Abstract
The in vitro and in vivo bioactivities of silibinin (SB), paclitaxel (PTX) and SB and PTX in combination (SB+PTX) against murine metastatic mammary 4T1 cancer cell line were investigated. Isobologram and combination index (CI) analyses showed that SB and PTX can function synergistically in the inhibition of 4T1 cell proliferation with a CI value < 1. Both SB and PTX alone or SB+PTX treatment inhibited 4T1 cell migration and motility possibly through downregulation of the serpin protease nexin-1 (PN-1) and N-cadherin expression, inhibition of matrix metalloprotease (MMP)-9 activity, and upregulation of E-cadherin. Flow cytometry and Western blot analyses demonstrated that both drugs deregulated cell-cycle mediators and induced apoptosis in 4T1 cells. A real-time in vivo bioluminescence imaging system to monitor the breast cancer cell metastasis in syngeneic BALB/c mice was established using a stable 4T1pGL-COX-2/Luc cell clone carrying a COX-2 promoter driven-luciferase reporter gene. In vivo study using the allograft 4T1pGL-COX-2/Luc metastatic mouse model indicated that SB co-treated with PTX can significantly suppress lung metastasis of 4T1 cells likely through inhibiting cell proliferation and angiogenesis. Together, this study demonstrates that SB could act synergistically with PTX in 4T1 cells, providing a therapeutic option for highly metastatic triple negative breast cancer.
Keywords: Silibinin, Paclitaxel, Triple negative breast cancer, Lung metastasis, Synergistic effect
Introduction
Breast cancer is the most common cancer in women, and the incidence, morbidity and mortality of the disease remain a major concern globally (Maxmen 2012). Immunohistochemical markers such as the expression of hormone receptors, e.g., estrogen receptor (ER) and progesterone receptors (PR) and the overexpression of HER2, provide therapeutic predictive value and clinical guidance in selection of breast cancer treatments. While effective targeted therapeutic modalities exist for women with hormone receptor positive and HER2-positive disease, chemotherapy is the only systemic therapy available for women with triple negative breast cancer (TNBC) (Lin et al., 2012).
Chemotherapy is used extensively in treating cancers, but its effectiveness, drug resistance and adverse side effects in patients are common concerns. For instance, the side effects of a commonly used anticancer drug paclitaxel (PTX) include pain, hair loss, diarrhea, nausea, vomiting and lowered white blood and red blood cell counts with increased risk of infection and anemia in patients (Salvinelli et al., 2003; Lee et al., 2012). This has led to the search for adjuvant therapy combining cytotoxic chemotherapeutic drugs with less toxic agent(s) with the potential to boost antitumor activity and reduce side effects that are considered to be an alternative approach for cancer treatment (Lin et al., 2012; Sarkar & Li 2006).
Silibinin (SB), the most active flavonolignan constituent present in the milk thistle (Silybum marianum L.) extract silymarin has demonstrated pleiotropic effects against a variety of cancers (Deep & Agarwal 2010). Silymarin was reported previously for its clinical properties in the management of hepatic disorders (Wellington & Jarvis 2001). Preclinical studies indicate that SB or silymarin are also effective against epithelial-type cancers, such as prostate, lung, ovarian, colon, and skin cancers (Colombo et al., 2011; Kaur & Agarwal 2007; Zhou et al., 2008). SB or silymarin has thus been considered a novel candidate for cancer chemotherapy and chemoprevention as it is well tolerated and is considered safe (Hoh et al., 2006), and can enhance chemosensitivity to select anticancer drugs, e.g., 5-fluorouracil, doxorubicin or PTX (Colombo et al., 2011; Zhou et al., 2008).
In the present study, we investigated the therapeutic effect and underlying mechanisms of SB, PTX and SB PTX cotreatment on triple negative breast cancer cells 4T1 in vitro and in an experimental lung metastatic mouse model. Our results demonstrate that SB and PTX can act synergistically by suppressing 4T1 cell activity through inhibiting migration, modulating cell-cycle machinery and inducing apoptosis. Both drugs also suppressed lung metastasis of 4T1 cells in syngeneic BALB/c mice.
Materials and Methods
Cell lines and culture conditions
The 4T1 murine metastatic breast cancer cell line was obtained from the American Type Culture Collection (ATCC, CRL-2539; Manassas, VA, USA). The TS/A murine adenocarcinoma cell line was a gift from Dr. Ning-Sun Yang of the Agriculture Biotechnology Research Center, Academia Sinica, Taiwan. The human normal breast epithelial cell line H184B5F5/M10 (BCRC 60197) was purchased from the Bioresource Collection and Research Center (BCRC, Hsinchu, Taiwan). All cell lines were cultured in indicated media supplemented with 10% fetal bovine serum (FBS) and penicillin-streptomycin (Invitrogen, Carlsbad, CA, USA) in a humidified 5% CO2 incubator at 37°C.
Animals
All animal care and experimental procedures adhered to the guidelines of Academia Sinica Institutional Animal Care and Utilization Committee. Female BALB/cByJNar1 mice (six-week-old) obtained from National Laboratory Animal Center, Taipei, Taiwan) were given a standard laboratory diet and distilled water ad libitum and kept on a 12 h light/dark cycle at 22 ± 2°C in the Animal Facility of Agricultural Biotechnology Research Center, Academia Sinica.
