ABSTRACT
WBN/Kob-Leprfa (fa/fa) rats have been identified as a new animal model of type 2 diabetes (T2DM), as they are characterized by impaired pancreatic insulin secretion and severe insulin resistance. Our previous study demonstrated impaired insulin secretion and its involvement in hyperglycemia in fa/fa rats. The present study was aimed at elucidating the role of insulin resistance in the development and progression of diabetes in these animals. Troglitazone (TGZ) was used as an insulin sensitizer. Insulin resistance and insulin secretory capacity were measured by a homeostasis model assessment of insulin resistance and the area under the blood concentration–time curve for plasma insulin levels after intravenous glucose tolerance testing, respectively. The fa/fa rats exhibited marked insulin resistance between 5 and 11 weeks of age, compared with age-matched Wistar rats. The insulin secretory capacity of fa/fa rats was higher than that of Wistar rats at 5 weeks of age, but decreased by 50% between 9 and 11 weeks of age. The fa/fa rats were fed a standard diet, with or without 0.2% w/w TGZ, for 4 weeks. Treatment with TGZ significantly improved insulin resistance, hyperglycemia and hypertriglyceridemia in both prophylactic and therapeutic study groups. These results suggest that insulin resistance is markedly involved in the development and progression of T2DM in fa/fa rats.
Keywords: insulin resistance, troglitazone, type 2 diabetes, WBN/Kob-Leprfa rat
Diabetes mellitus can be broadly classified into type 1 and type 2. While type 1 diabetes is believed to be caused by an absolute lack of insulin secretion associated with the destruction of pancreatic β cells, the major causes of type 2 diabetes mellitus (T2DM) are believed to be insulin resistance and impaired insulin secretion [5]. In recent years, the rapid increase in the prevalence of diabetes has become a serious problem worldwide [20]. This increase in diabetes warrants the development of new antidiabetic drugs. The use of animal models is essential when conducting pharmacological testing pertaining to drug efficacy and for elucidating the pathophysiology of diabetes itself.
Male WBN/Kob rats are known to develop a wide range of pathological conditions, such as pancreatic endocrine disorders and chronic pancreatitis that are not associated with obesity [12, 16]. Akimoto et al. [1] recently generated a fa/fa congenic rat strain by introducing the obesity gene (Leprfa) into wild-type WBN/Kob rats through mating, creating a new rat model of diabetes. These rats represent a multifactorial inherited model of diabetes: they develop diabetes spontaneously, are primarily obese and exhibit pathological conditions, such as hyperglycemia and pancreatitis. However, the contribution of impaired insulin secretion and insulin resistance to the development and progression of diabetes in these animals is not fully understood.
In a previous study, we demonstrated the involvement of impaired insulin secretion in the development of hyperglycemia in fa/fa rats [7]. The aim of the present study was to examine the role of insulin resistance in the pathogenesis and development of T2DM in fa/fa rats.
MATERIALS AND METHODS
Animals: Male fa/fa rats and age-matched male Wistar rats (Japan SLC, Inc., Hamamatsu, Japan) were housed in plastic cages and given a standard laboratory chow powdered diet (CRF-1; Oriental Yeast Co., Ltd., Tokyo, Japan). Water was provided ad libitum, and room temperature was controlled at 23 ± 3°C with the humidity being 55 ± 5%. The room was lighted between 0700 and 1900 daily. All rats were handled according to the experimental animal guidelines of Azabu University.
Age-related changes in insulin secretory capacity and insulin resistance: Intravenous glucose tolerance tests (IVGTTs) were performed, as described previously [7], in 5-, 9- and 11-week-old fa/fa and Wistar rats (5 rats per group). After a 16-hr overnight fast, the animals were anesthetized with pentobarbital sodium (50 mg/kg, ip). A fasting blood sample (0.2 ml) was collected from the jugular vein into a heparinized tube for measurement of baseline glucose and insulin, and 0.5 g/kg glucose (20% w/v; Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) was then injected into the femoral vein. Blood samples (0.2 ml) were taken 2, 5, 10 and 20 min after the glucose injection. Finally, a blood sample (3 ml) for chemical analysis was collected from the abdominal vena cava into a heparinized tube. Blood samples were centrifuged at 3,000 × g for 15 min at 4°C, and the plasma was removed and flash-frozen for performing analyses later.
