ABSTRACT
Chylomicrons from villous columnar epithelial cells are generally known to be transported only by central lymph vessels (CLV), whereas antigenic particulates derived from the intestinal lumen can also be transported by subepithelial blood capillaries (sBCs) in rat intestinal villi. The possibility of chylomicron absorption by sBCs was histoplanimetrically studied in the rat jejunum under a transmission electron microscope. The chylomicrons more abundantly presented in villous venules than in arterioles. The most frequent size (MFS) of chylomicrons was 75 to 90 nm in diameter in the areas near sBCs, while it was 45 to 60 nm in the epithelial intercellular spaces just above sBCs or the intermediate areas between sBCs. The MFS of chylomicrons was 45 to 60 nm in the intermediate areas between sBCs and in the epithelial intercellular spaces just above these areas. The MFS of chylomicrons in CLV was intermediate between that in the area adjacent to sBCs and that in the intermediate areas between sBCs. Chylomicrons were found in small vesicles in the endothelial cytoplasms of sBCs. No chylomicrons larger than 600 nm were observed in the lamina propria. These findings suggest that some of the chylomicrons smaller than 75 nm, which are probable intestinal very low-density lipoproteins (VLDL), are directly transported to the liver by hepatic portal blood in addition to CLV and that epithelial fat droplets larger than 600 nm are not discharged into lamina propria in rat jejunum under physiological conditions.
Keywords: chylomicrons, portal blood, small intestine, ultrastructure, VLDL
In the small intestinal lumen, fats in foods are generally degraded into glycerol and short-, medium- and long-chain fatty acids. The short-chain fatty acids are absorbed by villous columnar epithelial cells and are directly transported by subepithelial blood capillaries (sBCs), while long-chain fatty acids and some of the medium chain fatty acids are absorbed and reconstructed into fat droplets in the villous columnar epithelial cells. Newly synthesized fat droplets are discharged from villous columnar epithelial cells into the lamina propria as chylomicrons in the broad sense [4]. Chylomicrons are classified as plasma lipoproteins, i.e., spherical particles with widely ranging diameters, and were discovered in the blood of man and animals [4, 5].
Plasma lipoproteins are currently classified into chylomicrons in the narrow sense (more than 75 nm in diameter), very low-density lipoproteins (VLDL) (75 to 28 nm in diameter), low-density lipoproteins (LDL) (27 to 21 nm in diameter) and high-density lipoproteins (HDL) (12 to 7 nm in diameter) based on their diameters and specific gravities during centrifugation [10, 18]. The lipoproteins are generally composed of a hydrophobic core made up of triacylglycerides and cholesterol ester and surrounded by a 2 nm-thick shell. The shell consists of both phospholipids and apolipoproteins, which are classified as apoA, apoB, apoC, apoD and apoE. The common function of all apolipoproteins is to assist in the solubilization of neutral lipids in the blood circulation [18].
VLDL are generally produced by hepatocytes in the liver [9], but are also produced by epithelial cells in the small intestine [30]. Plasma VLDL possess apoB and apoE, but do not possess apoA-I and apoA-IV [20]. In contrast, intestinal VLDL possess apoA-I, apoA-IV, apoB and apoE, as well as chylomicrons [10]. Thus, the intestinal VLDL possess the same molecular structure as chylomicrons in the narrow sense. Therefore, the term “minute chylomicrons,” defined as chylomicrons less than 75 nm, is used instead of VLDL in this paper.
Chylomicrons discharged from the villous epithelial cells into the lamina propria are generally transported by central lymph vessels (CLV) and never by sBCs [2, 4]. On the other hand, a phenomenon known as “persorption,” by which various substances or particulates in the intestinal lumen are directly transported into the blood circulation without any digestion, has been reported in humans and various animal species [34]. In persorption, both specific antibodies against the ingested substances and epithelial receptors for IgG-Fc fragments probably play an important role [38,39,40]. Furthermore, the direct entry of luminal particulates into the sBCs has been histologically demonstrated in intestinal villi of the rat jejunum [40]. From these reports, the sBCs in the intestinal villi are assumed to be special blood vessels that can absorb and transport macromolecules or particulates, including chylomicrons, from the extravascular tissue. Therefore, the aim of the present study was to observe the direct transportation of some chylomicrons by hepatic portal blood in the rat jejunal villi by histoplanimetry and transmission electron microscopy.
MATERIALS AND METHODS
Experimental animals: A total of 4 male Wistar rats aged 7 weeks (Japan SLC Inc., Hamamatsu, Japan) were used. The animals were maintained under conventional laboratory housing conditions and permitted free access to water and food (Lab MR Stock; Nosan Corp., Yokohama, Japan). The animal facility was maintained under conditions of a 12-hr light/dark cycle at 23 ± 1°C and 50–60% humidity. Clinical and pathological examinations were performed for all animals, and no signs of disorder were observed. This study was approved by the Institutional Animal Care and Use Committee (Permission number: 17-04-05) and carried out according to the Kobe University Animal Experimentation Regulations.
Histological procedure: After cardiac exsanguination under deep anesthesia with i.p. injection of pentobarbital sodium (Dainippon Sumitomo Pharmaceuticals, Osaka, Japan) at AM0:00, small tissue blocks were obtained from a 5 cm segment that was 10 cm caudal from the duodenojejunal flexure. Then, the blocks were immediately immersed in 0.1 M phosphate-buffered 2.0% glutaraldehyde−2.0% paraformaldehyde solution at 4°C for 24 hr (pH 7.2). After fixation, the tissue blocks were immersed in 0.1 M phosphate-buffered 1.0% OsO4 (pH 7.2) for 90 min at room temperature (r.t.). Some of the blocks were then immersed in 1.0% imidazole−1.0% paraphenylenediamine solution for 2 hr at r.t. to confirm the lipid droplets with reference to the method of Nakao et al. [22]. After dehydration with an ethanol upgrade system, the tissue blocks were embedded in a QY-2 resin mixture. Semithin sections of 1 µm thickness were cut using an ultramicrotome (Sorvall MT-1; Dupont, Newton, CT, U.S.A.) and stained with 0.01 M phosphate-buffered 0.05% toluidine blue solution (pH 7.4). Ultrathin sections of 70 nm thickness were cut using the same ultramicrotome and stained with both 3% uranyl acetate solution and 3% lead citrate solution. The ultrathin sections were observed under a transmission electron microscope (H-7100; Hitachi, Tokyo, Japan) at an accelerating voltage of 75 kV.
