ABSTRACT
The intestinal helminth fauna of Tibetan sand foxes (Vulpes ferrilata) and red foxes (Vulpes vulpes) inhabiting in Qinghai, China, was evaluated by conducting necropsy of hunted foxes and fecal egg examination of field-collected feces. In northeast and south Qinghai, 36 foxes were necropsied, and the species of foxes and the parasites detected were identified by the DNA barcoding. In 27 red foxes and 9 Tibetan sand foxes examined, Mesocestoides litteratus (total prevalence: 64%), Toxascaris leonina (50%), Taenia pisiformis (8%) and Taenia crassiceps (8%) were found in both species of foxes. Echinococcus shiquicus (8%) and Taenia multiceps (6%) were found only in Tibetan sand foxes. Echinococcus multilocularis (3%) and Alaria alata (8%) were found only in red foxes. In the fecal egg examination of the rectal feces, 100% of taeniid cestodes, 73% of Toxascaris and 27% of Mesocestoides worm-positive samples showed egg-positive, indicating that coprological survey for parasite eggs could only provide partial information of intestinal parasite fauna. For field-collected feces, molecular identification of feces origins and fecal egg examination were performed. In 15 Tibetan sand fox and 30 red fox feces, we found E. multilocularis eggs in one feces of Tibetan sand fox. The present study indicated that the upper intestinal helminth fauna of the two fox species in Qinghai does not differ significantly and both species would play an important role in the maintenance of taeniid cestodes.
Keywords: China, helminth fauna, Qinghai, red foxes, Tibetan sand foxes
Qinghai province in China locating at the Qinghai-Tibetan plateau has the unique ecosystem formed by high-altitude adapted animals and plants. Two fox species, the Tibetan sand fox (Vulpes ferrilata) and the red fox (Vulpes vulpes), inhabit in Qinghai. It is considered that the former species is a diurnal specialist that thrives with pikas exclusively and the latter species is a nocturnal generalist that can thrive with a wide variety of foods [3, 7, 15, 19]. Although some general information is available, local ecology of the two fox species in this area, such as local distribution, habitat segregation, feeding habitat and its interaction, etc. has not been investigated in detail.
On the other hand, Qinghai is known to be one of the most endemic regions of echinococcosis in the world [4]. In this area, 3 species of Echinococcus, E. granulosus, E. multilocularis and E. shiquicus, are co-distributed [18, 20]. Domestic and wild canine animals, as the definitive host, play an important role in the maintenance of these Echinococcus species. Red foxes and Tibetan sand foxes are the main definitive host of E. multilocularis and E. shiquicus, respectively. For E. granulosus, domestic dogs are the main definitive host, however, foxes are possibly involved in the maintenance [16].
Several studies have been conducted to evaluate the prevalence of Echinococcus spp. in foxes in Qinghai. Post-mortem examination of hunted foxes was conducted in two studies: one study that necropsied 23 foxes hunted in southeast Qinghai performed fox and Echinococcus species identification [2], however, the other study that necropsied 149 foxes hunted in several regions of Qinghai did not identify fox and Echinococcus species, and thus, the information is less valuable [23]. Alternatively, test methods for coproantigen and copro-DNA detection in field-collected fox feces were developed as a non-invasive ecological method [5, 14, 17], and studies were conducted using such methods in southeast Qinghai [2, 23]. Unfortunately, all of the studies just focused on Echinococcus infection and were luck of the infection status of the other parasites in the two fox species.
This study was conducted to evaluate the intestinal helminth fauna of Tibetan sand foxes and red foxes inhabiting in northeast and south Qinghai by conducting necropsy of hunted foxes and fecal egg examination of field-collected feces. The validity of fecal egg examination for the investigation of intestinal helminths was evaluated by comparing the result of necropsy and egg examination of rectal feces. The grasp of the parasite fauna would be useful information not only to understand the biodiversity in the Qinghai-Tibetan plateau, but also to understand the long term feeding habitat of the two fox species that can be suspected from the intermediate hosts of the infected parasites. Furthermore, the grasp of infection status of zoonotic parasites, such as Echinococcus in the two fox species, would be necessary to design effective control measures.