Effects of TGZ on fa/fa rats: The fa/fa rats (n=30) were divided into 4 groups: (1) TGZ (n=8) and (2) control (n=8) groups in the prophylactic study; (3) TGZ (n=7) and control (n=7) groups in the therapeutic study. The TGZ rats were fed a diet of rat chow mixed with 0.2% w/w TGZ (Daiichi-Sankyo Co., Ltd., Tokyo, Japan) for 4 weeks, beginning at 7 weeks of age for the prophylactic group and at 12 weeks of age for the therapeutic group. Blood samples were collected weekly from the tail vein during the experimental period, and IVGTTs were performed at the end of each experiment.
Blood biochemical analysis: Levels of total cholesterol (T-Chol), triglycerides (TG) and non-esterified fatty acids (NEFA) in heparinized plasma were measured using an automatic analyzer (JCA-BM 2250; JEOL Ltd., Tokyo, Japan). Plasma immunoreactive insulin was measured using a rat insulin enzyme-linked immunosorbent assay kit (Morinaga Institute of Biological Science, Inc., Yokohama, Japan).
The area under the curve (AUC) for plasma glucose and plasma insulin, which represented the total glucose level and total insulin secretion, respectively, from 0 to 20 min during IVGTT, was determined according to the trapezoidal rule. The AUC of the blood insulin levels found during IVGTT was used as an index of the insulin secretory capacity of the pancreatic β cells. The fasting plasma glucose and insulin levels were used to calculate the homeostasis model assessment of insulin resistance (HOMA-IR), a measure of insulin-resistance status [10].
Statistical analysis: The results were calculated as the mean ± SEM. Student’s t-test was used for statistical analysis; the level of significance was set at P<0.05.
RESULTS
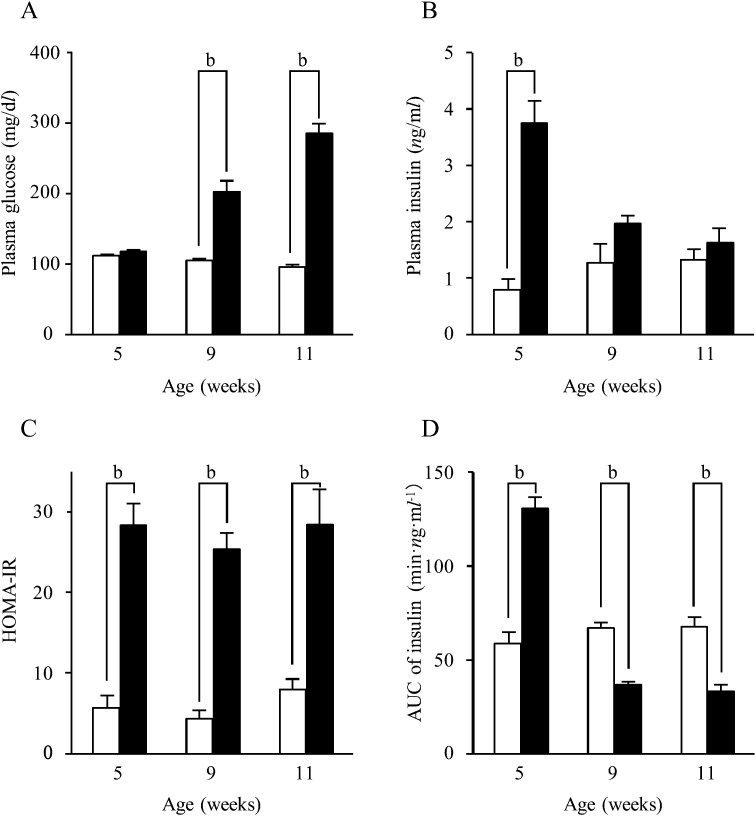
Comparison between fa/fa rats and Wistar rats: The plasma glucose levels of fa/fa rats were comparable to those of Wistar rats at 5 weeks of age, but while the levels remained constant in Wistar rats, they rose in fa/fa rats to become significantly higher between 9 and 11 weeks of age (P<0.01) (Fig. 1A). In contrast, the plasma insulin levels were significantly higher in fa/fa rats than in Wistar rats at 5 weeks of age, but there were no statistically significant differences at 9 and 11 weeks of age (Fig. 1B).