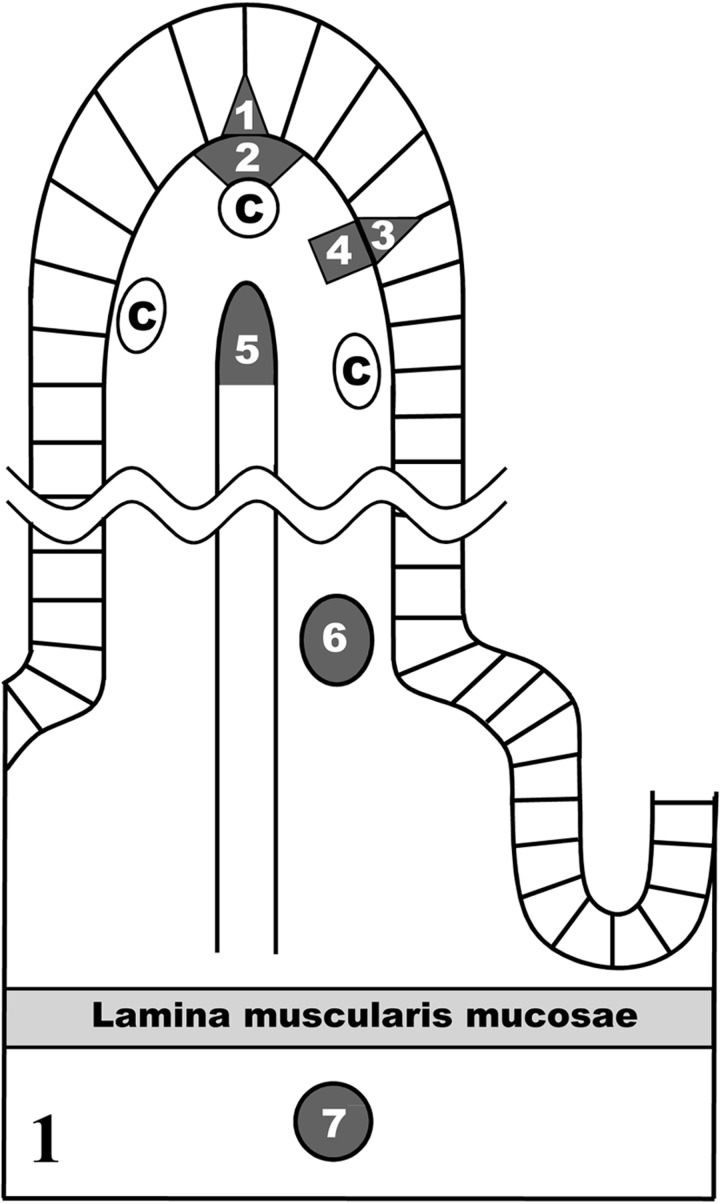
Quantitative ultrastructural observation: Five intestinal villi that were cut longitudinally along the villous axes were randomly selected from each animal under a transmission electron microscope. In each intestinal villus, the diameters of 300 chylomicrons were measured in each of 5 areas on the photographs, which were magnified 13,000 fold: the epithelial intercellular space just above sBCs, the narrow area adjacent to the sBCs, the epithelial intercellular space just above the intermediate area between sBCs, the intermediate area between sBCs and the lumen of CLV in the villous apex (Fig. 1). The mean diameters were calculated from the average of the major axis and the minor axis of each chylomicron. The size distribution of chylomicrons in each 15 nm was represented by the average of 4 animals, and the distributions from the 5 areas were compared.
Fig. 1.
The seven areas histoplanimetrically measured in this study: 1, the epithelial intercellular space just above subepithelial blood capillaries (sBCs); 2, the narrow area near sBCs; 3, the epithelial intercellular space just above the intermediate area between sBCs; 4, the intermediate area between sBCs; 5, the lumen of central lymph vessels (CLV) in the villous apex; 6, the lumen of the venules at the base of the intestinal villus; 7, the lumen of the arterioles in the submucosa. C, sBCs.
The number of chylomicrons in a unit area (6.0 × 105 nm2) was also measured in the lumina of the venules at the base of the intestinal villus and the arterioles in the submucosa (Fig. 1). The measured data were expressed as means ± SD. The size distribution of chylomicrons in each 15 nm was represented by the average of 4 animals. The Student’s t-test was used to compare the mean numbers of chylomicrons in the lumina of the arterioles and the venules. P values less than 0.05 were considered statistically significant.
RESULTS
Epithelial fat droplets: Numerous fat droplets were present in the cytoplasms of the villous columnar epithelial cells at the apical portions of intestinal villi. The amount of cytoplasmic fat droplets increased toward the villous apex (Fig. 2). The fat droplets varied in size with the largest being over 1 µm in diameter in villous columnar epithelial cells (Fig. 3). In the exfoliating epithelial cells at the villous apex, numerous large fat droplets were observed in the cytoplasms (Fig. 4).
Fig. 2.

