ABSTRACT
In this study, we attempted to express twelve glycoproteins of equine herpesvirus-1 (EHV-1) in 293T cells and to characterize these using monoclonal antibodies (MAbs) and horse sera against EHV-1. Expression of glycoprotein B (gB), gC, gD, gG, gI and gp2 was recognized by immunoblot analysis using horse sera, but that of gE, gH, gK, gL, gM and gN was not. Four MAbs recognized gB, four recognized gC and one recognized gp2. Two MAbs against gB cross-reacted with EHV-4. Interestingly, coexpression of gE and gI and gM and gN enhanced their antigenicity. Furthermore, immunoblot analysis of gp2 showed that different molecular masses of gp2 were recognized by the MAb against gp2 and horse sera against EHV-1. In this study, it was demonstrated that at least six glycoproteins were immunogenic to horses, and coexpression of gE and gI and gM and gN was important for enhancement of antigenicity.
Keywords: equine herpesvirus-1, glycoprotein, monoclonal antibody
Equine herpesvirus-1 (EHV-1) is a member of genus Varicellovirus, subfamily Alphaherpesvirinae, family Herpesviridae and order Herpesvirales. EHV-1 distributed worldwide [2] and is a major cause of respiratory disease in horses, resulting in serious economic losses in the horse industry. In addition, EHV-1 causes epidemic and sporadic abortion in mares and myeloencephalopathy [1, 9, 24].
Acquired immunity induced by natural infection or vaccination against EHV-1 is generally short-lived, and consequently, horses may suffer from repeated infections [28]. Herpesviral glycoproteins are essential in infection processes including virus adsorption, penetration and cell-to-cell spread and play significant roles in eliciting both humoral and cellular immune responses in horses [19, 20]. In EHV-1, eleven viral glycoproteins, gB (gp14), gC (gp13), gD (gp18), gE, gG, gH, gI, gK, gL, gM and gN are conserved in comparison with those of other alphaherpesviruses. However, glycoprotein2 (gp2) is encoded only in equine alphaherpesviruses, EHV-1, equine herpesvirus-4 (EHV-4) and asinine herpesvirus 3 (AHV-3) [20]. These twelve glycoproteins of EHV-1 are possible candidates for vaccine antigens. In some glycoproteins of herpesviruses, co-expression with different glycoproteins was often required for efficient expression and protein folding [10, 13, 15, 19, 21, 22], but the detail in EHV-1was still unclear. To characterize their immunogenicity and functions, twelve glycoproteins of EHV-1 were expressed in vitro and characterized using established monoclonal antibodies (MAbs) against EHV-1 and sera collected from EHV-1-infected horses.
MATERIALS AND METHODS
Cells: FHK-Tcl3.1 [3, 17] and 293T cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; GIBCO, Grand Island, NY, U.S.A.) supplemented with 10% heat-inactivated fetal calf serum (FCS; JR Scientific, Woodland, CA, U.S.A.), 100 units/ml penicillin and 100 µg/ml streptomycin (GIBCO) at 37°C under 5% CO2. The myeloma cell line P3U1 was maintained in RPMI1640 medium (GIBCO) supplemented with 10% heat-inactivated FCS, 100 units/ml penicillin, 100 µg/ml streptomycin and 55 µM of 2-mercaptoethanol (2-ME) (GIBCO) at 37°C under 5% CO2.
Viruses: EHV-1 strain 89c25 was isolated from a racehorse with respiratory disease caused by EHV-1 respiratory infection [18]. 89c25 was plaque-purified three times in primary fetal horse kidney (FHK) cells and designated as 89c25p. EHV-1 was propagated in FHK-Tcl3.1 cells. EHV-4 TH20p strain [11, 14] was propagated in FHK-Tcl3.1 cells and was used for indirect immunofluorescence assay (IFA).