MATERIALS AND METHODS
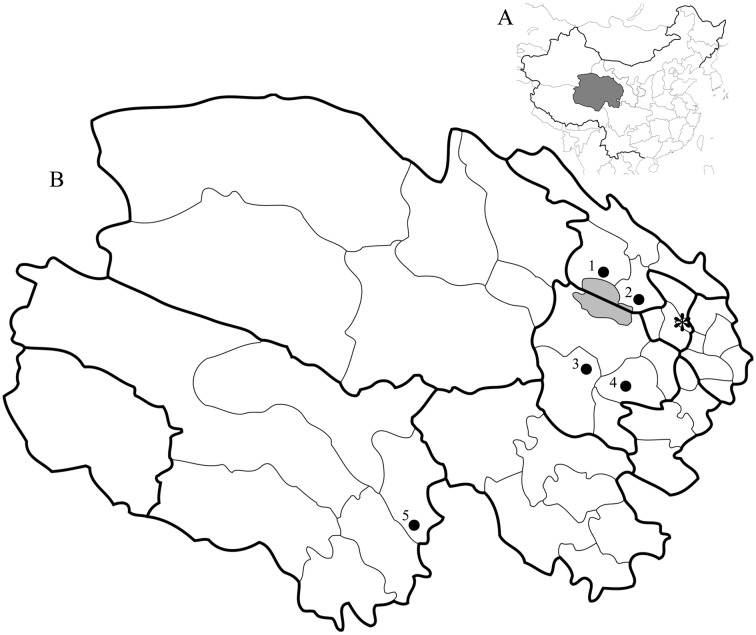
Study sites: This study was conducted in 5 counties located at northeast and south Qinghai, China (Fig. 1). In all sites, Tibetan and other minority ethnic groups conduct nomadism of yak and sheep in a vast expanse of grassland. The average altitudes of the study sites are 3,000–3,800 m in Haiyan, Gangcha, Guinan and Xinghai counties and 4,500 m in Chengduo county.
Fig. 1.
Study sites. A: Map of China with Qinghai province marked as dark area. B: Map of Qinghai province. Dark area in Map B indicates the Qinghai Lake. Bold line represents the district border, and thin line represents the county border. 1: Gangcha county, 2: Haiyan county, 3: Xinghai county (Heka town), 4: Guinan county, 5: Chengduo county, *: Xining (the provincial capital).
Hunting and dissection of foxes and parasite examination: Under the permission of the ministry of forestry, China for academic investigation on wild foxes, we asked local hunters to hunt foxes in Haiyan, Gangcha, Guinan and Chengduo counties in December 2010 to April 2011 and December 2011 to February 2012. Foxes were stored at −80°C for at least 10 days to inactivate Echinococcus eggs. Then, they were dissected and examined for their small intestinal helminths. The detected parasites were counted and stored in 70% ethanol for further examination. In addition, rectal feces were collected and stored at −20°C for fecal egg examination.
Collection of fox feces in the field: Fox feces were collected at grassland within 100 km apart from Heka town in Xinghai county in September 2010, August 2011 and August 2012. Collected feces were stored at −80°C for at least 10 days to inactivate Echinococcus eggs and then stored at −20°C until use.
Molecular identification of fox species by DNA barcording: A part of the liver and muscle of the dissected foxes were collected and stored in 70% ethanol. Then, DNA was extracted from the liver or muscle using QIAamp DNA Mini Kit (Qiagen, Tokyo, Japan). For the field-collected feces, fox rectum-derived cells distributed on the surface of feces were collected by washing the frozen feces with ASL buffer (QIAamp DNA Stool Mini Kit, Qiagen) following the method reported [12]. Then, DNA was extracted from the washing of frozen feces using QIAamp DNA Stool Mini Kit (Qiagen). On both DNA, PCR for the partial sequence of the D-loop region was performed with primers, prL (5’-CACCATTAGCACCCAAAGCT-3’) and prH (5’-CCTGAAGTAGGAACCAGATG-3’) as described previously [12], and DNA sequences of PCR products were read with a DNA sequencer (Model 3100, Applied Biosystems of Life Technologies Corporation, Tokyo, Japan) using Big-Dye terminator cycle sequencing kit v3.1 (Applied Biosystems of Life Technologies Corporation). Sequences obtained were applied to BLAST similarity search (Basic Local Alignment Search Tool) for species identification.
