ABSTRACT
The aim of this study was to evaluate MHC class I expression and prognosis using tumor tissues surgically removed from 9 dogs with mammary gland carcinomas and from 13 dogs with complex carcinomas. We assessed MHC class I expression and its correlation with tumor size, B2M expression, infiltration of lymphocytes, histological grade and prognosis. Hematoxylin and eosin-stained sections were histologically graded using the Elston and Ellis grading method. MHC class I expression on tumor cells was evaluated using the avidin-biotin peroxidase complex method. Loss of MHC class I expression from canine mammary gland carcinomas was significantly correlated with poor prognosis (P<0.05). Loss of MHC class I expression showed no association with poor prognosis in canine mammary gland complex carcinomas, because the data were not balanced. Only 1 of 13 (7.6%) canine mammary gland complex carcinomas showed loss of MHC class I expression. All 13 of these dogs showed good prognosis. Thus, the low frequency of MHC class I expression loss from canine mammary gland complex carcinomas may be associated with good prognosis. Taken together, these results suggest that loss of MHC class I expression may be associated with poor prognosis in canine mammary gland carcinomas.
Keywords: beta2-microglobulin, canine, mammary gland carcinoma, MHC class I
Major histocompatibility complex (MHC) class I antigens are composed of both variable chain MHC class I proteins and beta2-microglobulin (B2M) [18], an invariant chain essential for the structural stability and optimal function of these proteins [16]. In humans, MHC class I antigens are expressed on the surface of most nucleated cells [12]. Loss or downregulation of MHC class I expression has been reported in many types of cancer, including breast cancer [1, 2, 18]. The frequency of MHC class I expression loss and/or downregulation in breast cancer ranges from 37 to 88% [1, 18]. This loss or downregulation of MHC class I expression has been associated with disease progression and/or poor clinical outcome [2]. Cells with loss of these antigen proteins from the cell surface may escape recognition by CD8+ T lymphocytes [18]. Loss of MHC class I expression in breast cancer is caused by loss of B2M expression and/or function [4]. In dogs, mammary gland tumors are the second most commonly occurring neoplasms [13]. These tumors show different growth patterns and biological behavior, depending on whether the tumors are simple or complex carcinomas [9, 11]. Analysis of potentially important prognostic factors in canine mammary gland tumors has been the focus of many studies. Prognostic factors in dogs with these tumors include tumor size, histological type, evidence of metastasis at the time of diagnosis, clinical stage and histological grade [3, 8, 17]. Although canine and human mammary gland tumors have similar features, including histological appearance and biological behavior [20], no study to date has evaluated the relationship between loss or downregulation of MHC class I expression and prognosis in dogs with these tumors. This study therefore assessed MHC class I expression and its correlations with B2M expression, tumor size, infiltration of lymphocytes, histological grade and prognosis in dogs with mammary gland carcinoma and complex carcinomas.
This observational study involved 22 female dogs with mammary gland tumors, including 9 with mammary gland carcinomas and 13 with mammary gland complex carcinomas, admitted to the Veterinary Clinical Center of Osaka Prefecture University from June 2009 to October 2010. Histopathological findings were used to classify the tumors according to the criteria of a recently validated system [10]. Median dog age was 12 years (range 8 to 14 years). None of the dogs had metastases. Clinical data collected for all dogs included age at diagnosis, tumor size, treatment, relapse and survival. Dogs were followed-up for at least 2 years after surgical excision of the mammary gland tumors with assessment of no relapse or survival, rather than the disease-free interval. Since different types of treatment and deaths due to unrelated causes or euthanasia were considered confounders of survival time as an end point [6], dogs that died from unrelated causes or were euthanized were excluded. All dogs were treated by surgery only. Tumor tissue was removed and subjected to histopathological and immunohistochemical analysis. The study protocol was approved by the animal ethics review committee of Osaka Prefecture University.