Cell proliferation and cell cycle analyses
In cell proliferation assays, 4T1 cells were cultured in 96-well plates at 4 × 103 cells/well and allowed to adhere overnight. The cells were then treated for 24 h with 0.2% vehicle of dimethyl sulphoxide (DMSO) or indicated concentrations of silibinin (SB) and paclitaxel (PTX) (St. Louis, Mo, USA). Cell proliferation was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)-based colorimetric assay.
Analysis of cell cycle was carried out as described previously (Shyur et al., 2004). 4T1 cells (2 × 105 cells/mL) were synchronized by incubation in medium containing 1% FBS for 12 h. The low-serum (1% FBS) medium was then replaced by medium containing 10% FBS, and the 4T1 cells were treated with vehicle, SB, PTX, or SB+PTX for 6, 12, 24, and 48 h, respectively. Both adherent and floating cells were collected, washed with phosphate-buffered saline (PBS) and fixed with 1 mL of ice-cold 70% ethanol overnight at 4°C. Cells were stained with 0.2 mg/mL propidium iodide in darkness for 30 min at room temperature and analyzed by flow cytometry (FACS Calibular, BD Biosciences, Bedford, MA, USA).
Western blotting
Western blot analysis was performed following procedures described previously (Chiang et al., 2005). Protein content was measured by the Bradford method (Bio-Rad Laboratories, Hercules, CA, USA). Proteins were resolved by 5–20% gradient SDS-PAGE and then electrotransferred to polyvinylidene difluoride (PVDF) membrane (Immobilon, Millipore, Bedford, MA, USA). The blot was incubated in blocking buffer (3% skim milk in TBS buffer) for 30 min, and then incubated with antibodies against cleaved caspase 3, cleaved PARP (Cell Signaling, MA, USA), E-cadherin (Millipore Corp, Bedford, MA, USA), and cyclin D2, cyclin E, cdk 1, cdk 2, cyclin A, cyclin B1, cleaved PARP, N-cadherin, and actin (Santa Cruz Biochemicals, Santa Cruz, CA, USA) overnight at 4°C. After incubation with the appropriate secondary anti-rabbit or anti-mouse IgG conjugated to horseradish peroxidase, the reactive bands were visualized using enhanced chemiluminescence reagents (Amersham, Arlington Heights, IL, USA).
Analysis of synergistic effect of drugs combination
Isobologram analysis was used to determine the effects of SB and PTX in combination on 4T1 cells according to the Chou-Talalay method (Chou 2010). MTT assays were performed in 96 wells. Compound 1 (SB or PTX) was applied at a fixed concentration while compound 2 (PTX or SB, respectively) was applied at a range of concentrations in order to determine whether the effects were dose-dependent. The interaction of two compounds was quantified by determining the combination index (CI), according to the following classic isobologram equation: CI = (D)1/(Dx)1 + (D)2/(Dx)2., where Dx is the dose of one compound alone required to produce an effect, and (D)1 and (D)2 are the doses of compounds 1 (SB) and 2 (PTX), respectively, in combination that produces the same effect as the compound alone. From this analysis, the combined effects of the two drugs can be summarized as follows: CI = 1 indicates summation (additive and zero interaction); CI < 1 indicates synergism; and CI > 1 indicates antagonism.
Wound healing assay
4T1 cells were cultured in a 24-well plate until they formed a confluent monolayer. A wound (width 2 mm) was then created with a standard 2 mm wide strip. The cells were treated with indicated concentrations of SB, PTX or SB+PTX at 37 °C for 24 h and images were then taken by inverted microscopy (Axiovert 200M, Zeiss, Jena, Germany) at 100x, magnification. The cell numbers that migrated into the denuded area were counted.
Effect on MMP-2 and MMP-9 activities
The activities of the matrix metalloproteinase (MMP)-2 and MMP-9 were analyzed using zymography. After drug treatments, the conditioned media were collected and mixed with non-reducing sample buffer, then subjected to SDS-PAGE with 8% polyacrylamide gels copolymerized with 1 mg/mL gelatin. Gels were rinsed in washing buffer (50 mM Tris–HCl, pH 7.5, 2.5% Triton X-100) at room temperature for 1 h and incubated overnight at 37°C in zymogram development buffer (50 mM Tris–HCl, pH 7.5, 10 mM CaCl2 and 150 mM NaCl). Gels were fixed and stained with filtrated 0.1% Coomassie brilliant blue R250. After destaining, gelatinolytic signals were quantified by densitometry. Gelatinolytic activities of MMP-2 and MMP-9 were visualized as a clear band appearing at 72 and 92 kDa, respectively, against a dark gel background.
Stable 4T1pGL-COX-2/Luc cell line generation
A stable 4T1 cancer cell clone carrying a COX-2 promoter driven-luciferase (pGL-COX-2/Luc) reporter gene was established in this study. We previously constructed a gene plasmid in the pGL4.20-basic vector containing a luciferase reporter gene driven by the full length 5’ flanking promoter region (n1334/11) of the human COX-2 promoter (Hou et al., 2007). The plasmid was transfected into 4T1 cells using Lipofectamine PLUS (Invitrogen). Following several passages in the presence of puromycin (0.5 μg/mL), a stable 4T1 cell clone (designated 4T1pGL-COX-2/Luc) carrying the reporter gene and displaying the highest level of luciferase expression and activity among the selected transfected clones was isolated and used in the mouse lung metastasis experiments.
Effect on lung metastasis of 4T1 cells in BALB/c mice
The mouse tail vein was injected with 5 × 105 4T1 COX-2/Luc cells on day 0. Mice were assigned to five groups (n = 6 per group) at day 1, including a vehicle control, a tumor control, and SB-200 (200mg/kg), PTX-2 (2mg/kg), PTX-5 (5mg/kg), and SB-200+PTX-2 cotreatment groups. SB was fed by oral gavage and PTX was administered intraperitoneally (i.p.) every other day.
