ABSTRACT
We report here the clinical presentation, magnetic resonance imaging (MRI) findings and successful surgical management associated with triceps tendon avulsion in a dog. A definitive diagnosis of triceps tendon avulsion was made based on MRI with evidence of displacement of the triceps tendon. Surgical correction of triceps tendon avulsion was performed with two horizontal mattress sutures using polyester and two tunnels drilled in the olecranon to reattach the tendon to the proximal olecranon. At 9 months, there was no evidence of lameness on the left thoracic limb. This is the first case report to describe MRI evaluation for the diagnosis of the triceps tendon avulsion.
Keywords: canine, MRI, surgical correction, triceps tendon avulsion
Rupture or avulsion of the triceps tendon is a rarely reported tendinous injury in the dog and cat. Traumatic lacerations and avulsions are usually associated with tendon injuries [4, 7]. In humans, triceps tendon rupture represents less than 1% of all upper extremity tendon injuries [11]. To the authors’ knowledge, rupture or avulsion of the triceps tendon in the dog or cat has been described in only few case reports [1, 3, 7, 8]. The purpose of this case report is to describe the clinical presentation, magnetic resonance imaging (MRI) findings and successful surgical management associated with avulsion of triceps tendon in a dog. This is the first case report to describe MRI evaluation for the diagnosis of the triceps tendon avulsion.
A 3 kg, 3-year-old, intact female Pomeranian dog presented a sudden lameness after jumping off furniture in the left thoracic limb during two months. The lameness was not resolved with a 2-month course of a combination of non-steroidal anti-inflammatory drugs and Robert Jones bandage. Six weeks prior to presentation, the dog showed non-weight bearing. On physical examination, the dog showed a non-weight-bearing lameness of the left thoracic limb. Palpation of the left elbow and the distal humeral region revealed a painful, swollen elbow joint and a limited range of motion in the elbow joint with a firm mass proximal to the olecranon (Fig. 1). Atrophy of all heads of the triceps muscle was obvious. Neurological function was normal. Routine blood examination consisting of hemogram and serum biochemistry was unremarkable. Radiographic evaluation revealed swelling on the left elbow joint and a fleck sign on the left olecranon region of the insertion of the triceps tendon (Fig. 2). The dog was premedicated for magnetic resonance imaging (MRI) with midazolam (Vascam inj, Hana Pharm. Co., Ltd., Seoul, Korea) and butorphanol (Butorphan®, Myungmoon Pharm. Co., Ltd., Seoul, Korea), followed by anesthetic induction with propofol (Provive 1%®, Myungmoon Pharm. Co., Ltd.). The dog was intubated, and anesthesia was maintained with isoflurane (Isoflurane®; Choongwae. Co., Ltd., Seoul, Korea) and oxygen. Preoperative elbow MRI was performed on a 0.2-T (Vet-MR, Esaote, Florence, Italy) scanner using a dual phased array coil in the prone position with neutral posture. Imaging included sagittal T1-weighted sequences, sagittal fat-suppressed T1-weighted sequences and sagittal contrast-enhanced T1-weighted sequences. T1-weighted sequences were obtained with a spin-echo technique using 933/26 (TR range/TE range). Fat-suppressed T1-weighted sequences were obtained with a gradient echo technique using a 90° flip angle, 1,000/25 (TR range/TE range) and 75 inversion time. Sagittal T1-weighted images revealed complete rupture of the triceps tendon and olecranon bursitis that were characterized by the retracted distal triceps tendon from the olecranon and a large amount of edema in the adjacent subcutaneous tissue, respectively (Fig. 3). There was an evidence of high signal intensity around the elbow joint on sagittal fat-suppressed T1-weighted images (Fig. 3). A diagnosis of traumatic triceps tendon avulsion was made.
Fig. 1.