Construction of expression plasmids: For expression of glycoproteins of EHV-1, each gene was amplified from the genome of EHV-1 89c25p by polymerase chain reaction (PCR) using primers. PCR was performed by using Takara LA PCR Kit Ver. 2.1 (Takara, Otsu, Japan), and the amplified fragments were digested with restriction enzymes and were then cloned into the expression plasmid pCAGGS, which was kindly provided by Dr. Miyazaki (Osaka University). The nucleotide sequences of at least two constructed plasmids were determined using a Big Dye Terminator v3.1 kit (Applied Biosystems, Foster City, CA, U.S.A.). All plasmids were purified by QIAprep Spin Miniprep Kit (QIAGEN, Hilden, Germany) or QIAfilter plasmid Midi Kit (QIAGEN).
Expression in 293T cells: 293T cells were transfected with purified plasmids using polyethylenimine (PEI). Briefly, 3.2 µg of plasmid were mixed with 8 µl of PEI (2 mg/ml) and were then transfected to 293T cells in 6 wells plate (Sumitomo Bakelite, Tokyo, Japan), as reported previously [5].
Production of MAbs: BALB/c mice (female, aged six weeks) were intraperitoneally inoculated with 1 × 105 PFU of EHV-1 three times at an interval of three or four weeks. At 3 to 7 days after final immunization, splenocytes were collected and fused with P3U1 myeloma cells using 50% polyethylene glycol solution (Hybri-MaxTM; Sigma, St. Louis, MO, U.S.A.). Hybridoma cells were selected in GIT media (Wako, Osaka, Japan) containing hypoxanthine aminopterin thymidine supplement (HAT) (GIBCO), 10% BM-condimed H1 hybridoma cloning supplement (Roche Diagnostics, Mannheim, Germany) and 10% FCS for 7 days at 37°C under 5% CO2. Supernatants of hybridomas were screened by virus-neutralization (VN) test and/or IFA. Hybridoma cells secreting EHV-1-specific MAbs were cloned twice by limiting dilution method and were then inoculated into BALB/c mice pretreated with pristane (Sigma) for preparation of ascites.
VN test: In order to detect VN activity, supernatants of hybridoma or diluted ascites were mixed with EHV-1 for 60 min at 37°C, and then, the mixtures were directly inoculated into FHK-Tcl3.1 cells that were washed with DMEM without FCS. Control was carried out without hybridoma or ascites. After incubation for 60 min at 37°C under 5% CO2, cells were washed twice with DMEM without FCS and overlaid with 0.8% agarose (SeaPlaque GTG agarose; Lonza, Rockland, ME, U.S.A.) in DMEM containing 10% FCS. Plates were placed at 37°C in 5% CO2 for 3 days, and cells were fixed with 5% buffered formaldehyde. Agarose layers were removed, and cells were stained with crystal violet. The number of plaques was counted, and hybridoma or diluted ascites that reduced the number of plaques by more than 50% in comparison with the mean number of plaques in control wells was considered to be positive.
IFA: Virus-infected FHK-Tcl3.1 cells or plasmid-transfected 293T cells were collected, washed with PBS three times and placed on 24-well microscope slides (Matsunami Glass, Osaka, Japan). After fixation with cold acetone for 30 min, cells were incubated with MAbs for 60 min at 37°C. Cells were then washed three times in PBS and incubated with goat anti-mouse Ig(H+L)-FITC human-adsorbed (Southern Biotech, Birmingham, AL, U.S.A.) for 30 min at 37°C. After washing three times, fluorescence was observed under a Nikon Optiphot 2 EFD3 fluorescence phase contrast microscope (Nikon, Tokyo, Japan).
