lin K.H, Lan M.H, Wu L.Y, Chou D.S, Lin C.H, Su C.H, Sheu J.R. Antioxidative and hepatoprotective effects of Antrodia camphorata extract. J Agric Food Chem. 2003;51:3302–3308. doi: 10.1021/jf021159t. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh Y.C, Rao Y.K, Whang-Peng J, Huang C.Y, Shyue S.K, Hsu S.L, Tzeng Y.M. Antcin B and its ester derivative from Antrodia camphorata induce apoptosis in hepatocellular carcinoma cells involves enhancing oxidative stress coincident with activation of intrinsic and extrinsic apoptotic pathway. J Agric Food Chem. 2011;59:10943–10954. doi: 10.1021/jf202771d. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh Y.C, Rao Y.K, Wu C.C, Huang C.Y, Geethangili M, Hsu S.L, Tzeng Y.M. Methyl antcinate A from Antrodia camphorata induces apoptosis in human liver cancer cells through oxidant-mediated cofilin- and Bax-triggered mitochondrial pathway. Chem Res Toxicol. 2010;23:1256–1267. doi: 10.1021/tx100116a. [DOI] [PubMed] [Google Scholar]
- 21.Hsu Y.L, Kuo Y.C, Kuo P.L, Ng L.T, Kuo Y.H, Lin C.C. Apoptotic effects of extract from Antrodia camphorata fruiting bodies in human hepatocellular carcinoma cell lines. Cancer Lett. 2005;221:77–89. doi: 10.1016/j.canlet.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Hsu Y.L, Kuo P.L, Cho C.Y, Ni W.C, Tzeng T.F, Ng L.T, Kuo Y.H, Lin C.C. Antrodia Cinnamomea fruiting bodies extract suppresses the invasive potential of human liver cancer cell line PLC/PRF/5 through inhibition of nuclear factor kappaB pathway. Food Chem Toxicol. 2007;45:1249–1257. doi: 10.1016/j.fct.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Huang C.H, Chang Y.Y, Liu C.W, Kang W.Y, Lin Y.L, Chang H.C, Chen Y.C. Fruiting body of Niuchangchih (Antrodia camphorata) protects livers against chronic alcohol consumption damage. J Agric Food Chem. 2010a;58:3859–3866. doi: 10.1021/jf100530c. [DOI] [PubMed] [Google Scholar]
- 24.Huang G.J, Huang S.S, Lin S.S, Shao Y.Y, Chen C.C, Hou W.C, Kuo Y.H. Analgesic effects and the mechanisms of anti-inflammation of ergostatrien-3beta-ol from Antrodia camphorata submerged whole broth in mice. J Agric Food Chem. 2010b;58:7445–7452. doi: 10.1021/jf1013764. [DOI] [PubMed] [Google Scholar]
- 25.Jeong W.I, Park O, Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology. 2008;134:248–258. doi: 10.1053/j.gastro.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiménez-López J.M, Carrasco M.P, Segovia J.L, Marco C. Resistance of HepG2 cells against the adverse effects of ethanol related to neutral lipid and phospholipid metabolism. Biochem Pharmacol. 2002;63:1485–1490. doi: 10.1016/s0006-2952(02)00896-1. [DOI] [PubMed] [Google Scholar]
- 27.Knittel T, Mehde M, Grundmann A, Saile B, Scharf J.G, Ramadori G. Expression of matrix metalloproteinases and their inhibitors during hepatic tissue repair in the rat. Histochem Cell Biol. 2000;113:443–453. doi: 10.1007/s004180000150. [DOI] [PubMed] [Google Scholar]
- 28.Kumar K.J, Chu F.H, Hsieh H.W, Liao J.W, Li W.H, Lin J.C, Shaw J.F, Wang S.Y. Antroquinonol from ethanolic extract of mycelium of Antrodia Cinnamomea protects hepatic cells from ethanol-induced oxidative stress through Nrf-2 activation. J Ethnopharmacol. 2011;136:168–177. doi: 10.1016/j.jep.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 29.Lieber C.S, Jones D.P, Mendelson J, De Carli L.M. Fatty liver, hyperlipemia and hyperuricemia produced by prolonged alcohol consumption, despite adequate dietary intake. Trans Assoc Am Physicians. 1963;76:289–300. [Google Scholar]
- 30.Lin Y.W, Chiang B.H. 4-acetylantroquinonol B isolated from Antrodia Cinnamomea arrests proliferation of human hepatocellular carcinoma HepG2 cell by affecting p53, p21 and p27 levels. J Agric Food Chem. 2011;59:8625–8631. doi: 10.1021/jf2011326. [DOI] [PubMed] [Google Scholar]
- 31.Lin Y.W, Pan J.H, Liu R.H, Kuo Y.H, Sheen L.Y, Chiang B.H. The 4-acetylantroquinonol B isolated from mycelium of Antrodia Cinnamomea inhibits proliferation of hepatoma cells. J Sci Food Agric. 2010;90:1739–1744. doi: 10.1002/jsfa.4010. [DOI] [PubMed] [Google Scholar]
- 32.Lu Z.M, Tao W.Y, Xu H.Y, Ao Z.H, Zhang X.M, Xu Z.H. Further studies on the hepatoprotective effect of Antrodia camphorata in submerged culture on ethanol-induced acute liver injury in rats. Nat Prod Res. 2011;25:684–695. doi: 10.1080/14786410802525487. [DOI] [PubMed] [Google Scholar]