Hematoxylin and eosin (HE)-stained sections were histologically graded using the Elston and Ellis grading method as described previously [8, 19]. Grading was based on (1) gland tubules and acini formation, (2) pleomorphism of tumor cell nuclei and (3) mitotic counts with each feature scored from 1 to 3 points. Tumors with 3–5, 6–7 and 8–9 points were considered as having histological grades 1 (well differentiated), 2 (moderately differentiated) and 3 (poorly differentiated), respectively (Fig. 1). Infiltration of lymphocytes was evaluated using HE-stained sections semiquantitatively with − indicating no infiltration and + indicating mild, moderate or diffuse infiltration.
Fig. 1.
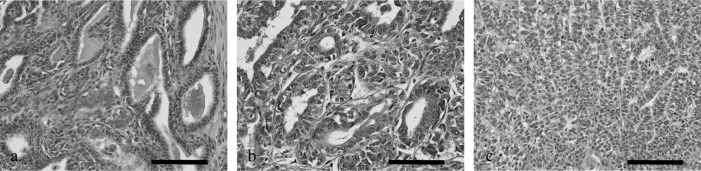
Representative images of canine mammary gland carcinomas and complex carcinomas of histological grades 1–3. (a) Grade 1, well-differentiated tumor; (b) Grade 2, moderately differentiated tumor; (c) Grade 3, poorly differentiated tumor. Scale bar: 100 µm.
MHC class I expression on tumor cells was assayed using mouse anti-dog MHC class I monoclonal antibody (VMRD, Inc., Pullman, WA, U.S.A., diluted 1:200) and a commercial streptavidin-biotin kit (LSAB+ Kit/HRP; Dako North America, Inc., Carpinteria, CA, U.S.A.). Briefly, sections, 1 cm in diameter, were embedded in optimal cutting temperature (OCT) embedding medium (Tissue Mount; Chiba Medical Co., Ltd., Saitama, Japan), frozen in liquid nitrogen and stored at −80°C until use. Cryostat sections were cut, air dried at room temperature, fixed in acetone for 10 min at room temperature and washed with 0.01 M phosphate-buffered saline (PBS; pH 7.4) for 5 min. The sections were incubated with 3% H2O2 at room temperature for 3 min, washed with PBS for 5 min and incubated with 1.5% skimmed milk in PBS at room temperature for 60 min. After washing, these sections were incubated with optimally diluted monoclonal antibodies for 30 min at room temperature and washed; isotypic IgG was used as a negative control. To detect MHC class I-positive cells, the sections were subsequently incubated with biotinylated anti-mouse IgG (Dako) at room temperature for 30 min, washed, incubated with streptavidin conjugated to horseradish peroxidase (HRP) at room temperature for 30 min, washed in PBS for 5 min and developed for 5 min using a 3,3’-diaminobenzidine chromogen (DAB) H2O2 solution. Color development was stopped by diluting in distilled deionized H2O, and the sections were counterstained with hematoxylin. Normal mammary gland or non-tumor lymph nodes of canine tissue were used as positive controls. MHC class I expression score was measured as described previously [18]. MHC class I-expressing tumor cells were analyzed in 20 different fields of each tumor, and the values reported represent the means of the area calculated. MHC class I expression was regarded as negative, if immunoreactivity was observed in <10% of tumor cells. MHC class I expression was regarded as positive, if >10% of tumor cells were stained (Fig. 2).
Fig. 2.
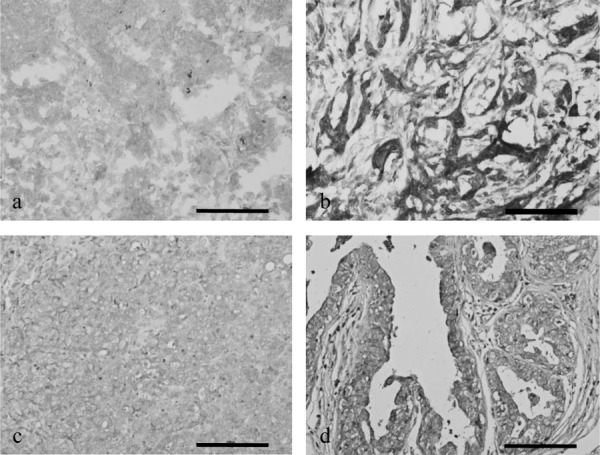
Representative images of (a) negative score for MHC class I expression on mammary gland carcinoma, (b) positive score for MHC class I expression on mammary gland carcinoma, (c) negative score for B2M expression on mammary gland carcinoma and (d) positive score for B2M expression on mammary gland carcinoma. Scale bar: 100 µm.