Fecal egg examination: Fecal egg examination was performed on 0.5 to 1.0 g of rectal feces or field-collected feces by the centrifugal sucrose flotation technique. When taeniid eggs were detected from the field-collected feces, the eggs were collected under a stereomicroscope for the molecular identification of their species.
Molecular identification of detected parasites: DNA was extracted either from the worms detected in the intestine or from the eggs detected in feces. For cestodes and trematodes, PCR for the partial sequence of mitochondrial cytochrome oxidase c subunit 1 (CO1) gene was performed with primers, 2575 (5’-TTTTTTGGGCATCCTGAGGTTTAT-3’) and 3021 (5’-TAAAGAAAGAACATAATGAAAATG-3’) following the method reported [1]. For nematodes, PCR for the partial sequence of internal transcribed spacer 2 (ITS2) region was performed with primers, LC1 (5’-CGAGTATCGATGAAGAACGCAGC-3’) and HC2 (5’-ATATGCTTAAGTTCAGCGGG-3’) following the method reported [10]. DNA sequences of PCR products were read as described above.
Statistics: The difference in the prevalence was statistically evaluated by the Fisher’s exact test and the Fisher-Freeman-Halton test (an extension of Fisher’s exact test from 2 × 2 tables to general row by column tables) using statistical software R [13]. P<0.05 was considered as significant.
RESULTS
Necropsy: A total of 36 foxes (27 red foxes and 9 Tibetan sand foxes) were examined. Both species were obtained in Haiyan and Gangcha counties, although more red foxes were obtained than Tibetan sand foxes. Only red foxes were obtained in Guinan county and only Tibetan sand foxes in Chengduo county.
From 32 foxes (89%), 8 different species of intestinal helminths were detected (Table 1). All sequences of the PCR products obtained from the parasite DNA were completely identical to those of the representative species registered in GenBank. There was no significant difference in the prevalence of intestinal helminths between two fox species (Fisher-Freeman-Halton test). Mesocestoides litteratus was most abundant and found in both fox species (overall prevalence in red foxes: 59% and that in Tibetan sand foxes: 78%). All of the Tibetan sand foxes obtained in Chengduo county (n=6) were infected with this species. In addition, Toxascaris leonina, Taenia pisiformis and Taenia crassiceps were found in both fox species with overall prevalence of 44, 7 and 4% in red foxes and 67, 11 and 11% in Tibetan sand foxes, respectively. Echinococcus multilocularis (3%) and Alaria alata (8%) were found only in red foxes, while Echinococcus shiquicus (8%) and Taenia multiceps (6%) were found only in Tibetan sand foxes.
Table 1. Fox species necropsied and their intestinal helminths at four counties in Qinghai province.