A light microscopic image of the jejunal villi. Numerous fat droplets (arrow) are visible in the villous columnar epithelial cells in the villous apex. IV, intestinal villus. Bar=50 µm. Block staining with 1.0% imidazole-1.0% paraphenylenediamine.
Fig. 3.

An electron microscopic figure of the epithelium of the villous apex. Highly electron dense fat droplets larger than 1 µm in diameter are visible in the villous columnar epithelial cells (Ep). Bar=10 µm. Block staining by 1.0% imidazole-1.0% paraphenylenediamine.
Fig. 4.
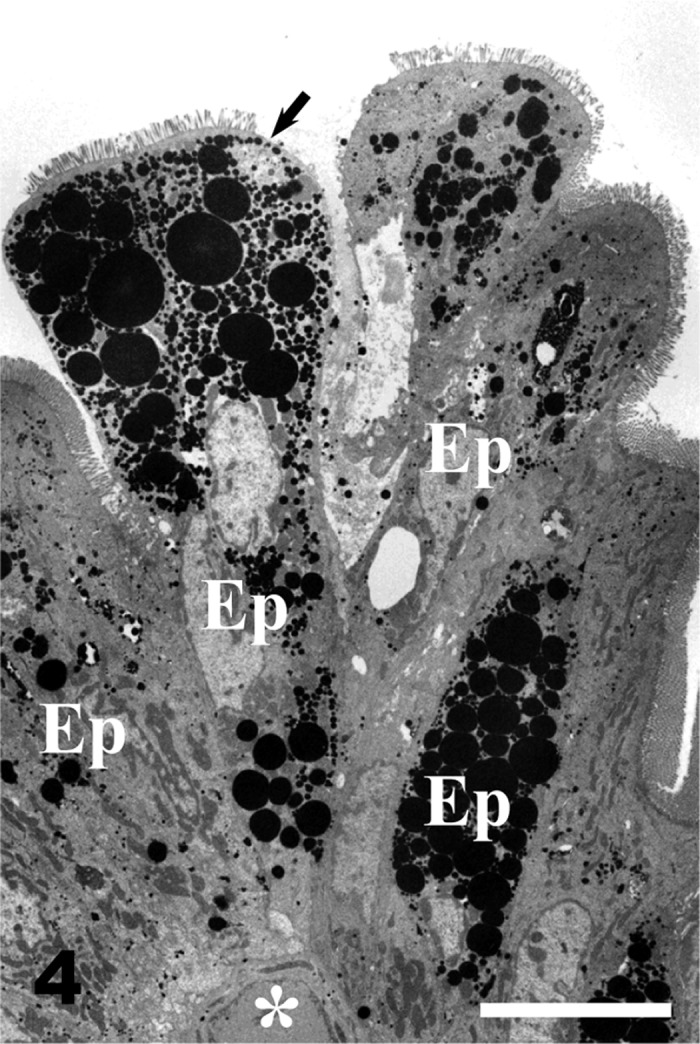
Villous columnar epithelial cells with numerous large fat droplets (arrow) are exfoliated from the apex of the intestinal villus. White asterisk, blood capillary; Ep, epithelial cells. Bar=10 µm. Block staining by 1.0% imidazole-1.0% paraphenylenediamine.
Qualitative distribution of chylomicrons: The epithelial intercellular spaces were markedly enlarged at the tops of the intestinal villi. The apical intercellular spaces contained a few chylomicrons (Fig. 5), whereas numerous chylomicrons were found in the basal epithelial intercellular spaces (Fig. 6). Chylomicrons were dispersed in the lamina propria just beneath the epithelial cells (Fig. 7). Chylomicrons were also occasionally found in the small vesicles in the endothelial cells of sBCs (Fig. 8). A few chylomicrons were found in the lumen of arterioles and venules, and all the chylomicrons in these locations were very minute (Figs. 9 and 10). On the other hand, numerous chylomicrons of various sizes were densely observed in the CLV (Fig. 11).
Fig. 5.

The apical portion of the epithelial intercellular space (EI) in the villous apex is enlarged and contains a few chylomicrons (arrows). Ep, epithelial cells. Bar=1 µm.
Fig. 6.
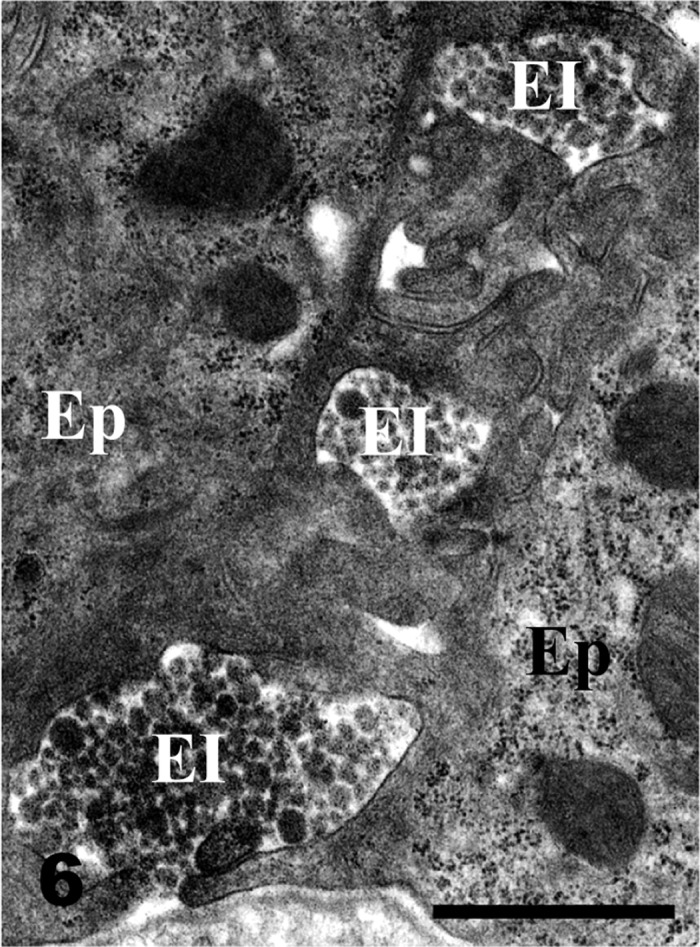
The basal portion of the epithelial intercellular space (EI) in the villous apex is more enlarged than the apical portion of the EI shown in Fig. 5. The EI is occupied by highly dense chylomicrons of various sizes. Ep, epithelial cells. Bar=1 µm.
