Abstract
This article will review selected herbal products used in traditional Chinese medicine, including medicinal mushrooms (巴西蘑菇 bā xī mó gū; Agaricus blazei, 雲芝 yún zhī; Coriolus versicolor, 靈芝 líng zhī; Ganoderma lucidum, 香蕈 xiāng xùn; shiitake, Lentinus edodes, 牛樟芝 niú zhāng zhī; Taiwanofungus camphoratus), Cordyceps (冬蟲夏草 dōng chóng xià cǎo), pomegranate (石榴 shí liú; Granati Fructus), green tea (綠茶 lǜ chá; Theae Folium Non Fermentatum), garlic (大蒜 dà suàn; Allii Sativi Bulbus), turmeric (薑黃 jiāng huáng; Curcumae Longae Rhizoma), and Artemisiae Annuae Herba (青蒿 qīng hāo; sweet wormwood). Many of the discussed herbal products have gained popularity in their uses as dietary supplements for health benefits. The review will focus on the active constituents of the herbs and their bioactivities, with emphasis on the most recent progress in research for the period of 2003 to 2011.
Keywords: Herbal Products, Medicinal Mushrooms, Dietary Supplements, Traditional Chinese Medicine (TCM)
Introduction
Herbal products have been used in traditional Chinese medicine (TCM) for thousands of years as foods to maintain good health and as drugs to treat disease. Because specific components in herbal products can exhibit different biological activities, the identification, characterization, and biological evaluations of these components can validate the traditional use of the herb, as well as also provide leads for new “single-component” drug discovery and development. In addition, the daily use of herbal dietary supplements to promote good health is increasing rapidly worldwide. Thus, ancient TCM remedies should be reinvestigated using modern scientific methods to assure their efficacy and safety, and develop them as first-class dietary supplements, as well as new medicines.
This article will reviews elected herbs used as functional foods or medicinal sources. Their folkloric use, chemical composition, and currently identified biological activities will be discussed with emphasis on research during the period of 2003-2011. The current review expands the prior article published in this journal on selected herbs classified as upper, middle, and lower classes in The Divine Husbandman's Herbal Foundation Canon (神農本草經 shén nóng běn cǎo jīng), as well as updates a prior similar review published by the author in 2003 (Lee et al., 2003).
Medicinal Mushrooms
Agaricus blazei Murill (巴西蘑菇 bā xī mó gū; Agaricaceae)
Agaricus blazei Murill (AbM) is an edible, medicinal mushroom of Brazilian origin. It has been cultivated commercially for the health food market. This mushroom has been used in folk medicine against various diseases, including cancer, chronic hepatitis, diabetes, arteriosclerosis, and hyperlipidemia (and Weis, 1999a). Interest in the use of this mushroom and/or its extracts as dietary supplements has increased significantly, in part because of the confirmed studies of its antitumor, anticarcinogenic, antiviral, anti-inflammatory, hypoglycemic, hypocholesterolemic, and antihypertensive effects (Fortes and Novaes, 2006; Wasser and Weis, 1999a). AbM mushroom extracts contain various active substances that promote stimulation of the immune system, in particular polysaccharides, such as proteoglycans, (1→3)-β- and (1→6)-β-D-glucans, (Ellertsen et al., 2006; Hetland et al., 2008; Kim et al., 2005; Lima et al., 2011).
Coriolus versicolor (雲芝 yún zhī; Polyporaceae)
Coriolus versicolor has a long history of medicinal use in China and Japan and is one of the most researched and respected of the medicinal mushrooms from Europe to the Far East (Kidd, 2000). These mushrooms contain large quantities of β-glucans that stimulate the immune system (Gorin and Barreto-Bergter, 1983). The most active medicinal components are protein-bound β-glucan polysaccharides (PSKs, also known as Krestin in Japan) and polysaccharide-peptides (PSPs, yún zhī in China). Several clinical assays have reported that PSKs exhibit antitumor activity and synergistic effects in combined therapies (Katoh and Ooshiro, 2007; Choi et al., 2007; Oba et al., 2007). Other studies have found antiviral activity against ectromelia virus and cytomegalovirus infections (Tsukagoshi et al., 1984), as well as cell-free human immunodeficiency virus (HIV) infection (Tochikura et al., 1987). PSKs also induce potent antimicrobial activity against Escherichia coli, Listeria monocytogenes, and Candida (Sakagami et al., 1991). PSPs have been more recently developed in China as anticancer and immunomodulatory agents (Ooi and Liu, 2000). PSPs also exhibit anti-oxidant properties, with strong scavenging effects on superoxide and hydroxyl radicals (Ooi and Liu, 2000).
Ganoderma (靈芝 líng zhī; Ganoderma lucidum, Polyporaceae)
Ganoderma (靈芝 líng zhī) has been widely used as a tonic and to treat various diseases, including chronic hepatopathy, hypertension, neurasthenia, insomnia, bronchitis, gastric ulcer, diabetes, and cancer in China, Japan, Korea, and other Asian countries for more than 2,000 years (Sanodiya et al., 2009). Because of Ganoderma's presumed health benefits and apparent absence of side effects, it has attained a reputation in the East as the ultimate herbal substance. G. lucidum contains numerous bioactive components, including triterpenoids, polysaccharides, nucleotides, sterols, steroids, fatty acids, proteins/peptides, and trace elements (Sanodiya et al., 2009). The active triterpenoids from ling zhī are mainly ganoderic acids (Figure 1) (Kim and Kim, 1999). They have been found to inhibit histamine release and cholesterol synthesis, as well as show antihypertensive, antitumor, and anti-HIV effects (Sandiya et al., 2009; Xu et al., 2010). G. lucidum also contains polysaccharides (β-D-glucans, heteropolysaccharides and glycoproteins), which can affect immune and immune-related cells, and show immunomodulatory, anti-angiogenic, and cytotoxic effects (Xu et al., 2011). In animal studies, polysaccharide fractions from G. lucidum have demonstrated potential hypoglycemic and hypolipidemic activities (Mohammed et al., 2007; Sanodiya et al., 2009).
Figure 1.
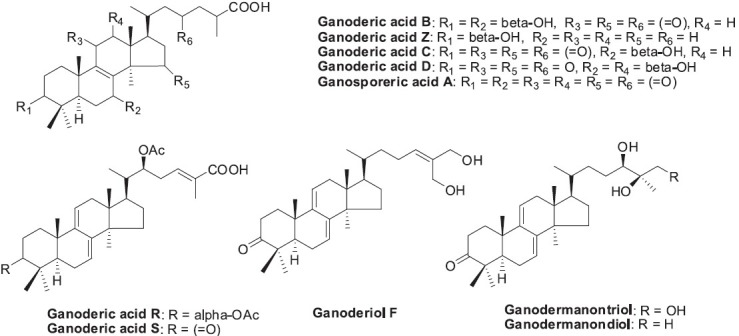
Structures of triterpenoids found in Ganoderma lucidum
Lentinus (香蕈 xiāng xùn; Shiitake, Lentinus edodes, Marasmiaceae)
Shiitake mushrooms are currently one of the five most cultivated edible mushrooms in the world (Chang, 1999). They have been used medicinally and as healthy food for thousands of years in Japan, China, and Korea, and are now becoming popular in nutritional and medicinal products throughout Europe and North America. Many health benefits have been ascribed to shiitake mushrooms and their constituents. The polysaccharide lentinan [β-(1→3)-D-glucan], shiitake mushroom mycelium, and culture media extracts (LEM, LAP, and KS-2) (Wasser, 2002) have demonstrated strong antitumor activities orally and by injection in both animals and humans. Lentinan is also effective against various bacterial, viral (including AIDS), and parasitic infections (Ngai and Ng, 2003). LEM is also useful in the treatment of AIDS, as studies have shown that it can inhibit HIV infections of cultured human T cells, and potentiate the effects of AZT against viral replication in vitro (Tochikura et al., 1988). Water-soluble lignins from EP3 and EPS4 from shiitake mushroom mycelium have shown antiviral and immunomodulating effects (Hanafusa et al., 1990). Centinamycins A and B have also been identified as antibacterial polyacetylene compounds in shiitake mushroom (Wasser, 2005). Eritadenine (Figure 2), a cholesterol-lowing compound found in shiitake mushrooms (Yang et al., 2002), also possesses antiviral properties (Wasser and Weis, 1999b).
Figure 2.
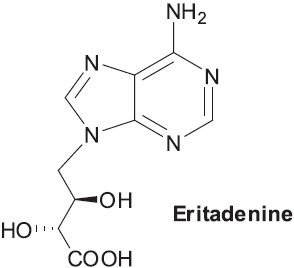
Structure of eritadenine found in Lentinus edodes
Taiwanofungus camphoratus (牛樟芝 niú zhāng zhī;Coriolaceae)
Taiwanofungus camphoratus is an indigenous mushroom in Taiwan, and has been used as a folkloric medicine since the 18th century for treating food and drug intoxications, diarrhea, abdominal pain, hypertension, itchy skin, and hepatoma (Wu et al., 1997). The reported biological activities for T. camphoratus include anticancer, antihepatotoxic, antihypertensive, anti-inflammatory, anti-oxidant, and neuroprotective. In addition, T. camphoratus produces a synergistic effect when combined with the drugs trichostatin A, lovastatin, and paclitaxel (Chen et al., 2010; Peng et al., 2008). Chemical constituents and their pharmacological properties are listed below.