B2M expression on tumor cells was assessed similarly using rabbit anti-dog B2M serum (10 µg/ml; diluted 1:500), the kind gift of Dr F. Hoshi (Kitasato University School of Veterinary Medicine, Towada, Japan) [14, 15] and a commercial streptavidin-biotin kit (LSAB+ Kit/HRP; Dako). To assay B2M expression, formalin-fixed paraffin-embedded sections of surgical specimens were deparaffinized and boiled for 15 min in a microwave oven for antigen retrieval. The sections were incubated with 3% H2O2 at room temperature for 10 min, washed with PBS for 5 min and incubated with 5% skimmed milk in PBS at room temperature for 60 min. The sections were subsequently incubated with optimally diluted rabbit anti-dog B2M serum at room temperature for 30 min, washed, incubated with biotinylated anti-mouse IgG (Dako) at room temperature for 15 min and again washed. The sections were incubated with streptavidin conjugated to HRP at room temperature for 15 min, washed in PBS for 5 min, developed for 3 min in DAB H2O2 and counterstained with hematoxylin. Normal mammary gland or non-tumor lymph nodes of dogs were used as positive controls. B2M expression score was measured as above (Fig. 2).
All statistical analyses were performed using R software (version 2.12.1). Continuous variables were assessed using Fisher’s exact test with statistical significance set at P<0.05. To assist in determining between-group differences, effect size statistics were calculated for each dependent variable. An effect size (Cramer’s coefficient of association) of 0.5 was defined as a meaningful between-group difference. MHC class I expression score was categorized as negative or positive; tumor size was classified as >3 cm or <3 cm; B2M expression was classified as negative or positive; infiltration of lymphocytes was classified as negative or positive; histological grade was classified as 1, 2 or 3; and disease-free interval was classified as >24 months or <24 months.
In 9 dogs with mammary gland carcinomas, the histological grade was 1 in 1 dogs (11%), 2 in 3 dogs (33%) and 3 in 5 dogs (56%). Immunohistochemical analysis of MHC class I expression in mammary gland carcinomas showed that 5 dogs had a positive score and 4 dogs had a negative score. Regarding MHC class I expression, immunoreactivity was observed in the membrane and cytoplasm. Tumors from 4 of the 9 dogs (44.4%) had lost MHC class I expression (Table 1). Of the 5 dogs with positive scores for MHC class I expression, 4 (80%) had tumors >3 cm in size, and 1 (20%) had a tumor <3 cm in size. Of the 4 dogs with negative scores for MHC class I expression, 3 (75%) and 1 (25%) had tumors >3 cm and <3 cm in size, respectively. Fisher’s exact test showed no significant association between MHC class I expression and tumor size (Table 1). Of the 5 tumors positive for MHC class I expression, 3 (60%) were positive, and 2 (40%) were negative for B2M expression. Regarding B2M expression, immunoreactivity was observed in the membrane and cytoplasm. All 4 tumors negative for MHC class I expression were also negative for B2M expression. Fisher’s exact test revealed no significant association between MHC class I and B2M expression (Table 1). However, the effect size statistic was large (0.63), suggesting that loss of MHC class I expression was significantly associated with loss of B2M expression (Table 1). Of the 5 tumors positive for MHC class I expression, 3 (60%) were positive, and 2 (40%) were negative for infiltration of lymphocytes. Of the 4 dogs with negative scores for MHC class I expression, 0 (0%) and 4 (100%) were positive and negative for infiltration of lymphocytes, respectively. Fisher’s exact test revealed no significant association between MHC class I and infiltration of lymphocytes (Table 1). However, the effect size statistic was large (0.63), suggesting that loss of MHC class I expression was significantly associated with infiltration of lymphocytes (Table 1). Of the 5 tumors positive for MHC class I expression, 1 (20%), 3 (60%) and 1 (20%) were of histological grades 1, 2 and 3, respectively, whereas the tumors of all 4 dogs negative for MHC class I expression were of histological grade 3. Fisher’s exact test showed that MHC class I expression was not significantly associated with histological grade (Table 1). However, the effect size statistic was large (0.8), suggesting that loss of MHC class I expression was associated with higher histological grade (Table 1). Staining intensity had no association with histological grade. All 5 dogs positive for MHC class I expression survived without relapse or shortened survival for 2 years after surgical treatment. In contrast, all 4 dogs negative for MHC class I expression relapsed or died during the 2 years after surgical treatment (median survival, 3.5 months; range, 1 to 6). Fisher’s exact test showed a significant relationship between loss of MHC class I expression and shorter event-free interval (Table 1, P<0.05).