Prior to take the in vivo bioluminescence imaging, the mice were anesthetized with isoflurane (USP, South Carolina, USA) in an acrylic chamber with 2.5% isoflurane/air mixture and i.p. injected with substrate D-luciferin solution (150 mg/kg) in PBS. After 10 minutes, images were taken in the live anesthetized mice using a bioluminescence IVIS Spectrum System (Caliper, CA, USA), which includes a cryogenic cooling unit and a data acquisition computer running Living Image software (Xenogen Corp.). The acquisition and overlay of a pseudocolor image was taken that represented the spatial distribution of detected photons emerging from active luciferase within the animals. Bioluminescent signals were quantified using Living Image 2.5 (Xenogen Corp.) as photons/sec/ROI (region of interest) at days 6, 10, 14 and 21 of treatment.
At day 21, the experimental endpoints, luciferin-injected animals were sacrificed, and lung organs were removed and imaged within 15 minutes of injection. The organs were then fixed with 10% buffered formalin and embedded in paraffin for haemotoxylin & eosin (H&E) staining and immunostaining with vascular endothelial growth factor (VEGF) and proliferating cell nuclear antigen (PCNA) antibody as described previously (Huang et al., 2010).
Data analysis
All data are expressed as mean ± SEM. Differences between treatments were determined by unpaired, two-tailed Student's t test or ANOVA. A P < 0.05 was considered statistically significant.
Results
Cytotoxic effect of silibinin and paclitaxel in breast cancer cells
The cytotoxic effect of silibinin (SB) (Figure. 1A) and paclitaxel (PTX) (Figure 1B) on ER(-) 4T1 and ER(+) TS/A murine mammary cancer cells were investigated. The test cells were exposed to a range of concentrations of SB (50-400 μM) or PTX (10-250 nM) for 24 h. SB was observed to induce a concentration-dependent loss of cell viability with similar IC50 values in both 4T1 and TS/A cells as measured by MTT assay (Figure 1). The viability of normal mammary epithelial M10 cells tested in parallel was not affected by SB treatment at concentrations up to 400 μM (Figure 1C), which is in agreement with studies showing that SB is relatively safe in clinical applications. On the contrary, PTX induced more modest inhibition (60-67%) in the 4T1 and TS/A cancer cell lines and in the normal M10 cells at concentrations up to 250 nM (Figure 1D). These results indicate that the chemotherapeutic drug PTX did not discriminate between normal and cancer cells. We therefore proceeded to investigate whether SB can sensitize the cells or function synergistically with PTX against the triple negative breast cancer line 4T1; currently, no systemic clinical treatment protocol for TNBC yet exists.
Figure 1.

Inhibitory effect on breast cancer cell proliferation of silibinin and paclitaxel. (A-B) Chemical structures of silibinin (SB) and paclitaxel (PTX). Two murine breast cancer cell lines 4T1 (ER-) and TS/A (ER+) and normal mammary epithelial M10 cells were treated with indicated concentrations of SB (C) and PTX (D), respectively. Cell viability was determined by MTT assay.
Combined effect of silibinin and paclitaxel on the growth of 4T1 cells
To investigate whether SB can act synergistically with PTX on 4T1 cell growth inhibition, cells were treated with SB and PTX alone or in combination for 6 h, 12 h, and 24 h, respectively, and cell viability was assessed by MTT assay. The concentrations used were 150 μM SB (SB-150), 450 μM SB (SB-450), 45 nM PTX (PTX-45), 180 nM PTX (PTX-180), and 150 μM SB plus 45 nM PTX (SB-150/PTX-45). We observed that the summation of percentage inhibition of SB-150 (14.9%) and PTX-45 (24.3%) was similar to that of SB-150/PTX-45 cotreatment (38.5%), and the combined treatment caused a significant (P < 0.05) decrease in cytotoxicity in the 4T1 cells compared to SB-150 or PTX-45 (Figure 2A). We therefore performed isobologram analysis (Chou 2010) to verify the combinational effect of SB and PTX against 4T1 cell activity. The 4T1 cells were challenged with different concentration combinations of SB (50-350 μM) and PTX (20-150 nM) for 24 h to determine the combined concentrations which can reduce 40% cell viability (IC40). The combined effect of SB and PTX was then be evaluated by plotting the six concentration combinations, deriving a curve that connects the pairs of drug concentrations expected to induce 40% growth inhibition (IC40), and determining the relationship between this curve and the theoretical additive (dashed) line connecting the IC40 for SB (325 μM) and the IC40 for PTX (125 nM) (Figure 2B). Our data produced a combination index (CI) < 1, indicating synergism of SB and PTX against 4T1 cells. Further, the range of CI values and combination inhibitory effects were calculated from the data obtained in Figure 2B fell between 0.43 and 0.96, and 22.3% and 58.5%, respectively (Figure 2C). These results indicate that SB and PTX indeed acted synergistically to inhibit the growth of breast cancer cells.
Figure 2.