Fig. 1.
Changes in values by age. Plasma glucose (A) and insulin (B) levels; homeostasis model assessment of insulin resistance (HOMA-IR) values (C); area under the curve (AUC) of blood insulin levels (D). □: Wistar rats; ■: fa/fa rats. Numerical values are given as the mean ± SEM; b: P<0.01.
The HOMA-IR, representing insulin resistance, showed a virtually constant value in both fa/fa and Wistar rats between 5 and 11 weeks of age. Throughout the experiments, however, the HOMA-IR values of the fa/fa rats were significantly higher than those of the Wistar rats (P<0.01) (Fig. 1C).
The AUC of the blood insulin levels, representing insulin secretory capacity, was significantly higher in fa/fa rats than in Wistar rats at 5 weeks of age (59.7 ± 4.8 min·ng·ml−1 vs. 130.7 ± 6.2 min·ng·ml−1; P<0.01). In Wistar rats, the AUC of insulin at 9 and 11 weeks of age was similar to the value at 5 weeks. However, the AUC decreased markedly in fa/fa rats: the values at 9 and 11 weeks of age were significantly lower than the values of age-matched Wistar rats (P<0.01) (Fig. 1D).
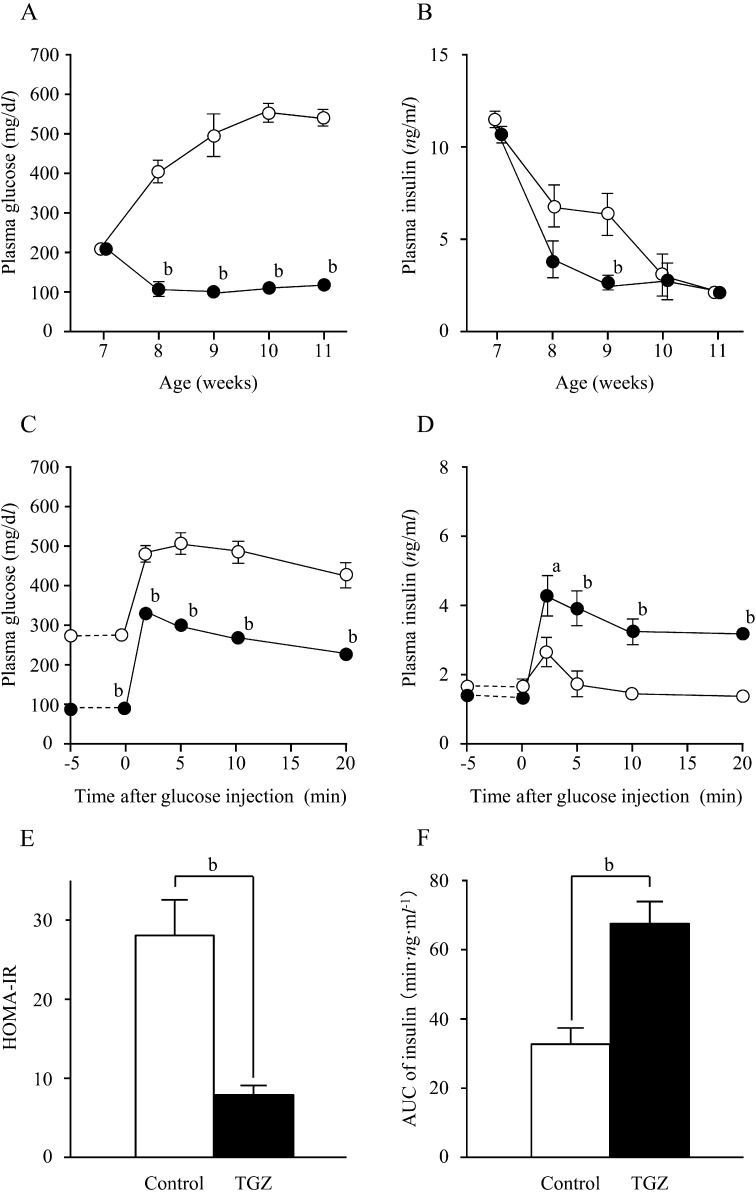
Prophylactic TGZ: The mean food intake of the TGZ and control groups was not significantly different (TGZ group, 23.1 ± 1.2 g/day; control group, 26.1 ± 1.5 g/day).