| County | Fox speciesa) | No. necropsied | No. foxes harboring parasite
speciesa) (range of No. parasites per fox) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mes. lit. | Tox. leo. | Ala. ala. | Ech. mul. | Ech. shi. | Tae. cra. | Tae. mul. | Tae. pis. | |||
| Haiyan | Vul. vul. | 19 | 11 (3-1,430) | 8 (2-20) | 3 (17-52) | 1 (116) | 0 | 2 (1-8) | 0 | 1 (1) |
| Vul. fer. | 2 | 1(87) | 1(6) | 0 | 0 | 0 | 1 (3) | 0 | 0 | |
| Gangcha | Vul. vul. | 4 | 1(4) | 2 (12-179) | 0 | 0 | 0 | 0 | 0 | 1 (3) |
| Vul. fer. | 1 | 0 | 1 (12) | 0 | 0 | 0 | 0 | 1(3) | 0 | |
| Guinan | Vul. vul. | 4 | 4 (4-654) | 2 (3-15) | 0 | 0 | 0 | 0 | 0 | 0 |
| Vul. fer. | 0 | NA | NA | NA | NA | NA | NA | NA | NA | |
| Chengduo | Vul. vul. | 0 | NA | NA | NA | NA | NA | NA | NA | NA |
| Vul. fer. | 6 | 6 (19-96) | 4 (4-34) | 0 | 0 | 3 (833 – 1,640) | 0 | 1 (1) | 1 (2) | |
| Total | Vul. vul. | 27 | 16 (3-1,430) | 12 (2-179) | 3 (17-52) | 1 (116) | 0 | 2 (1-8) | 0 | 2 (1-3) |
| Vul. fer. | 9 | 7 (19-96) | 6 (4-34) | 0 | 0 | 3 (833 – 1,640) | 1 (3) | 2 (1-3) | 1 (2) | |
a) Vul. vul.: Vulpes vulpes, Vul. fer.: Vulpes ferrilata, Mes. lit.: Mesocestoides litteratus, Tox. leo.: Toxascaris leonina, Ala. ala.: Alaria alata, Ech. mul.: Echinococcus multilocularis, Ech. shi.: Echinococcus shiquicus, Tae. cra.: Taenia crassiceps, Tae. mul.: Taenia multiceps, Tae. pis.: Taenia pisiformis. NA: Not applicable.
Mixed infection with multiple parasite species was found in 12 red foxes (44%) and 7 Tibetan sand foxes (78%). Mixed infection with different taeniid cestodes was found in one red fox in which E. multilocularis and T. crassiceps were detected.
There was no significant sex difference in the overall prevalence of M. litteratus (male: 9 infected/15 examined vs. female: 7/12 in red foxes and 4/5 vs. 3/4 in Tibetan sand foxes) or T. leonine (8/15 vs. 6/12 in red foxes and 3/5 vs. 3/4 in Tibetan sand foxes) in either species (Fisher’s exact test). Sex difference in the prevalence at each site or of other parasite infection was not evaluated due to the small sample size or the small number of infected animals.
Rectal feces examination: Rectal feces were able to be collected from 23 foxes out of 36 examined. The result of the fecal egg examination is shown in Table 2. When the result was compared with that of the necropsy, the specific parasite eggs could be detected in 100, 73 and 27% of samples from taeniid cestodes-, Toxascaris- and Mesocestoides-detected foxes, respectively.
Table 2. Comparison of worm-detection at necropsy and egg-detection in fecal egg examination.
| Examination | No. positive samples for parasite
speciesa) in each examination |
|||||||
|---|---|---|---|---|---|---|---|---|
| Mes. lit. | Tox. leo. | Ala. ala. | Ech. mul. | Ech. shi. | Tae. cra. | Tae. mul. | Tae. pis. | |
| Necropsy | 15 | 11 | 2 | –b) | 1 | 1 | 2 | 2 |
| Fecal egg examination | 4 | 8 | 0 | – | 1c) | 1c) | 2c) | 2c) |
a) Mes. lit.: Mesocestoides litteratus, Tox. leo.: Toxascaris leonina, Ala. ala.: Alaria alata, Ech. mul.: Echinococcus multilocularis, Ech. shi.: Echinococcus shiquicus, Tae. cra.: Taenia crassiceps, Tae. mul.: Taenia multiceps, Tae. pis.: Taenia pisiformis. b) Examination was not done, because no samples were obtained from foxes infected with the parasite species. c) Detected as morphologically species-indistinguishable taeniid eggs.
Field-collected feces examination: In the study site, 70 feces were collected. Feces origins (fox species) could be identified in 45 samples: 30 were of red foxes, and 15 were of Tibetan sand foxes. By the fecal egg examination, E. multilocularis eggs were detected in one feces of Tibetan sand fox, however, eggs of other parasites were not detected.