Fig. 7.

The area between the subepithelial blood capillaries in the villous apex contains numerous chylomicrons (arrows) of various sizes. Ep, epithelial cells. Bar=1 µm.
Fig. 8.
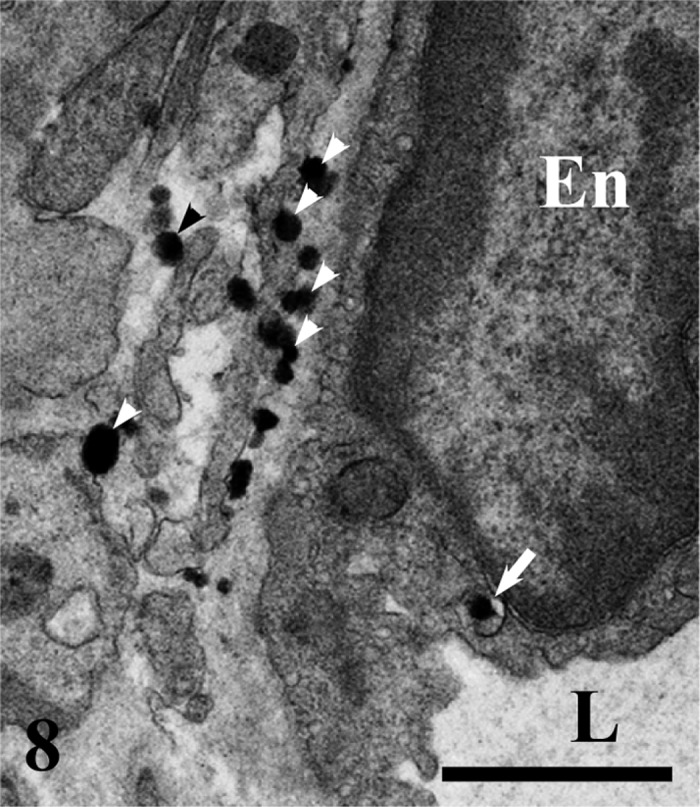
The epithelial side of a subepithelial blood capillary in the villous apex. Numerous chylomicrons (arrowheads) are located in a narrow space between the epithelial cell and the endothelial cell (En). A chylomicron in the small vesicle is visible in the endothelial cytoplasm (arrow). L, lumen. Bar=1 µm.
Fig. 9.
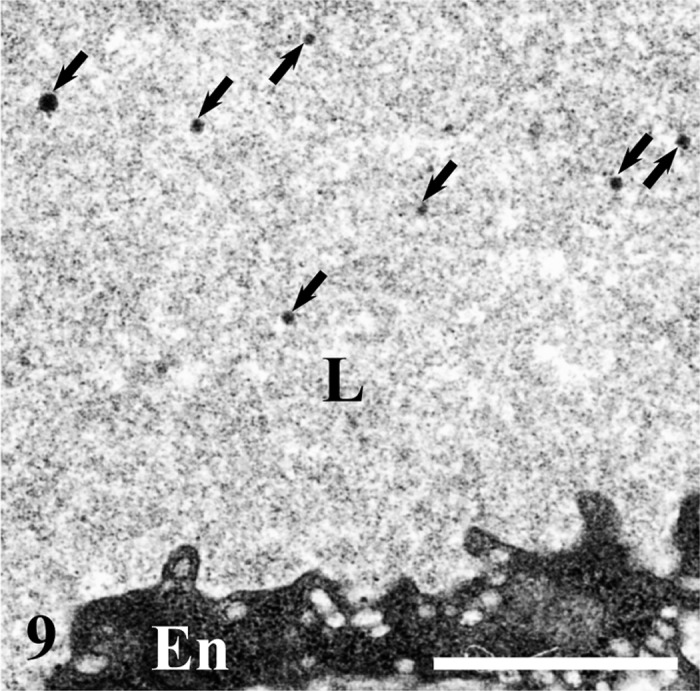
A few minute chylomicrons (arrows) are dispersed in the lumen (L) of a venule at the base of the intestinal villus. En, endothelial cells. Bar=1 µm.
Fig. 10.

The number of minute chylomicrons (arrows) in the lumen (L) of an arteriole in the submucosa is smaller than the number in a venule (Fig. 9). En, endothelial cells; Er, erythrocytes. Bar=1 µm.
Fig. 11.