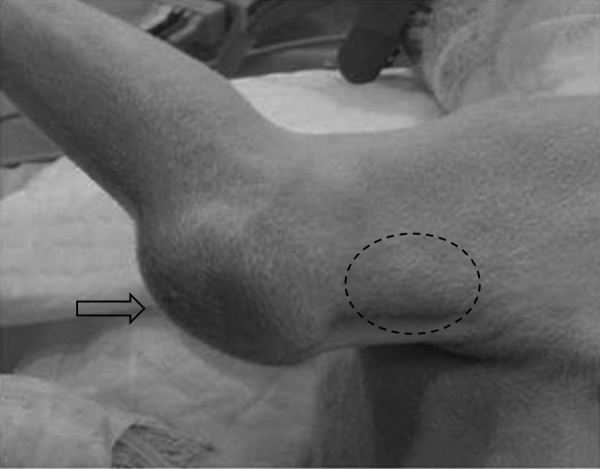
Preoperative findings of the left distal humeral region and proximal olecranon. A firm mass (dotted line) proximal to the olecranon and swollen elbow joint (arrow) are identified.
Fig. 2.
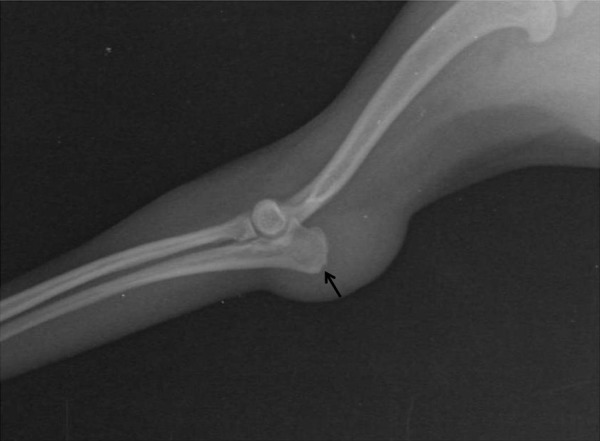
Mediolateral radiographic views of the left elbow joint. Note swelling on the elbow joint and a fleck sign (arrow) on the olecranon region of the insertion of the triceps tendon.
Fig. 3.
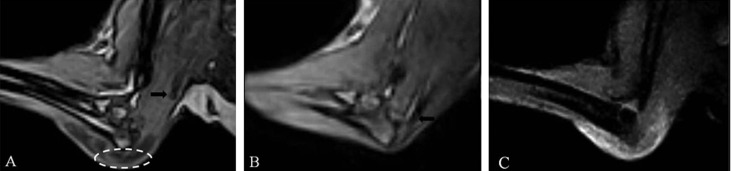
Preoperative magnetic resonance images of the left (A, C) and right (B) elbow joints. Sagittal T1-weighted imaging reveals olecranon bursitis (dotted line) and displacement of the triceps tendon (arrow) (A). Note normal insertion of the triceps tendon (arrow) into the proximal olecranon on sagittal T1-weighted imaging (B). There is an evidence of high signal intensity around the elbow joint on sagittal fat-suppressed T1-weighted image (C).
The elbow was approached surgically through a caudolateral skin incision to expose the triceps tendon and proximal olecranon. Careful blunt dissection of subcutaneous tissues revealed fibrous sac filled with serous fluid. Careful removal of the fibrous sac and serous fluid allowed visualization of the avulsed tendon end and proximal olecranon (Fig. 4a). The tendon ends were debrided using dry gauze. Two mediolateral holes with 1 mm diameter were drilled in the olecranon with a K-wire. Two horizontal mattress sutures using polyester (Ethibond Excel, Johnson & Johnson, Somerville, MA, U.S.A.) were placed through the triceps tendon and two tunnels in the olecranon, respectively, to reattach the tendon to the proximal olecranon (Fig. 4b). Additionally, several simple interrupted sutures using polyglyconate (Maxon, Covidien, Mansfield, MA, U.S.A.) were placed between the tendon and the remaining tissues on the olecranon. Subcutaneous tissue and skin closure was routine.
Fig. 4.