(1) Triterpenoids: Recently, 10 new triterpenoids, camphoratins A–J, and 13 known triterpenoids were isolated from the fruiting body of T. camphoratus (Shi et al., 2011; Wu et al., 2010). Camphoratins B-F and zhankuic acid A showed moderate to potent cytotoxicity, with EC50 values ranging from 0.3 to 3 μM against KB and KB-VIN human cancer cell lines (Wu et al., 2010). Zhankuic acids A and C and methyl antcinate B (Figure 3) displayed tumor-specific cytotoxicity against colon, breast, liver, and lung cancer cell lines. Furthermore, when these three compounds were combined at a concentration of 4 μM, a synergistic cytotoxic effect was seen against colon cancer HT-29 cells (Yeh et al., 2009). Camphoratins F and J, zhankuic acid A and its methyl ester (Figure 3), antcins A and C, methyl antcinate H, and methyl 4α-methylergost-8,24(28)-dien-3,11-dion-26-oate exhibited anti-inflammatory effects by inhibiting NO-production with IC50 values of less than 5 μM (Wu et al., 2010). These results may substantiate the use of T. camphoratus in TCM for the treatment of inflammation and cancer-related diseases, and prompt further investigation of its triterpenoid constituents as cancer chemotherapeutic agents or as anti-inflammatory drugs to treat NO-dependent neurodegenerative disorders.
(2)Benzenoids: In 2011, Shi et al. isolated five new benzenoids, benzocamphorins A–E and seven known benzenoids from the fruiting body of T. camphoratus (Shi et al., 2011). Antrocamphin A showed moderate cytotoxicity against MCF-7 cells with ED50 values of 3.4 μg/mL (threshold for activity is considered 4 μg/mL). Antrocamphin A, benzocamphorin B, and 2,2′,5,5′-tetramethoxy-3,4,3′,4′-bimethylenedioxy-6,6′-dimethylbiphenyl (Figure 3) also exhibited effective anti-inflammatory activity.
(3) Maleic and succinic acid derivatives: Tw o maleimide derivatives, antrodins B and C Figure 3, were isolated from the mycelium of T. camphoratus. They showed appreciable cytotoxic activity against the LLC tumor cell line (Nakamura et al., 2004).
(4) Polysaccharides: Polysaccharides from mycelia of T. camphoratus inhibited U937 cell growth by 55.3% via activation of mononuclear cells. Intraperitoneal and oral administration of T. camphoratus polysaccharides (100 and 200 mg/kg, respectively) significantly suppressed tumor growth to 69.1% and 58.8%, respectively, in sarcoma 180-bearing mice (Liu et al., 2004). T. camphoratus polysaccharides also inhibited cyclin D1 expression in endothelial cells by inhibiting vascular endothelial growth factor receptor signaling, and thereby suppressing angiogenesis (Cheng et al., 2005). In addition, the sulfated polysaccharides inhibited in vitro Matrigel tube formation in an angiogenesis model, and effectively prevented serum-deprived apoptosis, using a stress model of serum deprivation-induced apoptosis in neuronal-like PC12 cells (Cheng et al., 2009).
Figure 3.
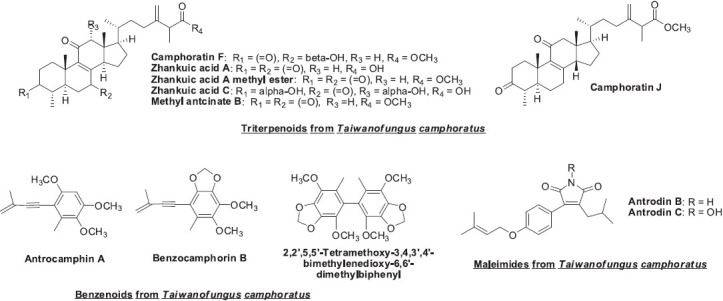
Structures of various chemical compounds found in Taiwanofungus camphoratus
Cordyceps (冬蟲夏草 dōng chóng xià cǎo; Cordyceps sinensis, Claviceptaceae)
Cordyceps sinensis (Bark) Sacc.Link (Claviceptaceae) (Ascomycetes) is a parasitic fungus. It invades and kills the larva of some Hepiaidae species, eventually forming a parasitic complex that contains the remains of the caterpillar and the stroma of the fungus. The parasitic complex is also known as “winter worm summer grass”, because of its appearance during different seasons. It is mostly found in Tibet, Qinghai, Sichuan, Yunnan and Gansu provinces of China. In TCM, Cordyceps is commonly used to “replenish the kidney and soothe the lung”, as well as to treat fatigue (Lull et al., 2005; Wojcikowski et al., 2006; Zhang and Wu, 2007). It also can be used to treat conditions such as night sweating, hyposexuality, hyperglycemia, hyperlipidemia, asthenia after severe illness, respiratory disease, and renal dysfunction or failure, arrhythmias and other heart and liver diseases (Liu and Shen, 2003). Although the pharmacologically active components of C.sinensis have not been fully elucidated, at least two chemical constituents, cordycepin and cordycepic acid (Figure 4), have been identified and proposed as important active components (Huang et al., 2003; Zhu et al, 1998). Cordycepic acid was originally identified as 1,3,4,5-tetrahydroxycyclohexane-1-carboxylic acid (Chatterjee et al., 1957), but later was re-identified as D-mannitol (Sprecher and Sprinson, 1963) (Figure 4). C. sinensis also contains polysaccharides, nucleotides, ergosterol crude protein and amino acids, fatty acids, and metal elements. The chemical components, pharmacological effects, and developmental products of Cordyceps fungi have been reviewed (Zhou et al., 2009).
Figure 4.
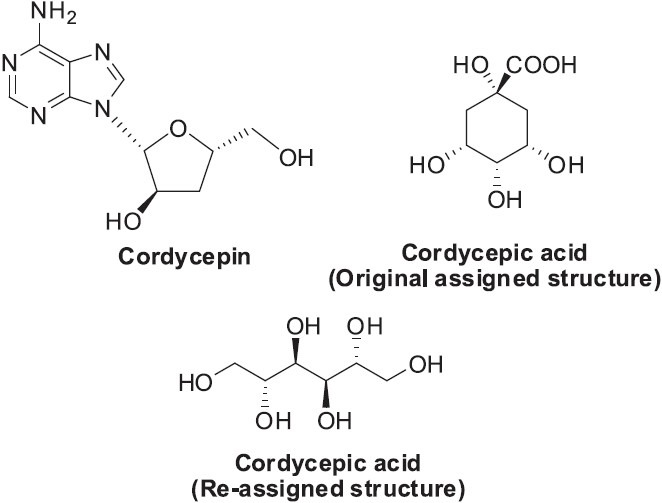
Structures of cordycepin and cordycepic acid found in Cordyceps sinensis
Pomegranate (石榴 shí liú; Granati Fructus, the fruit of Punica granatum, Punicaceae)
Pomegranate is the fruit of Punica granatum, and has long been cultivated and consumed by different cultures. Anatomically, this plant can be divided into seven parts, seed, juice, peel, leaf, flower, bark, and roots, and each part exhibits pharmacologic effects. The plant has been used as an antihelmintic and vermifuge, to eliminate parasites, to treat mouth ulcers, diarrhea, acidosis, dysentery, hemorrhage, microbial infections, and respiratory pathologies, and as an antipyretic. The primary chemical components and their potential beneficial effects have recently been reviewed (Lansky and Newman, 2007; Martos et al., 2010). For example, the flanovol quercetin inhibited lung cancer cell growth via G2/M arrest and apoptosis induction, while kaempferol reduced expression of TNF-α and interleukin 1β gene in tumor cells (Figure 5). The two compounds acted synergistically to inhibit breast cancer proliferation.
Figure 5.
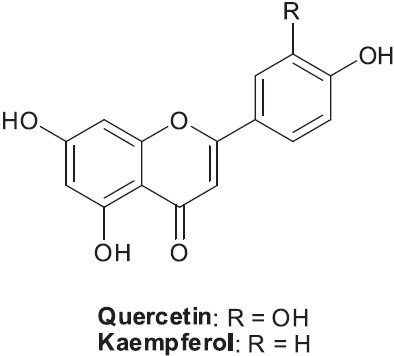
Structures of two active flavonols found in Punica granatum
Green tea (綠茶 lǜ chá; Theae Folium Non Fermentatum, Camellia sinensis, Theaceae)
Green tea is produced from the fresh nonfermented leaves of Camellia sinensis (Theaceae) and is a very popular beverage worldwide. Its bioactive components include green tea polyphenols (GTPs) (Figure 6), known as catechins, which are divided into four structural groups, (-)-epigallocatechin-3-gallate (EGCG), (-)-epigallocatechin (EGC), (-)-epicatechin-3-gallate (ECG), and (-)-epicatechin (EC) (Sano et al., 2001). GTPs have been studied for four main biological activities: cancer chemoprevention, antibacterial activity, antiviral effects, and neuroprotection.