DISCUSSION
As reported previously [19], two fox species, the Tibetan sand fox and the red fox, were found in the study sites. Although ecological characteristics of the two fox species are different, they shared the dominant parasite species, such as M. litteratus and T. leonina, and the statistical difference in parasite fauna of the small intestine was not elucidated in this study.
The most dominant species in the two fox species was M. litteratus. Although the lifecycle of the genus Mesocestoides has not been clarified in detail, this could indicate that both species of foxes intend to ingest the second intermediate host or possibly paratenic host of this parasite, such as birds, reptiles and small mammals [6,7,8]. It has not been cleared, if the parasite can infect to pikas that the Tibetan sand fox as specialist thrives on. Similarly, the finding of T. pisiformis, T. crassiceps and T. multiceps in Tibetan sand foxes may indicate that the fox species ingests hares, voles and sheep head as well, although the frequency of such feeding was supposed to be fewer than feeding on pika [7, 15]. It is known that Tibetan nomads do not eat sheep head, and thus, ingestion of sheep head thrown away may explain the infection of T. multiceps rather than hunting of sheep. On the other hand, other parasite species detected in red foxes indicate that the red foxes ingested frogs, voles and hares [15].
Five species of taeniid cestodes were detected in the foxes examined. Taenia crassiceps and T. pisiformis were found in both species at necropsy. Echinococcus multilocularis was found in a red fox at necropsy and its eggs were found in a feces of Tibetan sand fox, and thus, the parasite species could infect to both fox species. Infections with E. multilocularis in Tibetan sand foxes and in pikas were reported in the previous studies [2, 18], and thus, this parasite could be maintained efficiently by Tibetan sand fox and pika cycle as well as by red fox and vole cycle. In contrast, E. shiquicus was found exclusively in Tibetan sand foxes both in this study and in the previous studies [5]. Although the number of foxes examined was limited and more foxes should be examined to clarify, the red fox may not be a susceptible host for this parasite. On the other hand, as observed in other studies [2, 23], E. granulosus was not detected in either fox species in this study. Nevertheless, finding Taenia and Echinococcus species in the foxes would provide us a warning that surveys on field-collected feces with egg examination conducted in this area require a further method for the species discrimination of taeniid eggs.
Comparison of the results of necropsy and egg examination of rectal feces indicated that coprological survey for parasite eggs could provide valid but limited information of parasite fauna. Taeniid eggs were detected in 100% of foxes infected with taeniid cestodes. However, the observation could be a rare case, and an actual detection rate is considered to become lower because the previous studies showed that the detection rate of taeniid eggs from the rectal feces of red foxes infected with E. multilocularis was less than 50% [9, 21]. On the other hand, other parasite eggs, especially those of Mesocestoides, were detected in only part of the infected foxes. Mesocestoides eggs have thin eggshell and are stored and protected in a sac called paruterine organ of a gravid segment. Ignorance of detecting segment itself in the egg examination may explain the low rate of detection of the parasite eggs.
Various surveys on parasites examining animal feces have been conducted elsewhere [11, 14, 17]. Previous studies had some difficulty in identification of origins of feces collected, however, molecular identification methods for feces origins have been developed recently and applied to field surveys [5, 12]. In those studies, examination of parasites was conducted by detection of parasite eggs, coproantigen or copro-DNA [2, 17, 22]. However, the latter two detection methods are best applicable to surveys on the specific parasite, such as Echinococcus spp., and are not suitable for surveys on parasite fauna. In this study, we performed egg examination on field-collected feces in a town of Qinghai and found one positive sample that contained E. multilocularis eggs. Considering the above result, the observation indicated that the prevalence of intestinal parasites of foxes in the study site was quite low. For additional information, infection with trematodes, such as Alaria alata, could not be evaluated in this study, because fecal egg examination was performed by a floatation technique.
In conclusion, the present study revealed that the upper intestinal helminth fauna of the Tibetan sand fox and the red fox distributing in Qinghai is overlapped each other, and both fox species play an important role in the maintenance of taeniid cestodes. Therefore, the role of the foxes should be taken into consideration when countermeasures against echinococcosis or taeniosis (cysticercosis) are taken place.
