ABSTRACT
Antibiotics continue to be used as growth promoters in the poultry industry. Honeybee (Apis melifera) venom (HBV) possesses a number of beneficial biological activities, particularly for regulating the immune system. The aim of the present study was to evaluate the immunoprophylactic effects of HBV against Salmonella Gallinarum in broiler chicks as an initial step towards developing eco-friendly alternatives to reduce antibiotic use. HBV was administered using a spray technique. HBV improved body weight gain, particularly in the presence of infection. Moreover, HBV enhanced antibody production activity against formalin-killed S. Gallinarum. The CD4+:CD8+ T lymphocyte ratio, relative mRNA expression levels of interleukin-18 and interferon-γ, and serum lysozyme activity also increased following HBV administration before the infection period as well as during infection. HBV reinforced bacterial clearance and increased survivability against S. Gallinarum. Corresponding pathological analyses demonstrated that the HBV-sprayed group displayed mild and less severe abnormal changes compared with those in the control group. It was presumed that the prophylactic effects of HBV against S. Gallinarum were associated with its non-specific immune response stimulating activity. Thus, HBV may provide an alternative to reduce antibiotic use in the poultry industry.
Keywords: honeybee venom, immune enhancement, Salmonella Gallinarum
Antibiotics continue to be used as growth promoters in the poultry industry in many countries, although there is worldwide concern about the emergence and dissemination of antibiotic-resistant bacteria [9]. Hence, there is an urgent need to identify eco-friendly alternatives to reduce antibiotic use. One of the most promising methods of reducing antibiotics is to strengthen defense mechanisms of birds through prophylactic administration of natural immunostimulants [16].
Honeybee (Apis melifera) venom (HBV) has long been used as an alternative medicine to alleviate a variety of pathological conditions, such as pain and inflammation. This natural toxin is a complex mixture of proteins (phospholipase A2 and hyaluronidase), peptides (melittin, apamine, mast cell degranulating peptide 401 and adolapine) and low molecular components (histamine, dopamine and norepinephrine) [26]. It has recently been demonstrated that whole HBV and some of its components, particularly melittin, possess antinociceptive and anti-inflammatory activities in very small doses [20, 23]. Moreover, HBV possesses a number of beneficial biological effects, such as radioprotective [5], wound-healing [10] and anticancer activities [26]. Accumulating evidence has indicated that HBV plays an important role in regulating the immune system. Perrin-Cocon et al. [27] and Ramoner et al. [28] reported that HBV secretory phospholipase A2 induces maturation of dendritic cells and activates the dendritic cell immune response. Dendritic cells express receptors, such as members of the Toll-like family, which recognize pathogens through exogenous pathogen-associated molecular patterns [15]. These cells also express receptors for several cytokines, such as tumor necrosis factor and interferons (IFNs), which play an important role in host defense against infection by microbial pathogens [2, 29]. HBV increases the CD4+ T lymphocyte population and enhances IFN-γ expression in a mouse model [25]. These collective observations suggest that HBV may provide an alternative way to reduce antibiotic use by promoting immune activity and preventing disease.
Salmonella enterica serovar Gallinarum (S. Gallinarum) is the fowl typhoid causative agent in galliform birds of all ages. The major clinical signs of fowl typhoid are anorexia, diarrhea and dehydration, and the most frequent pathological findings are hepatospelnomegaly and intestinal tract hemorrhage [30]. Although fowl typhoid has been eradicated from Australia, North America and most European countries, it remains a significant problem in Africa, Asia and Central and South America [21, 30]. Outbreaks of fowl typhoid in Korea have been reported since 1992, and this disease has become a serious problem in the poultry industry [22].
In the present study, HBV was administered to broiler chicks using a spray technique, which is one of the most technically efficient and economically feasible methods in the poultry industry [6]. The aim of the present study was to evaluate the immunoprophylactic effects of HBV against S. Gallinarum in broiler chicks as an initial step towards developing an eco-friendly alternative to reduce antibiotic use. The present study conducted three independent experiments with formalin-killed S. Gallinarum and two different infective doses of the pathogenic live bacteria after three HBV spray administrations. Antibody production, T lymphocyte subpopulations, relative cytokines and lysozyme mRNA expression levels were evaluated. In addition, body weight gain, bacterial clearance, survivability and pathological changes were also monitored.
MATERIALS AND METHODS
Source and preparation of HBV for spray administration: The HBV was provided by Wissen Co., Ltd. (Daejeon, Korea). It is formulated fractions purified from the bee venom obtained by Large Quantiti Bee-Venom Collector (P10-1003672, Wissen Co., Ltd.).
The fine HBV powder was dissolved in a solvent consisting of 95.7% distilled water, 3.5% ethanol and 0.8% propylene glycol, by volume, for spray administration. Its concentration was 2.1 mg/ml, which was the optimal concentration determined in our preliminary experiments [14].
Chicks and administration of HBV spray: One-day-old Ross broiler chicks with no history of salmonellosis were obtained from a local hatchery. After a 2-day acclimation period, the chicks were randomly divided into two groups consisting of 60 chicks each. The control group was exposed only to the spray solvent. The experimental group (HBV-sprayed group) was exposed to the HBV spray in solvent. As the chicks grew, 30, 60 and 90 ml solvent volumes were administered to each group at 3, 9 and 15 days of age, respectively. After three sprays, chicks were subjected to three independent experiments including an evaluation of antibody production against formalin-killed S. Gallinarum (Experiment 1), an evaluation of immune response and bacterial clearance against a sublethal S. Gallinarum dose [1 × 109 colony forming units (cfu)] (Experiment 2) and an evaluation of survivability and pathological changes against a low lethal dose (LD25, 5 × 109 cfu) of the bacteria (Experiment 3). All birds were housed in separate air-controlled rooms by group and allowed free access to nutritionally complete antibiotic-free chick feed and tap water. All animal procedures were approved by the Institutional Animal Care and Use Committee of Chonnam National University (approval number: CNU IACUC-YB-2010-1).
