ABSTRACT
We evaluated diurnal variation and hyperferritinemia as factors that influence the values of serum iron concentration in dogs, using the International Committee for Standardization in Hematology (ICSH) colorimetric method. Serum iron levels were significantly higher in the morning than in the evening in 6 clinically healthy beagle dogs, and the maximum decrease in serum iron concentration was 47.3%. Moreover, the change in serum iron concentrations in 22 clinical canine cases with various serum ferritin levels was evaluated by immunoprecipitation of ferritin. The rate of decline in the serum iron concentrations positively correlated with serum ferritin levels (r=0.48, P=0.024). These results show that it is necessary to consider the sampling time and serum ferritin level for accurate interpretation of serum iron concentrations in dogs.
Keywords: canine, diurnal variation, ferritin, serum iron
Serum iron concentration is measured in veterinary medicine to evaluate transport compartments of iron in blood. Iron is the essential metallic element for oxygen delivery to tissues and is a cofactor for several enzymes involved in DNA synthesis and energy metabolism [6]. It is absorbed from food in the small intestine, is released into the plasma and is bound to transferrin, a carrier protein for iron [6]. In normal serum, most iron exists as transferrin-binding iron (TBI); therefore, serum iron is commonly indicated as TBI.
The serum iron concentration is generally increased in animals with hemolytic anemia [7], dyserythropoiesis [15], iron overload [14] and glucocorticoid administration [8]. Serum iron concentration is generally decreased in iron deficiency [12] and inflammation [3]. However, factors affecting the measurement of serum iron concentration in companion animals are not known. In human medicine, the prevailing opinion is that serum iron levels are higher in the morning than in the afternoon or evening [2]. Therefore, it is thought that for accurate evaluation of serum iron concentration, the sampling time should be considered.
On the other hand, ferritin, an iron storage protein, is known to be present in serum; it is increased in various diseases, such as neoplasms and inflammatory diseases, in humans and dogs [4, 20]. Ferritin-bound iron is a form of non-transferrin-bound iron (NTBI) in serum, and it has been reported to interfere in the measurement of serum iron concentrations when using the method of the International Committee for Standardization in Hematology (ICSH) [10]. Watanabe et al. reported that the iron/protein ratio of canine serum ferritin is 0.112 ± 0.017 [18], and Worwood et al. reported this ratio in four human patients with iron overload serum ferritin as 0.023–0.067 [21]. Therefore, canine serum ferritin is thought to bind more iron than that of humans, and thus, it can be expected that interference of canine serum ferritin in the measurement of serum iron concentration would be higher than in humans.
The purpose of this study was to examine the influence of diurnal changes and serum ferritin on serum iron concentration, in order to provide data for improving the reliability of canine serum iron measurement.
Healthy male (n=2) and female (n=3) beagle dogs (mean body weight, 9.0 kg) aged 2–6 years were used in this study (Crea Japan, Inc., Tokyo, Japan). The dogs were maintained in a temperature- and light-controlled environment and were given standard laboratory food, CD-5M (Crea Japan, Inc.), once a day (at 9:00 AM), with free access to water. Blood was drawn with iron-free disposable 5-ml syringes (TERUMO, Tokyo, Japan) from the jugular vein. It was collected at 8:00 AM, 12:00 AM, 4:00 PM and 8:00 PM on the same day. Samples were left at room temperature for 30 min and then were centrifuged (1,560 × g, 5 min) to separate the serum for laboratory analysis. The obtained serum samples were preserved at −20°C until usage.
Serum samples from 22 client-owned dogs with various values of serum ferritin concentration were non-randomly selected and evaluated in this study. The dogs visited Kitasato University Veterinary Teaching Hospital Small Animal Medical Center between 2010 and 2012. All dogs were diagnosed by a veterinarian through histopathological examination after surgical resection or autopsy, direct Coombs test, fine needle aspiration biopsy and/or other clinicopathological examinations. Serum samples from clinical cases were collected and preserved by the method described above. All dog owners provided written informed consent for participation in this study.
Both studies were approved by the Kitasato University Small Animal Committee (approval number: 13-090 and 13-091).