Immunoblot analysis: Virus-infected FHK-Tcl3.1 cells or plasmid-transfected 293T cells were extracted with RIPA [25mM Tris-HCl (pH 7.6), 150 mM sodium chloride (NaCl), 1% sodium dodecyl sulfate (SDS), 1% sodium deoxycholate and 1% Triton X-100] and then dissolved in 2×SDS sample buffer (125 mM Tris-HCl, 4% SDS, 40% glycerol and 0.002% bromophenol blue) with or without 2-ME or directly dissolved in 1×SDS sample buffer with or without 5% 2-ME. After boiling for 3 min, samples were loaded on SDS-polyacrylamide gel (PAGE), and electrophoresis was carried out in SDS buffer (25 mM Tris, 192 mM glycine and 0.1% SDS). Then, proteins were transferred to polyvinylidene difluoride (PVDF) membrane (Immobillon; Millipore, Billerica, MA, U.S.A.) by semi-dry blotting apparatus (Biocraft, BE-310, Tokyo, Japan). After membranes were reacted with 3% gelatin (Bio-rad, Hercules, CA, U.S.A.) in TBS (20 mM Tris-HCl and150 mM NaCl, pH 7.5) for 30 min at 37°C, they were then washed three times with TBS containing 0.05% Tween 20. After washing, the membrane was incubated with diluted horse sera or MAbs for 1 hr at 37°C as primary antibody, followed by incubation with the peroxidase-conjugated F(ab)2 fragment of anti-horse IgG(H&L) goat (Rockland, Gilbertsville, PA, U.S.A.) or peroxidase-conjugated goat affinity purified antibody against mouse imunoglobulins IgG, IgA and IgM (Cappel, Solon, OH, U.S.A.) for 30 min at 37°C as secondary antibody. All antibodies were diluted with TBS containing 0.05% Tween 20 and 1% gelatin. The reaction was visualized using 0.03% diaminobenzidine (Wako) and 0.009% H2O2 (Wako) in TBS.
Sera: Sera were collected from three foals that were experimentally infected with EHV-1 89c25p [27]. Serum specific to EHV-1 gE was collected from BALB/c mice immunized with fusion protein of glutathione S-transferase and gE(169-201) [4].
Immunoglobulin class and subclass: Immunoglobulin class and subclass of MAbs were determined using mouse monoclonal antibody isotyping kit (Roche).
RESULTS
Sequence analysis of each glycoprotein of EHV-1 strain 89c25p: Nucleotide sequences of at least two cloned genes encoding each glycoprotein of 89c25p were determined and compared with those of EHV-1 strain Ab4 [26]. The results showed that the amino acid sequences of gC, gD, gE, gG, gH, gI, gK, gL and gN of 89c25p were identical to those of Ab4, but those of gp2, gB and gM were different at positions 95 (Serine to Phenylalanine), 938 (Lysine to Glutamic acid) and 84 (Methionine to Lysine), respectively (data not shown). These plasmids were designated as pCAG-gp2-1 pCAG-gB-1, pCAG-gC-1, pCAG-gD-1, pCAG-gE-1 pCAG-gG-1, pCAG-gH-1, pCAG-gI-1, pCAG-gK-1, pCAG-gL-1, pCAG-gM-1 and pCAG-gN-1.
Expression of EHV-1 glycoproteins in 293T cells: Twelve expression plasmids encoding each glycoprotein were transfected into 293T cells, and expression was examined by immunoblot analysis using horse sera. Expressed gp2, gB, gC, gD, gG and gI were detected, but gE, gH, gK, gL, gM and gN were not. Three bands with molecular masses of approximately 230, 65 and 42 kilo Daltons (kDa) were detected in pCAG-gp2-1-transfected cells, two bands of 148 and 135 kDa were detected in pCAG-gB-1-transfected cells, two bands of 150 and 68–75 kDa were detected in pCAG-gC-1-transfected cells, one band of 50 kDa was detected in pCAG-gD-1-transfected cells, two bands of 100 and 50 kDa were detected in pCAG-gG-1-transfected cells, and two bands of 140 and 65 kDa were detected in pCAG-gI-1-transfected cells (data not shown).