ACKNOWLEDGMENTS
We are grateful to the staff of the Laboratory of Veterinary Parasitic Diseases, Department of Veterinary Sciences, Faculty of Agriculture, University of Miyazaki and the staff of Academy of Animal and Veterinary Medicine, University of Qinghai for their valuable support. This work was supported by the Strategic Japanese-Chinese Cooperative Program of Japan Science and Technology Agency (JST), and by the Integrated Research Project for Human and Veterinary Medicine in University of Miyazaki funded by the Ministry of Education, Culture, Sports, Science and Technology in Japan.
REFERENCES
- 1.Guo Z. H., Kubo M., Kudo M., Nibe K., Horii Y., Nonaka N.2011. Growth and genotypes of Echinococcus granulosus found in cattle imported from Australia and fattened in Japan. Parasitol. Int. 60: 498–502. doi: 10.1016/j.parint.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 2.Han X., Wang H., Qiu J., Ma X., Cai H., Liu P., Ding Q., Dai N., Ito A., Craig P. S.2006. Epidemiological study on Echinococcus multilocularis and E. granulosus in Banma county of Qinghai province. Chin. J. Zoonoses 22: 189–190 (in Chinese). [Google Scholar]
- 3.Harris R. B., Wang Z. H., Zou J. K., Liu Q. X.2008. Notes on biology of the Tibetan fox (Vulpes ferrilata). Canid News 11: 1–7 [Google Scholar]
- 4.Jenkins D. J., Romig T., Thompson R. C. A.2005. Emergence/re-emergence of Echinococcus spp.—a global update. Int. J. Parasitol. 35: 1205–1219. doi: 10.1016/j.ijpara.2005.07.014 [DOI] [PubMed] [Google Scholar]
- 5.Jiang W., Liu N., Zhang G., Renqing P., Xie F., Li T., Wang Z., Wang X.2012. Specific detection of Echinococcus spp. from the Tibetan fox (Vulpes ferrilata) and the red fox (V. vulpes) using copro-DNA PCR analysis. Parasitol. Res. 111: 1531–1539. doi: 10.1007/s00436-012-2993-8 [DOI] [PubMed] [Google Scholar]
- 6.Kugi G.1973. Studies on life-history of Mesocestoides (2). On tetrathyridium of Mesocestoides litteratus found from Japanese pheasant and copper pheasant. J. Vet. Med. 586: 265–267 (JVM). [Google Scholar]
- 7.Liu Q., Harris R. B., Wang X.2010. Food habits of the Tibetan fox (Vulpes ferrilata) in the Kunlun Mountains, Qinghai Province, China. Mamm. Biol. 75: 283–286 [Google Scholar]
- 8.Martinez-Carrasco C., Ruiz de Ybanez M. R., Sagarminaga J. L., Garijo M. M., Moreno F., Acosta I., Hernandez S., Alonso F. D.2007. Parasites of the red fox (Vulpes vulpes Linnaeus, 1758) in Murchia, southeast Spain. Revue Med. Vet. 158: 331–335 [Google Scholar]
- 9.Morishima Y., Tsukada H., Nonaka N., Oku Y., Kamiya M.1999. Evaluation of coproantigen diagnosis for natural Echinococcus multilocularis infection in red foxes. Jpn. J. Vet. Res. 46: 185–189 [PubMed] [Google Scholar]
- 10.Navajas M., Cotton D., Kreiter S., Gutierrez J.1992. Molecular approach in spider mites (Acari: Tetranychidae): preliminary data on ribosomal DNA sequences. Exp. Appl. Acarol. 15: 211–218. doi: 10.1007/BF01246563 [DOI] [PubMed] [Google Scholar]