In 7-week-old fa/fa rats, blood glucose levels of the TGZ and control groups were comparable (TGZ group, 208.1 ± 21.2 mg/dl; control group, 205.3 ± 32.6 mg/dl). In the control group, blood glucose levels increased rapidly after 8 weeks of age and were as high as 541.1 ± 6.0 mg/dl at 11 weeks of age. In contrast, in the TGZ group, there was no increase in blood glucose levels after 7 weeks of age. Instead, the levels decreased to approximately 100 mg/dl, and the decreased levels were maintained until 11 weeks of age (Fig. 2A). At 7 weeks of age, there was no significant difference in insulin levels between the TGZ and control groups (TGZ group, 10.6 ± 0.7 ng/ml; control group, 11.4 ± 0.6 ng/ml). By 8 weeks of age, insulin levels decreased in both groups and were significantly lower in the TGZ group than in the control group at 9 weeks of age (P<0.01) (Fig. 2B); these values were the same in both groups at 10 and 11 weeks of age.
Fig. 2.
Changes in plasma glucose (A) and insulin (B) levels in fa/ fa rats during the prophylactic study; changes in plasma glucose (C) and insulin (D) levels on intravenous glucose tolerance testing (IVGTT); HOMA-IR values (E) and AUC of blood insulin levels (F) at the end of the prophylactic study. ○, □: control group; ●, ■: troglitazone (TGZ) group. Numerical values are given as the mean ± SEM; a: P<0.05; b: P<0.01.
Blood glucose levels peaked in both the control and TGZ groups 2 to 5 min after glucose loading. The blood glucose levels in the TGZ group were consistently and significantly lower than those in the control group, both before and after glucose loading (P<0.01) (Fig. 2C). In response to the increase in blood glucose levels, insulin levels peaked 2 min after glucose loading in both groups and decreased slowly thereafter. There was no significant difference between groups with respect to insulin levels before glucose loading, but the insulin levels in the TGZ group were significantly higher after glucose loading (P<0.05) (Fig. 2D). There were no significant differences in glucose and insulin levels between Wistar rats and TGZ-treated fa/fa rats in the IVGTTs (data not shown). The HOMA-IR values in the TGZ group were significantly lower than that in the control group (P<0.01) (Fig. 2E). The AUC of insulin was significantly higher in the TGZ group than in the control group (P<0.01) (Fig. 2F).
The results of the prophylactic study revealed that TG and NEFA levels were significantly lower in the TGZ group (P<0.01), whereas the T-Chol levels were significantly higher in the TGZ group (P<0.01) (Table 1A).
Table 1. Effects of troglitazone (TGZ) on blood lipid levels in prophylactic (A) and therapeutic (B) studies.
| Control group | TGZ group | |
|---|---|---|
| (A) Prophylactic study | ||
| Number of rats | 8 | 8 |
| TG (mg/dl) | 160 ± 17 | 46 ± 5 b |
| T-Chol (mg/dl) | 94 ± 4 | 162 ± 5 b |
| NEFA (μEq/l) | 1,526 ± 280 | 421 ± 45 b |
| (B) Therapeutic study | ||
| Number of rats | 7 | 7 |
| TG (mg/dl) | 191 ± 72 | 50 ± 8 b |
| T-Chol (mg/dl) | 114 ± 43 | 161 ± 6 b |
| NEFA (μEq/l) | 1,301 ± 492 | 1,111 ± 62 b |
Numerical values are given as the mean ± SEM. TG: triglyceride; T-Chol: total cholesterol; NEFA: non-esterified fatty acids. b: P<0.01 vs. control group.