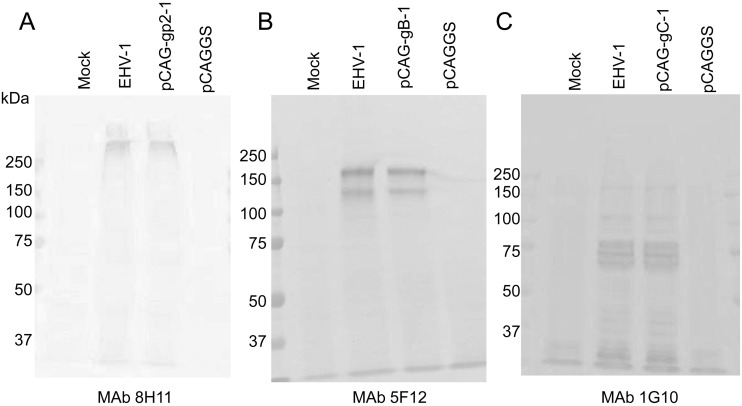
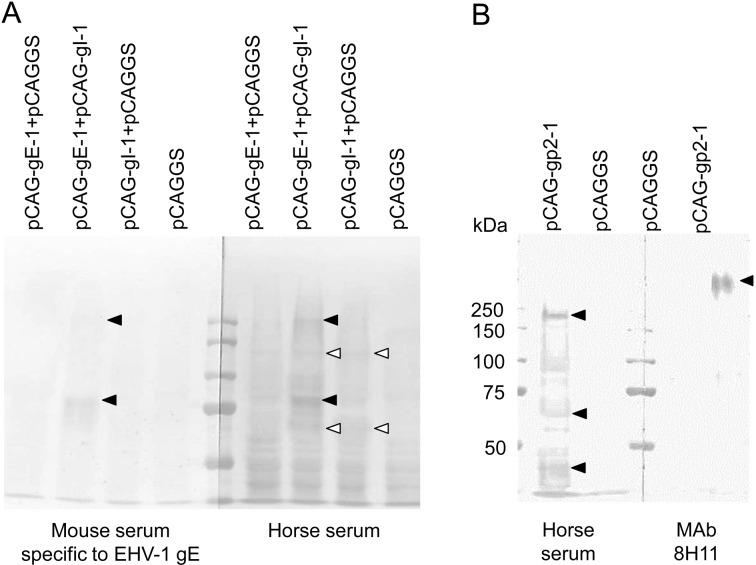
Establishment and characterization of MAbs: In order to characterize EHV-1 glycoproteins, nine MAbs against EHV-1 strain 89c25p were produced (Table 1). Four MAbs, 5F8, 1G10, 8H4 and 8B2, had VN activity against EHV-1, and all were specific to gC (Table 1, Fig. 1C). Four MAbs, 5F12, 6F4, 6G12 and 7E11, were specific to gB (Table 1, Fig.1B), and two, 6F4 and 6G12, cross-reacted with EHV-4 (Table 1). One MAb 8H11 was specific to gp2 (Table 1, Fig. 1A). All MAbs did not recognize glycoproteins on immunoblot analysis under reducing conditions (data not shown). In addition, the molecular masses of gp2, gB and gC expressed in 293T cells were similar to those in EHV-1-infected cells (Fig. 1A–C). Interestingly, the molecular mass of gp2 detected by MAb 8H11 was over 250 kDa and was different from those detected in horse sera, 230, 65 and 42 kDa (Figs. 1A and 3B).
Table 1. Characterization of MAbs against EHV-1.
| MAbs | Isotype | Proteins | VN titer | IFA |
Immunoblot analysis a) | |
|---|---|---|---|---|---|---|
| EHV-1 | EHV-4 | |||||
| 8H11 | IgG2a | gp2 | <1:10 | 1:1,280 | <1:100 | + |
| 5F8 | IgM | gC | 1:80 | 1:1,600 | <1:100 | + |
| 1G10 | IgM | gC | 1:640 | 1:800 | <1:100 | + |
| 8H4 | IgM | gC | 1:1,280 | 1:800 | <1:100 | + |
| 8B2 | IgM | gC | 1:3,200 | 1:1,600 | <1:100 | – |
| 5F12 | IgM | gB | <1:10 | 1:1,280 | <1:100 | + |
| 7E11 | IgM | gB | <1:10 | 1:1,280 | <1:100 | + |
| 6F4 | IgG2a | gB | <1:10 | 1:20,480 | 1:800 | + |
| 6G12 | IgM | gB | <1:10 | 1:1,280 | 1:800 | + |
a) Immunoblot analysis was carried out under non-reducing conditions.