Figure 6.
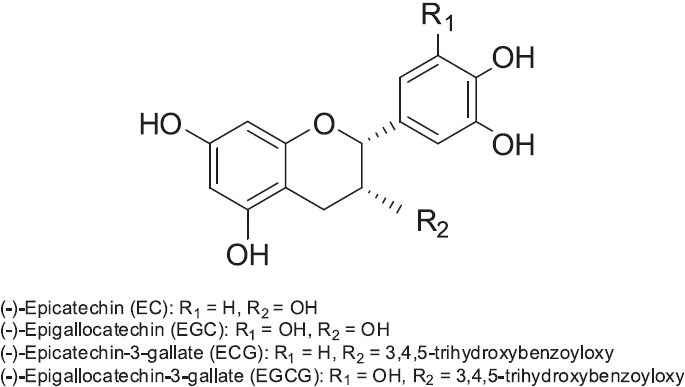
Structures of green tea polyphenols (GTPs) found in Green Tea (Camellia sinensis)
(1) Cancer, Chemoprevention: GTPs been linked to the prevention of many cancers, including lung, colon, esophagus, mouth, stomach, small intestine, kidney, pancreas, and mammary glands (Chacko et al., 2010). EGCG, the major GTP, showed potent cancer-preventive effects both in vitro and in vivo (Yang et al., 2000). EGCG modulated the function of certain transcription factors, namely NF-κB and activator protein (AP-1) in cell culture and animal studies (Dong et al., 1997; Gupta et al., 2004; Nomura et al., 2000). NF-κB facilitated the transcription of genes involved in inflammation, immunity, and carcinogenesis. EGCG also modulated other multiple signaling pathways at various cellular levels, ultimately leading to apoptosis, cell cycle arrest, growth inhibition, anti-angiogenesis, and metastasis inhibition. Which of EGCG's multiple molecular targets are most critical for its chemopreventive actions has not been fully established. Kim et al. recently summarized the molecular targets modulated by EGCG (Kim et al., 2010).
(2) Antibacterial Activity: EGCG has possible use as an adjuvant in antibacterial therapy, because it dramatically enhanced tetracycline activity in Staphylococci by reducing tetracycline efflux from bacterial cells (Sudano Roccaro et al., 2004).
(3) Antiviral Activity: GTPs, in particular EGCG, have been studied against various viruses. EGCG interrupted the HIV-1 life cycle with a destructive effect on the viral particles. Post-adsorption entry and reverse transcriptase in THP-1 cells were significantly inhibited at EGCG concentrations over 1 μM, and protease kinetics were suppressed at a concentration higher than 10 μM in a cell-free study (Yamaguchi et al., 2002). EGCG also inhibited influenza virus by binding to hemagglutinin (HA) spike proteins, thus, blocking viral attachment to the target cell receptors (Mukoyama et al., 1991). Against adenovirus infection and the viral protease adenain, EGCG displayed an IC50 of 25 μM and a therapeutic index of 22 in Hep2 cells (Weber et al., 2003). In other studies, rotavirus, poliovirus, and herpes simplex virus were also inhibited by EGCG and tea extracts (Mukoyama et al., 1991; Yamaguchi et al., 2002).
(4) Neuroprotective Activity: In studies by Weinreb et al., EGCG appeared to affect the mortality of neuronal cells, and thus, possibly have an effect on neurodegenerative diseases, such as Alzheimer 's and Parkison's diseases, which are associated with oxidative damage and iron accretion. Because mitochondria play a central role in oxidative stress–induced apoptosis, EGCG-mediated inhibition of apoptosis might implicate mitochondrial targets (Weinreb et al., 2004).
Garlic (大蒜 dà suàn; Allii Sativi Bulbus, Allium sativum, Liliaceae)
Garlic (Allium sativum), belonging to the family Liliaceae, is highly regarded throughout the world. Originally from Central Asia, garlic is one of the earliest cultivated plants and is used for both culinary and medicinal purposes. It has been used to treat infections and wounds, due to its antibacterial properties (Goncagul and Ayaz, 2010) and various disease states, including diarrhea, rheumatism, diabetes, and heart disease (Aviello et al., 2009). Its beneficial effects on the cardiovascular system, particularly the prevention and treatment of artherosclerosis, include antithrombotic, blood pressure lowering, and hypolipidemic properties (El-Sabban and Abouazra, 2008; Ginter and Simko, 2010). Additionally, antiviral, anti-oxidant, free radical scavenging, hypoglycemic, and anticancer properties have also been demonstrated (Bongiorno et al., 2008; Tripathi, 2009). Organic sulfur compounds found in garlic may promote early mitotic arrest followed by apoptotic cell death without affecting normal cells (Ban et al., 2007; Jakubikova and Sedlak, 2006). Modulation of NF-κB (Ban et al., 2007) and alteration of the microtubule network (Cerella et al., 2011) have been found as possible mechanisms for these activities. Taken together, these findings indicate a promising potential for the use of garlic-derived sulfur compounds in chemoprevention and chemotherapy, as well as an immune booster (Butt et al., 2009). The two most studied chemical constituents are allicin (produced from the alliin in fresh garlic by the enzymatic action of alliinase when garlic is chopped) and ajoene (Figure 7). Other bioactive components are diallyl disulfide and triallyl trisulfide (Lei et al., 2010), as well as S-allylmercaptocysteine and thiacremonone (Ban et al., 2007) (Figure 7).
Figure 7.
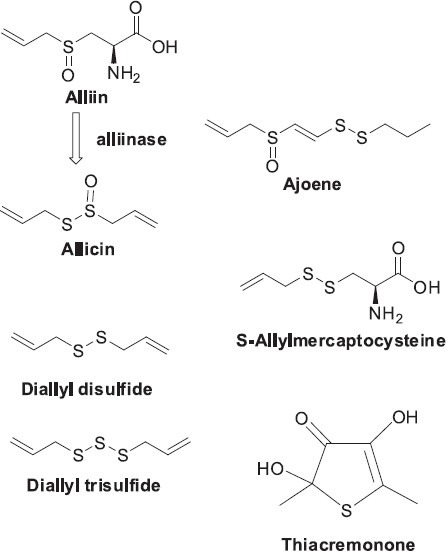
Structures of organic sulfur compounds found in Garlic (Allium sativum)
Turmeric (薑黃 jiāng huáng; Curcumae Longae Rhizoma, Curcuma longa, Zingiberaceae)
Curcuma longa (Zingiberaceae) is a plant in the ginger family and is heavily used as a spice (turmeric) to flavor food. Its main constituent is the natural product curcumin [diferuloylmethane, 1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione], which is responsible for much of turmeric's flavor, yellow color, and bioactivity. Besides curcumin, other curcuminoids, such as demethoxycurcumin, bisdemethoxycurcumin, cyclocurcumin (Kiuchi et al., 1993), and calebin A (Kim et al., 2001) are also found in C. longa (Figure 8).
Figure 8.
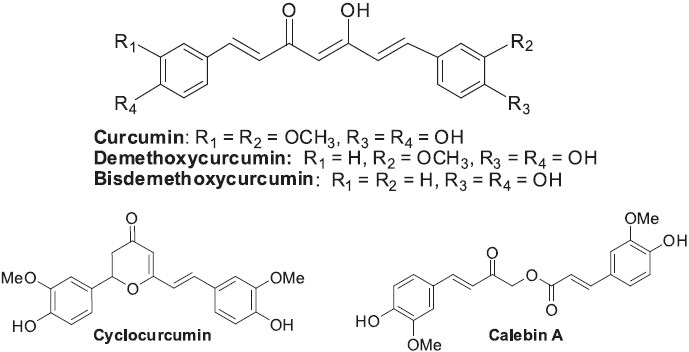
Structures of curcumin and other natural curcuminoids from Curcuma longa
Known as jiāng huáng in Chinese, C. longa rhizome has been used as a safe and non-toxic traditional medicine in Asia for centuries to treat diarrhea,intermittent fever, edema, bronchitis, colds, worms, leprosy, kidney inflammation, and cystitis, and as an anticancer treatment (Aggarwal et al., 2003; Anand et al., 2008; Cheng et al., 2001; Uehara et al., 1992). The unique structure of curcumin also makes this molecule biologically distinctive. Modern scientific research in mammalian cells and animals has confirmed its broad scope of biological activities and numerous therapeutic effects, including anti-oxidant (Ruby et al., 1995), anti-inflammatory (Aggarwal and Harikumar, 2009; Itokawa et al., 2008; Kohli et al., 2005), and particularly, anticancer effects (Ravindran et al., 2009).