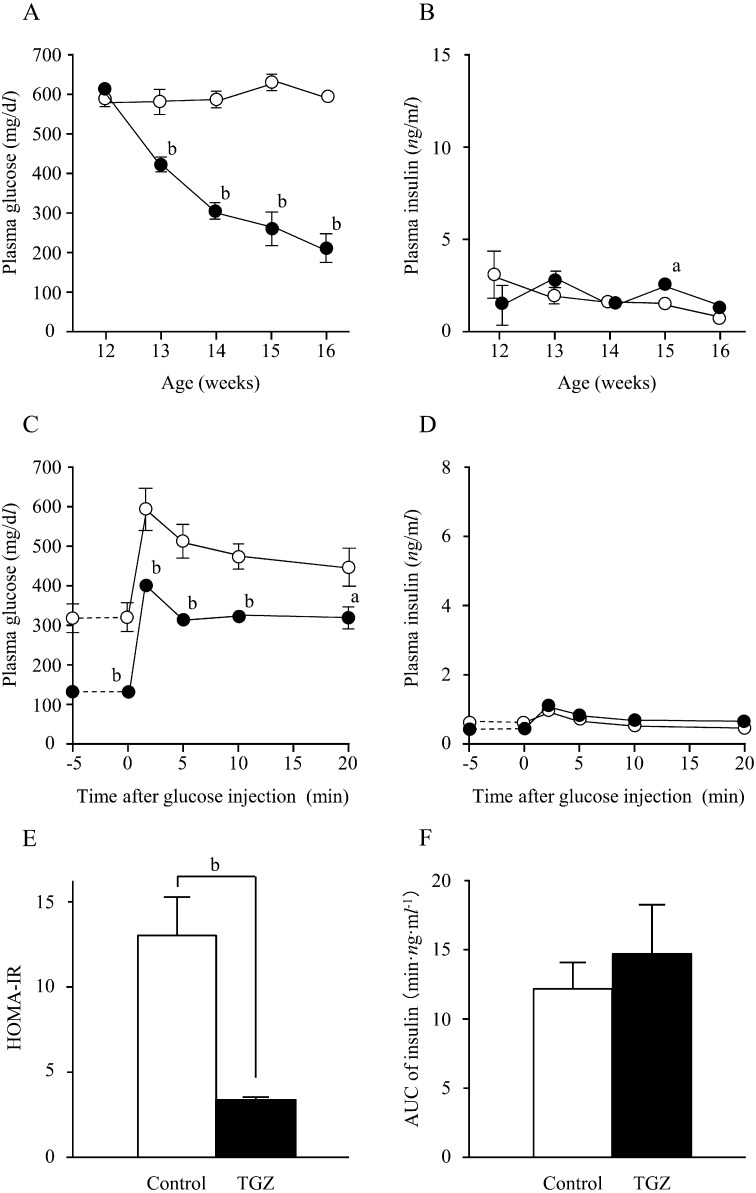
Therapeutic TGZ: The average food intake of the TGZ and control groups did not differ significantly (TGZ group, 26.5 ± 0.5 g/day; control group, 26.0 ± 0.9 g/day).
High blood glucose levels were observed in both the TGZ and control groups at 12 weeks of age, before TGZ administration (TGZ group, 610.1 ± 18.1 mg/dl; control group, 576.9 ± 28.8 mg/dl; P>0.05). The high blood glucose levels persisted in the control group, while the TGZ group demonstrated decreased blood glucose levels from 13 weeks of age, most noticeably 1 week after TGZ administration (P<0.01). The blood glucose levels in the TGZ group tended to decrease continuously over the study period (Fig. 3A). Insulin levels remained within the same range in both groups; no significant difference was found except at 15 weeks of age (3 weeks after TGZ administration) (Fig. 3B).
Fig. 3.
Changes in plasma glucose (A) and insulin (B) levels in fa / fa rats during the therapeutic study, changes in plasma glucose (C) and insulin (D) levels on IVGTT; HOMA-IR values (E) and AUC of blood insulin levels (F) at the end of the therapeutic study. ○, □: control group; ●, ■: TGZ group. Numerical values are given as the mean ± SEM; a: P<0.05; b: P<0.01.
In both groups, blood glucose levels peaked 2 min after glucose loading and decreased gradually thereafter; the blood glucose levels in the TGZ group were significantly lower than those in the control group, both before and after glucose loading (P<0.01) (Fig. 3C). In both groups, insulin levels peaked 2 min after glucose loading in response to a rise in blood glucose levels induced by glucose loading, but there was no significant difference between groups before or after glucose loading (Fig. 3D). However, the insulin levels in both groups were much lower than those seen in the 11-week-old fa/fa and Wistar rats in the initial experimentation groups. There were no significant differences in glucose and insulin levels between Wistar rats and TGZ-treated fa/fa rats in the IVGTTs (data not shown). The HOMA-IR was significantly lower in the TGZ group than in the control group (P<0.01) (Fig. 3E). The AUC of the insulin level in the TGZ and control groups was 14.5 ± 3.6 and 12.1 ± 1.8 min·ng·ml−1, respectively (P>0.05) (Fig. 3F).