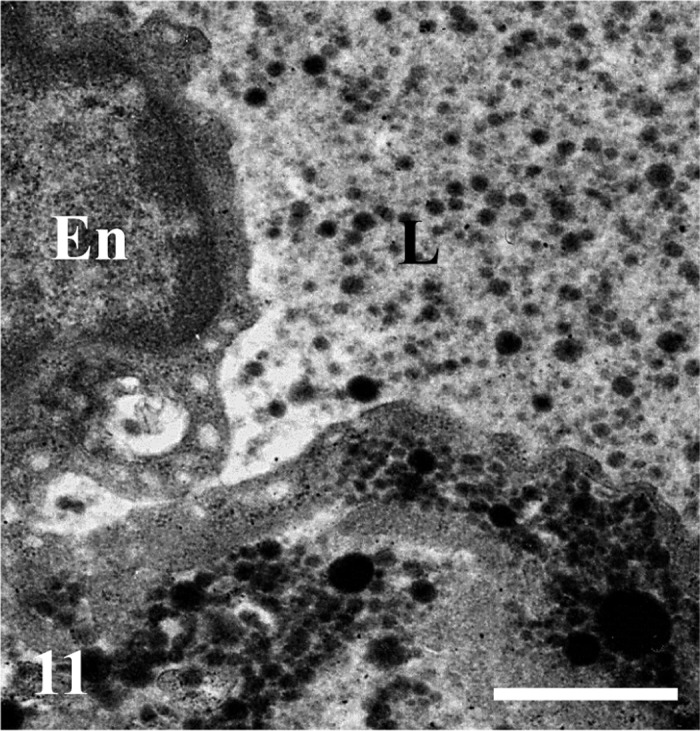
Numerous chylomicrons of various sizes are visible in the lumen (L) of the central lymph vessels and are also accumulated just under the endothelium (En). Bar=1 µm.
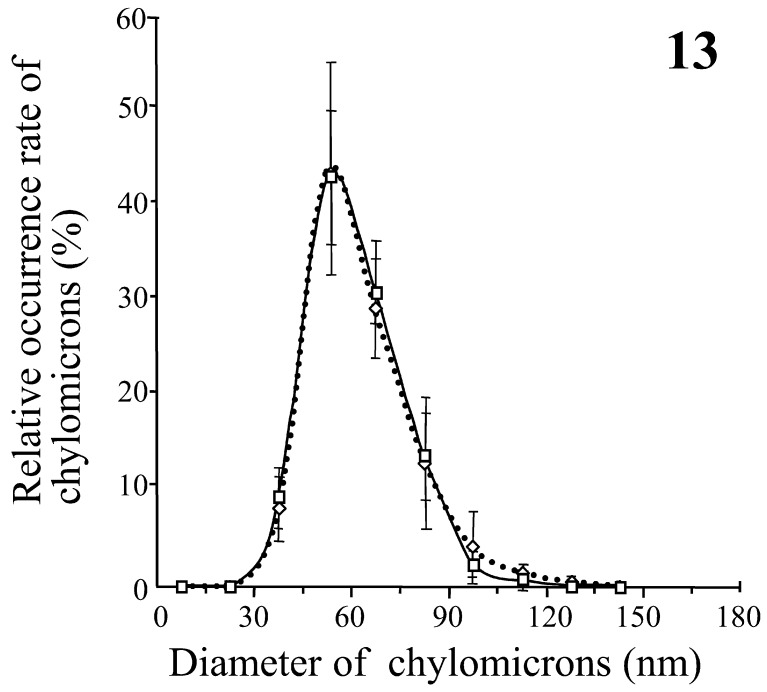
Quantitative distribution of chylomicrons: There were no chylomicrons larger than 600 nm in diameter, and almost all the chylomicrons were less than 180 nm in diameter in all areas measured. The most frequent size (MFS) of chylomicrons in the epithelial intercellular space situated just above the sBCs was in the range from 45 to 60 nm and was smaller than the MFS (75 to 90 nm) in the area near the sBCs (Fig. 12). The MFS of chylomicrons in the CLV was in the range from 60 to 75 nm and was an intermediate size between those in the area near the sBCs and those in the epithelial intercellular space situated just above the sBCs (Fig. 12). On the other hand, the MFS of chylomicrons in the intermediate area between sBCs was 45 to 60 nm and was almost the same as that in the epithelial intercellular spaces just above the intermediate area between sBCs (Fig. 13). In addition, the number of chylomicrons in a unit area was significantly larger in the lumen of venules at the base of the intestinal villus than in the lumen of arterioles in the submucosa (Fig. 14). The MFS of chylomicrons was 45 to 60 nm in diameter in both the venules and arterioles, which was smaller than the range in the CLV (Figs. 12 and 14).
Fig. 12.
Comparison of the relative frequencies of appearance of chylomicrons among the epithelial intercellular space just above the subepithelial blood capillaries (sBCs) (black circles with a thin line), the areas near the sBCs (triangles with a dotted line) and the lumen of central lymph vessels (white circles with a thick line). Each value is the average of the percentages of chylomicrons in each of the 15 diameter and represents the mean ± SD.
Fig. 13.
Comparison of the relative frequencies of appearance of chylomicrons between the epithelial intercellular spaces just above the intermediate areas between sBCs (squares with a thick line) and the intermediate areas between sBCs (white diamonds with a dotted line). Each value is the average of the percentages of chylomicrons in each of the 15 diameter and represents the mean ± SD.
Fig. 14.
Comparison of the number of chylomicrons between the lumina of venules at the base of the intestinal villus (white diamonds with a thick line) and the lumina of arterioles in the submucosa (triangles with a dotted line). Each value is the number of chylomicrons in a unit area 6.0 × 105 nm2 and represents the mean ± SD. Asterisks, P<0.05.
DISCUSSION
In villous columnar epithelial cells, fatty acids and monoglycerides taken through the epithelial microvilli are synthesized into triglyceride droplets which are discharged into the epithelial intercellular spaces as chylomicrons. This process from the fatty acid absorption to the discharge of fat droplets occurs within a few minutes [1, 3, 11]. It has been reported that the size range of chylomicrons in the epithelial intercellular space was from 120 to 480 nm in diameter after gastric intubation of corn oil in the rat jejunum [26]. Moreover, in fasting rats, the most frequent size of chylomicrons in the mesenteric lymph ranges from 20 to 40 nm in diameter, while in fat feeding rats, the sizes of almost all the chylomicrons in the lymph range from 20 to 200 nm [12]. In the present ultrastructural study, there were no chylomicrons larger than 600 nm in diameter in all areas measured. However, the exfoliating epithelial cells of villous apices possessed fat droplets larger than 1 µm in diameter. Therefore, these findings suggest that large chylomicrons over 600 nm in diameter are not discharged into the lamina propria by villous columnar epithelial cells and are expelled with epithelial cells into the lumen in the rat jejunum under physiological conditions.