Experiment 1—Evaluation of antibody production activity against formalin-killed S. Gallinarum: On day 1 after the last spray, 10 chicks were randomly selected from each group and isolated in separate rooms by group. In this experiment, 1 × 109 cfu/ml formalin-killed S. Gallinarum (SG3001) was used as the antigen. All chicks received two subcutaneous injections (0.3 ml each time) with a 1-week interval between injections. The first inoculation was given with Freund’s complete adjuvant (Sigma-Aldrich, St. Louis, MO, U.S.A.) at 16 days of age, and the second was given with Freund’s incomplete adjuvant (Sigma-Aldrich) at 23 days of age. Two blood samples were collected from the wing vein into microcentrifuge tubes. One was collected before immunization, and the other was collected 1 week after the last immunization. Serum was obtained by centrifugation at 2,000 × g for 10 min at 4°C, and the serum was inactivated at 56°C for 30 min. Antibody production was measured by enzyme-linked immunosorbent assay (ELISA), as described previously [16]. Briefly, 96-well plates (Iwaki, Tokyo, Japan) were coated with 100 µl of a solution containing 20 µg whole formalin-killed S. Gallinarum (used as antigen) in 1 ml of 0.1 M carbonate-bicarbonate buffer (pH 9.6) and left overnight at 4°C. After three washes with PBS containing 0.05% Tween 20 (PBS-T), the wells were saturated with 200 µl of 5% skim milk (BD Biosciences, Franklin Lakes, NJ, U.S.A.). The wells were washed three times with PBS-T after a 2 hr incubation at room temperature. Inactivated serum samples were diluted 1:160 with PBS-T. The diluted samples were added to wells and incubated at room temperature for 1 hr. After three washes with PBS-T, 100 µl of a 1:5,000 dilution of horseradish peroxidase-conjugated rabbit anti-chicken IgY (Jackson ImmunoResearch, West Grove, PA, U.S.A.) was added to each well. After a 1 hr incubation at room temperature, the plates were washed, and 100 µl of a substrate consisting of 0.05 M citrate buffer (pH 4.0), 2-2’-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (Bio Basic, Markham, ON, Canada) and 30% hydrogen peroxide was added to each well. After a 10 min incubation at room temperature in the dark, the reaction was stopped with 5% sodium dodecyl sulfate (AppliChem, Darmstadt, Germany). Reactions were read at an optical density of 405 nm using an ELISA plate reader (Thermo Labsystems, Helsinki, Finland). Pre-immunized chick serum was used as a negative control, and each sample was tested in duplicate.
Experiment 2—Evaluation of immune responses and bacterial clearance against a sublethal dose of S. Gallinarum: Thirty chicks were randomly selected from each group and isolated in separate rooms by group at 1 day after the last spray administration. Ten chicks from each isolated group were randomly sacrificed for analysis of the immune response in non-infected chicks, and the remaining 20 chicks in each isolated group were orally inoculated with 1 × 109 cfu (sublethal dose) S. Gallinarum (SG3001). Five infected chicks from each isolated group were randomly sacrificed for analysis of the immune response and bacterial clearance against a sublethal dose of S. Gallinarum at 3, 7, 10 and 14 days post-infection (DPI).
Experiment 2.1—Determination of T lymphocyte subpopulation in the spleen: The spleen was obtained from each sacrificed chick at 0 DPI (the day of infection but before infection) and at 3, 7, 10 and 14 DPI, and single-cell suspensions were prepared by forcing the tissue through 40-µm nylon mesh (BD Biosciences). Isolated cells were analyzed to determine CD4+CD8− T lymphocyte and CD4−CD8+ T lymphocyte percentages, as described previously [16]. The cells were stained with both fluorescein isothiocyanate (FITC)-conjugated mouse anti-chicken CD4 (Southern Biotech, Birmingham, AL, U.S.A.) and phycoerythrin (PE)-conjugated mouse anti-chicken CD8 (Southern Biotech). After a 30 min incubation at room temperature in the dark, the cells were washed twice with PBS, and the lymphocyte subpopulations were analyzed using a FACSort flow cytometer (BD Biosciences). Viable lymphocytes were gated by forward and side-scatter characteristics (FSC/SSC), and 10,000 events were analyzed for FITC or PE positive staining. Results of each lymphocyte subpopulation are expressed as percentages of events in the FSC/SSC lymphocyte gate.
Experiment 2.2—Evaluation of relative IL-18 and IFN-γ mRNA expression levels in spleen: Total RNA was extracted from splenocytes using the RNeasy Mini kit (Qiagen, Valencia, CA, U.S.A.), and target RNA was reverse transcribed using the QuantiTect® Reverse Transcription kit (Qiagen), according to the manufacturer’s instructions. All samples were transcribed simultaneously to minimize variations in reverse transcriptase efficiency. The chicken IL-18, IFN-γ and GAPDH primer sets were designed as described previously [4], and their sequences are shown in Table 1. IL-18 and IFN-γ mRNA levels were determined by real-time polymerase chain reaction (PCR) assay using MyiQTM2 (Bio-Rad Laboratories, Hercules, CA, U.S.A.) with the iQTM SYBR® Green Supermix (Bio-Rad Laboratories), according to the manufacturer’s instructions. Each sample was amplified in duplicate in the same 96-well PCR plate, and each procedure was repeated twice. The relative quantitative PCR conditions were 5 min at 95°C followed by 45 cycles of 30 sec at 94°C, 30 sec at 57°C and 45 sec at 72°C. A melting program was run after amplification to verify the presence of only one PCR product. The threshold cycle (Ct; the cycle number at which the amount of amplified gene of interest reached a fixed threshold) was determined subsequently. Relative quantification of IL-18 and IFN-γ mRNA expression was calculated by the comparative Ct method, as described previously [24]. The relative quantification values of the target genes (IL-18 and IFN-γ) were normalized to an endogenous control gene (GAPDH) and relative to a calibrator. The values were expressed as 2−ΔΔCt(fold), where ΔCt=Ct of target gene − Ct of endogenous control gene and ΔΔCt=ΔCt of samples for target gene − ΔCt of the target gene calibrator.
Table 1. Primer used for the relative real-time polymerase chain reaction (PCR).
| Nucleotide sequence (5’→3’) | Accession number a) | ||
|---|---|---|---|
| IL-18 | FW | AGGTGAAATCTGGCAGTGGAAT | NM_204608 |
| RV | TGAAGGCGCGGTGGTTT | ||
| IFN-γ | FW | GCTCCCGATGAACGACTTGA | NM_205149 |
| RV | TGTAAGATGCTGAAGAGTTCATTCG | ||
| GAPDH | FW | CCTAGGATACACAGAGGACCAGGTT | NM_204305 |
| RV | GGTGGAGGAATGGCTGTCA | ||
FW, forward primer; RV, reverse primer; IL-18, interleukin-18; IFN-γ, interferon-gamma; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. a) cDNA Genbank accession number and corresponding genes are available online at http://www.ncbi.nlm.nih.gov/.
Experiment 2.3—Determination of serum lysozyme activity: Blood was collected from the wing vein of each sacrificed chick at 0, 3, 7, 10 and 14 DPI. Serum was obtained by centrifugation at 2,000 × g for 10 min at 4°C and used to determine lysozyme activity [16]. Briefly, a standard dilution series was produced by dissolving crystalline lysozyme (Sigma-Aldrich) in a phosphate buffer (pH 6.2) to concentrations of 1, 2, 3, 4, 5 or 6 µg/ml. Twenty microliters of each solution was added to two wells of a 96-well microtiter plate. Two hundred microliters of phosphate buffer was added to one well, and 200 µl of a Micrococcus lysodeikticus solution (600 mg/l phosphate buffer; Sigma-Aldrich) was added to the other well. The absorbance was determined at 540 nm after 15, 30, 45 and 60 min incubations at 41°C. The regression coefficient (b) between absorbance and time was calculated for each concentration. Serum samples were treated in a manner similar to the standard solutions. Lysozyme activity (lysis of M. lysodeikticus) was determined based on b and the lysozyme concentration. Each sample was assayed in duplicate.