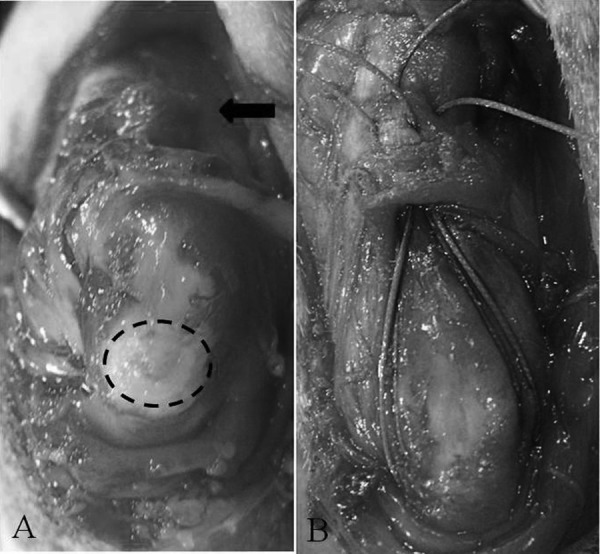
Intraoperative views of the left elbow joint. Careful removal of the fibrous sac and serous fluid allows visualization of the avulsed tendon end (arrow) and proximal olecranon (dotted line) (A). Two horizontal mattress sutures using polyester are placed through the triceps tendon and two tunnels drilled in the olecranon to reattach the tendon to the proximal olecranon (B).
The limb was supported in a spica splint for 4 weeks. After splint removal, the amount of exercise was gradually increased with leash walks for another two weeks. Muscle atrophy still was present; however, palpation of the left elbow joint revealed no pain and a good range of motion. Normal limb function was regained within 7 weeks postoperatively. At 9 months, no evidence of lameness was noted on the left thoracic limb.
Previous case reports with triceps tendon avulsion describe trauma associated with a fall, dog bite or automobile accident or local corticosteroid injection as the cause of tendon injury [1, 3, 7, 8]. This trauma can be associated with a partial or complete tendon rupture that will cause various grades of lameness. Weight bearing lameness and non-weight bearing may be suggestive of a partial and complete rupture, respectively [7]. In the present report, progression of the clinical symptoms from the persistent limping to non-weight bearing lameness suggested that the rupture of the triceps tendon might develop from a partial injury to complete avulsion in six weeks prior to presentation.
Clinical signs associated with triceps tendon avulsion involve affected limb lameness, pain, swelling, a limited range of motion in the elbow joint, triceps muscle atrophy and a firm mass proximal to the olecranon [1, 8]. Radiographic finding includes calcific opacity in the area of the triceps tendon [5]. In previous case reports with triceps tendon avulsion, tentative diagnosis was based on clinical signs and radiographic findings, followed by surgical exploration [1, 3, 7, 8]. A firm mass proximal to the olecranon and calcific opacity in the area of the triceps tendon evident on physical examination and radiographic evaluation respectively should be necessary for the diagnosis of triceps tendon avulsion [1, 6]. In the present report, however, no evidence of calcific opacity in the area of the triceps tendon was identified on radiographs and MRI. A fleck sign was identified on the region of the insertion of the triceps tendon; however, bone fragment was not found proximal to the olecranon. In previous case reports with triceps tendon avulsion, calcific opacity was evident on radiographs obtained in relatively short term (less than 4 weeks) after traumatic incident [7, 8]. Magnetic resonance imaging may be a helpful diagnostic tool to the cases, which have not presented these suggestive physical and imaging evidences to diagnose olecranon bursitis and displacement of the triceps tendon.
There are a few surgical techniques described in the literature related to the reattachment of the triceps tendon to the olecranon [1, 2, 7, 8, 10]. Presently available options include a tension band fixation, horizontal mattress suture with drilled holes in the proximal olecranon, a modified three-loop pulley suture and a Bunnell-Mayer suture [1, 2, 7, 8, 10]. Tension band fixation has been described for the management of a cat with an avulsion fracture of the proximal ulnar epiphysis [7]. Horizontal mattress suture technique has been described for the management of a 20 kg dog with a complete avulsion of the triceps tendon [7]. In this technique, nonabsorbable sutures were placed through the tendon and holes drilled in the proximal olecranon for the reattachment of the triceps tendon to the proximal olecranon. A modified three-loop pulley suture technique has been described for the management of a cat with a rupture of the triceps tendon [8]. In this technique, three-loop suture was placed through the triceps tendon proximally and anchored through the tunnel in the olecranon distally with additional horizontal mattress sutures between the tendon and the remaining tissues on the olecranon. A Bunnell-Mayer suture technique has been described for the management of a 10 kg dog with a complete avulsion of the triceps tendon [1]. In this technique, two holes were drilled in the olecranon from proximally to distally, and a Bunnell-Mayer suture pattern was placed in the distal tendon and threaded through the holes in the olecranon. In the present report, modified horizontal mattress suture technique was performed. Two horizontal mattress sutures with two drilled holes in the proximal olecranon provided adequate strength to maintain reattachment of the triceps tendon to the proximal olecranon.