Effects of silibinin and paclitaxel alone or in combination on the growth of 4T1 cells. (A) Percentage inhibition of 150 μM SB, 450 μM SB, 45 nM PTX, 180 nM PTX, and 150 μM SB+45 nM PTX on 4T1 cell growth in different treatment times. Data are mean ± SD of three independent experiments. (B) Classic isobologram analysis of combined effect of SB and PTX producing 40% inhibition (IC40) in 4T1 cells. Cells (4000 cell/well) were treated with SB and PTX for 24 h. Values were the means of three independent experiments. The dashed line indicates the zero interaction of isobole. The CI <1 indicates synergy; CI = 1 indicates an additive effect; and CI > 1 indicates antagonism. (C) Combination index plot of SB and PTX cotreatment.
SB/PTX induces cell cycle perturbation and apoptosis
Flow cytometry analysis revealed that 12-h treatment decreased the G1 phase DNA content from 54.5% in the control group to 43.3% with SB-150 treatment, 32.7% with PTX-45 treatment, and 13.0% in cells treated with both SB-150 and PTX-45 (Figure 3A). Further, the G2/M phase DNA content of the control group was 29.2%, and increased to 42.6% with SB-150 treatment, 45.2% with PTX-45 treatment, and 64.1% SB-150/PTX-45 treatment. Apoptotic sub-G1 DNA was only identified in cells treated with PTX-45 and SB-150/PTX-45, with 12.4% and 13.5%, respectively (Figure 3A).
Figure 3.

Silibinin and paclitaxel induce cell-cycle arrest in 4T1 cells. (A) Flow cytometry analysis of DNA distribution in propidium iodide-stained cells. The 4T1 cells were treated with 0.2% DMSO (control), 150 μM SB (SB-150), 45 nM PTX (PTX-45) and 150 μM SB+45 nM PTX (SB-150/ PTX-45) for 12 h. The percentages of G1, S, G2/M, and sub-G1 cells were calculated. (B) Western blot analysis of protein expression levels of cellcycle mediators and apoptotic hallmark PARP. Western blot analysis of protein expression levels of cell-cycle mediators and apoptotic hallmark PARP in 4T1 cells treated with indicated concentrations and times of vehicle or drugs. Signal intensities of the band were determined by using the ImageJ software. Protein levels were normalized to the level of actin.
Next, we examined expression levels of key cell cycle mediators in synchronous 4T1 cells treated with SB and PTX (Figure 3B). Expression of the G1 phase mediators cyclin D2 and cyclin E decreased over the time course (6, 12, and 24 h) in 4T1 cells following treatment with SB-150, PTX-45, and SB-150/PTX-45. Expression of the G1/S phase mediator cdk2 slightly decreased in SB-150 and SB-150/PTX-45 treated cells, but remained unchanged in PTX-45 treated cells, compared to vehicle control cells. Cyclin A, involved in S and G2 phase transition, also decreased in all treatments groups. The expression of cyclin B1 and cdk1, proteins involved in the G2/M phase, increased over the time course in all treatments. Additionally cells receiving the combined SB-150/PTX-45 treatment also exhibited an increase in the cleaved form of PARP, a hallmark of apoptosis.
Effects of SB and PTX on metastatic activity of 4T1 cells
The horizontal cell migration capacity of 4T1 cells was evaluated by wound healing assay as described previously (Huang et al., 2010). Cells were treated with 0.2% DMSO (control), SB-150, SB-450, PTX-45, PTX-180 and SB-150/PTX-45 for 24 h, and the number of cells that had migrated into the wound (lesion) area was counted. The DMSO-treated control group had 702 ± 35 cells/field, while all treatment groups significantly inhibited cell migration (SB-150: 424 ± 20 cells/field; SB-450: 218 ± 20 cells/field; PTX-45: 201 ± 21 cells/field; PTX-180: 111 ± 17 cells/field; and SB-150/PTX-45: 141 ± 20 cells/field) (Figure 4A).
Figure 4.
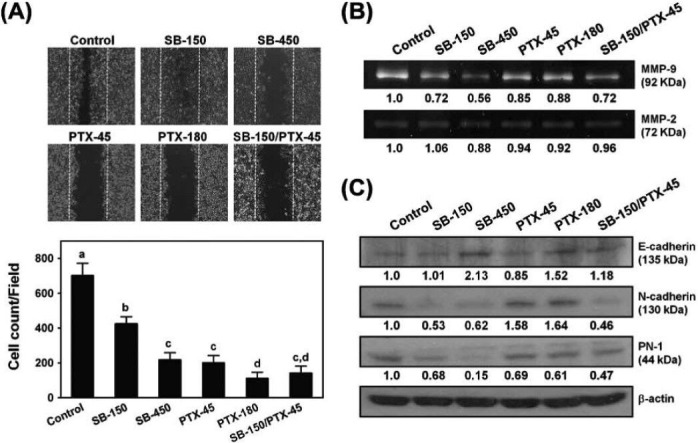
Silibinin and paclitaxel inhibit wound healing and MMP-9 activity in 4T1 cells. (A) Inhibitory effect on cell migration was assessed by wound healing assay. The effect on the movement of 4T1 cells into the denuded area by treatment with indicated concentrations of SB, PTX or SB+PTX at 37 °J for 24 h was quantified by counting the numbers of cells. (B) Zymography analysis of MMP-9 and MMP-2 activity in 4T1 cells. The gelatinolytic bands corresponding to MMP-9 and MMP-2 proteins are indicated. The ratios of active MMP-9 and MMP-2 bands relative to that in vehicle control were determined by densitometry. (C) Western blot analysis of E-cadherin, N-cadherin and PN-1 proteins in 4T1 cells with vehicle or drug treatments for 24 h.