- 33.Lu Z.M, Tao W.Y, Zou X.L, Fu H.Z, Ao Z.H. Protective effects of mycelia of Antrodia camphorata and Armillariella tabescens in submerged culture against ethanol-induced hepatic toxicity in rats. J Ethnopharmacol. 2007;110:160–164. doi: 10.1016/j.jep.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 34.Marrero J.A. Hepatocellular carcinoma. Curr Opin Gastroenterol. 2006;22:248–253. doi: 10.1097/01.mog.0000218961.86182.8c. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura N, Hirakawa A, Gao J.J, Kakuda H, Shiro M, Komatsu Y, Sheu C.C, Hattori M. Five new maleic and succinic acid derivatives from the mycelium of Antrodia camphorata and their cytotoxic effects on LLC tumor cell line. J Nat Prod. 2004;67:46–48. doi: 10.1021/np030293k. [DOI] [PubMed] [Google Scholar]
- 36.Shao Y.Y, Chen C.C, Wang H.Y, Chiu H.L, Hseu T.H, Kuo Y.H. Chemical constituents of Antrodia camphorate submerged whole broth. Nature Product Research. 2008;22:1151–1157. doi: 10.1080/14786410601132410. [DOI] [PubMed] [Google Scholar]
- 37.Sharma H.S, Kilpatrick M, Lyons G. Prediction of potential mushroom yield by visible and near-infrared spectroscopy using fresh phase II compost. Appl Spectrosc. 2005;59:1054–1059. doi: 10.1366/0003702054615269. [DOI] [PubMed] [Google Scholar]
- 38.Shen Y.C, Wang Y.H, Chou Y.C, Chen C.F, Lin L.C, Chang T.T, Tien J.H, Chou C.J. Evaluation of the antiinflammatory inflammatory activity of zhankuic acids isolated from the fruiting bodies of Antrodia camphorata. Planta Med. 2004;70:310–314. doi: 10.1055/s-2004-818941. [DOI] [PubMed] [Google Scholar]
- 39.Shen C.C, Wang Y.H, Chang T.T, Lin L.C, Don M.J, Hou Y.C, Liou K.T, Chang S, Wang W.Y, Ko H.C, Shen Y.C. Anti-inflammatory ergostanes from the basidiomata of Antrodia salmonea. Planta Med. 2007;73:1208–1213. doi: 10.1055/s-2007-981591. [DOI] [PubMed] [Google Scholar]
- 40.Song T.Y, Hsu S.L, Yeh G.C. Induction of apoptosis in human hepatoma cells by mycelia of Antrodia camphorata in submerged culture. J Ethnopharmacol. 2005a;100:158–167. doi: 10.1016/j.jep.2005.02.043. [DOI] [PubMed] [Google Scholar]
- 41.Song T.Y, Hsu S.L, Yeh C.T, Yen G.C. Mycelia from Antrodia camphorata in Submerged culture induce apoptosis of human hepatoma HepG2 cells possibly through regulation of Fas pathway. J Agric Food Chem. 2005b;53:5559–5564. doi: 10.1021/jf050329+. [DOI] [PubMed] [Google Scholar]
- 42.Tipoe G.L, Leung T.M, Liong E.C, Lau T.Y, Fung M.L, Nanji A.A. Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology. 2010;273:45–52. doi: 10.1016/j.tox.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Tu S.H, Wu C.H, Chen L.C, Huang C.S, Chang H.W, Chang C.H, Lien H.M, Ho Y.S. In vivo antitumor effects of 4,7-dimethoxy-5-methyl-1,3-benzodioxole isolated from the fruiting body of Antrodia camphorata through activation of the p53-mediated p27/Kip1 signaling pathway. J Agric Food Chem. 2012;60:3612–3618. doi: 10.1021/jf300221g. [DOI] [PubMed] [Google Scholar]
- 44.Wong F.W, Chan W.Y, Lee S.S. Resistance to carbon tetrachloride-induced hepatotoxicity in mice which lack CYP2E1 expression. Toxicol Appl Pharmacol. 1998;153:109–118. doi: 10.1006/taap.1998.8547. [DOI] [PubMed] [Google Scholar]
- 45.Wu M.F, Peng F.C, Chen Y.L, Lee C.S, Yang Y.Y, Yeh M.Y, Liu C.M, Chang J.B, Wu R.S, Yu C.C, Lu H.F, Chung J.G. Evaluation of genotoxicity of Antrodia cinnamomea in the Ames test and the in vitro chromosomal aberration test. In Vivo. 2011;25:419–423. [PubMed] [Google Scholar]
- 46.Xiao J, Liong E.C, Ching Y.P, Chang R.C, So K.F, Fung M.L, Tipoe G.L. Lycium barbarum polysaccharides protect mice liver from carbon tetrachloride-induced oxidative stress and necroinflammation. J Ethnopharmacol. 2012;139:462–470. doi: 10.1016/j.jep.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 47.Yoon J.H, Gores G.J. Death receptor-mediated apoptosis and the liver. Hepatology. 2002;37:400–410. doi: 10.1016/s0168-8278(02)00209-x. [DOI] [PubMed] [Google Scholar]
- 48.You M, Rogers C.Q. Adiponectin: a key adipokine in alcoholic fatty liver. Exp Biol Med (Maywood) 2009;234:850–859. doi: 10.3181/0902-MR-61. [DOI] [PubMed] [Google Scholar]
- 49.You M, Liang X, Ajmo J.M, Ness G.C. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am J Physiol Gastrointest Liver Physiol. 2008;294:G892–898. doi: 10.1152/ajpgi.00575.2007. [DOI] [PubMed] [Google Scholar]
- 50.You M, Matsumoto M, Pacold C.M, Cho W.K, Crabb D.W. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. 2004;127:1798–1808. doi: 10.1053/j.gastro.2004.09.049. [DOI] [PubMed] [Google Scholar]