Experiment 2.4—Viable bacterial counts in liver, the bursa of Fabricius and cecum: Viable bacteria cell counts were carried out for the liver, bursa of Fabricius and cecum collected from each sacrificed chick at 3, 7, 10 and 14 DPI as follows. A 10% (w/v) suspension of each tissue sample was prepared in sterile PBS. The pool was homogenized using a Precellys®24 (Bertin Technologies, Montigny-le-Bretonneux, France). One hundred microliters of the homogenate was serially diluted 10-fold in PBS, and 100 µl of each dilution was spread onto a xylose lysine deoxycholate agar plate and incubated at 37°C for 24 hr. Characteristic black-colored colonies were counted and expressed as log cfu/g tissue, but only for those colonies with counts of 30–300 per plate. In addition, representative colonies were subjected to Gram staining and biochemical tests for identification [13]. Each sample was tested in duplicate.
Experiment 3—Evaluation of survivability and pathological changes against a low lethal dose of S. Gallinarum: Twenty chicks were randomly selected from each group and isolated in separate rooms by group at 1 day after the last spray administration. All chicks were orally inoculated with 5 × 109 cfu (LD25) S. Gallinarum (SG3001). Mortality in each group was recorded for 17 days after bacterial infection. All remaining chicks were euthanized at 17 DPI. Necropsy examinations were carried out on all dead and euthanized chicks, and any pathological changes associated with fowl typhoid were observed.
Statistical analysis: Data are expressed as mean ± standard deviation. Student’s t-test was performed for the statistical analysis of all data. All statistical analyses were performed using SigmaPlot® version 10.0 software (Systat Software, San Jose, CA, U.S.A.). A P<0.05 was considered significant.
RESULTS
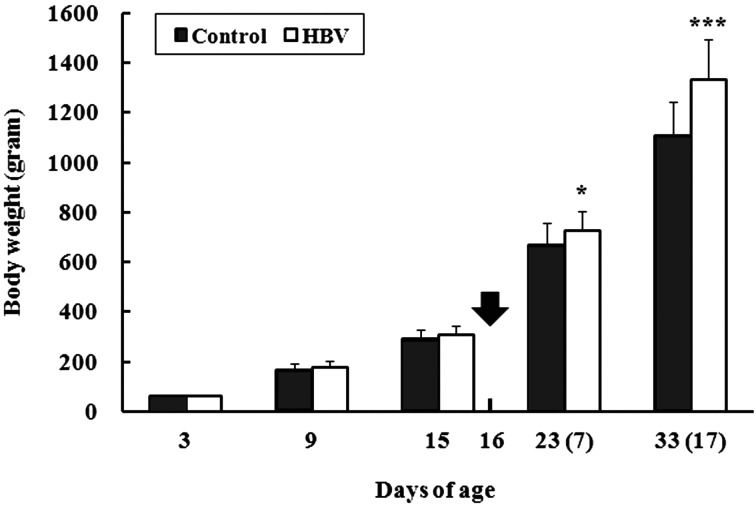
Body weight changes: Body weight was recorded at 3, 9 and 15 days of age in all chicks and at 23 (7 DPI) and 33 (17 DPI) days of age in surviving chicks infected with a low lethal dose of S. Gallinarum (Fig. 1). The initial chick body weight did not differ between the groups at 3 days of age (control group and HBV-sprayed group: 62.0 ± 2.4 and 62.1 ± 2.4 g, respectively). However, the HBV-sprayed group showed a tendency for increasing body weight compared with that in the control group at 9 and 15 days of age, but the difference was not significant (P=0.060 and 0.053 at 9 and 15 days of age, respectively). This increase in body weight reached significance at 23 (P<0.05) and 33 days of age (P<0.001).
Fig. 1.
Effect of honeybee venom (HBV) on body weight changes in broiler chicks. Body weight was recorded at 3, 9 and 15 days of age in all chicks and was also recorded at 23 and 33 days of age in surviving chicks after infection with a low lethal dose of Salmonella Gallinarum. Arrow indicates the day of the experimental S. Gallinarum infection. Numbers in parentheses refer to days post-infection. Control, control group; HBV, HVB-sprayed group. Data are mean ± standard deviation (3–15 days of age: n=60/group, 23 days of age: Control group and HBV-sprayed group: n=17 and 19, respectively, 33 days of age: Control group and HBV-sprayed group: n=15 and 17, respectively). *P<0.05 vs. control, ***P<0.001 vs. control.
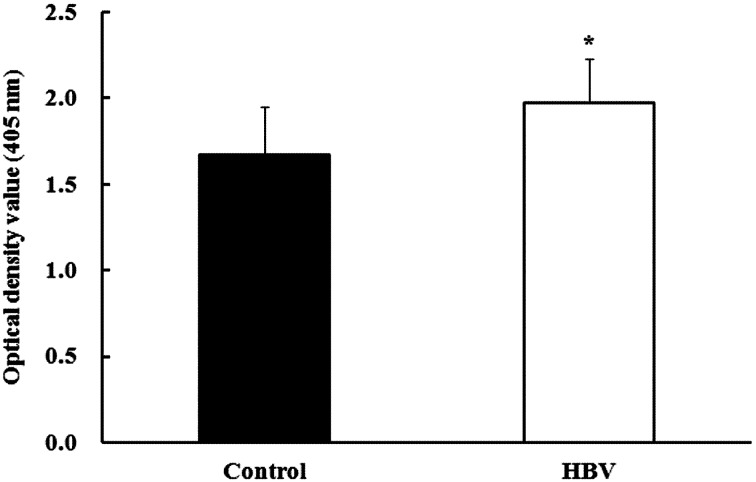
Experiment 1—Antibody production activity against formalin-killed S. Gallinarum: Antibody production activity (determined at OD405 nm) against formalin-killed S. Gallinarum in the HBV-sprayed group (1.97 ± 0.26) increased significantly compared to that in the control group (1.67 ± 0.27; P<0.05) (Fig. 2). Sera of pre-immunized chicks displayed a clear negative reaction (control group and HBV-sprayed group: 0.35 ± 0.10 and 0.34 ± 0.08, respectively) against the S. Gallinarum antigen.
Fig. 2.
Effect of honeybee venom (HBV) on antibody production activity in broiler chicks. Chicks received two subcutaneous injections of formalin-killed Salmonella Gallinarum. Serum antibody production was determined (OD405 nm) using an enzyme-linked immunosorbent assay plate reader. Serum antibody production levels in the HBV-sprayed group were significantly higher compared to those in the control group. Control, control group; HBV, HVB-sprayed group. Data are mean ± standard deviation (n=10/group). *P<0.05 vs. control.