Many in vitro studies have reported that curcumin is effective against various cancer cell lines, including breast (hormone-dependent, hormone-independent, and multidrug-resistant) (Anand et al., 2008), gastric (Anand et al., 2008), head and neck squamous (Anand et al., 2008; Wilken et al., 2011), pancreatic (Kamohara et al., 2007), colorectal (Anand et al., 2008; Villegas et al., 2008), urinary bladder (Kamat et al., 2007; Sun et al., 2004), kidney (Jiang et al., 1996; Jung et al., 2005), cervical (Anand et al., 2008; Roy et al., 2002), ovarian (Wahl et al., 2007), lung (Lee et al., 2005), oral (Atsumi et al., 2005; Atsumi et al., 2006), and thymic (Bhattacharyya et al., 2007). It also can inhibit cellular proliferation and enhance apoptosis (Reuter et al., 2008; Skommer et al., 2006; Wei et al., 2004). In prostate cancer cells, such as LNCaP, DU145, C4-2B, and PC3, curcumin up-regulated the expression of the androgen receptor (AR), AP-1, cyclin D1, NF-κB and cAMP response element binding (CREB) protein and EGFR-TK activity (Anand et al., 2008). Recently, some curcumin derivatives (such as 1-4 in Table 1) were reported to be more potent anti-prostate cancer agents than curcumin and to act by inducing or enhancing AR degradation (Lin et al., 2006; Ohtsu et al., 2003). Curcumin can also modulate/activate numerous target proteins, including transcription factors (e.g., NF-κB, STAT-3, AP-1, NFR-2, PPR-γ, HIF-1), receptors (e.g., MMP, iNOS, GST, ATPase), and growth factors (e.g., EGF, NGF, HGF, PDGF) (Goel et al., 2008; Hatcher et al, 2006).
Table 1.
Reduction of AR protein expression in prostate cancer cells and cytotoxicity of curcumin analoguesa
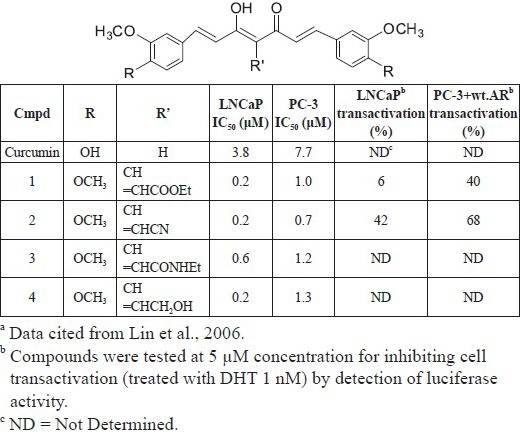
One significant advantage of curcumin as an antitumor and chemopreventive agent is its differential effects on normal cells, such as endothelial cells, lymphocytes, hepatocytes, fibroblasts, thymocytes, and mammary epithelial cells (Santel et al, 2008; Watson et al., 2008; Uddin et al., 2005). The reason why curcumin selectively kills tumor cells but not normal cells still remains unclear; however, some suggestions have been proposed: (1) cellular uptake of curcumin is higher in tumor cells than in normal cells (Kunwar et al., 2008), (2) the glutathione levels in tumor cells tend to be lower than normal cells, which promotes the sensitivity of tumor cells to curcumin (Syng-Ai et al., 2004), and (3) most tumor cells express constitutively active NF-κB and mediate their survival (Lin et al., 2007).
In 1985, Kuttan et al. reported the first in vivo study showing the anticancer activity of curcumin in an ascites mice model of Dalton's lymphoma (Kuttan et al, 1985). Since then, extensive in vivo studies have investigated the chemopreventive, as well as anticancer, effects of curcumin. Their results revealed that curcumin has potential against many cancers, such as lymphoma/ leukemia, colon, esophageal, liver, lung, oral, skin, breast, and prostate cancers, etc., in animal and tumor models (Goel et al., 2008).
Clinical trials of curcumin in cancer patients have been conducted worldwide for many years. Several reports demonstrated that curcumin is effective against myeloma, colorectal, pancreatic, and prostate cancers in humans (Anand et al., 2008; Anuchapreeda et al., 2006; Goel et al., 2008). In a recent phase I/II study, curcumin was administrated orally in combination with gemcitabine to treat gemcitabine-resistant pancreatic cancer. The study demonstrated that this combination chemotherapy is safe and feasible in pancreatic cancer patients (Kanai et al., 2011).
Artemisiae Annuae Herba (青蒿 qīng hāo; sweet wormwood, Artemisia annua, Asteraceae)
Artemisia annua Linn. (sweet wormwood; Chinese wormwood) is a member of the Asteraceae family of plants (formerly Compositae) and is the source of the Chinese medicine Qinghao. A. annua contains various terpenoids (particulary highly oxygenated sesquiterpene lactones), flavonoids, coumarins, and other shikimate metabolites. The presence of numerous terpene allylic hydroperoxides and endoperoxides is a characteristic phytochemical feature of this species (Brown, 2010). Artemisinin is one such highly oxygenated sesquiterpene lactone found uniquely in A. annua. It is a well known antimalarial drug, with no known side effects. The unique 1,2,4-trioxane ring structure found in artemisinin and its analogues, such as artemether, arteether, and artesunate, (Figure 9) is necessary for the antimalarial activity. This important compound was isolated in the early 1970s based on information recorded long ago about Qinghao in the TCM archive, “Emergency Prescriptions Kept Up One's Sleeve”, by the famous physician Ge Hong (284-363) (Hsu, 2006). Although artemisinins also show cytotoxic activity against cancer cells by various mechanisms of action (Efferth, 2006), the antimalarial activity is the prominent medicinal use (Klayman et al., 1984; Sriram et al., 2004). It should be noted that the Lasker Foundation awarded the 2011 Lasker~DeBakey Clinical Medical Research Award to Tu Youyou (China Academy of Chinese Medical Sciences, Beijing), who first dove back into the ancient literature and led to the isolation of qinghaosu as a potent antimalarial drug through a group effort in China (http://www.laskerfoundation.org/awards/2011_c_description.htm). It should also be mentioned that a significant contribution was made by Dr. Yi Zhao with regard to the elucidation of the pharmacological effect as well as the mechanism of action of qinghaosu as evidenced in his academic papers entitled Studies on Artemisia annua L./Qinghaosu, Guangxi TCM University, China (Zhao et al., 1986; Zhao et al., 1987).
Figure 9.
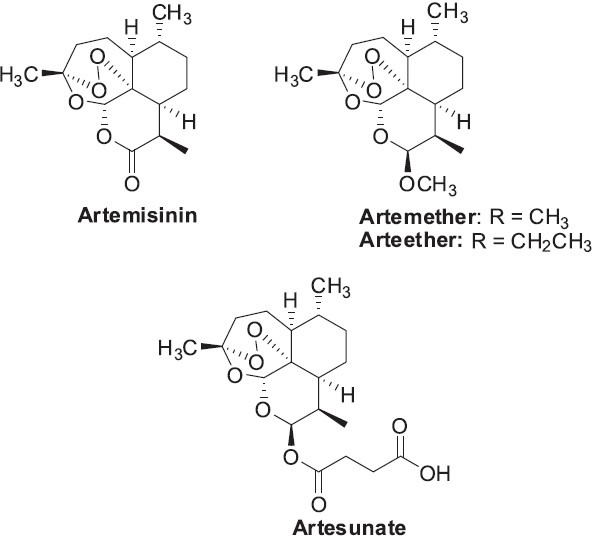
Structures of the antimalarial drug artemisinin from Artemisia annua and some synthetic analogues
Conclusion
As part of the TCM and other folk medicine systems, many plant and fungal species are used as foods and dietary supplements to promote good health, as well as to prevent or treat many diseases. Relatively nontoxic herbal products are particularly desireable for alleviating chronic health effects. However, the pharmacological effects of TCM and other natural products on the human body must be ascertained, toxicological studies must be performed, authenticity must be verified, and quality control standards must be maintained to ensure both maximal safety and efficacy to treat both chronic and acute conditions. Modern scientific methodology and medicinal chemistry approaches will continue to identify and evaluate the bioactive components found in TCM prescriptions, and validate their use as sources of new, effective, and safe world-class new medicines and dietary supplements.
Acknowledgement
We would like to thank the Taiwan Department of Health Clinical Trial and Research Center of Excellence (DOH100-TD-B-111-0004) for partial support.