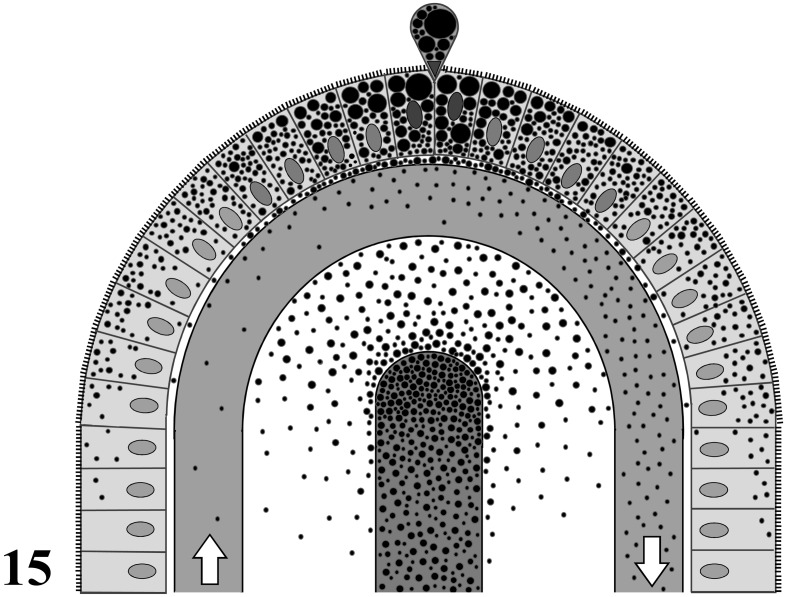
The exchange of both water and small lipid-insoluble molecules across the endothelium of blood capillaries is largely regulated by the balance between hydrostatic and colloid osmotic pressures in animal tissues [28]. The macromolecules, such as proteins permeated from blood capillaries, are generally collected by lymphatic capillaries in the interstitial tissues and finally returned back to the blood circulation [24]. On the other hand, the persorption of macromolecules and particulates from the intestinal lumen to the systemic circulation has been reported in various vertebrates—i.e., fishes [21], chickens [33], mice [14], rats [17, 27, 37], rabbits [31], dogs [13, 36, 37] and humans [15, 35]. Various substances are persorbed from the intestinal lumen into the systemic circulation, including pollens and spores [32], starch particles [13, 33], coal particles [23], polyvinyl chloride particles and metallic iron particles [33, 36], colloidal gold particles [14], bovine serum albumin (BSA) [38] and BSA-coated sheep erythrocytes [39]. The number of persorbed particulates from the intestinal lumen is significantly greater in the portal blood than in the systemic circulating blood in rats [39]. Moreover, the direct crossing of the endothelium by sBCs has been histologically demonstrated by luminal administration of IgG-Fc fragment-coated particulates into the rat jejunum [40]. In the present study, the number of chylomicrons in the venules of the intestinal villi was greater than that in the arterioles in the submucosa. This finding suggests that the chylomicrons are directly added into the hepatic portal blood in the intestinal villi. In addition, in the present study, the MFS (75 to 90 nm) of chylomicrons in the areas near sBCs was larger than that (45 to 60 nm) in the epithelial intercellular spaces and the intermediate areas between sBCs, while the MFS of chylomicrons in the epithelial intercellular spaces was almost the same as the MFS in the intermediate areas between sBCs (45 to 60 nm). This finding suggests that minute chylomicrons less than 75 nm had partially disappeared from the areas near sBCs. Moreover, in the present study, the MFS (60 to 75 nm) of chylomicrons in the CLV was intermediate between the above two MFS values. As a result, these findings suggest that some of the minute chylomicrons with diameters of less than 75 nm are directly transported to the liver by hepatic portal blood, and the rest are transported to the systemic circulation by the CLV in the rat jejunum under physiological conditions. The transportation route of chylomicrons is summarized in Fig. 15.
Fig. 15.
A schema of transportation routes of chylomicrons in an apex of rat jejunal villus. A central blind canal is a central lymph vessel. White arrows indicate the direction of blood flow in a subepithelial blood capillary. Black dotts, lipid droplets or chylomicrons.
Some apolipoprotein receptors in the cell surface membranes recognize free chylomicrons in the broad sense. Rabbit VLDL receptors bind with high affinity to apoE-containing lipoproteins, such as chylomicrons [29]. The mouse LDL receptor pathway plays a significant role in the chylomicron remnant metabolism [16]. LDL receptor-related protein derived from the rat liver also recognizes rabbit LDL and rat LDL [19]. ApoB48 receptors mediate the rapid high-affinity uptake of chylomicrons in murine and human macrophages [7, 8]. The present ultrastructural observation suggests the direct transportation of chylomicrons encapsulated by cell membranes across the endothelial cells of subepithelial blood capillaries. Therefore, some of the apolipoprotein receptors probably participate in the transportation across the endothelial cells into subepithelial blood capillaries in the rat jejunal villi under physiological conditions. In addition, the size of minute chylomicrons transported by sBCs was less than 75 nm in diameter in the present study. Lipoproteins less than 75 nm in diameter are classified as VLDL, LDL or HDL [10, 18]. On the other hand, the VLDL receptor is expressed in the small intestine of 15-day-old rats and in the enterocytes from the small intestine of 1-month-old rats [6]. It has also been reported that the LDL receptor is expressed in the human small intestine [25]. Therefore, VLDL receptors or LDL receptors may participate in the transportation of minute chylomicrons by sBCs. Identification of the receptors for minute chylomicrons is now in progress.