Because of the significant effects induced by SB and PTX on cell migration, we next evaluated the effects of these drugs on MMP-2 and MMP-9 enzymatic activities in 4T1 cells using gelatin zymography. Little or no effect on MMP-2 activity was detected in the different treatment groups. However, cells treated with SB and PTX alone or together exhibited more reduced band intensities of MMP-9 relative to the control, indicating a reduction in MMP-9 activity (Vehicle: 1.0; SB-150: 0.72; SB-45: 0.56; PTX-45: 0.85; PTX-180: 0.88; SB-150/PTX-45: 0.72) (Figure 4B).
The expression of three other proteins involved in breast cancer cell motility, invasion and metastasis, i.e., E-cadherin, N-cadherin and PN-1, were examined. Both drugs alone or in combination caused the increase of E-cadherin and the decrease of PN-1 protein expressions, but the decrease of N-cadherin protein observed in SB and SB/PTX treated cells were not observe in PTX treated cells (Figure 4C).
Effect of SB and PTX on lung metastasis of 4T1 cells in BALB/c mice
To further investigate the influence of SB and PTX on 4T1 cancer cell metastasis in vivo, we established a stable 4T1pGL-COX-2/Luc cell line carrying a COX-2 promoter-driven luciferase reporter gene for in situ and live monitoring of cancer cell migration and location in test animals. The 4T1pGL-COX-2/Luc cells were introduced by intravenous (i.v.) injection into BALB/c mice at mammary fat pad areas, and 21 days later bioluminescence imaging was performed to determine the degree of lung metastasis of the cancer cells in test animals (Figure 5A). Significant inhibition of 4T1 cell metastasis into the lungs was detected in all treatment groups, i.e., PTX-2, PTX-5, SB-200, and SB-200/PTX-2 compared to the tumor group (P < 0.05). However, no statistical difference was observed among the treatment groups (Figure 5A).
Figure 5.

Silibinin and paclitaxel inhibit lung metastasis of 4T1 cells in syngeneic BALB/c mice. (A)Bioluminescence imaging of lung organs of female BALB/c mice 21 days after 4T1pGL-COX-2/Luc cell implementation, and treatment with vehicle, 200 mg/kg SB (SB-200), 2 mg/kg PTX (PTX-2), 5 mg/kg PTX (PTX-5), and 200 mg/kg SB+2 mg/kg PTX (SB-200/PTX-2) SB. Fluorescent (Light emission) intensities of metastatic cancer cells in lung tissues were acquired using the IVIS Spectrum System (Xenogen Corp.). Quantification of photon counts representing degree of metastasis is shown. *: Significant difference with P < 0.05 as determined by one-way ANOVA. (B) Haemotoxylin and eosin (H&E) staining and immunohistochemical analysis. The representative levels of vascular endothelial growth factor (VEGF) and proliferating cell nuclear antigen (PCNA) in lung tissues of sham control, tumor control, 200 mg/kg SB (SB-200), 2 mg/kg PTX (PTX-2), 5 mg/kg PTX (PTX-5), and 200 mg/kg SB+2 mg/kg PTX (SB-200/PTX-2) treatment groups are shown. Scale bar = 200 μM.
The lung tissues from the mice were sectioned and subjected to pathological examination by H&E staining, or immunohistochemical analysis by antibodies against PCNA and VEGF. The percentage of cells positive for VEGF in the tumor group was 71.8 ± 2.9%, which was markedly higher than those detected in the SB-200 (46.5 ± 2.6%), PTX-2 (22.5 ± 3.8%), PTX-5 (15.8 ± 3.5%), and SB-200/PTX-2 (8.0 ± 1.1%) groups. The differences within the tumor and treatment groups were all highly significant with P < 0.05 (one-way ANOVA). Similarly, the percentage of positive PCNA tumor cells in the untreated tumor group was 85.8 ± 1.9% which was greater than those detected in SB-200 (48.3 ± 5.2%), PTX-2 (60.5 ± 4.4%), PTX-5 (56.8 ± 4.0%), and SB-200/PTX-2 (25.5 ± 3.4%) groups (P < 0.05, one-way ANOVA) (Figure 5B). Notably, SB and PTX co-treatment resulted in more substantial inhibition of both PCNA and VEGF expression, which is in good agreement with the in vitro data (Figure 2), suggesting synergism of SB and PTX on cancer cell activity.
Discussion
The outcome of breast cancer treatment has improved steadily over the last fifty years. However, the cure rates are unlikely to be raised further with current therapies, especially for patients with metastatic breast cancer. Chemotherapeutic drugs, such as paclitaxel, are usually cytotoxic and pose adverse effects to patients. Moreover, paclitaxel can lead to development of drug resistance in tumor cells, thereby limiting the clinical success of general chemotherapies (Ribeiro et al., 2012; Lee et al., 2012). In an effort to develop effective alternative strategies that can increase the therapeutic efficacy while minimizing the systemic toxicity of chemotherapeutic agents, the use of phytoagents in combination with anticancer drugs may provide alternative and promising treatment options (Lee et al., 2012; Hsan et al., 2010).