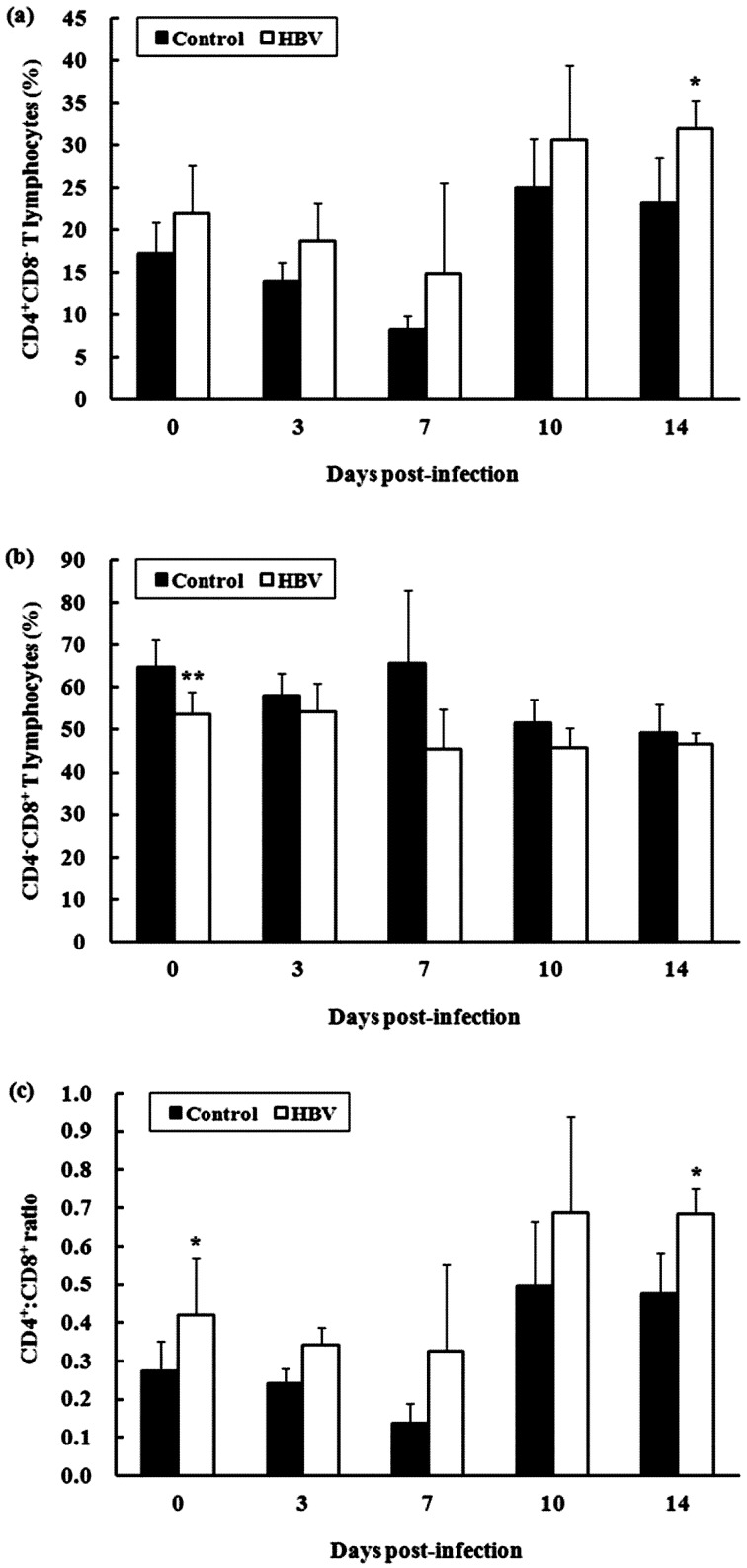
Experiment 2.1—T lymphocyte subpopulation in spleen: The percentage of CD4+CD8− T lymphocytes in the spleen of the HBV-sprayed group tended to increase during the entire experimental period compared with that in the control group (Fig. 3a). In particular, the difference in the percentage of CD4+CD8− T lymphocytes was significant between the groups at 14 DPI (P<0.05). In contrast, the percentage of CD4−CD8+ T lymphocytes in the spleen of the HBV-sprayed group tended to decrease during the entire experimental period compared with that in the control group (Fig. 3b). The difference in the percentage of CD4−CD8+ T lymphocyte was significant between the groups at 0 DPI (P<0.01). Consequently, the CD4+:CD8+ ratio in the spleen of the HBV-sprayed group tended to increase during the entire experimental period compared with that in the control group (Fig. 3c). In particular, the difference in the CD4+:CD8+ cell ratio was significant between the groups at 0 and 14 DPI (P<0.05).
Fig. 3.
Effect of honeybee venom (HBV) on T lymphocyte subpopulations in the spleen of broiler chicks. (a) The percentage of CD4+CD8− T lymphocyte in the spleen of the HBV-sprayed group tended to increase compared with that in the control group. (b) Conversely, the percentage of CD4−CD8+ T lymphocytes in spleen of the HBV-sprayed group tended to decrease compared with that in the control group. (c) Consequently, the CD4+:CD8+ ratio in the spleen of the HBV-sprayed group tended to increase during the entire experimental period compared with that in the control group. 0 days post-infection (DPI) is the day of infection, but before infection. Control, control group; HBV, HVB-sprayed group. Data are mean ± standard deviation (0 DPI: n=10/group, 3–14 DPI: n=5/group). *P<0.05 vs. control.
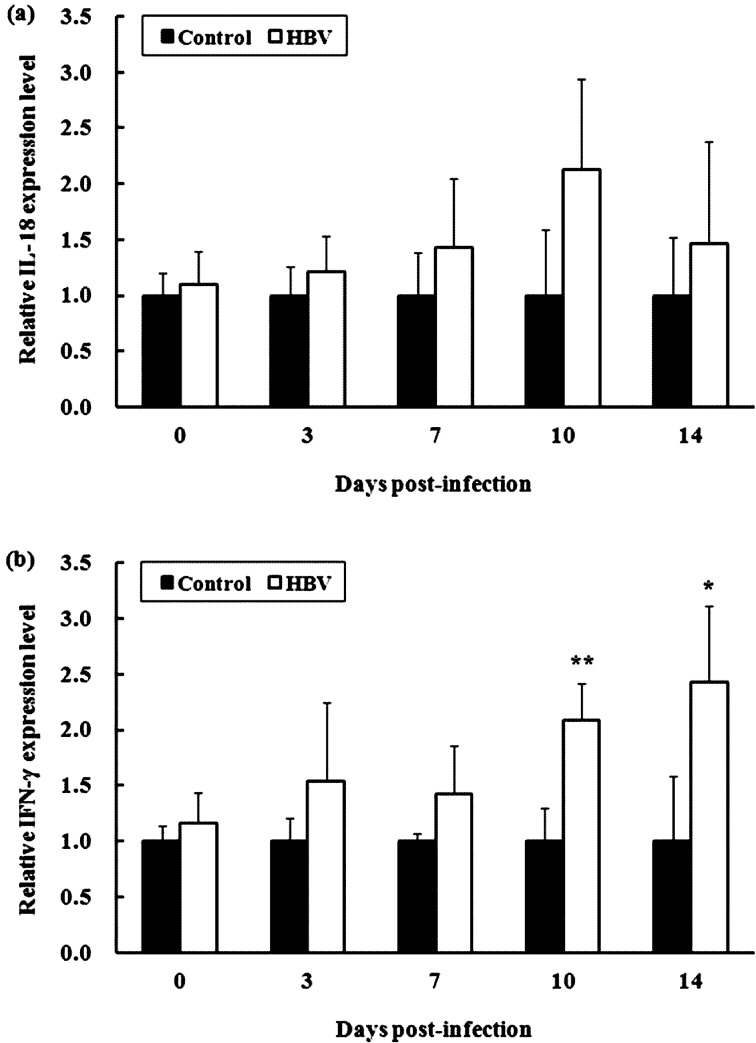
Experiment 2.2—Relative IL-18 and IFN-γ mRNA expression levels: The relative IL-18 and IFN-γ mRNA expression levels in spleen of the HBV-sprayed group tended to increase during the entire experimental period compared with those in the control group (Fig. 4). Notably, the relative IFN-γ mRNA expression levels in spleen of the HBV-sprayed group increased significantly compared with those in the control group at 10 (P<0.01) and 14 DPI (P<0.05).