Fig. 1.
Comparison of gp2, gB and gC expressed in plasmid-transfected 293T cells and EHV-1-infected FHK-Tcl3.1 cells. Immunoblot analysis was carried out under non-reducing conditions. MAbs, 8H11(A), 5F12 (B) and 1G10 (C) were used as primary antibodies.
Fig. 3.
(A) Comparison of co-expressed gE and gI by immunoblot analysis using mouse sera specific to gE (169-201) and horse sera specific to EHV-1. Closed arrowheads show gE-specific bands and open arrowheads do gI-specific bands. (B) Comparison of molecular masses of gp2 detected by MAb 8H11 and horse sera specific to EHV-1. Immunoblot analysis was carried out under non-reducing conditions. Closed arrowheads show gp2-specific bands.
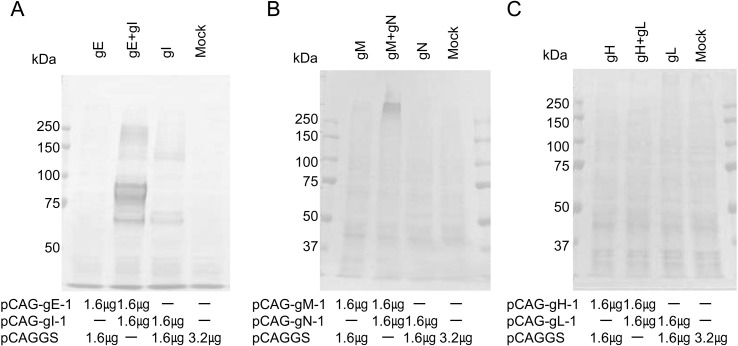
Co-expression of glycoproteins: EHV-1 gE and gI, gM and gN and gH and gL were co-expressed in 293T cells, meaning that co-expressed gE and gI, and gM and gN reacted strongly with horse sera, but co-expressed gH and gL did not (Fig. 2). With co-expression of gE and gI, two additional bands other than gI-specific bands with molecular masses of 80–90 and 250 kDa were seen (Figs. 2A and 3A). Furthermore, mouse antibody specific to gE also recognized two bands with molecular masses of 80–90 and 250 kDa with co-expressed gE and gI, but not with individually expressed gE and gI (Fig. 3A). With co-expression of gM and gN, one band with a molecular mass of over 250 kDa was observed (Fig.2B).
Fig. 2.
Co-expression of EHV-1 glycoproteins. Plasmids pCAG-gE-1 and pCAG-gI-1 (A), pCAG-gM-1 and pCAG-gN-1 (B) and pCAG-gH-1 and pCAG-gL-1 (C) were co-transfected into 293T cells. Immunoblot analysis was carried out under non-reducing conditions. Sera collected from horses experimentally infected with EHV-1 were used as primary antibody. The amount of plasmid DNA transfected into 293T cells in 35 mm dishes is noted under each figure.
DISCUSSION
In this study, twelve glycoproteins in EHV-1 were analyzed using horse sera against EHV-1 and established MAbs. In expression systems for single glycoproteins, gp2, gB, gC, gD, gG and gI reacted strongly with horse sera (data not shown). Although high immunogenicity has been reported for gp2, gB, gC, gD and gG [6,7,8], it is interesting that the immunogenicity of gI was also high. Furthermore, gp2, gB and gC expressed in 293T cells were similar to those expressed in EHV-1-infected cells (Fig. 1), indicating that these proteins were mature when expressed in vitro.
We reported that EHV-1 gE is able to induce antibodies in horses and that the 20 amino acids of gE at positions 169 to188 were a useful antigen for ELISA to differentiate EHV-1 and EHV-4 infections [4]. However, gE expressed in 293T cells was not recognized by horse sera (Figs. 2A and 3A). After co-expression with gI, gE strongly reacted with horse sera against EHV-1 and mouse antibody specific to gE (Fig. 3A), indicating that gI might be required for efficient expression of gE. In EHV-4, gE (95kDa) was co-precipitated with gI (75kDa) by immunoprecipiration using anti-gI serum, but not by anti-gE serum [12]. It is unknown whether two bands, 80–90 and 250 kDa, in co-expressed gE and gI contain only gE or complex of gE and gI. Further experiment will be required to clarify the role of gI in expression of gE.