Medicinal herbs are a promising source of new lead compound discovery and future medicine development (Kinghorn et al., 2011; Koop 2002). More than 60% of new chemical entities approved between 1982 and 2006 were from natural products or their derivatives (Cragg et al., 2009). Silibinin (SB), the natural flavonolignan identified from the medicinal milk thistle plant is well-known for its hepatoprotective properties, and is also a potentially chemopreventive agent against various cancers by targeting proliferation, apoptosis, and metastasis (Deep & Agarwal 2010). Recent reports showed that SB could be used in combination with chemotherapeutics, e.g., PTX, 5-fluorouracil or doxorubicin, to increase the chemosensitivity and lower the systemic toxicity of the anti-cancer drugs, or to synergistically inhibit tumor progression in vitro or in vivo of the ovarian carcinoma cells (Zhou et al., 2008), renal carcinoma cells (Chang et al., 2011), or multidrug-resistant colon cancer cells (Colombo et al., 2011). In the present study, we observed that SB inhibited proliferation of both 4T1 (ER-) and TS/A (ER+) breast cancer cell lines with a similar IC50 value (Figure 1). Aggressive and recurrent metastasis is often a hallmark of triple-negative breast cancers (TNBC), and specific treatment options are limited in these tumors (Foulkes et al., 2010). We therefore used the triple negative 4T1 breast cancer cell line derived from a syngeneic mouse that exhibits a similar aggressive phenotype to the human disease to assess the anticancer effects and modes of action of SB alone and in combination with PTX. We determined that co-treatment of SB and PTX exerted synergistic antiproliferative (Figure 2) and antimigratory (Figure 4) effects in vitro, suggesting SB enhanced chemosensitivity of 4T1 cells to PTX.
It has been revealed that aberrant activity of cell cycle protein kinases is a hallmark of hyperproliferative cancer cells. Cyclin dependent kinases, a family of serine/threonine kinases, regulate the transition from one cell cycle phase to another that are activated at specific checkpoint of the cell cycle (Vermeulen et al., 2003). In this study, we observed that antiproliferative effects of SB or in combination with PTX on 4T1 cells were possibly through modulating the activity of cyclin B1/cdk1 (responsible for G2/M transition), and cyclin E/cdk2 or cyclin A/cdk2 (G1/S mediators).
The poor clinical outcome of the vast majority of all cancers is mainly due to the invasion and metastasis of metastatic tumor cells and the outgrowth of secondary tumors at distant sites. MMPs play crucial roles in the processes of invasion and metastasis and have been found to be of prognostic significance in breast cancers (Kohrmann et al., 2009). In our studies, SB treatment only inhibited the expression of MMP-9 in a dose-dependent manner (Figure 4B). Another metastasis-associated protein, protease nexin 1 (PN-1), a serine protease inhibitor (serpin), has an expression profile that varies significantly between non-metastatic and highly metastatic breast cancer cells and has therefore been suggested to be a useful marker for the degree of tumor progression (Candia et al., 2006). The overexpression of PN-1 in 4T1 cells has also been previously reported for correlating directly to the increase of MMP-9 expression and enzymatic activity and to the lung metastatic behavior of 4T1 tumors (Fayard et al., 2009). We thus propose that SB treatment results in deregulated PN-1 expression, which then induces a decrease of MMP-9 activity and ultimately a decrease in lung metastasis of 4T1 cells (Figure 4 and 5).
Several studies have shown a correlation between the action of E-cadherin and N-cadherin on tumor cell adhesion and migration. Specifically, E-cadherin functions as a suppressor of metastasis, and loss of E-cadherin correlates with advanced tumor grade and poor outcome in breast patients (Heimann et al., 2000; Oka et al., 1993; Siitonen et al., 1996). On the other hand, gain of N-cadherin action in breast cancer enhances cell motility and facilitates tumor cell interaction with host stromal cells (Nieman et al., 1999). Our results showing that treatment with SB alone or in combination with PTX resulted in increased E-cadherin expression and reduced N-cadherin expression (Figure 4C). It is known that the ratio of relative E-cadherin and N-cadherin protein level can refer to the degree of metastasis to be inhibited; when the ratio is higher the metastatic activity is inhibited greater. Based on the Western blotting data, we obtained that the relative E-cadherin/N-cadherin ratio in SB-150, SB-450, PTX-45, PTX-180, and SB-150/PTX-45 treated cells were 1.91, 3.43, 0.54, 0.93, and 2.57, respectively, indicating the SB-150/PTX/45 cotreatment showed highest inhibition on 4T1 cell metastasis with synergism. This data maybe relatable to a mechanism for the inhibition of 4T1 cell migration and metastasis observed in vivo by SB and PTX treatment. Additionally, we examined focal-adhesion kinase (FAK), a non-receptor tyrosine kinase acting as biosensors or integrator to control cell motility (Mitra et al., 2005) and observed significantly decreased levels of both the unphosphorylated and phosphorylated forms in cells treated with SB-150/PTX-45 treatment (data not shown), suggesting the drug treatment attenuated cancer cell motility.
To investigate the in vivo effects of SB alone and in combination with PTX, a metastatic 4T1pGL-COX-2/Luc cell clone containing COX-2 promoter-driven luciferase reporter gene was established and introduced into a syngeneic BALB/c mouse model. The lung metastasis of 4T1pGL-COX-2/Luc cancer cells in mice was readily observed as examined by the IVIS imaging system. We observed the decrease of lung bioluminescence intensities in the SB, PTX and SB+PTX treatment groups, which were significantly lower than that of the tumor control group (P < 0.05), indicating the treatments can all effectively suppress 4T1 cells metastasized to the lungs in test mice. Furthermore, immunostaining of the lungs of mice that received SB and PTX together revealed more significantly reduction on the expression of PCNA and VEGF proteins than those found in mice treated with SB or PTX alone (Figure 5B). VEGF is a critical molecule involved in angiogenesis, an important process for tumor growth and metastasis. Our data suggest that SB in combination with PTX inhibited VEGF expression, which may then prevents angiogenesis in 4T1 tumor-bearing mice. A similar mechanism has been previously described in colorectal cancer, where SB that was implicated in the deregulation of VEGF and inhibition of proliferation and angiogenesis (Singh et al., 2008).