Fig. 4.
Effect of honeybee venom (HBV) on relative interleukin (IL)-18 and interferon (IFN)-γ mRNA expression levels in the spleen of broiler chicks. The relative mRNA expression levels of IL-18 (a) and IFN-γ (b) in spleen of the HBV-sprayed group tended to increase during the entire experimental period compared with those in the control group. 0 days post-infection (DPI) is the day of infection, but before infection. Control, control group; HBV, HVB-sprayed group. Data are mean ± standard deviation (0 DPI: n=10/group, 3–14 DPI: n=5/group). *P<0.05 vs. control, **P<0.01 vs. control.
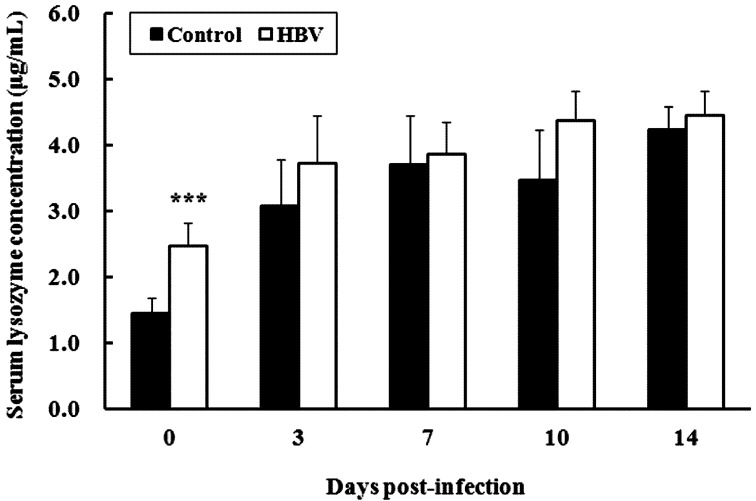
Experiment 2.3—Serum lysozyme activity: Serum lysozyme concentration in the HBV-sprayed group (2.47 ± 0.35 µg/ml) was significantly higher than that in the control group (1.45 ± 0.22 µg/ml) before the experimental S. Gallinarum infection (P<0.001). Moreover, serum lysozyme concentration in the HBV-sprayed group tended to increase during the entire experimental infection period compared with that in the control group, although the difference was not significant (Fig. 5).
Fig. 5.
Effect of honeybee venom (HBV) on serum lysozyme activity in broiler chicks. Serum lysozyme concentration in the HBV-sprayed group was significantly higher than that in the control group before the experimental Salmonella Gallinarum infection (0 days post infection [DPI]). Moreover, serum lysozyme concentration in the HBV-sprayed group tended to increase during the entire experimental infection period compared with that in the control group. 0 DPI is the day of infection, but before infection. Control, control group; HBV, HVB-sprayed group. Data are mean ± SD (0 DPI: n=10/group, 3–14 DPI: n=5/group). ***P<0.001 vs. control.
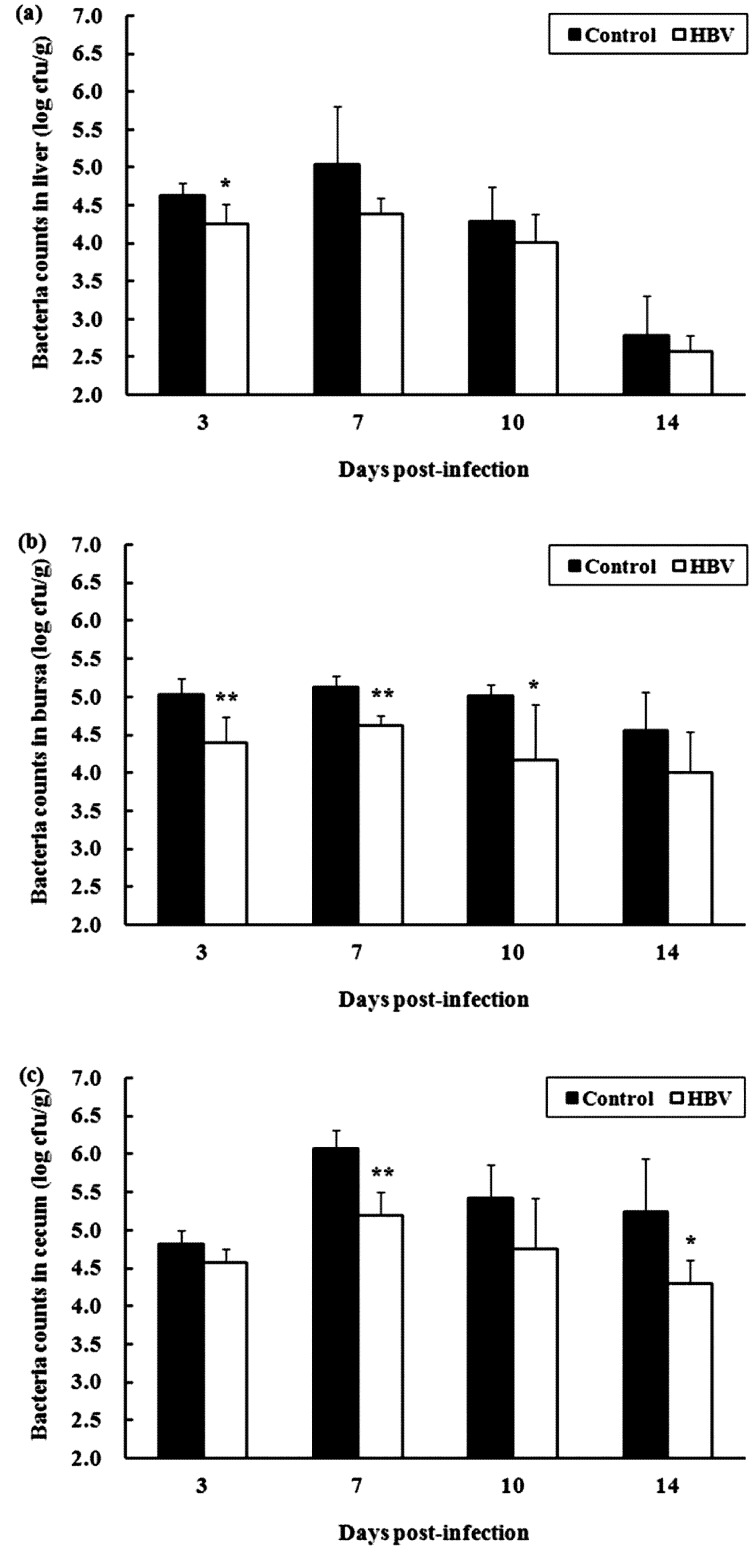
Experiment 2.4—Bacterial clearance in chicks infected with a sublethal dose of S. Gallinarum: Viable bacterial cells in the liver, bursa of Fabricius and cecum of chicks in both groups were maximum at 7 DPI, after which there was a moderate decline until 14 DPI (Fig. 6). The number of viable bacterial cells in those tissues in the HBV-sprayed group tended to decrease during the entire experimental infection period compared with that in the control group. Differences in the viable bacterial cell counts were significant at 3 DPI (P<0.05) in the liver (Fig. 6a), at 3 (P<0.01), 7 (P<0.01) and 10 DPI (P<0.05) in the bursa of Fabricius (Fig. 6b) and at 7 (P<0.01) and 14 DPI (P<0.05) in the cecum (Fig. 6c).