As shown in Fig. 2B, over 250 kDa proteins in co-expressed gM and gN strongly reacted with horse sera against EHV-1, indicating that gM/gN complex is important for antigenicity in horses. In a previous study, it was reported that gN is necessary and sufficient for functional processing of gM [21]. Therefore, we speculated that immature gM and gN are expressed in a single expression system and that coexpression of gM and gN induces maturation of gM and enhances the antigenicity. However, it is still unknown whether over 250 kDa protein contains only gM or both gM and gN.
In EHV-1, it is reported that co-expression of gH and gL is important for efficient expression and for induction of protective immunity [15, 23]. Therefore, coexpression of gH and gL was examined, but reactions with horse sera were not observed (Fig. 2C). It is unknown whether antigenicity of EHV-1 gH and gL against horse is low or whether another protein is required for maturation of EHV-1 gH and gL.
Horse sera against EHV-1 and MAb against gp2 recognized different molecular masses of gp2, 230, 65 and 42 kDa and over 250 kDa, respectively (Figs. 1A and 3B). EHV-1 gp2 is rich in serine and threonine residues and is a heavily O-glycosylated protein with a molecular mass in the range of 192 to 400 kDa [25, 26, 29]. Furthermore, it has been reported that gp2 is cleaved into a highly glycosylated N-terminal subunit (over 200 kDa) and C-terminal subunit (42 kDa) [16]. Therefore, horse sera against EHV-1 and MAb 8H11 might recognize different positions of gp2, the C-terminal subunit and glycosylated N-terminal subunit, respectively.
In conclusion, our results showed that efficient expression and/or maturation of two glycoproteins, gE and gM, requires co-expression of gI and gN, respectively. In addition, these materials, expression plasmids and MAbs, may be useful for further analysis of EHV-1 glycoproteins.
ACKNOWLEDGMENTS
This study was supported by a grant from the Japan Racing Association. H.Y.A.H.M. received a financial support from the Egyptian government (Faculty of Veterinary Medicine, South Valley University) in the form of a scholarship for his PhD degree.
References
- 1.Allen G. P., Bryans J. T.1986. Molecular epizootiology, pathogenesis, and prophylaxis of equine herpesvirus-1 infections. Prog. Vet. Microbiol. Immunol. 2: 78–144 [PubMed] [Google Scholar]
- 2.Allen G. P., Kydd J. H., Slater J. D., Smith K. C.1999. Advances in understanding of the pathogenesis, epidemiology and immunological control of equine herpesvirus abortion. pp. 129–146. In: Equine Infectious Diseases VIII, Proceedings of the Eighth International Conference (Wernery, U., Wade, J. A., Mumford, J. A. and Kaaden, O.R. eds.), R&W Publications, Newmarket.