Table 1. MHC class I expression, tumor size, B2M expression, infiltration of lymphocytes, histological grade and event-free intervals in dogs with mammary gland and mammary gland complex carcinomas.
| MHC class I expressionin mammary gland
carcinoma |
MHC class I expressionin mammary gland
complex carcinoma |
|||||
|---|---|---|---|---|---|---|
| Positiven (%) | Negativen (%) | PEffect size | Positiven (%) | Negativen (%) | PEffect size | |
| Tumor size | ||||||
| >3 cm | 4 (80) | 3 (75) | 0.72 | 6 (50) | 1 (100) | 1 |
| <3 cm | 1 (20) | 1 (25) | 0.05 | 6 (50) | 0 (0) | 0.26 |
| B2M expression | ||||||
| Positive | 3 (60) | 0 (0) | 0.12 | 11 (92) | 1 (100) | 1 |
| Negative | 2 (40) | 4 (100) | 0.63 | 1 (8) | 0 (0) | 0.08 |
| Infiltration of lymphocytes | ||||||
| Positive | 3 (60) | 0 (0) | 0.12 | 10 (83) | 0 (0) | 0.23 |
| Negative | 2 (40) | 4 (100) | 0.63 | 2 (17) | 1 (100) | 0.53 |
| Histological grade | ||||||
| 1 | 1 (20) | 0 (0) | 0.09 | 6 (50) | 1 (100) | 1 |
| 2 | 3 (60) | 0 (0) | 0.8 | 4 (33) | 0 (0) | 0.26 |
| 3 | 1 (20) | 4 (100) | 2 (17) | 0 (0) | ||
| Event-free interval | ||||||
| >24 months | 5 (100) | 0 (0) | 0.008 | 12 (100) | 1 (100) | 1 |
| <24 months | 0 (0) | 4 (100) | 1 | 0 (0) | 0 (0) | - |
P was calculated using Fisher’s exact test with statistical significance set at P<0.05. An effect size of 0.5 was identified as a meaningful difference between groups.