The therapeutic study showed that plasma TG and NEFA levels were significantly lower in the TGZ group (P<0.01), whereas the T-Chol level was significantly higher in the TGZ group (P<0.01) (Table 1B).
DISCUSSION
T2DM is a disease primarily characterized by chronic hyperglycemia due to an imbalance between insulin secretion and sensitivity. The present study examines the involvement of insulin resistance in the onset and progression of diabetes in fa/fa rats.
A comparative study with Wistar rats revealed that fa/fa rats have higher insulin resistance. This insulin profile is consistent with our previous data [7] and with past studies reporting that Zucker fatty rats with Leprfa exhibit obesity, insulin resistance and hyperinsulinemia [3, 8, 21]. The level of insulin resistance is constant from 5 to 11 weeks of age. The fa/fa rats also have high plasma insulin levels at 5 weeks of age with decreased levels at 9 and 11 weeks of age. This reduction in plasma insulin might be due to pancreatic β cell dysfunction similar to that observed in WBN/Kob rats [1, 16]. The insulin profile found in our study suggests that the metabolic abnormalities observed in fa/fa rats meet the definition of T2DM. Compared to the parent strains, fa/fa rats are characterized by an earlier onset of diabetes and more severe pancreatic dysfunction. The latter feature distinguishes fa/fa rats from Zucker diabetic fatty (ZDF) rats, which are widely used as an animal model of T2DM with obesity. Future studies investigating the genetic background of fa/fa rats are necessary to further differentiate fa/fa rats from other animal models of T2DM, including ZDF rats.
Next, we examined the contribution of insulin resistance to the onset and progression of T2DM in fa/fa rats using TGZ, an insulin sensitizer. TGZ is a prototype thiazolidinedione derivative that is reported to improve insulin resistance through its action on peroxisome proliferator-activated receptor gamma (PPARγ) [4]. Previous studies have suggested that TGZ promotes the apoptosis of hypertrophic/large adipocytes and the differentiation of preadipocytes into small adipocytes [6, 13, 19]. Large adipocytes produce significant quantities of resistin, free fatty acids and tumor necrosis factor alpha, which impair insulin signaling in the liver and skeletal muscles, causing insulin resistance [13].
In the present study, TGZ treatment of fa/fa rats resulted in the significant inhibition of hyperglycemia and a decrease in insulin resistance in both prophylactic and therapeutic studies. The TGZ dose used in this study is equivalent to 130–140 mg/kg and has been frequently used in rat pharmacologic studies [4, 9, 17]. Treatment using TGZ in fa/fa rats reduced plasma TG and T-Chol levels in the present study; these plasma-lipid effects are consistent with previously reported data from both human and animal experiments [11, 18]. In addition, TGZ treatments resulted in a significant increase in body weight (data not shown), which is a typical adverse effect of thiazolidinedione derivatives [14, 15].
The current and previous [7] studies showed that fa/fa rats exhibit hyperinsulinemia at 5 and 7 weeks of age, but that plasma insulin levels gradually decrease thereafter. The high early insulin levels might represent compensation for high insulin resistance with hyperglycemia resulting when plasma insulin levels eventually drop. It was previously reported that impaired pancreatic β cells precede the onset of hyperglycemia [1, 2]. Insulin secretary capacity, as measured by the IVGTT, was enhanced by TGZ in our prophylactic study; however, plasma insulin levels in TGZ-treated rats at 8 and 9 weeks of age, when plasma insulin levels were sharply decreasing, were lower than those seen in the control rats. The insulin levels at 7, 10 and 11 weeks of age were comparable. The precise mechanism responsible for this observation is not clear. It is unlikely that the higher insulin secretory capacity observed in TGZ-treated fa/fa rats is due to the direct effects of TGZ on pancreatic β cells, which prevent pancreatitis/necrosis, as TGZ does not have a direct anti-inflammatory or anti-necrosis action. One possible explanation is that the improvement of insulin resistance by TGZ results in a decrease in the amount of insulin required to maintain normal blood glucose levels, thereby protecting pancreatic β cells from exhaustion of insulin secretion.