- 3.Andoh K., Kai K., Matsumura T., Maeda K.2009. Further development of an equine cell line that can be propagated over 100 times. J. Equine Sci. 20: 11–14. doi: 10.1294/jes.20.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andoh K., Takasugi M., Mahmoud H. Y. A., Hattori S., Terada Y., Noguchi K., Shimoda H., Bannai H., Tsujimura K., Matsumura T., Kondo T., Maeda K.2013. Identification of a major immunogenic region of equine herpesvirus-1 glycoprotein E and its application to enzyme-linked immunosorbent assay. Vet. Microbiol. 164: 18–26. doi: 10.1016/j.vetmic.2013.01.033 [DOI] [PubMed] [Google Scholar]
- 5.Boussif O., Lezoualc’h F., Zanta M. A., Mergny M. D., Scherman D., Demeneix B., Behr J. P.1995. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. U.S.A. 92: 7297–7301. doi: 10.1073/pnas.92.16.7297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crabb B. S., Allen G. P., Studdert M. J.1991. Characterization of the major glycoproteins of equine herpesviruses 4 and 1 and asinine herpesvirus 3 using monoclonal antibodies. J. Gen. Virol. 72: 2075–2082. doi: 10.1099/0022-1317-72-9-2075 [DOI] [PubMed] [Google Scholar]
- 7.Crabb B. S., Studdert M. J.1993. Epitopes of glycoprotein G of equine herpesviruses 4 and 1 located near the C termini elicit type-specific antibody responses in the natural host. J. Virol. 67: 6332–6338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crabb B. S., MacPherson C. M., Reubel G. H., Browning G. F., Studdert M. J., Drummer H. E.1995. A type-specific serological test to distinguish antibodies to equine herpesviruses 4 and 1. Arch. Virol. 140: 245–258. doi: 10.1007/BF01309860 [DOI] [PubMed] [Google Scholar]
- 9.Crabb B. S., Studdert M. J.1995. Equine herpesviruses 4 (equine rhinpneumonitis virus) and 1(equine abortion virus). Adv. Virus Res. 45: 153–190. doi: 10.1016/S0065-3527(08)60060-3 [DOI] [PubMed] [Google Scholar]
- 10.Crump C. M., Bruun B., Bell S., Pomeranz L. E., Minson T., Browne H. M.2004. Alphaherpesvirus glycoprotein M causes the relocalization of plasma membrane proteins. J. Gen. Virol. 85: 3517–3527. doi: 10.1099/vir.0.80361-0 [DOI] [PubMed] [Google Scholar]
- 11.Damiani A. M., Matsumura T., Yokoyama N., Maeda K., Miyazawa T., Kai C., Mikami T.1998. Nucleotide sequences of glycoprotein I and E genes of equine herpesvirus type 4. J. Vet. Med. Sci. 60: 219–225. doi: 10.1292/jvms.60.219 [DOI] [PubMed] [Google Scholar]
- 12.Damiani A. M., Matsumura T., Jang H. K., Izumiya Y., Mikami T., Takahashi E.2000. Identification of the products of the equine herpesvirus type 4 gI and gE genes. Arch. Virol. 145: 1489–1496. doi: 10.1007/s007050070106 [DOI] [PubMed] [Google Scholar]
- 13.Farnsworth A., Goldsmith K., Johnson D. C.2003. Herpes simplex virus glycoproteins gD and gE/gI serve essential but redundant functions during acquisition of the virion envelope in the cytoplasm. J. Virol. 77: 8481–8494. doi: 10.1128/JVI.77.15.8481-8494.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawakami Y., Kaji T., Ishizaki R., Shimizu T., Matumoto M.1962. Etiologic study on an outbreak of acute respiratory disease among colts due to equine rhinopneumonitis virus. Jpn. J. Exp. Med. 32: 211–229 [PubMed] [Google Scholar]
- 15.Kukreja A., Love D. N., Whalley J. M., Field H. J.1998. Study of the protective immunity of co-expressed glycoprotein H and L of equineherpesvirus-1 in a murine intranasal infection model. Vet. Microbiol. 60: 1–11. doi: 10.1016/S0378-1135(97)00201-0 [DOI] [PubMed] [Google Scholar]