In the 13 dogs with mammary gland complex carcinomas, the histological grades was 1 in 7 dogs (54%), 2 in 4 dogs (31%) and 3 in 2 dogs (15%). Immunohistochemical analysis of the 13 mammary gland complex carcinomas showed that 12 (92.4%) were positive and 1 (7.6%) was negative for MHC class I expression. Regarding MHC class I expression, immunoreactivity of luminal epithelial cells was observed in the membrane and cytoplasm. Immunoreactivity of myoepithelial cells was the same as that of luminal epithelial cells. Of the 12 tumors positive for MHC class I expression, 6 (50%) each had tumors >3 cm and <3 cm in size, although the tumor negative for MHC class I expression was >3 cm in size. Fisher’s exact test showed that the association between MHC class I expression and tumor size was not statistically significant (Table 1). Immunohistochemical analysis of the 12 tumors positive for MHC class I expression showed that 11 (92%) were positive for B2M expression and 1 (8%) was negative. Regarding B2M expression, immunoreactivity of luminal epithelial cells was observed in the membrane and cytoplasm. Immunoreactivity of myoepithelial cells was the same as that of luminal epithelial cells. The tumor negative for MHC class I expression was positive for B2M expression. Fisher’s exact test and the small effect size statistic (0.08) indicated that MHC class I expression and B2M expression were not significantly associated (Table 1). Of the 12 tumors positive for MHC class I expression, 10 (83%) were positive, and 2 (17%) were negative for infiltration of lymphocytes. The 1 dog with negative scores for MHC class I expression was negative for infiltration of lymphocytes. Fisher’s exact test revealed no significant association between MHC class I and infiltration of lymphocytes (Table 1). However, the effect size statistic was large (0.53), suggesting that loss of MHC class I expression was significantly associated with infiltration of lymphocytes (Table 1). Of the 12 tumors positive for MHC class I expression, 6 (50%), 4 (33%) and 2 (17%) were of histological grades 1, 2 and 3, respectively. The tumor negative for MHC class I expression was of histological grade 1. Fisher’s exact test and the small effect size statistic (0.26) indicated no significant association between MHC class I expression and histological grade (Table 1). Staining intensity had no association with histological grade. All 12 dogs with tumors positive for MHC class I expression and the 1 dog with a tumor negative for MHC class I expression survived without relapse for 2 years after surgical treatment. Fisher’s exact test showed no significant association between loss of MHC class I expression and the event-free interval (Table 1).
Previously reported prognostic factors in dogs with mammary gland tumors included tumor size, histological type, evidence of metastasis at the time of diagnosis, clinical stage and histological grading using the Elston and Ellis grading method [3, 8, 17]. This study showed that loss of MHC class I expression from canine mammary gland carcinomas was associated with loss of B2M expression and prognosis. In human cancers, abnormalities in MHC class I expression can be caused by alterations in the MHC class I processing machinery, including TAP1, TAP2, tapasin, LMP2, LMP7 and B2M [2]. In human breast cancer, loss of MHC class I expression is caused by loss of B2M expression and/or function [4]. Tumor cells that do not express MHC class I proteins on their surfaces can escape recognition by CD8+ T lymphocytes [18]. Thus, loss or downregulation of MHC class I expression has been associated with disease progression and/or poor clinical outcome [2]. Our study showed that positive expression of MHC class I and infiltration of lymphocytes in canine mammary gland carcinoma and complex carcinoma were correlated. Moreover, infiltration by CD8+ T-cells in canine mammary gland carcinoma has been found to be a marker of good prognosis [5]. Therefore, in dogs with mammary gland carcinoma, loss of MHC class I expression may correlate with poor prognosis. Loss of MHC class I expression was observed in canine mammary gland carcinomas of histological grade 3. Histological grading is related to prognosis, being worse in dogs with grade 3 (poorly differentiated) than in those with grade 1 (well differentiated) and 2 (moderately differentiated) mammary gland carcinomas [8]. MHC class I expression may be lost in poorly differentiated mammary gland carcinomas. Therefore, MHC class I expression loss may be correlated with poor prognosis.
In this study, dogs with mammary gland complex carcinoma had a good prognosis. However, only 1 of these 13 dogs showed loss of MHC class I expression. Because of this imbalance, loss of MHC class I expression from these tumors was not associated with loss of B2M expression, histological grade or prognosis. Tumors in 12 of the 13 dogs with mammary gland complex carcinoma (12/13) showed expression of MHC class I, and all of these dogs had a good prognosis. Thus, the prognosis of dogs with mammary gland tumors is associated with both histological grade and tumor type. Dogs with carcinomas had a poorer prognosis (death rate 67.3%) than dogs with complex carcinomas (death rate 0%) [8]. The low frequency of loss of MHC class I expression in dogs with mammary gland complex carcinoma may be associated with the absence of relapse or prolonged survival after surgical treatment. This study had some limitations, including the small study sample and the imbalanced data in dogs with mammary gland complex carcinoma. Further work is needed to evaluate the association between loss of MHC class I expression and loss of B2M expression, histological grade, infiltration of CD8+ T lymphocytes and prognosis in a larger and better balanced population of dogs with mammary gland complex carcinomas.