References
- 1.Aggarwal B.B., Harikumar K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against egenerative, cardiovascular, pulmonary, metabolic, autoimmune, and neoplastic diseases. International Journal of Biochemistry & Cell Biology. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal B., Kumar A., Bharti A.C. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Research. 2003;23:363–398. [PubMed] [Google Scholar]
- 3.Anand P., Sundaram C., Jhurani S., Kunnumakkara A.B., Aggarwal B.B. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Letters. 2008;267:133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 4.Anuchapreeda S., Thanarattanakorn P., Sittipreechacharn S., Tima S., Chanarat P., Limtrakul P. Inhibitory effect of curcumin on MDR1 gene expression in patient leukemic cells. Archives of Pharmaceutical Research. 2006;10:866–873. doi: 10.1007/BF02973907. [DOI] [PubMed] [Google Scholar]
- 5.Atsumi T., Fujisawa S., Tonosaki K. Relationship between intracellular ROS production and membrane mobility in curcumin and tetrahydrocurcumin treated human gingival fibroblasts and human submandibular gland carcinoma cells. Oral Diseases. 2005;11:236–242. doi: 10.1111/j.1601-0825.2005.01067.x. [DOI] [PubMed] [Google Scholar]
- 6.Atsumi T., Tonosaki K., Fujisawa S. Induction of early apoptosis and ROS-generation activity in human gingival fibroblasts (HGF) and human submandibular gland carcinoma (HSG) cells treated with curcumin. Archives of Oral Biology. 2006;51:913–921. doi: 10.1016/j.archoralbio.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Aviello G., Abenavoli L., Borrelli F., Capasso R., Izzo A.A., Lembo F., Romano B., Cappasso F. Garlic: empiricism or science? Natural Product Communications. 2009;4:1785–1796. [PubMed] [Google Scholar]
- 8.Ban J.O., Yuk D.Y., Woo K.S., Kim T.M., Lee U.S., Jeong H.S., Kim D.J., Chung Y.B., Hwang B.Y., Oh K.W., Hong J.T. Inhibition of cell growth and induction of apoptosis via inactivation of NF-κB by a sulfur compound isolated from garlic in human colon cancer cells. Journal of Pharmacological Sciences. 2007;104:374–383. doi: 10.1254/jphs.fp0070789. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharyya S., Mandal D., Sen G.S., Pal S., Banerkee S., Lahiry L., Finke J.H., Tannenbaum C.S., Das T., Sa G. Tumor- induces oxidative stress perturbs nuclear factor-kappaB activity- augmenting tumor necrosis factor-alpha-mediated T-cell death: Protection by curcumin. Cancer Research. 2007;67:362–370. doi: 10.1158/0008-5472.CAN-06-2583. [DOI] [PubMed] [Google Scholar]
- 10.Bongiorno P.B., Fratellone P.M., Lo Giudice P. Potential health benefits of garlic (Allium sativum) Journal of Complementary and Integrative Medicine. 2008;5:1–24. [Google Scholar]
- 11.Brown G.D. The biosynthesis of artemisinin (Qinghaosu) and the phytochemistry of Artemisia annua L (Qinghao) Molecules. 2010;15:7603–7698. doi: 10.3390/molecules15117603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butt M.S., Sultan M.T., Butt M.S., Iqbal J. Garlic: nature's protection against physiological threats. Critical Reviews in Food Science and Nutrition. 2009;49:538–551. doi: 10.1080/10408390802145344. [DOI] [PubMed] [Google Scholar]
- 13.Cerella C., Dicata M., Jacob C., Diederich M. Chemical properties and mechanism determing the anti-cancer action of garlic-derived organic sulfur compounds. Anticancer Agents in Medicinal Chemistry. 2011;11:267–271. doi: 10.2174/187152011795347522. [DOI] [PubMed] [Google Scholar]
- 14.Chacko S.M., Thambi P.T., Kuttan R., Nishigaki I. Beneficial effects of green tea: a literature review. Chinese Medicine. 2010;5:13. doi: 10.1186/1749-8546-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang S.T. World production of cultivated edible and medicinal mushrooms in 1997 with emphasis on Lentinus edodes (Berk) Sing in China. International Journal of Medicinal Mushrooms. 1999;1:387–409. [Google Scholar]
- 16.Chatterjee R., Srinivasan K.S., Maiti P.C. Cordyceps sinensis (Berkeley) Saccardo: structure of cordycepic acid. Journal of American Pharmaceutical Association. 1957;46:114–118. doi: 10.1002/jps.3030460211. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y.C., Ho H.O., Su C.H., Sheu M.T. Anticancer effects of Taiwanofungus camphoratus extracts, isolated compounds and its combinational use. Journal of Experimental & Clinical Medicine. 2010;2:274–281. [Google Scholar]
- 18.Cheng A.L., Hsu C.H., Lin J.K., Hsu M.M., Ho Y.F., Shen T.S., Ko J.Y., Lin J.T., Lin B.R., Ming-Shiang W., Yu H.S., Jee S.H., Chen G.S., Chen T.M., Chen C.A., Lai M.K., Pu Y.S., Pan M.H., Wang Y.J., Tsai C.C., Hsieh C.Y. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high- risk or pre-malignant lesions. Anticancer Research. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 19.Cheng J.J., Huang N.K., Chang T.T., Ling Wang D, Lu M.K. Study for anti-angiogenic activities of polysaccharides isolated from Antrodia cinnamomea in endothelial cells. Life Sciences. 2005;76:3029–3042. doi: 10.1016/j.lfs.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 20.Cheng J.J., Huang N.K., Lur H.S., Kuo C.I., Lu M.K. Characterization and biological functions of sulfated polysaccharides from sulfated-salt treatment of Antrodia cinnamomea Process. Biochemistry. 2009;44:453–459. [Google Scholar]
- 21.Choi J.H., Kim Y.B., Lim H.Y., Park J.S., Kim H.C., Cho Y.K., Han S.W., Kim M.W., Joo H.J. 5-Fluorouracil mitomycin-C, and polysaccharide-K adjuvant chemoimmunotherapy for locally advanced gastric cancer: the prognostic significance of frequent perineural invasion. Hepatogastroenterology. 2007;54:290–297. [PubMed] [Google Scholar]
- 22.Dong Z., Ma W., Huang C., Yang C.S. Inhibition of tumor promoterinduced activator protein 1 activation and cell transformation by tea polyphenols, (−)-epigallocatechin gallate, and theaflavins. Cancer Research. 1997;57:4414–4419. [PubMed] [Google Scholar]
- 23.Efferth T. Molecular pharmacology and pharmacogenomics of artemisinin and its derivatives in cancer cells. Current Drug Targets. 2006;7:407–421. doi: 10.2174/138945006776359412. [DOI] [PubMed] [Google Scholar]
- 24.Ellertsen L.K., Hetland G., Johnson E., Grinde B. Effect of a medicinal extract from Agaricus blazei Murill on gene expression in a human monocyte cell line as examined by microarrays and immuno assays. International Immunopharmacology. 2006;6:133–143. doi: 10.1016/j.intimp.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 25.El-Sabban F., Abouazra H. Effect of garlic on atherosclerosis and its factors. Eastern Mediterranean Health Journal. 2008;14:195–205. [PubMed] [Google Scholar]
- 26.Fortes R.C., Novaes M.R.C.G. Effects of dietary supplementation with Agaricales mushrooms and other medicinal fungus on therapy against the cancer. Revista Brasileira de Cancerologia. 2006;52:363–371. [Google Scholar]
- 27.Ginter E., Simko V. Garlic (Allium sativum L) and cardiovascular diseases. Bratisl Lek Listy. 2010;111:452–456. [PubMed] [Google Scholar]
- 28.Goel A., Kunnumakkare A.B., Agarwal B.B. Curcumin as “curecumin”: from kitchen to clinic. Biochemical Pharmacology. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Goncagul G., Ayaz E. Antimicrobial effect of garlic (Allium sativum) Recent Patents in Antiinfectious. Drug Discovery. 2010;5:91–93. doi: 10.2174/157489110790112536. [DOI] [PubMed] [Google Scholar]
- 30.Gorin P.A.J, Barreto-Bergter E. In: The Polysaccharides. Aspinall G.O., editor. Vol. 2. Orlando, Florida, USA: Academic Press; 1983. pp. 365–409. [Google Scholar]
- 31.Gupta S., Hastak K., Afaq F., Ahmad N., Mukhtar H. Essential role of caspases in epigallocatechin-3-gallate-mediated inhibition of nuclear factor κB and induction of apoptosis. Oncogene. 2004;23:2507–2522. doi: 10.1038/sj.onc.1207353. [DOI] [PubMed] [Google Scholar]