ACKNOWLEDGMENTS
This work was financially supported in part by a Grant-in-Aid for Scientific Research (no. 23580403) from the Japan Society for the Promotion of Science.
REFERENCES
- 1.Cartwright I. J., Plonné D., Higgins J. A.2000. Intracellular events in the assembly of chylomicrons in rabbit enterocytes. J. Lipid Res. 41: 1728–1739 [PubMed] [Google Scholar]
- 2.Dobbins W. O.1969. Morphologic aspects of lipid absorption. Am. J. Clin. Nutr. 22: 257–265 [DOI] [PubMed] [Google Scholar]
- 3.Friedman H. I., Cardell R. R., Jr1972. Morphological evidence for the release of chylomicra from intestinal absorptive cells. Exp. Cell Res. 75: 57–62. doi: 10.1016/0014-4827(72)90519-8 [DOI] [PubMed] [Google Scholar]
- 4.Friedman H. I., Nylund B.1980. Intestinal fat digestion, absorption, and transport. Am. J. Clin. Nutr. 33: 1108–1139 [DOI] [PubMed] [Google Scholar]
- 5.Gage S. H.1920. The free granules (chylomicrons) of the blood as shown by the dark field microscope. Cornell Vet. 10: 154–155 [Google Scholar]
- 6.García-Miranda P., Peral M. J., Ilundain A. A.2010. Rat small intestine expresses the reelin-Disabled-1 signalling pathway. Exp. Physiol. 95: 498–507. doi: 10.1113/expphysiol.2009.050682 [DOI] [PubMed] [Google Scholar]
- 7.Gianturco S. H., Lin A. H.-Y., Hwang S.-L. C., Young J., Brown S. A., Via D. P., Bradley W. A.1988. Distinct murine macrophage receptor pathway for human triglyceride-rich lipoproteins. J. Clin. Invest. 82: 1633–1643. doi: 10.1172/JCI113775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gianturco S. H., Ramprasad M. P., Lin A. H.-Y., Song R., Bradley W. A.1994. Cellular binding site and membrane binding proteins for triglyceride-rich lipoproteins in human monocyte-macrophages and THP-1 monocytic cells. J. Lipid Res. 35: 1674–1687 [PubMed] [Google Scholar]
- 9.Gibbons G. F.1990. Assembly and secretion of hepatic very-low-density lipoprotein. Biochem. J. 268: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green P. H. R., Glickman R. M.1981. Intestinal lipoprotein metabolism. J. Lipid Res. 22: 1153–1173 [PubMed] [Google Scholar]
- 11.Green P. H. R., Riley J. W.1981. Lipid absorption and intestinal lipoprotein formation. Aust. N. Z. J. Med. 11: 84–90. doi: 10.1111/j.1445-5994.1981.tb03746.x [DOI] [PubMed] [Google Scholar]
- 12.Hayashi H., Fujimoto K., Cardelli J. A., Nutting D. F., Bergstedt S., Tso P.1990. Fat feeding increases size, but not number, of chylomicrons produced by small intestine. Am. J. Physiol. 259: 709–719 [DOI] [PubMed] [Google Scholar]
- 13.Herbst G.1844. Das lymphgefäßsystem und seine verrichtung. pp. 333–337. In: Nach Eigenen Untersuchungen Dargestellt. Verlag von Vandenhoeck und Ruprecht, Göttingen (in German). [Google Scholar]
- 14.Hillyer J. F., Albrecht R. M.2001. Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J. Pharm. Sci. 90: 1927–1936. doi: 10.1002/jps.1143 [DOI] [PubMed] [Google Scholar]
- 15.Husby S., Jensenius J. C., Svehag S.E.1986. Passage of undegraded dietary antigen into the blood of healthy adults. Further characterization of the kinetics of uptake and the size distribution of the antigen. Scand. J. Immunol. 24: 447–455. doi: 10.1111/j.1365-3083.1986.tb02133.x [DOI] [PubMed] [Google Scholar]
- 16.Ishibashi S., Perrey S., Chen Z., Osuga J., Shimada M., Ohashi K., Harada K., Yazaki Y., Yamada N.1996. Role of the low density lipoproteins (LDL) receptor pathway in the metabolism of chylomicron remnants. J. Biol. Chem. 271: 22422–22427. doi: 10.1074/jbc.271.37.22422 [DOI] [PubMed] [Google Scholar]
- 17.Jani P., Halbert G. W., Langridge J., Florence A. T.1990. Nanoparticle uptake by the rat gastrointestinal mucosa: quantitation and particle size dependency. J. Pharm. Pharmacol. 42: 821–826. doi: 10.1111/j.2042-7158.1990.tb07033.x [DOI] [PubMed] [Google Scholar]
- 18.Jonas A.2002. Lipoprotein structure. pp. 483–504. In: Biochemistry of Lipids, Lipoproteins and Membranes. 4th ed. (Vance, D. E. and Vance, J. E. eds.), Elsevier Science B. V., Amsterdam. [Google Scholar]
- 19.Lund H., Takahashi K., Hamilton R. L., Havel R. J.1989. Lipoprotein binding and endosomal itinerary of the low density lipoprotein receptor-related protein in rat liver. Proc. Natl. Acad. Sci. U.S.A. 86: 9318–9322. doi: 10.1073/pnas.86.23.9318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahley R. W., Innerarity T. L., Rall Jr S. C., Weisgraber K. H. Plasma lipoprotein structure and function. J. Lipid Res. 25: 1277–1294 [PubMed] [Google Scholar]
- 21.McLean E., Ash R.1987. The time-course of appearance and net accumulation of horseradish peroxidase (HRP) presented orally to rainbow trout Salmo gairdneri (Richardson). Comp. Biochem. Physiol. A. Comp. Physiol. 88: 507–510. doi: 10.1016/0300-9629(87)90072-7 [DOI] [PubMed] [Google Scholar]