Taken together, the data in the current study and previous studies suggest that fa/fa rats exhibit insulin resistance from an early age, but the occurrence of hyperglycemia is prevented through compensatory enhanced insulin secretion. Although direct evidence is lacking, it is assumed that as the rats age, the burden imposed by the compensatory hypersecretion of insulin exhausts the pancreatic β cells, leading to impaired insulin secretion and the development of diabetes. After the onset of diabetes, chronic hyperglycemia develops as a result of severe insulin resistance and impaired insulin secretion. Furthermore, the burden on the pancreas causes irreversible changes in the β cells, which further exacerbate the pathophysiological mechanisms involved in diabetes. Pathological analysis is needed to confirm our hypothesis.
In summary, our study suggests that fa/fa rats exhibit insulin resistance and impaired insulin secretion and that insulin resistance is decidedly involved in the onset and progression of diabetes in these rats. The fa/fa rats as models of T2DM are useful for elucidating the pathophysiological mechanisms of T2DM that are associated with advanced pancreatic dysfunction and obesity as well as with the development of new antidiabetic agents.
ACKNOWLEDGMENTS
This study was partially supported by a Grant-in-aid for Scientific Research (Startup) 20880029 from the Japan Society for Promotion of Science, Ministry of Education, Culture, Sports, Science and Technology of Japan and by a research project grant awarded by Azabu University. The authors wish to thank Dr. Hiroyoshi Horikoshi for his insightful discussions.
REFERENCES
- 1.Akimoto T., Nakama K., Katsuta Y., Zhang X. J., Ohsuga M., Ishizaki M., Sawai N., Ozawa H.2008. Characterization of a novel congenic strain of diabetic fatty (WBN/Kob-Leprfa) rat. Biochem. Biophys. Res. Commun. 366: 556–562. doi: 10.1016/j.bbrc.2007.12.003 [DOI] [PubMed] [Google Scholar]
- 2.Akimoto T., Terada M., Shimizu A.2012. Progression of pancreatitis prior to diabetes onset in WBN/Kob-Leprfa rats. J. Vet. Med. Sci. 74: 65–70. doi: 10.1292/jvms.11-0168 [DOI] [PubMed] [Google Scholar]
- 3.Chua S. C., Jr, White D. W., Wu-Peng X. S., Liu S. M., Okada N., Kershaw E. E., Chung W. K., Power-Kehoe L., Chua M., Tartaglia L. A., Leibel R. L.1996. Phenotype of fatty due to Gln269Pro mutation in the leptin receptor (Lepr). Diabetes 45: 1141–1143. doi: 10.2337/diabetes.45.8.1141 [DOI] [PubMed] [Google Scholar]
- 4.Fujiwara T., Yoshioka S., Yoshioka T., Ushiyama I., Horikoshi H.1988. Characterization of new oral antidiabetic agent CS-045. Studies in KK and ob/ob mice and Zucker fatty rats. Diabetes 37: 1549–1558. doi: 10.2337/diabetes.37.11.1549 [DOI] [PubMed] [Google Scholar]
- 5.Gupta D., Krueger C. B., Lastra G.2012. Over-nutrition, obesity and insulin resistance in the development of β-cell dysfunction. Curr. Diabetes Rev. 8: 76–83. doi: 10.2174/157339912799424564 [DOI] [PubMed] [Google Scholar]
- 6.Kadowaki T.2000. Insights into insulin resistance and type 2 diabetes from knockout mouse models. J. Clin. Invest. 106: 459–465. doi: 10.1172/JCI10830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaji N., Okuno A., Ohno-Ichiki K., Oki H., Ishizawa H., Shirai M., Asai F.2012. Plasma profiles of glucose, insulin and lipids in male WBN/Kob-Leprfa rat, a new model of type 2 diabetes with obesity. J. Vet. Med. Sci. 74: 1185–1189. doi: 10.1292/jvms.12-0045 [DOI] [PubMed] [Google Scholar]