Fig. 6.
Effect of honeybee venom (HBV) on bacterial clearance in chicks infected with a sublethal dose of Salmonella Gallinarum. Viable bacteria cell counts were carried out in the liver (a), bursa of Fabricius (b) and cecum (c) collected from each sacrificed chick at 3, 7, 10 and 14 days post-infection (DPI). Control, control group; HBV, HBV-sprayed group. Data are mean ± SD (n=5 in each group). *P<0.05 vs. control, **P<0.01 vs. control.
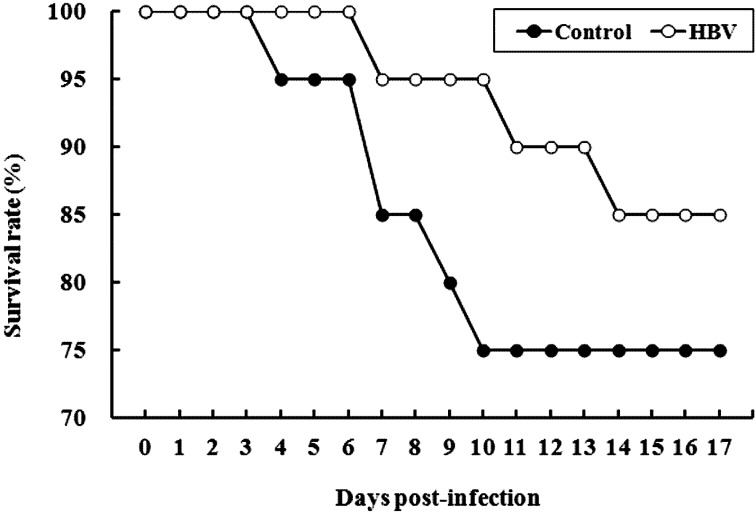
Experiment 3.1—Survivability against a low lethal dose of S. Gallinarum: Mortality was first observed at 4 DPI in the control group, whereas mortality in the HBV-sprayed group was delayed for 3 days compared with that in the control group. Survival rates in the HBV-sprayed group remained higher throughout the experimental infection period than those in the control group. Survival rates were 75% (15 of 20 chicks) in the control group and 85% (17 of 20 chicks) in the HBV-sprayed group by 17 DPI (Fig. 7).
Fig. 7.
Survival rate trends in chicks infected with a low lethal dose of Salmonella Gallinarum. Mortality was first observed at 4 days post-infection (DPI) in the control group, whereas mortality in the honey bee venom (HBV)-sprayed group was delayed for 3 days compared with that in the control group. Survival rates in the HBV-sprayed group remained higher throughout the experimental infection period than those in the control group. Survival rates were 75% (15 of 20 chicks) in the control group and 85% (17 of 20 chicks) in the HBV-sprayed group by 17 DPI. Control, control group; HBV, HVB-sprayed group.
Experiment 3.2—Pathological changes in chicks infected with a low lethal dose of S. Gallinarum: Chicks infected with a low lethal dose of S. Gallinarum displayed typical gross lesions of fowl typhoid at necropsy. Congested subcutaneous blood vessels and dark brown skeletal muscles were observed in septicemic carcasses. Petechial hemorrhaging and necrotic foci were common features in the liver, spleen and myocardium. In addition, hepatosplenomegaly, hydropericardium, nephropathy and intestinal tract congestion were also observed. Although these pathological changes were observed in both groups, the control group showed more severe petechial hemorrhaging and necrotic foci in the liver (Fig. 8a) and also displayed more severe fibrinous inflammation in the liver, hydropericardium and intestinal tract congestion (Fig. 8b) compared with those in the HBV-sprayed group (Fig. 8c).
Fig. 8.
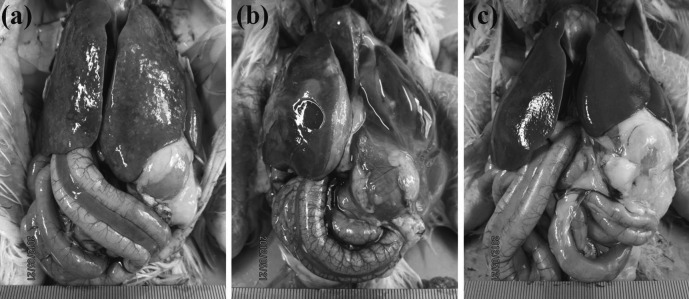
Gross findings in chicks infected with a low lethal dose of Salmonella Gallinarum. The control group showed more severe petechial hemorrhaging and necrotic foci in the liver (a) and also displayed more severe fibrinous inflammation in the liver and hydropericardium as well as intestinal tract congestion (b) compared with those in the honeybee venom (HBV)-sprayed group (c).
DISCUSSION
Antibiotics are used as feed or drinking water additives to improve growth performance and to prevent subclinical disease challenge in the poultry industry [9]. Therefore, the present study monitored body weight changes and evaluated whether HBV is an effective immunostimulant that can protect against S. Gallinarum, which remains a significant problem in many countries [21, 22].
Our results demonstrated that administering HBV by spray had a beneficial effect on body weight gain in broiler chicks. This finding was similar to previous reports showing that subcutaneous injections of HBV and acupuncture increase body weight gain in young pigs [11], and HBV supplementation via drinking water improves body weight gain and feed intake in broiler chicks [12]. Notably, we showed that the increase in body weight following HBV spray administration was more pronounced in broiler chicks infected with a low lethal dose of S. Gallinarum. These results suggest that administering HBV by spray could help improve growth performance in the absence of infection as well as in environments with a risk of exposure to contaminating pathogens, such as conventional farms.