- 22.Nakao I., Okada H., Senzaki H., Nishimura R., Fujita N., Shikata N., Mori S.1992. A mixture of paraphenylenediamine and imidazole: its effect on the extraction of lipid droplets during electron microscopy staining. Biotech. Histochem. 67: 219–223. doi: 10.3109/10520299209110069 [DOI] [PubMed] [Google Scholar]
- 23.Oesterlen F.1846. Ueber den Eintritt von Kohle und andern unlöslichen Stoffen vom Darmcanal aus in die Blutmasse. Z. Rationelle Med. 5: 434–439 (in German). [Google Scholar]
- 24.Pepper M. S., Skobe M.2003. Lymphatic endothelium: morphological, molecular and functional properties. J. Cell Biol. 163: 209–213. doi: 10.1083/jcb.200308082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rudling M. J., Reihnér E., Einarsson K., Ewerth S., Angelin B.1990. Low density lipoprotein receptor-binding activity in human tissues: quantitative importance of hepatic receptors and evidence for regulation of their expression in vivo. Proc. Natl. Acad. Sci. U.S.A. 87: 3469–3473. doi: 10.1073/pnas.87.9.3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabesin S. M., Frase S.1977. Electron microscopic studies of the assembly, intracellular transport, and secretion of chylomicrons by rat intestine. J. Lipid Res. 18: 496–511 [PubMed] [Google Scholar]
- 27.Simon L., Shine G., Dayan A. D.1995. Translocation of particulates across the gut wall—a quantitative approach. J. Drug Target. 3: 217–219. doi: 10.3109/10611869509015948 [DOI] [PubMed] [Google Scholar]
- 28.Starling E. H.1896. On the absorption of fluids from the connective tissue spaces. J. Physiol. 19: 312–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi S., Kawarabayasi Y., Nakai T., Sakai J., Yamamoto T.1992. Rabbit very low density lipoprotein receptor: A low density lipoprotein receptor-like protein with distinct ligand specificity. Proc. Natl. Acad. Sci. U.S.A. 89: 9252–9256. doi: 10.1073/pnas.89.19.9252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tso P., Drake D. S., Black D. D., Sabesin S. M.1984. Evidence for separate pathways of chylomicron and very low-density lipoprotein assembly and transport by rat small intestine. Am. J. Physiol. 247: 599–610 [DOI] [PubMed] [Google Scholar]
- 31.Udall J. N., Pang K., Fritze L., Kleinman R., Walker W. A.1981. Development of gastrointestinal mucosal barrier. I. The effect of age on intestinal permeability to macromolecules. Pediatr. Res. 15: 241–244. doi: 10.1203/00006450-198103000-00008 [DOI] [PubMed] [Google Scholar]
- 32.Volkheimer G.1964. The passage of small, solid particles from the intestinal canal into the chyle and blood. Wien. Med. Wochenschr. 114: 915–923 [PubMed] [Google Scholar]
- 33.Volkheimer G.1977. Persorption of particles: Physiology and pharmacology. Adv. Pharmacol. Chemother. 14: 163–187. doi: 10.1016/S1054-3589(08)60188-X [DOI] [PubMed] [Google Scholar]
- 34.Volkheimer G.1993. Persorption von mikropartikeln. Pathologe 14: 247–252 [PubMed] [Google Scholar]
- 35.Volkheimer G., Schulz F. H.1968. The phenomenon of persorption. Digestion 1: 213–218. doi: 10.1159/000196856 [DOI] [PubMed] [Google Scholar]
- 36.Volkheimer G., Schulz F. H., Lindenau A., Beitz U.1969. Persorption of metallic iron particles. Gut 10: 32–33. doi: 10.1136/gut.10.1.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wells C. L., Maddaus M. A., Erlandsen S. L., Simmons R. L.1988. Evidence for the phagocytic transport of intestinal particles in dogs and rats. Infect. Immun. 56: 278–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuji M., Tsubata M., Chin K., Onishi S., Inamoto T., Qi W.-M., Warita K., Yokoyama T., Hoshi N., Kitagawa H.2006. Persorption of luminal antigenic molecule and its specific antibody via apoptotic epithelial cells of intestinal villi and Peyer’s patches into peripheral blood in rats. J. Vet. Med. Sci. 68: 1297–1305. doi: 10.1292/jvms.68.1297 [DOI] [PubMed] [Google Scholar]
- 39.Yuji M., Fujimoto M., Miyata H., Inamoto T., Qi W.-M., Yamamoto K., Warita K., Yokoyama T., Hoshi N., Kitagawa H.2007. Persorption mechanisms of luminal antigenic particulates via apoptotic epithelial cells of intestinal villi into systemic blood circulation in orally immunized rats. J. Vet. Med. Sci. 69: 339–346. doi: 10.1292/jvms.69.339 [DOI] [PubMed] [Google Scholar]
- 40.Yuji M., Fujimoto M., Qi W.-M., Takahara E., Mantani Y., Udayanga K. G. S., Takeuchi T., Warita K., Yokoyama T., Hoshi N., Kitagawa H.2012. Persorption of IgG-Fc-coated particulates from intestinal lumen into portal blood via villous columnar epithelial cells in rat small intestine. J. Vet. Med. Sci. 74: 1447–1452. doi: 10.1292/jvms.12-0111 [DOI] [PubMed] [Google Scholar]