- 32.Hanafusa T., Yamazaki S., Okubo A., Toda S., Suzuki K., Nakajima E., Yasukawa Y. Intestinal absorption and tissue distribution of immunoactive and antiviral water-soluble (14C) lignins in rats. Drug Metabolism and Pharmacokinetics. 1990;5:661–674. [Google Scholar]
- 33.Hatcher H., Planalp R., Cho J., Torti F.M., Torti S.V. Curcumin: from ancient medicine to current clinical trials. Cellular and Molecular Life Sciences. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hetland G., Johnson E., Lyberg T., Bernardshaw S., Tryggestad A.M.A, Grinde B. Effects of the medicinal mushroom Agaricus blazei Murill on immunity, infection and cancer Scandinavian. Journal of Immunology. 2008;68:363–70. doi: 10.1111/j.1365-3083.2008.02156.x. [DOI] [PubMed] [Google Scholar]
- 35.Hsu E. Reflections on the ‘discovery’ of the antimalarial qinghao. British Journal of Clinical Pharmacology. 2006;61:666–670. doi: 10.1111/j.1365-2125.2006.02673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang L.F., Liang Y.Z., Guo F.Q., Zhou Z.F., Cheng B.M. Simultaneous separation and determination of active components in Cordyceps sinensis and Cordyceps militarris by LC/ESI-MS. Journal of Pharmaceutical and Biomedical Analysis. 2003;33:1155–1162. doi: 10.1016/s0731-7085(03)00415-1. [DOI] [PubMed] [Google Scholar]
- 37.Itokawa H., Shi Q., Akiyama T., Morris-Natschke S.L., Lee K.H. Recent advances in the investigation of curcuminoids. Chinese Medicine. 2008;3:11. doi: 10.1186/1749-8546-3-11. http://www.cmjournal.org/content/3/1/11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jakubikova J., Sedlak J. Garlic-derived organosulfides induce cytotoxicity, apoptosis, cell cycle arrest and oxidative stress in human colon carcinoma cell lines. Neoplasma. 2006;53:191–199. [PubMed] [Google Scholar]
- 39.Jiang M.C., Yang-Yen H.F., Yen J.J., Lin J.K. Curcumin induces apoptosis in immortalized NIH 3T3 and malignant cancer cell lines. Nutrition and Cancer. 1996;26:111–120. doi: 10.1080/01635589609514468. [DOI] [PubMed] [Google Scholar]
- 40.Jung E.M., Lim J.H., Lee T.J., Park J.W., Choi K.S., Kwon T.K. Curcumin sensitizes tumor necrosis factor-related apoptosis- inducing ligand (TRAIL)-induced apoptosis through reactive oxygen species-mediated upregulation of death receptor-5 (DR5) Carcinogenesis. 2005;26:1905–1913. doi: 10.1093/carcin/bgi167. Roy. [DOI] [PubMed] [Google Scholar]
- 41.Kamat A.M., Sethi G., Aggarwal B.B. Curcumin potentiates the apoptotic effects of chemotherapeutic agents and cytokines through down-regulation of nuclear factor-kappaB and nuclear factor-kappaB-regulated gene products in IFN-alpha-sensitive and IFN-alpha-resistant human bladder cancer cells. Molecular Cancer Therapeutics. 2007;6:1022–1030. doi: 10.1158/1535-7163.MCT-06-0545. [DOI] [PubMed] [Google Scholar]
- 42.Kamohara H., Takahashi M., Ishiko T., Ogawa M., Baba H. Induction of interleulin-8 (CXCL-8) by tumor necrosis factor- alpha and leukemia inhibitory factor in pancreatic carcinoma cells: Impact of CXCL-8 as an autocrine growth factor. International Journal of Oncology. 2007;31:627–632. [PubMed] [Google Scholar]
- 43.Kanai M., Yoshimura K., Asada M., Imazumi A., Suzuki C., Matsumoto S., Nishimura T., Mori Y., Masui T., Kawaguchi Y., Yanagihara K., Yazumi S., Chiba T., Guha S., Aggarwal B.B. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemotherapy and Pharmacology. 2011;68:157–164. doi: 10.1007/s00280-010-1470-2. [DOI] [PubMed] [Google Scholar]
- 44.Katoh R., Ooshiro M. Enhancement of antitumor effect of tegafur/uracil (UFT) plus leucovorin by combined treatment with protein-bound polysaccharide, PSK, in mouse models. Cellular & Molecular Immunology. 2007;4:295–299. [PubMed] [Google Scholar]
- 45.Kidd P.M. The use of mushroom glucans and proteoglycans in cancer treatment. Alternative Medicine Review. 2000;5:4–27. [PubMed] [Google Scholar]
- 46.Kim D.S., Kim J.Y., Kim J.H. Total synthesis of calebin-A, preparation of its analogues, and their neuronal cell protectivity against beta-amyloid insult. Bioorganic & Medicinal Chemistry Letters. 2001;11:2541–2543. doi: 10.1016/s0960-894x(01)00489-9. [DOI] [PubMed] [Google Scholar]
- 47.Kim G.Y., Lee M.Y., Lee H.J., Moon D.O., Lee C.M., Jin C.Y., Choi Y.H., Jeong Y.K., Chung K.T., Lee J.Y., Choi I.H., Park Y.M. Effect of water-soluble proteoglycan isolated from Agaricus blazei on the maturation of murine bone marrow-derived dendritic cells. International Pharmacology. 2005;5:1523–1532. doi: 10.1016/j.intimp.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 48.Kim H.W., Kim B.K. Biomedical triterpenoids of Ganoderma lucidum (Curt: Fr) P Karst (Aphyllophoromycetideae) International Journal of Medicinal Mushrooms. 1999;1:121–138. [Google Scholar]
- 49.Kim J.W., Amin A.R., Shin D.M. Chemoprevention of head and neck cancer with green tea polyphenols. Cancer Prevention Research. 2010;3:900–909. doi: 10.1158/1940-6207.CAPR-09-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kiuchi F., Goto Y., Sugimoto N., Akao N., Kondo K., Tsuda Y. Nematocidal activity of turmeric: synergistic action of curcuminoids. Chemical & Pharmaceutical Bulletin (Tokyo) 1993;41:1640–1643. doi: 10.1248/cpb.41.1640. [DOI] [PubMed] [Google Scholar]
- 51.Klayman D.L., Lin A.J., Acton N., Scovili J.P., Hoch J.M., Milhous W.K., Theoharides A.D. Isolation of artemisinin (Qinghaosu) from Artemisia annua growing in the US. Journal of Natural Products. 1984;47:715–717. doi: 10.1021/np50034a027. [DOI] [PubMed] [Google Scholar]
- 52.Kohli K., Ali J., Ansari M.J., Raheman Z. Curcumin: a natural antiinflammatory agent. Indian Journal of Pharmacology. 2005;37:141–147. [Google Scholar]
- 53.Kunwar A., Barik A., Mishra B., Rathinasamy K., Pandey R., Priyadarsini K.I. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochimica et Biophysica Acta. 2008;1780:673–679. doi: 10.1016/j.bbagen.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 54.Kuttan R., Bhanumathy P., Nirmala K., George M.C. Potential anticancer activity of turmeric (Curcuma longa) Cancer Letters. 1985;29:197–202. doi: 10.1016/0304-3835(85)90159-4. [DOI] [PubMed] [Google Scholar]
- 55.Lansky E.P., Newman R.A. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. Journal of Ethnopharmacology. 2007;109:177–206. doi: 10.1016/j.jep.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 56.Lee J., Im Y.H., Jung H.H., Kim J.H., Park J.O., Kim K., Kim W.S., Ahn J.S., Jung C.W., Park Y.S., Kang W.K., Park K. Curcumin inhibits interferon-alpha induced NF-kappaB and COX-2 in human A549 non-small cell lung cancer cells. Biochemical and Biophysical Research Communications. 2005;334:313–318. doi: 10.1016/j.bbrc.2005.06.093. [DOI] [PubMed] [Google Scholar]
- 57.Lee K.H., Itokawa H., Kozuka M. Oriental herbal products: the basis for development of dietary supplements and new medicines in the 21st century. In: Ho C.T., Lin J.K., Zheng Q.Y., editors. Oriental Foods and Herbs - Chemistry and Health Effects. Washington DC: American Chemical Society Press; 2003. pp. 2–31. [Google Scholar]
- 58.Lei Y.P., Liu C.T., Sheen L.Y., Chen H.W., Lii C.K. Diallyl disulfide and diallyl trisulfide protect endothelial nitric oxide synthase against damage by oxidized low-density lipoprotein. Molecular Nutrition & Food Research. 2010;54(Suppl 1):S42–S52. doi: 10.1002/mnfr.200900278. [DOI] [PubMed] [Google Scholar]
- 59.Lima C.U.J.O, Cordova C.O.D.A, Nobrega O.D.T, Funghetto S.S., Karnikowski M.G.D.O. Does the Agaricus blazei Murill mushroom have properties that affect the immune system? An integrative review. Journal of Medicinal Food. 2011;14:2–8. doi: 10.1089/jmf.2010.0017. [DOI] [PubMed] [Google Scholar]