- 8.Leonard B. L., Watson R. N., Loomes K. M., Phillips A. R., Cooper G. J.2005. Insulin resistance in the Zucker diabetic fatty rat: a metabolic characterisation of obese and lean phenotypes. Acta Diabetol. 42: 162–170. doi: 10.1007/s00592-005-0197-8 [DOI] [PubMed] [Google Scholar]
- 9.Marra F., DeFranco R., Robino G., Novo E., Efsen E., Pastacaldi S., Zamara E., Vercelli A., Lottini B., Spirli C., Strazzabosco M., Pinzani M., Parola M.2005. Thiazolidinedione treatment inhibits bile duct proliferation and fibrosis in a rat model of chronic cholestasis. World J. Gastroenterol. 11: 4931–4938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., Turner R. C.1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419. doi: 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 11.Min K. M., Park S. W., Cho K. Y., Song M. S., Kim D. K., Park G. S., Lee M. K.2002. Troglitazone improves blood flow by inhibiting neointimal formation after balloon injury in Otsuka Long-Evans Tokushima fatty rats. Metabolism 51: 998–1002. doi: 10.1053/meta.2002.34027 [DOI] [PubMed] [Google Scholar]
- 12.Ohashi K., Kim J. H., Hara H., Aso R., Akimoto T., Nakama K.1990. WBN/Kob rats. A new spontaneously occurring model of chronic pancreatitis. Int. J. Pancreatol. 6: 231–247 [PubMed] [Google Scholar]
- 13.Okuno A., Tamemoto H., Tobe K., Ueki K., Mori Y., Iwamoto K., Umesono K., Akanuma Y., Fujiwara T., Horikoshi H., Yazaki Y., Kadowaki T.1998. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J. Clin. Invest. 101: 1354–1361. doi: 10.1172/JCI1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross S. A., Dzida G., Vora J., Khunti K., Kaiser M., Ligthelm R. J.2011. Impact of weight gain on outcomes in type 2 diabetes. Curr. Med. Res. Opin. 27: 1431–1438. doi: 10.1185/03007995.2011.585396 [DOI] [PubMed] [Google Scholar]
- 15.Stein S. A., Lamos E. M., Davis S. N.2013. A review of the efficacy and safety of oral antidiabetic drugs. Expert Opin. Drug Saf. 12: 153–175. doi: 10.1517/14740338.2013.752813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuchitani M., Saegusa T., Narama I., Nishikawa T., Gonda T.1985. A new diabetic strain of rat (WBN/Kob). Lab. Anim. 19: 200–207. doi: 10.1258/002367785780893575 [DOI] [PubMed] [Google Scholar]
- 17.Wendel A. A., Belury M. A.2006. Effects of conjugated linoleic acid and troglitazone on lipid accumulation and composition in lean and Zucker diabetic fatty (fa/fa) rats. Lipids 41: 241–247. doi: 10.1007/s11745-006-5093-7 [DOI] [PubMed] [Google Scholar]
- 18.Willi S. M., Kennedy A., Wallace P., Ganaway E., Rogers N. L., Garvey W. T.2002. Troglitazone antagonizes metabolic effects of glucocorticoids in humans: effects on glucose tolerance, insulin sensitivity, suppression of free fatty acids, and leptin. Diabetes 51: 2895–2902. doi: 10.2337/diabetes.51.10.2895 [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi T., Kamon J., Waki H., Murakami K., Motojima K., Komeda K., Ide T., Kubota N., Terauchi Y., Tobe K., Miki H., Tsuchida A., Akanuma Y., Nagai R., Kimura S., Kadowaki T.2001. The mechanisms by which both heterozygous peroxisome proliferator-activated receptor γ (PPARγ) deficiency and PPARγ agonist improve insulin resistance. J. Biol. Chem. 276: 41245–41254. doi: 10.1074/jbc.M103241200 [DOI] [PubMed] [Google Scholar]
- 20.Wild S., Roglic G., Green A., Sicree R., King H.2004. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27: 1047–1053. doi: 10.2337/diacare.27.5.1047 [DOI] [PubMed] [Google Scholar]
- 21.Zucker L. M.1965. Hereditary obesity in the rat associated with hyperlipemia. Ann. N.Y. Acad. Sci. 131: 447–458. doi: 10.1111/j.1749-6632.1965.tb34810.x [DOI] [PubMed] [Google Scholar]