- 16.Learmonth G. S., Love D. N., Wellington J. E., Gilkerson J. R., Whalley J. M.2002. The C-terminal regions of the envelope glycoprotein gp2 of equine herpesviruses 1 and 4 are antigenically distinct. Arch. Virol. 147: 607–615. doi: 10.1007/s007050200010 [DOI] [PubMed] [Google Scholar]
- 17.Maeda K., Yasumoto S., Tsuruda A., Andoh K., Kai K., Otoi T., Matsumura T.2007. Establishment of a novel equine cell line for isolation and propagation of equine herpesviruses. J. Vet. Med. Sci. 69: 989–991. doi: 10.1292/jvms.69.989 [DOI] [PubMed] [Google Scholar]
- 18.Matsumura T., Sugiura T., Imagawa H., Fukunaga Y., Kamada M.1992. Epizootiological aspects of type 1 and type 4 equine herpesvirus infections among horse populations. J. Vet. Med. Sci. 54: 207–211. doi: 10.1292/jvms.54.207 [DOI] [PubMed] [Google Scholar]
- 19.Norrild B.1985. Humoral responses to herpes simplex virus infections. pp. 69–86. In: The Herpesviruses 4 (Roizman, B. and Lopez, C. eds.), Plenum Press, New York. [Google Scholar]
- 20.Paillot R., Case R., Ross J., Newton R., Nugent J.2008. Equine herpes virus-1: virus, immunity and vaccines. Open Vet. Sci. J. 2: 68–91 [Google Scholar]
- 21.Rudolph J., Osterrieder N.2002. Equine herpesvirus type 1devoid of gM and gp2 is severely impaired in virus egress but not direct cell-to-cell spread. Virology 293: 356–367. doi: 10.1006/viro.2001.1277 [DOI] [PubMed] [Google Scholar]
- 22.Rudolph J., Seyboldt C., Granzow H., Osterrieder N.2002. The gene 10 (UL49.5) product of equine herpesvirus 1 is necessary and sufficient for functional processing of glycoprotein M. J. Virol. 76: 2952–2963. doi: 10.1128/JVI.76.6.2952-2963.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stokes A., Alber D. G., Greensill J., Amellal B., Carvalho R., Taylor L. A., Doel T. R., Killington R. A., Halliburton I. W., Meredith D. M.1996. The expression of the proteins of equine herpesvirus 1 which share homology with herpes simplex virus 1 glycoproteins H and L. Virus Res. 40: 91–107. doi: 10.1016/0168-1702(95)01256-7 [DOI] [PubMed] [Google Scholar]
- 24.Studdert M. J., Hartley C. A., Dynon K., Sandy J. R., Slocombe R. F., Charles J. A., Milne M. E., Clarke A. F., El-Hage C.2003. Outbreak of equine herpesvirus type 1 myeloencephalitis: new insights from virus identification by PCR and the application of an EHV-1-specific antibody detection ELISA. Vet. Rec. 153: 417–423. doi: 10.1136/vr.153.14.417 [DOI] [PubMed] [Google Scholar]
- 25.Sun Y., MacLean A. R., Dargan D., Brown S. M.1994. Identification and characterization of the protein product of gene 71 in equine herpesvirus1. J. Gen. Virol. 75: 3117–3126. doi: 10.1099/0022-1317-75-11-3117 [DOI] [PubMed] [Google Scholar]
- 26.Telford E. A., Watson M. S., McBride K., Davison A. J.1992. The DNA sequence of equine herpesvirus-1. Virology 189: 304–316. doi: 10.1016/0042-6822(92)90706-U [DOI] [PubMed] [Google Scholar]
- 27.Tsujimura K., Shiose T., Yamanaka T., Nemoto M., Kondo T., Matsumura T.2009. Equine hepesvirus type 1 mutant defective in glycoprotein E gene as candidate vaccine strain. J. Vet. Med. Sci. 71: 1439–1448. doi: 10.1292/jvms.001439 [DOI] [PubMed] [Google Scholar]
- 28.Turtinen L. W., Allen G. P.1982. Identification of the envelope surface glycoproteins of equine herpesvirus type 1. J. Gen. Virol. 63: 481–485. doi: 10.1099/0022-1317-63-2-481 [DOI] [PubMed] [Google Scholar]
- 29.Whittaker G. R., Wheldon L. A., Giles L. E., Stocks J. M., Halliburton I. W., Killington R. A., Meredith D. M.1990. Characterization of the high Mr glycoprotein (gP300) of equine herpesvirus type 1 as a novel glycoprotein with extensive O-linked carbohydrate. J. Gen. Virol. 71: 2407–2416. doi: 10.1099/0022-1317-71-10-2407 [DOI] [PubMed] [Google Scholar]