The triceps brachii muscle consists of four heads (long, lateral, medial and accessory heads) with a common insertional tendon to the olecranon in dogs; however, in human, the medial head of triceps has a separate insertion that is positioned deep to the common tendon of the lateral and long heads [5, 9]. In human, MRI has been described for determining the affected tendon and preoperative planning, since it shows whether rupture is complete or partial [11]. If the injury is partial, the patient can be treated non-operatively with splint protection for about 4 weeks at 30° flexion [6]. In the present MRI, it was only considered whether the rupture was complete or partial, since dogs have one insertional common tendon to the olecranon. Therefore, the preoperative applications of MRI to the affected canine cases will provide the effective information for whether these cases should be treated by various types of the surgical techniques.
REFERENCES
- 1.Anson L. W., Betts C. W.1989. Triceps tendon avulsion in a dog: surgical management and xeroradiographic evaluation. J. Am. Anim. Hosp. Assoc. 25: 655–658 [Google Scholar]
- 2.Berg R. J., Egger E. L.1986. In vitro comparison of the three loop pulley and locking loop suture patterns for repair of canine weightbearing tendons and collateral ligaments. Vet. Surg. 15: 107–110. doi: 10.1111/j.1532-950X.1986.tb00187.x [DOI] [Google Scholar]
- 3.Davies J. V., Clayton Jones D. G.1982. Triceps tendon rupture in the dog following corticosteroid injection. J. Small Anim. Pract. 23: 779–787. doi: 10.1111/j.1748-5827.1982.tb03505.x [DOI] [Google Scholar]
- 4.Dueland R., Quenin J.1980. Triceps tenotomy: biomechanical assessment of healing strength. J. Am. Anim. Hosp. Assoc. 16: 507–512 [Google Scholar]
- 5.Evans H. E.1993. Muscles of the thoracic limb. pp 321–349. In: Miller’s Anatomy of the Dog, 3rd ed. (Evans, H. E. ed.), WB Saunders, Philadelphia. [Google Scholar]
- 6.Farrar E. L., 3rd, Lippert F. G., 3rd1981. Avulsion of the triceps tendon. Clin. Orthop. 161: 242–246 [PubMed] [Google Scholar]
- 7.Gilmore D. R.1984. Triceps tendon avulsion in the dog and cat. J. Am. Anim. Hosp. Assoc. 20: 239–242 [Google Scholar]
- 8.Liehmann L., Lorinson D.2006. Traumatic triceps tendon avulsion in a cat. J. Small Anim. Pract. 47: 94–97. doi: 10.1111/j.1748-5827.2006.00037.x [DOI] [PubMed] [Google Scholar]
- 9.Madsen M., Marx R. G., Millett P. J., Rodeo S. A., Sperling J. W., Warren R. F.2006. Surgical anatomy of the triceps brachii tendon: anatomical study and clinical correlation. Am. J. Sports Med. 34: 1839–1843. doi: 10.1177/0363546506288752 [DOI] [PubMed] [Google Scholar]
- 10.Moores A. P., Comerford E. J., Tarlton J. F., Owen M. R.2004. Biomechanical and clinical evaluation of a modified 3-loop pulley suture pattern for reattachment of canine tendons to bone. Vet. Surg. 33: 391–397. doi: 10.1111/j.1532-950X.2004.04057.x [DOI] [PubMed] [Google Scholar]
- 11.Sharma S. C., Singh R., Goel T., Singh H.2005. Missed diagnosis of triceps tendon rupture: a case report and review of literature. J. Orthop. Surg. (Hong Kong) 13: 307–309 [DOI] [PubMed] [Google Scholar]