Serum iron concentrations were measured by the method on the ICSH, as described previously [9, 10]. The ICSH method is a reference method for serum iron measurement in humans [10, 17]. Briefly, serum iron was separated from its binding proteins, and ferric iron (Fe3+) was reduced to ferrous iron (Fe2+) by a mixed acid reagent containing 2 M HCl, 0.6 M trichloroacetic acid and 0.3 M thioglycolic acid and left for 30 min at room temperature. Then, it was centrifuged at 9,300 × g for 10 min. The free ferrous iron in the supernatant was then reacted with a ferrozine (monosodium 3-2-pyridyl-5,6-bis-4-phenylsulfonic acid-1,2,4-triazine), which is a chromogen, yielding a pink ferrous chromogen complex that could be quantitated spectrophotometrically using a U-5100 spectorophotometer (Hitachi High-Technologies Corporation, Tokyo, Japan) at 562 nm [16]. The iron standard solution (100.5 mg/l) used for atomic absorption spectrometry was of chemical analysis grade and was obtained from Kanto Chemical (Tokyo, Japan). Serum ferritin concentration was measured by sandwich enzyme-linked immunosorbent assay (ELISA) using purified rabbit anti-canine heart ferritin polyclonal antibody, as described in our previous report [1]. TIBC was measured by the 2-nitroso-5-(N-propyl-N-sulfopropylamino)-phenol (nitroso-PSAP) method at SRL Inc. (Tokyo, Japan).
Ferritin immunoprecipitation was performed by adding 5 µl (containing 7 µg of antibody) of purified rabbit anti-canine heart ferritin polyclonal antibody, which was used same antibody in ELISA [1], to 300 µl of canine serum. After incubation overnight at 4°C, ferritin absorbed in the serum supernatant was collected by centrifugation at 9,300 × g for 10 min, and the supernatant then measured as serum. We found that the serum ferritin concentration after immunoprecipitation of ferritin was less than 7.8 ng/ml, which is the minimum detection limit of this ELISA [1]. We measured serum iron concentrations before and after immunoprecipitation and calculated the rate of decline between the 2 values.
Serum iron, ferritin and TIBC concentrations were evaluated by repeated measures analysis of variance (ANOVA), and when this showed a significant difference, we used Bonferroni’s correction. Pearson’s correlation test was used to analyze the correlation between the serum ferritin concentration and rate of decline in the serum iron concentration after immunoprecipitation of ferritin. Values were considered statistically significant when the p-value was less than 0.05. All data were analyzed using statistical software in Excel (ystat 2008; Igaku Tosho Shuppan, Tokyo, Japan).