Canine mammary gland tumors >3 cm in size have been associated with poor prognosis [17]. In this study, loss of MHC class I expression was not associated with tumor size in dogs with mammary gland and complex carcinomas. In humans, loss of MHC class I expression from tumor cells occurs when the primary tumor penetrates the basal membranes, invading surrounding tissues and/or starts to metastasize to regional lymph nodes or distant organs [7]. None of the dogs in our study showed any evidence of metastasis. Therefore, loss of MHC class I expression may be independent of tumor size.
This study is limited by the small sample size. In future studies, a larger number of mammary gland tumors should be evaluated with regard to loss of MHC class I expression as an independent prognostic factor by multivariate analysis, and the mechanism of MHC class I expression loss should also be explored. In conclusion, our findings suggested that loss of MHC class I expression may be a factor associated with poor prognosis in dogs with mammary gland carcinoma.
REFERENCES
- 1.Aptsiauri N., Cabrera T., Garcia-Lora A., Lopez-Nevot M. A., Ruiz-Cabello F., Garrido F.2007. MHC class I antigens and immune surveillance in transformed cells. Int. Rev. Cytol. 256: 139–189. doi: 10.1016/S0074-7696(07)56005-5 [DOI] [PubMed] [Google Scholar]
- 2.Chang C. C., Campoli M., Ferrone S.2003. HLA class I defects in malignant lesions: what have we learned? Keio J. Med. 52: 220–229. doi: 10.2302/kjm.52.220 [DOI] [PubMed] [Google Scholar]
- 3.Chang S. C., Chang C. C., Chang T. J., Wong M. L.2005. Prognostic factors associated with survival two years after surgery in dogs with malignant mammary tumors: 79 cases (1998–2002). J. Am. Vet. Med. Assoc. 227: 1625–1629. doi: 10.2460/javma.2005.227.1625 [DOI] [PubMed] [Google Scholar]
- 4.Chen H. L., Gabrilovich D., Virmani A., Ratnani I., Girgis K. R., Nadaf-Rahrov S., Fernandez-Viña M., Carbone D. P.1996. Structural and functional analysis of β2 microglobulin abnormalities in human lung and breast cancer. Int. J. Cancer 67: 756–763 [DOI] [PubMed] [Google Scholar]
- 5.Estrela-Lima A., Araújo M. S., Costa-Neto J. M., Teixeira-Carvalho A., Barrouin-Melo S. M., Cardoso S. V., Martins-Filho O. A., Serakides R., Cassali G. D.2010. Immunophenotypic features of tumor infiltrating lymphocytes from mammary carcinomas in female dogs associated with prognostic factors and survival rates. BMC Cancer 10: 256. doi: 10.1186/1471-2407-10-256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilbertson S. R., Kurzman I. D., Zachrau R. E., Hurvitz A. I., Black M. M.1983. Canine mammary epithelial neoplasms: biologic implications of morphologic characteristics assessed in 232 dogs. Vet. Pathol. 20: 127–142 [DOI] [PubMed] [Google Scholar]
- 7.Hsieh C. H., Hsu Y. J., Chang C. C., Liu H. C., Chuang K. L., Chuang C. K., Pang S. T., Hasumi K., Ferrone S., Liao S. K.2009. Total HLA class I loss in a sarcomatoid renal carcinoma cell line caused by the coexistence of distinct mutations in the two encoding beta2-microglobulin genes. Cancer Immunol. Immunother. 58: 395–408. doi: 10.1007/s00262-008-0565-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karayannopoulou M., Kaldrymidou E., Constantinidis T. C., Dessiris A.2005. Histological grading and prognosis in dogs with mammary carcinomas: application of a human grading method. J. Comp. Pathol. 133: 246–252. doi: 10.1016/j.jcpa.2005.05.003 [DOI] [PubMed] [Google Scholar]