- 60.Lin L., Shi Q., Nyarko A.K., Bastow K.F., Wu C.C., Su C.Y., Shih C.C.Y, Lee K.H. Antitumor agents 250 Design and synthesis of new curcumin analogues as potential anti-prostate cancer agents. Journal of Medicinal Chemistry. 2006;49:3963–3972. doi: 10.1021/jm051043z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin Y.G., Kunnumakkara A.B., Nair A., Merritt W.M., Han L.Y., Armaiz-Pena G.N., Kamat A.A., Spannuth W.A., Gershenson D.M., Lutgendorf S.K., Aggarwal B.B., Sood A.K. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clinical Cancer Research. 2007;13:3423–3430. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- 62.Liu J.J., Huang T.S., Hsu M.L., Chen C.C., Lin W.S., Lu F.J., Chang W.H. Antitumor effects of the partially purified polysaccharides from Antrodia camphorata and the mechanism of its action. Toxicology and Applied Pharmacology. 2004;201:186–193. doi: 10.1016/j.taap.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 63.Liu Y.K., Shen W. Inhibitive effect of cordyceps sinensis on experimental hepatic fibrosis and its possible mechanism. World Journal of Gastroenterology. 2003;9:529–33. doi: 10.3748/wjg.v9.i3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lull C., Wichers H.J., Savelkoul H.F. Antiinflammatory and immunomodulating properties of fungal metabolites. Mediators of Inflammation. 2005;2:63–80. doi: 10.1155/MI.2005.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martos M.V., Lopez J.F., Alvarez J.A.P. Pomegranate and its many functional components as related to human health: A review. Comprehensive Reviews in Food Science and Food Safety. 2010;9:635–654. doi: 10.1111/j.1541-4337.2010.00131.x. [DOI] [PubMed] [Google Scholar]
- 66.Mohammed A., Adelaiye A.B., Abubakar M.S., Abdurahman E.M. Effects of aqueous extract of Ganoderma lucidum on blood glucose levels of normoglycemic and alloxaninduced diabetic wistar rats. Journal of Medicinal Plants Research. 2007;1:34–37. [Google Scholar]
- 67.Mukoyama A., Ushijima H., Nishimura S., Koike H., Toda M., Hara Y., Shimamura T. Inhibition of rotavirus and enterovirus infections by tea extracts. Japanese Journal of Medical Science & Biology. 1991;44:181–186. doi: 10.7883/yoken1952.44.181. [DOI] [PubMed] [Google Scholar]
- 68.Nakamura N., Hirakawa A., Gao J.J., Kakuda H., Shiro M., Komatsu Y., Sheu C.C., Hattori M. Five new maleic and succinic acid derivatives from the mycelium of Antrodia camphorata and their cytotoxic effects on LLC tumor cell line. Journal of Natural Products. 2004;67:46–48. doi: 10.1021/np030293k. [DOI] [PubMed] [Google Scholar]
- 69.Ngai P.H.K, Ng T.B. Lentin a novel and potent antifungal protein from shitake mushroom with inhibitory effects on activity of human immunodeficiency virus-1 reverse transcriptase and proliferation of leukemia cells. Life Sciences. 2003;73:3363–3374. doi: 10.1016/j.lfs.2003.06.023. [DOI] [PubMed] [Google Scholar]
- 70.Nomura M., Ma W., Chen N., Bode A.M., Dong Z. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced NF-κB activation by tea polyphenols, (−)-epigallocatechin gallate and theaflavins. Carcinogenesis. 2000;21:1885–1890. doi: 10.1093/carcin/21.10.1885. [DOI] [PubMed] [Google Scholar]
- 71.Oba K., Teramukai S., Kobayashi M., Matsui T., Kodera Y., Sakamoto J. Efficacy of adjuvant immunochemotherapy with polysaccharide K for patients with curative resections of gastric cancer. Cancer Immunology, Immunotherapy. 2007;56:905–911. doi: 10.1007/s00262-006-0248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ohtsu H., Itokawa H., Xiao Z., Su C.Y., Shih C.C.Y, Chiang T., Chang E., Lee Y., Chiu S.Y., Chang C., Lee K.H. Antitumor agents. 222. Synthesis and anti-androgen activity of new diarylheptanoids. Bioorganic & Medicinal Chemistry. 2003;11:5083–5090. doi: 10.1016/j.bmc.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 73.Ooi V.E.C, Liu R. Immunomodulation and anti-cancer activity of polysaccharide-protein complexes. Current Medicinal Chemistry. 2000;7:715–729. doi: 10.2174/0929867003374705. [DOI] [PubMed] [Google Scholar]
- 74.Peng R.Y., Chyau C.C., Su C.H., Chen C.C. Review on the unique Formosan medicinal mushroom Taiwanofungus camphoratus (Antrodia camphorata) Recent Progress in Medicinal Plants. 2008;20:189–214. [Google Scholar]
- 75.Ravindran J., Prasad S., Aggarwal B.B. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? The AAPS Journal. 2009;11:495–510. doi: 10.1208/s12248-009-9128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reuter S., Eifes S., Dicato M., Aggarwal B.B., Diederich M. Modulation of anti-apoptotic and survival pathways by curcumin as a strategy to induce apoptosis in cancer cells. Biochemical Pharmacology. 2008;76:1340–1351. doi: 10.1016/j.bcp.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 77.Roy M., Chakraborty S., Siddiqi M., Bhattacharya R.K. Induction of apoptosis in tumor cell by natural phenolic compounds Asian Pacific. Journal of Cancer Prevention. 2002;3:61–67. [PubMed] [Google Scholar]
- 78.Ruby A.J., Kuttan G., Babu K.D., Rajasekharan K.N., Kuttan R. Antitumor and oxidant activity of natural curcuminoids. Cancer Letters. 1995;94:79–83. doi: 10.1016/0304-3835(95)03827-j. [DOI] [PubMed] [Google Scholar]
- 79.Sakagami H., Aoki T., Simpson A., Tanuma S.I. Induction of immunopotentiation activity by a protein-bound polysaccharide, PSK (review) Anticancer Research. 1991;11:993–1000. [PubMed] [Google Scholar]
- 80.Sandiya B.S., Thakur G.S., Baghel R.K., Prasad G.B.K.S, Bisen P.S. Ganoderma lucidum: a potent pharmacological macrofungus. Current Pharmaceutical Biotechnology. 2009;10:717–742. doi: 10.2174/138920109789978757. [DOI] [PubMed] [Google Scholar]
- 81.Sano M., Tabata M., Suzuki M., Degawa M., Miyase T., Maeda-Yamamoto M. Simultaneous determination of twelve tea catechins by high-performance liquid chromatography with electrochemical detection. Analyst. 2001;126:816–820. doi: 10.1039/b102541b. [DOI] [PubMed] [Google Scholar]
- 82.Sanodiya B.S., Thakur G.S., Baghel R.K., Prasad G.B., Bisen P.S. Ganoderma lucidum: a potent pharmacological macrofungus. Curr Pharm Biotechnol. 2009;10:717–742. doi: 10.2174/138920109789978757. [DOI] [PubMed] [Google Scholar]
- 83.Santel T., Pflug G., Hemdan N.Y., Schäfer A., Hollenbach M., Buchold M., Hintersdorf A., Lindner I., Otto A., Bigl M., Oerlecke I., Hutschenreuther A., Sack U., Huse K., Groth M., Birkemeyer C., Schellenberger W., Gebhardt R., Platzer M., Weiss T., Vijayalakshmi M.A., Drüger M., Birkenmeier G. Curcumin inhibits glyoxalase 1: a possible link to its anti- inflammatory and anti-tumor activity. PLoS ONE. 2008;3:e3508. doi: 10.1371/journal.pone.0003508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shi L.S., Chao C.H., Shen D.Y., Chan H.H., Chen C.H., Liao Y.R., Wu S.J., Leu Y.L., Shen Y.C., Kuo Y.H., Lee E.J., Qian K., Wu T.S., Lee K.H. Biologically active constituents from the fruiting body of Taiwanofungus camphoratus. Bioorganic & Medicinal Chemistry. 2011;19:677–683. doi: 10.1016/j.bmc.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Skommer J., Wlodkowic D., Pellonen J. Cellular foundation of curcurmin-induced apoptosis in follicular lymphoma cell lines. Experimental Hematology. 2006;34:463–467. doi: 10.1016/j.exphem.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 86.Sprecher M., Sprinson D.B. A reinvestigation of the structure of ‘cordycepic acid’. Journal of Organic Chemistry. 1963;28:2490–2491. [Google Scholar]
- 87.Sriram D., Rao V.S., Chandrasekhar K.V.G, Yogeeswari P. Progress in the research of artemisinin and its analogues as antimalarials: an update. Natural Product Research. 2004;18:503–527. doi: 10.1080/14786410310001620556. [DOI] [PubMed] [Google Scholar]
- 88.Sudano Roccaro A, Blanco A.R., Giuliano F., Rusciano D., Enea V. Epigallocatechin-gallate enhances the activity of tetracycline in staphylococci by inhibiting its efflux from bacterial cells. Antimicrobial Agents and Chemotherapy. 2004;48:1968–1973. doi: 10.1128/AAC.48.6.1968-1973.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun M., Yang Y., Li H., Su B., Lu Y., Wei Q., Fan T. The effect of curcumin on bladder cancer cell line EJ in vitro. Zhong Yao Cai. 2004;27:848–850. [PubMed] [Google Scholar]