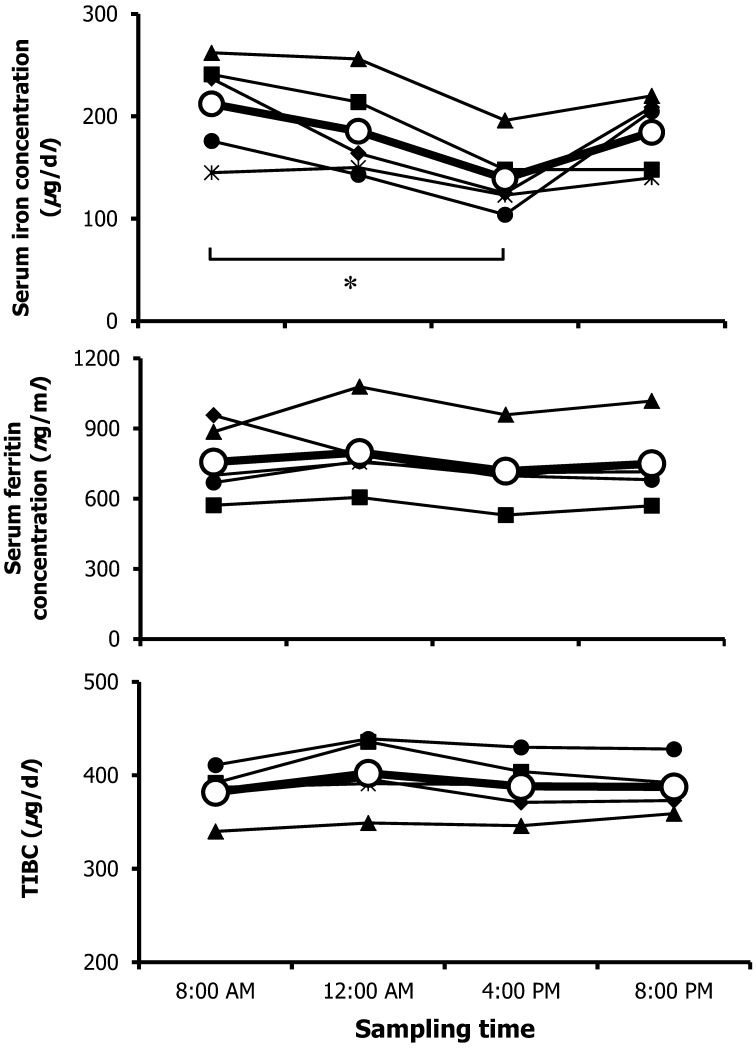
To evaluate the diurnal changes in serum iron, TIBC and ferritin levels in 5 normal dogs, we measured these levels at 8:00 am, 12:00 AM, 4:00 AM and 8:00 PM on the same day (Fig. 1). The highest mean serum concentration in all dogs (212.2 µg/dl) was found at 8:00 AM, while that at 4:00 PM was the lowest (139.2 µg/dl); these values were significantly different (P<0.05). The serum ferritin concentration and TIBC did not change during the day. These results showed that similar to humans, normal dogs exhibit diurnal variation of serum iron levels with a higher value in the morning than in the afternoon [2]. To exclude the influence of multiple blood drawing events, we measured the serum iron concentration in the same beagle dogs at 8:00 AM and 4:00 PM on another day, and the result was similar to that found in the present study (data not shown). This implied that drawing blood multiple times did not have a marked influence on the results. However, the detailed mechanism of the diurnal change in serum iron concentration is unknown, but proliferative activity in the bone marrow, which was evaluated by DNA synthesis and mitotic index, and serum erythropoietin, which has a central role in the maintenance of erythrocyte mass, were also reported to show diurnal variation [11, 19]. In addition, it was reported that hepcidin, an iron-regulatory hormone that mediates the homeostasis of extracellular iron concentrations, showed a diurnal increase in serum level at noon and in the evening compared with in the morning [5]. Therefore, because the quantities of available iron for erythropoiesis change during the day, diurnal variation may be observed. We did not evaluate them in this study.
Fig. 1.
Diurnal changes in serum iron, TIBC and ferritin levels in 5 normal dogs. Individual and mean values (open circle) are shown for each sampling time. * P<0.05.
The reference range for canine serum iron concentrations was reported to be 33.0–147.0 µg/dl, but this report did not include information about the sampling time [6]. It was thought that it was necessary to consider sampling time for accurate interpretation of serum iron concentrations in dogs. To the authors’ knowledge, this is the first report to describe the diurnal variation of serum iron in dogs. In this study, because we used normal dogs, we did not evaluate whether the serum iron concentration changed during the day in dogs with iron metabolism abnormalities (i.e., iron deficiency, anemia due to chronic disease and iron overload). Further studies are necessary in this regard.