Despite some limitations in our study, data from MMP levels in vitro (Figure 4B) and lung metastasis in vivo (Figure 5A) were not synergy. Overall, combination of SB and PTX still have enough evidences to prove the synergistic effects on 4T1 model via the data of PN-1, the ratio of E-cadherin/N-cadherin (Figure 4C), and immunohistochemical analysis of PCNA and VEGF (Figure 5B).
Taken together, our data shows that SB synergistically cooperates with the toxic anti-cancer drug PTX to inhibit proliferation, migration, and motility of 4T1 cells in vitro and to reduce metastatic efficacy in vivo, which provides a foundation for a novel therapeutic option in triple negative, highly metastatic breast tumor cells.
Acknowledgments
This study was supported by grants from National Science Council (NSC 101-2325-B-001-007) and from Academia Sinica, Taiwan, R.O.C. We thank Miss Sheue-Fen Tzeng and Dr. Wai-Leng Lee for their technical assistance, and Dr. AndreAna Peña, English Editor's Office of the Agricultural Biotechnology Research Center and Institute of Molecular Biology, Academia Sinica, Taiwan, for English editorial assistance.
References
- 1.Candia B.J, Hines W.C, Heaphy C.M, Griffith J.K, Orlando R.A. Protease nexin-1 expression is altered in human breast cancer. Cancer Cell International. 2006;6:16. doi: 10.1186/1475-2867-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang H.R, Chen P.N, Yang S.F, Sun Y.S, Wu S.W, Hung T.W, Lian J.D, Chu S.C, Hsieh Y.S. Silibinin inhibits the invasion and migration of renal carcinoma 786-O cells in vitro, inhibits the growth of xenografts in vivo and enhances chemosensitivity to 5-fluorouracil and paclitaxel. Molecular Carcinogenesis. 2011;50:811–823. doi: 10.1002/mc.20756. [DOI] [PubMed] [Google Scholar]
- 3.Chiang Y.M, Lo C.P, Chen Y.P, Wang S.Y, Yang N.S, Kuo Y.H, Shyur L.F. Ethyl caffeate suppresses NF-kappaB activation and its downstream inflammatory mediators, iNOS, COX-2, and PGE2 in vitro or in mouse skin. British Journal of Pharmacology. 2005;146:352–363. doi: 10.1038/sj.bjp.0706343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Research. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 5.Colombo V, Lupi M, Falcetta F, Forestieri D, D’Incalci M, Ubezio P. Chemotherapeutic activity of silymarin combined with doxorubicin or paclitaxel in sensitive and multidrug-resistant colon cancer cells. Cancer Chemotherapy and Pharmacology. 2011;67:369–379. doi: 10.1007/s00280-010-1335-8. [DOI] [PubMed] [Google Scholar]
- 6.Cragg G.M, Grothaus P.G, Newman D.J. Impact of natural products on developing new anti-cancer agents. Chemical Reviews. 2009;109:3012–3043. doi: 10.1021/cr900019j. [DOI] [PubMed] [Google Scholar]
- 7.Deep G, Agarwal R. Antimetastatic efficacy of silibinin: molecular mechanisms and therapeutic potential against cancer. Cancer and Metastasis Reviews. 2010;29:447–463. doi: 10.1007/s10555-010-9237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fayard B, Bianchi F, Dey J, Moreno E, Djaffer S, Hynes N.E, Monard D. The serine protease inhibitor protease nexin-1 controls mammary cancer metastasis through LRP-1-mediated MMP-9 expression. Cancer Research. 2009;69:5690–5698. doi: 10.1158/0008-5472.CAN-08-4573. [DOI] [PubMed] [Google Scholar]
- 9.Foulkes W.D, Smith I.e, Reis-Filho J.S. Triple-negative breast cancer. The New England Journal of Medicine. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 10.Heimann R, Lan F, Mc Bride R, Hellman S. Separating favorable from unfavorable prognostic markers in breast cancer: the role of E-cadherin. Cancer Research. 2000;60:298–304. [PubMed] [Google Scholar]
- 11.Hoh C, Boocock D, Marczylo T, Singh R, Berry D.P, Dennison A.R, Hemingway D, Miller A, West K, Euden S, Garcea G, Farmer P.B, Steward W.P, Gescher A.J. Pilot study of oral silibinin, a putative chemopreventive agent, in colorectal cancer patients: silibinin levels in plasma, colorectum, and liver and their pharmacodynamic consequences. Clinical Cancer Research. 2006;12:2944–2950. doi: 10.1158/1078-0432.CCR-05-2724. [DOI] [PubMed] [Google Scholar]
- 12.Hou C.C, Chen Y.P, Wu J.H, Huang C.C, Wang S.Y, Yang N.S, Shyur L.F. A galactolipid possesses novel cancer chemopreventive effects by suppressing inflammatory mediators and mouse B16 melanoma. Cancer Research. 2007;67:6907–6915. doi: 10.1158/0008-5472.CAN-07-0158. [DOI] [PubMed] [Google Scholar]