- 90.Syng-Ai C., Kumari A.L., Khar A. Effect of curcumin on normal and tumor cells: role of glutathione and bcl-2. Molecular Cancer Therapeutics. 2004;3:1101–1108. [PubMed] [Google Scholar]
- 91.Tochikura T.S., Nakashima H., Kaneko Y., Kobayashi N., Yamamoto N. Suppression of human immunodeficiency virus replication by 3‘-azido-3’-deoxythymidine in various human hematopoietic cell lines in vitro: augmentation of the effect by lentinan. Japanese Journal of Cancer Research. 1987;78:583–589. [PubMed] [Google Scholar]
- 92.Tochikura T.S., Nakashima H., Ohashi Y., Yamamoto N. Inhibition (in vitro) of replication and of the cyptopathic effect of human immunodeficiency virus by an extract of the culture medium of Lentinus edodes mycelia. Medical Microbiology and Immunology. 1988;177:235–244. doi: 10.1007/BF00189409. [DOI] [PubMed] [Google Scholar]
- 93.Tripathi K. A review - garlic, the spice of life (part-1) Asian Journal of Research in Chemistry. 2009;2:8–13. [Google Scholar]
- 94.Tsukagoshi S., Hashimoto Y., Fujii G., Kobayashi H., Nomoto K., Orita K. Krestin (PSK) Cancer Treatment Reviews. 1984;11:131–155. doi: 10.1016/0305-7372(84)90005-7. [DOI] [PubMed] [Google Scholar]
- 95.Uddin S., Hussain A.R., Manogaran P.S., Al-Hussein K., Platanias L.C., Gutierrez M.I., Bhatia K.G. Curcumin suppresses growth and induces apoptosis in primary effusion lymphoma. Oncogene. 2005;24:7022–7030. doi: 10.1038/sj.onc.1208864. [DOI] [PubMed] [Google Scholar]
- 96.Uehara S., Yasuda I., Takeya K., Itokawa H. Comparison of the commercial turmeric and its cultivated plant by their constituents. Shoyakugaku Zasshi. 1992;46:55–61. [Google Scholar]
- 97.Villegas I., Sanchez-Fidalgo S., de la Alarcon L.C. New mechanisms and therapeutic potential of curcumin for colorectal cancer. Molecular Nutrition & Food Research. 2008;52:1040–1061. doi: 10.1002/mnfr.200700280. [DOI] [PubMed] [Google Scholar]
- 98.Wahl H., Tan L., Griffith K., Choi M., Liu J.R. Curcumin enhances Apo21/TRAIL-induced apoptosis in chemoresistant ovarian cancer cells. Gynecologic Oncology. 2007;105:289–297. doi: 10.1016/j.ygyno.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 99.Wasser S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Applied Microbiology and Biotechnology. 2002;60:258–274. doi: 10.1007/s00253-002-1076-7. [DOI] [PubMed] [Google Scholar]
- 100.Wasser S.P. Coates P.M., editor. Shiitake (Lentinus edodes) Encyclopedia of Dietary Supplements. 2005:653–664. [Google Scholar]
- 101.Wasser S.P., Weis A.L. Medicinal properties of substances occurring in higher Basidiomycetes mushrooms: current perspectives (Review) International Journal of Medicinal Mushrooms. 1999b;1:31–62. [Google Scholar]
- 102.Wasser S.P., Weis A.L. Therapeutic effects of substances occurring in higher Basidiomycetes mushrooms: a modern perspective. Critical Reviews in Immunology. 1999a;19:65–96. [PubMed] [Google Scholar]
- 103.Watson J.L., Hill R., Lee P.W., Giacomantonio C.A., Hoskin D.W. Curcumin induces apoptosis in HCT-116 human colon cancer cells in a p21-independent manner. Experimental and Molecular Pathology. 2008;84:230–233. doi: 10.1016/j.yexmp.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 104.Weber J.M., Ruzindana-Umunyana A., Imbeault L., Sircar S. Inhibition of adenovirus infection and adenain by green tea catechins. Antiviral Research. 2003;58:167–173. doi: 10.1016/s0166-3542(02)00212-7. [DOI] [PubMed] [Google Scholar]
- 105.Wei S.C., Lin Y.S., Tsao P.N., Wu-Tsai J.J., Wu C.H., Wong J.M. Comparison of the anti-proliferation and apoptosis-induction activities of sulindac, celecoxib, curcumin, and nifedipine in mismatch repair-deficient cell lines. Journal of the Formosan Medical Association. 2004;103:599–606. [PubMed] [Google Scholar]
- 106.Weinreb O., Mandel S., Amit T., Youdim M.B.H. Neurological mechanisms of green tea polyphenols in Alzheimer's and Parkinson's diseases. Journal of Nutritional Biochemistry. 2004;15:506–516. doi: 10.1016/j.jnutbio.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 107.Wilken R., Veena M.S., Wang M.B., Srivatsan E.S. Curcumin: a review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Molecular Cancer. 2011;10:12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wojcikowski K., Johnson D.W., Gobe G. Herbs or natural substances as complementary therapies for chronic kidney disease: ideas for future studies. Journal of Laboratory and Clinical Medicine. 2006;147:160–166. doi: 10.1016/j.lab.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 109.Wu S.H., Ryvarden L., Chang T.T. Antrodia camphorata (“niu-chang-chih”), new combination of a medicinal fungus in Taiwan. Botanical Bulletin Academia Sinica (Taiwan) 1997;38:273–275. [Google Scholar]
- 110.Wu S.J., Leu Y.L., Chen C.H., Chao C.H., Shen D.Y., Chan H.H., Lee E.J., Wu T.S., Wang Y.H., Shen Y.C., Qian K., Bastow K.F., Lee K.H. Camphoratins A–J, potent cytotoxic and anti-inflammatory triterpenoids from the fruiting body of Taiwanofungus camphoratus. Journal of Natural Products. 2010;73:1756–1762. doi: 10.1021/np1002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu J.W., Zhao W., Zhong J.J. Biotechnological production and application of ganoderic acids. Applied Microbiology and Biotechnology. 2010;87:457–466. doi: 10.1007/s00253-010-2576-5. [DOI] [PubMed] [Google Scholar]
- 112.Xu Z.T., Chen X.P., Zhong Z.F., Chen L.D., Wang Y.T. Ganoderma lucidum polysaccharides: immunomodulation and potential anti-tumor activities. The American Journal of Chinese Medicine. 2011;39:15–27. doi: 10.1142/S0192415X11008610. [DOI] [PubMed] [Google Scholar]
- 113.Yamaguchi K., Honda M., Ikigai H., Hara Y., Shimamura T. Inhibitory effects of (−)-epigallocatechin gallate on the life cycle of human immunodeficiency virus type 1 (HIV-1) Antiviral Research. 2002;53:19–34. doi: 10.1016/s0166-3542(01)00189-9. [DOI] [PubMed] [Google Scholar]
- 114.Yang B.K., Kim D.H., Jeong S.C., Das S., Choi Y.S., Shin J.S., Lee S.C., Song C.H. Hypoglycemic effect of a Lentinus edodes exo-polymer produced from a submerged mycelial culture. Bioscience Biotechnology and Biochemistry. 2002;66:937–942. doi: 10.1271/bbb.66.937. [DOI] [PubMed] [Google Scholar]
- 115.Yang C.S., Chung J.Y., Yang G., Chhabra S.K., Lee M.J. Tea and tea polyphenols in cancer prevention. Journal of Nutrition. 2000;130:472–478. doi: 10.1093/jn/130.2.472S. [DOI] [PubMed] [Google Scholar]
- 116.Yeh C.T., Rao Y.K., Yao C.J., Yeh C.F., Li C.H., Chuang S.E., Luong J.H., Lai G.M., Tzeng Y.M. Cytotoxic triterpenes from Antrodia camphorata and their mode of action in HT-29 human colon cancer cells. Cancer Letters. 2009;285:73–79. doi: 10.1016/j.canlet.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 117.Zhang Q.X., Wu J.Y. Cordyceps sinensis mycelium extract induces human premyelocytic leukemia cell apoptosis through mitochondrion pathway. Experimental Biology and Medicine (Maywood) 2007;232:52–57. [PubMed] [Google Scholar]
- 118.Zhao Y., Hanton W.K., Lee K.H. Antimalarial agents, 2 Artesunate an inhibitor of chytochrom oxidase activity in Plasmodium berghei. Journal of Natural Products. 1986;49:139–142. doi: 10.1021/np50043a018. [DOI] [PubMed] [Google Scholar]
- 119.Zhao Y., Hall I.H., Oswald C.B., Yokoi T., Lee K.H. Antimalarial agents III Mechanism of action of artesunate against Plasmodium berghei infection. Chemical and Pharmaceutical Bulletin. 1987;35:2052–2061. doi: 10.1248/cpb.35.2052. [DOI] [PubMed] [Google Scholar]
- 120.Zhou X., Gong Z., Su Y., Lin J., Tang K. Cordyceps fungi: natural products, pharmacological functions and developmental products. Journal of Pharmacy and Pharmacology. 2009;61:279–291. doi: 10.1211/jpp/61.03.0002. [DOI] [PubMed] [Google Scholar]
- 121.Zhu J.S., Halpern G.M., Jones K. The scientific rediscovery of a precious ancient Chinese herbal regimen: Cordyceps sinensis: part II. Journal of Alternative and Complementary Medicine. 1998;4:429–457. doi: 10.1089/acm.1998.4.429. [DOI] [PubMed] [Google Scholar]