Antibody production against formalin-killed S. Gallinarum increased significantly in the HBV-sprayed group compared with that in the control group. This finding was similar to a previous report that acupuncture with natural honey bee stings enhances antibody production against the classical swine fever virus vaccine, mycoplasma hyopneumoniae vaccine and the atrophic rhinitis vaccine in pigs [19]. The antibody titer is a humoral immunity indicator [31]. Therefore, our result indicates that humoral immunity was enhanced by administering HBV to broiler chicks.
The CD4+:CD8+ ratio is used as a measure of immune function and response. Low CD4+:CD8+ ratios are usually observed in individuals with acute viral diseases and hemophilia [3], whereas high ratios have been associated with an increase in chick immunofunctional ability [1, 16, 17]. We showed that the CD4+:CD8+ ratio in the spleen of the HBV-sprayed group increased during the entire experimental period compared with that in the control group, suggesting that the HBV spray administration conferred a benefit on broiler chick immune function. This finding echoed that of Nam et al. [25], who found that an intraperitoneal injection of HBV increases the percentage of CD4+CD8− T lymphocytes and decreases the percentage of CD4−CD8+ T lymphocytes in the spleen of BALB/c mice.
The relative IL-18 and IFN-γ mRNA expression levels in spleen of the HBV-sprayed group tended to increase during the entire experimental period compared with those in the control group. Of note, the enhanced relative IFN-γ expression following administration of the HBV spray was significant in broiler chicks infected with a sublethal dose of S. Gallinarum. This observation agreed with the finding of Nam et al. [25], who noted that an intraperitoneal injection of HBV enhances IFN-γ expression in BALB/c mice. IL-18 and IFN-γ are mainly produced by activated macrophages and stimulated CD4+ T lymphocytes [7, 29]. These cytokines play an important role in the host defense against infection by microbial pathogens and induce a variety of physiologically significant responses that contribute to immunity [7, 29]. These results suggest that the HBV spray could improve host immunity.
Lysozyme, which is secreted by some phagocytes, such as macrophages and polymorphonuclear leukocytes, is highly active against bacteria. Lysozyme destroys glucosidic bonds in the cell walls of Escherichia coli and Staphylococcus as a result of its phagocytic activity [8]. Therefore, high serum lysozyme activity is associated with high phagocyte destructive activity [18]. In the present study, the serum lysozyme concentration in the HBV-sprayed group was significantly higher than that in the control group before the experimental S. Gallinarum infection. Moreover, serum lysozyme concentration in the HBV-sprayed group tended to increase during the entire experimental infection period compared to that in the control group. These results suggest that the HBV spray may enhance destructive activity of phagocytes.
The number of viable bacterial cells in all tested tissues of the HBV-sprayed group tended to decrease compared with that in the control group after infection with a sublethal dose of S. Gallinarum. Moreover, mortality in the HBV-sprayed group was delayed 3 days compared with that in the control group, and the final survival rate in the HBV-sprayed group was 10% higher than that in the control group after the experimental infection with a low lethal dose of S. Gallinarum. Additionally, the necropsy revealed that the HBV-sprayed group showed mild and less severe abnormal gross lesion pathological changes compared with those in the control group, indicating that the HBV spray reinforced bacterial clearance and increased survivability against S. Gallinarum. Previous studies have shown that improving the general immune status of birds through prophylactic administration of natural immunostimulants helps alleviate clinical symptoms and increases survivability against S. Gallinarum [16, 17]. Therefore, it can be presumed that the prophylactic effects of administering the HBV against S. Gallinarum are also associated with stimulation of the non-specific immune response.
Taken together, our findings suggest that the HBV spray improved body weight gain, particularly in the presence of infection and enhanced protective immune activity against S. Gallinarum. Hence, the HBV spray may provide an alternative way to decrease antibiotic use, particularly in chicks. However, the exact mechanisms of the HBV spray on body weight gain and bacterial clearance were not determined. Therefore, these mechanisms of the HBV spray must be assessed in a future study. In addition, confirmation of the protective efficacy of the HBV spray is required in naturally occurring fowl typhoid. These studies are currently in progress.
ACKNOWLEDGMENTS
This study was supported by the Technology Development Program for Agriculture and Forestry, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea. The authors acknowledge a graduate fellowship provided by the Korean Ministry of Education and Human Resources Development through the Brain Korea 21 project.
REFERENCES
- 1.Abdukalykova S. T., Zhao X., Ruiz-Feria C. A.2008. Arginine and vitamin E modulate the subpopulations of T lymphocytes in broiler chickens. Poult. Sci. 87: 50–55. doi: 10.3382/ps.2007-00315 [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J., Steinman R. M.1998. Dendritic cells and the control of immunity. Nature 392: 245–252. doi: 10.1038/32588 [DOI] [PubMed] [Google Scholar]
- 3.Chakravarti A.1995. The CD4/CD8 ratio: message in a bottle? Nat. Med. 1: 1240–1241. doi: 10.1038/nm1295-1240 [DOI] [PubMed] [Google Scholar]
- 4.Cox C. M., Stuard L. H., Kim S., McElroy A. P., Bedford M. R., Dalloul R. A.2010. Performance and immune responses to dietary beta-glucan in broiler chicks. Poult. Sci. 89: 1924–1933. doi: 10.3382/ps.2010-00865 [DOI] [PubMed] [Google Scholar]
- 5.Gajski G., Garaj-Vrhovac V.2009. Radioprotective effects of honeybee venom (Apis mellifera) against 915-MHz microwave radiation-induced DNA damage in wistar rat lymphocytes: in vitro study. Int. J. Toxicol. 28: 88–98. doi: 10.1177/1091581809335051 [DOI] [PubMed] [Google Scholar]
- 6.Ganapathy K., Bufton A., Pearson A., Lemiere S., Jones R. C.2010. Vaccination of commercial broiler chicks against avian metapneumovirus infection: a comparison of drinking-water, spray and oculo-oral delivery methods. Vaccine 28: 3944–3948. doi: 10.1016/j.vaccine.2010.03.065 [DOI] [PubMed] [Google Scholar]
- 7.Göbel T. W., Schneider K., Schaerer B., Mejri I., Puehler F., Weigend S., Staeheli P., Kaspers B.2003. IL-18 stimulates the proliferation and IFN-gamma release of CD4+ T cells in the chicken: conservation of a Th1-like system in a nonmammalian species. J. Immunol. 171: 1809–1815 [DOI] [PubMed] [Google Scholar]
- 8.Guo Y. M., Chen S. Y., Xia Z. G., Yuan J. M.2004. Effects of different types of polyunsaturated fatty acids on immune function and PGE2 synthesis by peripheral blood leukocytes of laying hens. Anim. Feed. Sci. Tech. 116: 249–258. doi: 10.1016/j.anifeedsci.2004.07.011 [DOI] [Google Scholar]
- 9.Gyles C. L.2008. Antimicrobial resistance in selected bacteria from poultry. Anim. Health. Res. Rev. 9: 149–158. doi: 10.1017/S1466252308001552 [DOI] [PubMed] [Google Scholar]
- 10.Han S., Lee K., Yeo J., Kim W., Park K.2011. Biological effects of treatment of an animal skin wound with honeybee (Apis mellifera. L) venom. J. Plast. Reconstr. Aesthet. Surg. 64: e67–e72. doi: 10.1016/j.bjps.2010.08.022 [DOI] [PubMed] [Google Scholar]
- 11.Han S. M., Lee K. G., Yeo J. H., Hwang S. J., Jang C. H., Chenoweth P. J., Pak S. C.2009. Effects of bee venom treatment on growth performance of young pigs. Am. J. Chin. Med. 37: 253–260. doi: 10.1142/S0192415X09006813 [DOI] [PubMed] [Google Scholar]
- 12.Han S. M., Lee K. G., Yeo J. H., Oh B. Y., Kim B. S., Lee W., Baek H. J., Kim S. T., Hwang S. J., Pak S. C.2010. Effects of honeybee venom supplementation in drinking water on growth performance of broiler chickens. Poult. Sci. 89: 2396–2400. doi: 10.3382/ps.2010-00915 [DOI] [PubMed] [Google Scholar]
- 13.Huberman Y. D., Terzolo H. R.2008. Fowl typhoid: assessment of a disinfectant oral dose to reduce horizontal spread and mortality. Avian Dis. 52: 320–323. doi: 10.1637/8105-090307-ResNote.1 [DOI] [PubMed] [Google Scholar]
- 14.Hyun P. M., Jeon J. W., Lee K. J., Park J. K.2011. PEF-treated bee venom has different activities both in vitro and vivo p. 241. In: 42th International Apicultural Congress.