- 13.Hsan K.M, Chen C.C, Shyur L.F. Current research and development of chemotherapeutic agents for melanoma. Cancers. 2010;2:397–419. doi: 10.3390/cancers2020397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C, Lo C.P, Chiu C.Y, Shyur L.F. Deoxyelephantopin a novel multifunctional agent, suppresses mammary tumour growth and lung metastasis and doubles survival time in mice. British Journal of Pharmacology. 2010;159:856–871. doi: 10.1111/j.1476-5381.2009.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaur M, Agarwal R. Silymarin and epithelial cancer chemoprevention: how close we are to bedside? Toxicology and Applied Pharmacology. 2007;224:350–359. doi: 10.1016/j.taap.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinghorn A.D, Pan L, Fletcher J.N, Chai H. The relevance of higher plants in lead compound discovery programs. Journal of Natural Products. 2011;74:1539–1555. doi: 10.1021/np200391c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohrmann A, Kammerer U, Kapp M, Dietl J, Anacker J. Expression of matrix metalloproteinases (MMPs) in primary human breast cancer and breast cancer cell lines: New findings and review of the literature. BMC cancer. 2009;9:188. doi: 10.1186/1471-2407-9-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koop C.E. The future of medicine. Science. 2002;295:233. doi: 10.1126/science.295.5553.233. [DOI] [PubMed] [Google Scholar]
- 19.Lee W.L, Shiau J.Y, Shyur L.F. Taxol, camptothecin, and beyond for cancer therapy. In: Shyur L.F, Lau, Allan S.Y, editors. Advances in Botanical Research. volume 62. Recent Trends in Medicinal Plants Research; 2012. pp. 133–178. [Google Scholar]
- 20.Lin C.H, Lee W.L, Shyur L.F. An overview of the current development of phytoremedies for breast cancer. In: Cho, William C.S, editors. Materia Medica for Various Cancers (volume 2): Evidence-based Anticancer Complementary and Alternative Medicine. The Netherlands: Springer Press; 2012. pp. 47–67. [Google Scholar]
- 21.Maxmen A. The hard facts. Nature. 2012;485:S50–S51. doi: 10.1038/485S50a. [DOI] [PubMed] [Google Scholar]
- 22.Mitra S.K, Hanson D.A, Schlaepfer D.D. Focal adhesion kinas: in command and control of cell motility. Nature Reviews in Molecular Cell Biology. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 23.Nieman M.T, Prudoff R.S, Johnson K.R, Wheelock M.J. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. The Journal of Cell Biology. 1999;147:631–643. doi: 10.1083/jcb.147.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oka H, Shiozaki H, Kobayashi K, Inoue M, Tahara H, Kobayashi T, Takatsuka Y, Matsuyoshi N, Hirano S, Takeichi M, et al. Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Research. 1993;53:1696–1701. [PubMed] [Google Scholar]
- 25.Ribeiro J.T, Macedo L.T, Curigliano G, Fumagalli L, Locatelli M, Dalton M, Quintela A, Carvalheira J.B, Manunta S, Mazzarella L, Brollo J, Goldhirsch A. Cytotoxic drugs for patients with breast cancer in the era of targeted treatment: back to the future. Annals of Oncology. 2012;23:547–555. doi: 10.1093/annonc/mdr382. [DOI] [PubMed] [Google Scholar]
- 26.Salvinelli F, Casale M, Vincenzi B, Santini D, Di Peco V, Firrisi L, Onori N, Greco F, Tonini G. Bilateral irreversible hearing loss associated with the combination of carboplatin and paclitaxel chemotherapy: a unusual side effect. Journal of Experimental & Clinical Cancer Research. 2003;22:155–158. [PubMed] [Google Scholar]
- 27.Sarkar F.H, Li Y. Using chemopreventive agents to enhance the efficacy of cancer therapy. Cancer Research. 2006;66:3347–3350. doi: 10.1158/0008-5472.CAN-05-4526. [DOI] [PubMed] [Google Scholar]
- 28.Shyur L.F, Chen C.H, Lo C.P, Wang S.Y, Kang P.L, Sun S.J, Chang C.A, Tzeng C.M, Yang N.S. Induction of apoptosis in MCF-7 human breast cancer cells by phytochemicals from Anoectochilus formosanus. Journal of Biomedical Science. 2004;11:928–939. doi: 10.1159/000081840. [DOI] [PubMed] [Google Scholar]
- 29.Siitonen S.M, Kononen J.T, Helin H.J, Rantala I.S, Holli K.A, Isola J.J. Reduced E-cadherin expression is associated with invasiveness and unfavorable prognosis in breast cancer. American Journal of Clinical Pathology. 1996;105:394–402. doi: 10.1093/ajcp/105.4.394. [DOI] [PubMed] [Google Scholar]
- 30.Singh R.P, Gu M, Agarwal R. Silibinin inhibits colorectal cancer growth by inhibiting tumor cell proliferation and angiogenesis. Cancer Research. 2008;68:2043–2050. doi: 10.1158/0008-5472.CAN-07-6247. [DOI] [PubMed] [Google Scholar]
- 31.Vermeulen K, Van Bockstaele D.R, Berneman Z.N. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Proliferation. 2003;36:131–149. doi: 10.1046/j.1365-2184.2003.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wellington K, Jarvis B. Silymarin: a review of its clinical properties in the management of hepatic disorders. BioDrugs. 2001;15:465–489. doi: 10.2165/00063030-200115070-00005. [DOI] [PubMed] [Google Scholar]
- 33.Zhou L, Liu P, Chen B, Wang Y, Wang X, Chiriva Internati M, Wachtel M.S, Frezza E.E. Silibinin restores paclitaxel sensitivity to paclitaxel-resistant human ovarian carcinoma cells. Anticancer Research. 2008;28:1119–1127. [PubMed] [Google Scholar]