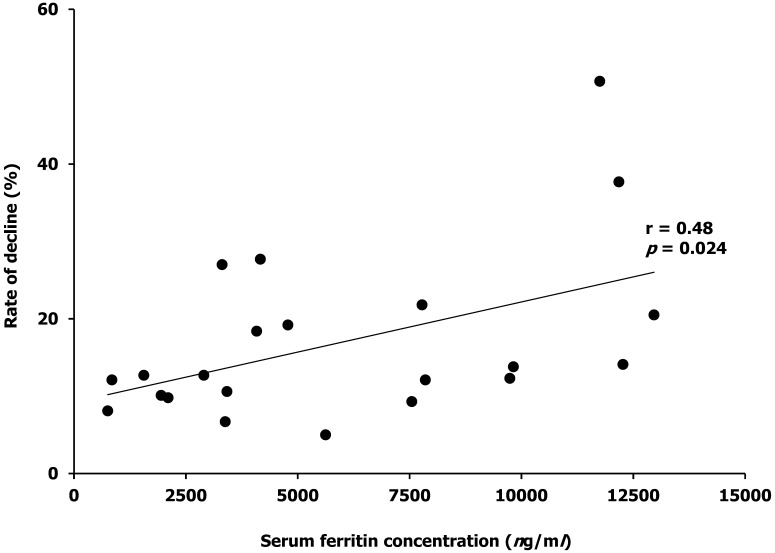
To examine the influence of serum ferritin, we also measured the serum iron concentration before and after immunoprecipitation of ferritin in the sera with various values of serum ferritin concentration in clinical cases. Samples were collected from 22 dogs (11 male and 11 female dogs; mean body weight ± SD, is 20.8 ± 13.6 kg) enrolled in this study. Diagnoses in these dogs included hemangiosarcoma, autoimmune hemolytic anemia, histiocytic sarcoma, lymphoma, hemangioma, mast cell tumor, lung adenocarcinoma, immune mediated polyarthritis and acute pneumonia. The serum ferritin range of these dogs was 754–12,962 ng/ml (Table 1). We had previously confirmed that there was no change in serum TIBC value after immunoprecipitation processing (data not shown). There was a positive correlation between the serum ferritin level and rate of decline in serum iron concentration after immunoprecipitation of ferritin (r=0.48, P=0.024, Fig. 2).
Table 1. Individual serum iron concentration data before and after immunoprecipitation of ferritin in 22 clinical cases.
| Case No. | Breed | Diagnosis | Initial ferritin concentration (ng/ml) |
Serum iron concentration before and after immunoprecipitation (μg/dl) |
Rate of decline (%) |
|
|---|---|---|---|---|---|---|
| Before | After | |||||
| 1 | Golden retriever | Hemangiosarcoma | 12,962 | 127 | 101 | 20.5 |
| 2 | Beagle | Autoimmune hemolytic anemia | 12,268 | 142 | 122 | 14.1 |
| 3 | Golden retriever | Histiocytic sarcoma | 12,178 | 101 | 73 | 37.7 |
| 4 | Beagle | Hemangiosarcoma | 11,750 | 73 | 36 | 50.7 |
| 5 | Bernese mountain dog | Histiocytic sarcoma | 9,820 | 189 | 163 | 13.8 |
| 6 | Golden retriever | Autoimmune hemolytic anemia | 9,740 | 358 | 314 | 12.3 |
| 7 | Beagle | Lymphoma | 7,853 | 215 | 189 | 12.1 |
| 8 | Welsh corgi | Histiocytic sarcoma | 7,779 | 124 | 97 | 21.8 |
| 9 | Welsh corgi | Hemangioma | 7,550 | 236 | 214 | 9.3 |
| 10 | Shetland sheepdog | Mast cell tumor | 5,623 | 199 | 189 | 5.0 |
| 11 | Flat-coated retriever | Lung adenocarcinoma | 4,780 | 208 | 168 | 19.2 |
| 12 | Shiba | Immune-mediated polyarthritis | 4,165 | 112 | 81 | 27.7 |
| 17 | Golden retriever | Histiocytic sarcoma | 4,082 | 76 | 62 | 18.4 |
| 13 | Bernese mountain dog | Lymphoma | 3,419 | 179 | 160 | 10.6 |
| 14 | Pug | Acute pneumonia | 3,380 | 165 | 154 | 6.7 |
| 15 | Akita | Autoimmune hemolytic anemia | 3,310 | 122 | 89 | 27.0 |
| 16 | Golden retriever | Mast cell tumor | 2,901 | 158 | 138 | 12.7 |
| 18 | Mongrel | Lymphoma | 2,103 | 143 | 129 | 9.8 |
| 19 | Pomeranian | Immune-mediated polyarthritis | 1,948 | 276 | 248 | 10.1 |
| 20 | Golden retriever | Lymphoma | 1,560 | 102 | 89 | 12.7 |
| 21 | Beagle | Lymphoma | 848 | 58 | 51 | 12.1 |
| 22 | Golden retriever | Lymphoma | 754 | 111 | 102 | 8.1 |
Fig. 2.