- 15.Joffre O., Nolte M. A., Spörri R., Reis e Sousa C.2009. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol. Rev. 227: 234–247. doi: 10.1111/j.1600-065X.2008.00718.x [DOI] [PubMed] [Google Scholar]
- 16.Jung B. G., Ko J. H., Lee B. J.2010. Dietary supplementation with a probiotic fermented four-herb combination enhances immune activity in broiler chicks and increases survivability against Salmonella Gallinarum in experimentally infected broiler chicks. J. Vet. Med. Sci. 72: 1565–1573. doi: 10.1292/jvms.10-0152 [DOI] [PubMed] [Google Scholar]
- 17.Jung B. G., Lee J. A., Nam K. W., Lee B. J.2012. Oxygenated drinking water enhances immune activity in broiler chicks and increases survivability against Salmonella Gallinarum in experimentally infected broiler chicks. J. Vet. Med. Sci. 74: 341–346. doi: 10.1292/jvms.11-0316 [DOI] [PubMed] [Google Scholar]
- 18.Kreukniet M. B., Nieuwland M. G., van der Zijpp A. J.1995. Phagocytic activity of two lines of chickens divergently selected for antibody production. Vet. Immunol. Immunopathol. 44: 377–387. doi: 10.1016/0165-2427(94)05304-B [DOI] [PubMed] [Google Scholar]
- 19.Kwon Y. B., Lee J. H.2001. Effect on antibody titer to hog cholera, Mycoplasma hyopneumoniae and atropic rhinitis vaccines after bee venom acupuncture in pigs. J. Vet. Med. Biotechnol. 1: 65–77 [Google Scholar]
- 20.Kwon Y. B., Lee H. J., Han H. J., Mar W. C., Kang S. K., Yoon O. B., Beitz A. J., Lee J. H.2002. The water-soluble fraction of bee venom produces antinociceptive and anti-inflammatory effects on rheumatoid arthritis in rats. Life Sci. 71: 191–204. doi: 10.1016/S0024-3205(02)01617-X [DOI] [PubMed] [Google Scholar]
- 21.Kwon Y. K., Kim A., Kang M. S., Her M., Jung B. Y., Lee K. M., Jeong W., An B. K., Kwon J. H.2010. Prevalence and characterization of Salmonella Gallinarum in the chicken in Korea during 2000 to 2008. Poult. Sci. 89: 236–242. doi: 10.3382/ps.2009-00420 [DOI] [PubMed] [Google Scholar]
- 22.Lee Y. J., Mo I. P., Kang M. S.2007. Protective efficacy of live Salmonella gallinarum 9R vaccine in commercial layer flocks. Avian Pathol. 36: 495–498. doi: 10.1080/03079450701691278 [DOI] [PubMed] [Google Scholar]
- 23.Lee J. H., Kwon Y. B., Han H. J., Mar W. C., Lee H. J., Yang I. S., Beitz A. J., Kang S. K.2001. Bee venom pretreatment has both an antinociceptive and anti-inflammatory effect on carrageenan-induced inflammation. J. Vet. Med. Sci. 63: 251–259. doi: 10.1292/jvms.63.251 [DOI] [PubMed] [Google Scholar]
- 24.Livak K. J., Schmittgen T. D.2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 25.Nam S., Ko E., Park S. K., Ko S., Jun C. Y., Shin M. K., Hong M. C., Bae H.2005. Bee venom modulates murine Th1/Th2 lineage development. Int. Immunopharmacol. 5: 1406–1414. doi: 10.1016/j.intimp.2005.03.011 [DOI] [PubMed] [Google Scholar]
- 26.Oršolić N.2012. Bee venom in cancer therapy. Cancer Metastasis Rev. 31: 173–194. doi: 10.1007/s10555-011-9339-3 [DOI] [PubMed] [Google Scholar]
- 27.Perrin-Cocon L., Agaugué S., Coutant F., Masurel A., Bezzine S., Lambeau G., André P., Lotteau V.2004. Secretory phospholipase A2 induces dendritic cell maturation. Eur. J. Immunol. 34: 2293–2302. doi: 10.1002/eji.200324797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramoner R., Putz T., Gander H., Rahm A., Bartsch G., Schaber C., Thurnher M.2005. Dendritic-cell activation by secretory phospholipase A2. Blood 105: 3583–3587. doi: 10.1182/blood-2004-08-3001 [DOI] [PubMed] [Google Scholar]
- 29.Samuel C. E.2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14: 778–809. doi: 10.1128/CMR.14.4.778-809.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shivaprasad H. L.2000. Fowl typhoid and pullorum disease. Rev. Sci. Tech. 19: 405–424 [DOI] [PubMed] [Google Scholar]
- 31.Yang L., Hu Y., Xue J., Wang F., Wang D., Kong X., Li P., Xu W.2008. Compound Chinese herbal medicinal ingredients can enhance immune response and efficacy of RHD vaccine in rabbit. Vaccine 26: 4451–4455. doi: 10.1016/j.vaccine.2008.06.075 [DOI] [PubMed] [Google Scholar]