- 11.Nonaka N., Nakamura S., Inoue T., Oku Y., Katakura K., Matsumoto J., Mathis A., Chembesofu M., Phiri I. G. K.2011. Coprological survey of alimentary tract parasites in dogs from Zambia and evaluation of a coproantigen assay for canine echinococcosis. Ann. Trop. Med. Parasitol. 105: 521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nonaka N., Sano T., Inoue T., Arumua M. T., Fukui D., Katakura K., Oku Y.2009. Multiplex PCR system for identifying the carnivore origins of faeces for an epidemiological study on Echinococcus multilocularis in Hokkaido, Japan. Parasitol. Res. 106: 75–83. doi: 10.1007/s00436-009-1629-0 [DOI] [PubMed] [Google Scholar]
- 13.R Development Core Team. 2012. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna (cited 2013 April 2). Available from http://www.R-project.org/
- 14.Raoul F., Deplazes P., Nonaka N., Piarroux R., Vuitton D. A., Giraudoux P.2001. Assessment of the epidemiological status of Echinococcus mulitlocularis in foxes in France using ELISA coprotests on fox faeces collected in the field. Int. J. Parasitol. 31: 1579–1588. doi: 10.1016/S0020-7519(01)00280-6 [DOI] [PubMed] [Google Scholar]
- 15.Schaller G. B.1998. Wildlife of the Tibetan Steppe, University Chicago Press, Chicago. [Google Scholar]
- 16.Thompson R. C. A., McManus D. F.2001. Aetiology: parasistes and life-cycle. pp. 1–16. In: WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern (Eckert, J., Gemmell, M. A., Meslin, F. X. and Pawlowski, Z. S. eds.), World Organization for Animal Health, Paris. [Google Scholar]
- 17.Tsukada H., Hamazaki K., Ganzorig S., Iwaki T., Konno K., Lagapa J. T., Matsuo K., Ono A., Shimizu M., Sakai H., Morishima Y., Nonaka N., Oku Y., Kamiya M.2002. Potential remedy against Echinococcus multilocularis in wild red foxes using baits with anthelmintic distributed around fox breeding dens in Hokkaido, Japan. Parasitology 125: 119–129. doi: 10.1017/S0031182002001968 [DOI] [PubMed] [Google Scholar]
- 18.Wang Z., Wang X., Liu X.2008. Echinococcosis in China, a review of the epidemiology of Echinococcus spp. EcoHealth 5: 115–126. doi: 10.1007/s10393-008-0174-0 [DOI] [PubMed] [Google Scholar]
- 19.Wozencraft W. C.2008. Family Canidae. pp. 416–422. In: A Guide to the Mammals of China (Smith, A. T. and Xie, Y. eds.), Princeton University Press, Princeton. [Google Scholar]
- 20.Xiao N., Qiu J., Nakao M., Li T., Yang W., Chen X., Schantz P. M., Craig P. S., Ito A.2005. Echinococcus shiquicus n. sp., a taeniid cestode from Tibetan fox and plateau pika in China. Int. J. Parasitol. 35: 693–701. doi: 10.1016/j.ijpara.2005.01.003 [DOI] [PubMed] [Google Scholar]
- 21.Yimam A. E., Nonaka N., Oku Y., Kamiya M.2002. Prevalence and intensity of Echinococcus multilocularis in red foxes (Vulpes vulpes schrencki) and raccoon dogs (Nyctereutes procyonoides albus) in Otaru city, Hokkaido, Japan. Jpn. J. Vet. Res. 49: 287–296 [PubMed] [Google Scholar]
- 22.Yu S. H., Wang H., Wu X. H., Ma X., Liu P. Y., Liu Y. F., Zhao Y. M., Morishima Y., Kawanaka M.2008. Cystic and alveolar echinococcosis: an epidemiological survey in a Tibetan population in Southeast Qinghai, China. Jpn. J. Infect. Dis. 61: 242–246 [PubMed] [Google Scholar]
- 23.Zhang J. X., Wang H.2007. Epidemiological survey on Echinococcus infection in animals in Qinghai Province. Chin. J. Parasitic Dis. 25: 350–352 (in Chinese with English abstract). [PubMed] [Google Scholar]