Correlation between serum ferritin concentration and rate of decline in serum iron concentration after immunoprecipitation of ferritin. The correlation coefficient and P value shown in the figure. Serum ferritin concentrations were plotted against the rate of decline in serum iron concentration.
The ICSH method that we used in this study is a reference method for the measurement of serum iron in human blood [9, 10]. This method is sensitive and suitable for assaying serum iron; however, it is reported to suffer from interference by high ferritin levels in human patients [13, 22, 23]. This effect is thought to be due to the liberation of iron from ferritin by the deproteinization step [23]. In humans, the ICSH method showed a decrease of more than 5%, especially in patients with a ferritin concentration exceeding 2,000 ng/ml [23]. In this study, even the cases with normal serum ferritin concentrations (approximately 500–1,000 ng/ml) showed about a 10% decline in the serum iron concentration after immunoprecipitation of ferritin. Therefore, serum iron measurement may be affected by ferritin in dogs more than in humans. This was thought to be because of the higher iron content of serum ferritin in dogs than humans [18, 21]. Therefore, we propose that it is necessary to consider the serum ferritin level when measuring the canine serum iron concentration using the ICSH method. It has been reported that hyperferritinemia does not influence measurement of serum iron by a direct method that does not require deproteinization [23]. However, protein precipitation plays an important role in the separation of the analyte from interferences in the matrix of serum specimens (e.g., hemoglobin and bilirubin) [17]. Therefore, further investigation is necessary to establish a reference assay for canine serum iron measurement.
We concluded that diurnal changes and interference by serum ferritin are factors that affect measurement of the serum iron concentration in dogs when using the ICSH colorimetric assay. It may be necessary to reexamine the reference range of the serum iron concentration in dogs by standardizing the sampling time and the assay.
REFERENCES
- 1.Chikazawa S., Hori Y., Hoshi F., Kanai K., Ito N., Sato J., Orino K., Watanabe K., Higuchi S.2013. Development of a sandwich enzyme-linked immunosorbent assay to detect and measure serum levels of canine ferritin. J. Vet. Med. Sci. 75: 515–517. doi: 10.1292/jvms.12-0324 [DOI] [PubMed] [Google Scholar]
- 2.Dale J. C., Burritt M. F., Zinsmeister A. R.2002. Diurnal variation of serum iron, iron-binding capacity, transferrin saturation, and ferritin levels. Am. J. Clin. Pathol. 117: 802–808. doi: 10.1309/2YT4-CMP3-KYW7-9RK1 [DOI] [PubMed] [Google Scholar]
- 3.Feldman B. F., Kaneko J. J., Farver T. B.1981. Anemia of inflammatory disease in the dog: clinical characterization. Am. J. Vet. Res. 42: 1109–1113 [PubMed] [Google Scholar]
- 4.Friedrichs K. R., Thomas C., Piler M., Andrews G. A., Chavey P. S., Young K. M.2010. Evaluation of serum ferritin as a marker for canine histiocytic sarcoma. J. Vet. Intern. Med. 24: 904–911. doi: 10.1111/j.1939-1676.2010.0543.x [DOI] [PubMed] [Google Scholar]
- 5.Ganz T., Olbina G., Girelli D., Nemeth E., Westerman M.2008. Immunoassay for human serum hepcidin. Blood 112: 4292–4297. doi: 10.1182/blood-2008-02-139915 [DOI] [PubMed] [Google Scholar]
- 6.Harvey J. W.2008. Iron metabolism and its disorders. pp.259–285. In: Clinical Biochemistry of Domestic Animals, 6th ed. (Kaneko, J. J., Harvey, J. W. and Bruss, M. L. eds.) Elsevier, Massachusetts. [Google Scholar]