- 9.Misdorp W., Hart A. A.1976. Prognostic factors in canine mammary cancer. J. Natl. Cancer Inst. 56: 779–786 [DOI] [PubMed] [Google Scholar]
- 10.Misdorp W., Else R. W., Hellmén E., Lipscomb T. P.1999. Histological classification of mammary tumors of the dog and the cat. pp. 11–29. In: Armed Forces Institute of Pathology and the American Registry of Pathology and the World Health Organization Collaborating Center for Worldwide Reference on Comparative Oncology, vol. 7, Washington, D.C. [Google Scholar]
- 11.Misdorp W., Cotchin E., Hampe J. F., Jabara A. G., Von Sandersleben J.1973. Canine malignant mammary tumors. 3. Special types of carcinomas, malignant mixed tumors. Vet. Pathol. 10: 241–256. doi: 10.1177/030098587301000307 [DOI] [PubMed] [Google Scholar]
- 12.Morrison L. A., Lukacher A. E., Braciale V. L., Fan D. P., Braciale T. J.1986. Differences in antigen presentation to MHC class I- and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J. Exp. Med. 163: 903–921. doi: 10.1084/jem.163.4.903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moulton J. E.1990. Tumor of the mammary gland. pp. 518–552. In: Tumors in Domestic Animals. 3rd ed. (Moulton, J. E. ed.), Univ. California Press, Berkeley. [Google Scholar]
- 14.Nakajima Y., Hoshi F., Higuchi S., Kawamura S.1999. The complete amino acid sequence of dog beta2-microglobulin. J. Vet. Med. Sci. 61: 517–521. doi: 10.1292/jvms.61.517 [DOI] [PubMed] [Google Scholar]
- 15.Nakajima Y., Hoshi F., Higuchi S., Kawamura S.2001. Determination of canine beta2-microgloblin in plasma and urine by enzyme-linked immunosorbent assay. J. Vet. Med. Sci. 63: 343–345. doi: 10.1292/jvms.63.343 [DOI] [PubMed] [Google Scholar]
- 16.Pedersen L. O., Hansen A. S., Olsen A. C., Gerwien J., Nissen M. H., Buus S.1994. The interaction between beta 2-microglobulin (beta 2m) and purified class-I major histocompatibility (MHC) antigen. Scand. J. Immunol. 39: 64–72. doi: 10.1111/j.1365-3083.1994.tb03341.x [DOI] [PubMed] [Google Scholar]
- 17.Philibert J. C., Snyder P. W., Glickman N., Glickman L. T., Knapp D. W., Waters D. J.2003. Influence of host factors on survival in dogs with malignant mammary gland tumors. J. Vet. Intern. Med. 17: 102–106. doi: 10.1111/j.1939-1676.2003.tb01330.x [DOI] [PubMed] [Google Scholar]
- 18.Redondo M., Garcia J., Villar E., Rodrigo I., Perea-Milla E., Serrano A., Morell M.2003. Major histocompatibility complex status in breast carcinogenesis and relationship to apoptosis. Hum. Pathol. 34: 1283–1289. doi: 10.1016/j.humpath.2003.06.001 [DOI] [PubMed] [Google Scholar]
- 19.Saleh F., Abdeen S.2007. Pathobiological features of breast tumours in the State of Kuwait: a comprehensive analysis. J. Carcinog. 6: 12. doi: 10.1186/1477-3163-6-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uva P., Aurisicchio L., Watters J., Loboda A., Kulkarni A., Castle J., Palombo F., Viti V., Mesiti G., Zappulli V., Marconato L., Abramo F., Ciliberto G., Lahm A., La Monica N., de Rinaldis E.2009. Comparative expression pathway analysis of human and canine mammary tumors. BMC Genomics 10: 135. doi: 10.1186/1471-2164-10-135 [DOI] [PMC free article] [PubMed] [Google Scholar]