- 7.Harvey J. W., Smith J. E.1994. Haematology and clinical chemistry of English springer spaniel dogs with phosphofructokinase deficiency. Comp. Haematol. Int. 4: 70–75. doi: 10.1007/BF00368272 [DOI] [Google Scholar]
- 8.Harvey J. W., Levin D. E., Chen C. L.1987. Potential effects of glucocorticoids on serum iron concentration in dogs. Vet. Clin. Pathol. 16: 46–50. doi: 10.1111/j.1939-165X.1987.tb00461.x [DOI] [PubMed] [Google Scholar]
- 9.International Committee for Standardization in Hematology. 1978. Recommendations for measurements of serum iron in human blood. Br. J. Haematol. 38: 291–294. doi: 10.1111/j.1365-2141.1978.tb01045.x [DOI] [PubMed] [Google Scholar]
- 10.Iron panel of the International Committee for Standardization in Hematology. 1990. Revised recommendations for the measurements of the serum iron in human blood. Br. J. Haematol. 75: 615–616. doi: 10.1111/j.1365-2141.1990.tb07808.x [DOI] [PubMed] [Google Scholar]
- 11.Mauer A. M.1965. Diurnal variation of proliferative activity in the human bone marrow. Blood 26: 1–7 [PubMed] [Google Scholar]
- 12.Naigamwalla D. Z., Webb J. A., Giger U.2012. Iron deficiency anemia. Can. Vet. J. 53: 250–256 [PMC free article] [PubMed] [Google Scholar]
- 13.Pootrakul P., Josephson B., Huebers H. A., Finch C.1988. Quantitation of ferritin iron in plasma, an explanation for non-transferrin iron. Blood 71: 1120–1123 [PubMed] [Google Scholar]
- 14.Sprague W. S., Hackett T. B., Johnson J. S., Swardson-Olver C. J.2003. Hemochromatosis secondary to repeated blood transfusions in a dog. Vet. Pathol. 40: 334–337. doi: 10.1354/vp.40-3-334 [DOI] [PubMed] [Google Scholar]
- 15.Steffen D. J., Elliott G. S., Leipold H. W., Smith J. E.1992. Congenital dyserythropoiesis and progressive alopecia in polled Hereford calves: hematologic, biochemical, bone marrow cytologic, electrophoretic, and flow cytometric findings. J. Vet. Diagn. Invest. 4: 31–37. doi: 10.1177/104063879200400108 [DOI] [PubMed] [Google Scholar]
- 16.Stookey L. L.1970. Ferrozine—a new spectrophotometric reagent for iron. Anal. Chem. 42: 779–781. doi: 10.1021/ac60289a016 [DOI] [Google Scholar]
- 17.Tietz N. W., Rinker A. D., Morrison S. R.1994. When is a serum iron really a serum iron? The status of serum iron measurements. Clin. Chem. 40: 546–551 [PubMed] [Google Scholar]
- 18.Watanabe K., Muranishi N., Murata Y., Orino K., Okano S., Yamamoto S.2000. Biochemical properties of canine serum ferritin: iron content and nonbinding to concanavalin A. BioMetals 13: 319–324. doi: 10.1023/A:1009276323707 [DOI] [PubMed] [Google Scholar]
- 19.Wide L., Bengtsson C., Birgegard G.1989. Circadian rhythm of erythropoietin in human serum. Br. J. Haematol. 72: 85–90. doi: 10.1111/j.1365-2141.1989.tb07657.x [DOI] [PubMed] [Google Scholar]
- 20.Worwood M.1990. Ferritin. Blood Rev. 4: 259–269. doi: 10.1016/0268-960X(90)90006-E [DOI] [PubMed] [Google Scholar]
- 21.Worwood M., Dawkins S., Wagstaff M., Jacobs A.1976. The purification and properties of ferritin from human serum. Biochem. J. 157: 97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamanishi H., Iyama S., Amino N.1996. Ferritin iron interference with recommended method for measuring serum iron. Clin. Chem. 42: 2042–2043 [PubMed] [Google Scholar]
- 23.Yamanishi H., Iyama S., Fushimi R., Amino N.1996. Interference of ferritin in measurement of serum iron concentrations: comparison by five methods. Clin. Chem. 42: 331–332 [PubMed] [Google Scholar]