ABSTRACT
Quantitative contrast enhanced ultrasound is a major breakthrough for ultrasound imaging in recent years. However, contrast enhancement of the pancreas is brief with bolus injection. To assess if continuous infusion of Sonazoid® can prolong the duration of pancreatic enhancement over bolus injections, eight adult dogs received bolus injection and continuous infusion of Sonazoid® on separate days. Contrast enhanced ultrasound of the pancreatic parenchyma and proximal descending duodenum was performed, and time intensity curves reflecting tissue perfusions were generated. Perfusion parameters- time to initial upslope, peak time, time to wash-out and peak intensity were calculated and evaluated. Fast wash-in to intense peak, followed by rapid wash-out was observed for time intensity curves of bolus injection. With continuous infusion, contrast wash-in to peak intensity was gradual, followed by long plateau and slow wash-out. Median contrast enhancement durations of the pancreas and duodenum were significantly prolonged by continuous infusion from 11 sec (range, 10 to 23 sec) and 16 sec (range, 3 to 43 sec) at bolus injection to 205 sec (range, 170 to 264 sec, P<0.01) and 193 sec (range, 169 to 216 sec, P<0.05), respectively. Median peak intensity of the pancreas was 100.9 MPV (range, 80.2 to 124.3 MPV) at bolus injection and 77.6 MPV (range, 58.2 to 99.5 MPV, P<0.05) at continuous infusion. Prolonged continuous imaging is afforded by continuous infusion of contrast agent. Peak intensity of the pancreas was slightly diminished in continuous infusion, but offered adequate imaging subjectively.
Keywords: canine, contrast ultrasound, pancreas, perfusion
Contrast enhanced ultrasound (CEUS) utilizes microbubbles as contrast agent and contrast-specific imaging modalities for real time perfusion imaging of organs [4]. Sonazoid®, a second generation contrast agent, consists of low solubility perfluorobutane gas microspheres encapsulated by a stable external shell [25]. When exposed to intermediate acoustic power (0.1<Mechanical index<0.5), it develops nonlinear resonance, resulting in harmonic signals with minimal destruction [4, 27]. For these reasons, second generation contrast agents have high stability in vivo and can provide continuous real-time imaging of longer duration [4, 27].
CEUS is used most extensively in human medicine for cardiac [28] and liver imaging and has expanded to non-liver structures, such as the pancreas, kidney, spleen, thyroid, prostate, gall bladder, bile duct and breast [29]. In veterinary medicine, CEUS is used mainly for the liver [12, 16, 20, 30], spleen [9, 19, 21] and kidney [8, 26]. Other studies exist for the canine pancreas, duodenum, jejunum and adrenal glands [10, 11, 23].
Detection and characterization of pancreatic diseases in human, such as acute pancreatitis, pancreatic adenocarcinoma and insulinoma, can be improved with CEUS [2, 13, 24]. Bolus injection of microbubbles intravenously is the method commonly employed for abdominal CEUS. The typical tissue-intensity curve for the pancreas following bolus injection shows a rapid increase followed by a short peak of strong enhancement with fast wash-out [6, 11]. This pattern of enhancement means that the duration of diagnostically useful enhancement is brief, and repeat bolus injections may be required resulting in several short, interrupted periods of enhancement. The canine pancreas is V-shaped and consists of two limbs, emerging from the body [14]. Due to its anatomy, the canine pancreas cannot be viewed entirely in a single ultrasound view [7]. These considerations allow us to foresee the difficulties in continuous imaging of multiple regions of the pancreas with the bolus injection method. The rapidity of enhancement may also result in perfusional parameter changes that are too minute (e.g. msec) to be detected. Slow bolus injection on another hand results in an infusion speed may be inconsistent due to different operators or different days. Continuous infusion method using an infusion pump may overcome the time limitation faced with bolus injection or the inconsistencies with slow bolus injection. It has been demonstrated that not only the duration of enhancement can be greatly prolonged, but also a constant plateau of enhancement throughout the duration of the infusion can be achieved with continuous infusion [1, 15, 22].
Thus, the goal of this study was to (1) characterize image enhancement of the normal canine pancreas with duodenum as reference using bolus injection and continuous infusion with contrast agent Sonazoid® and to (2) assess if continuous infusion method can prolong the duration of pancreatic enhancement over that with bolus injection.
MATERIALS AND METHODS
Eight mixed-breed adult dogs, aged between 1 and 6 years old and weighing between 11 and 18 kg, were studied. The dogs were healthy based on physical examination and exhibited no clinical signs or biochemical laboratory abnormalities (amylase, lipase and C-reactive protein) suggesting pancreatic disease. Prior to CEUS studies, fundamental B-mode US was performed on the pancreas and duodenum of each dog, and no evidence of focal or diffused abnormalities was observed. All procedures were approved by the Hokkaido University Animal Care and Use Committee.
An US scanner (Aplio XG, Toshiba Medical Systems, Tochigi, Japan) with a 5–11 MHz broadband linear probe (PLT-704 AT, Toshiba Medical Systems) suitable for pulse subtracting imaging was used. Adjustable parameters were optimized during preliminary studies and maintained for all imaged dogs. Mechanical index was set at 0.21 to minimize microbubble destruction. Focus depth was placed below the pancreas. The B-mode and contrast imaging gain were set at 100 and 75 dB, respectively. US imaging was set at 30–31 frames/sec, and the images were recorded in 40-sec cine-loops to a hard disk for off-line analysis. Perfusion of the pancreatic parenchyma and duodenal mucosa was evaluated after intravenous bolus injection and continuous infusion of microbubble contrast agent (Sonazoid®, Daiichi-Sankyo, Tokyo, Japan).
For bolus injection, we estimated that a microbubble contrast agent dose of 0.01 ml/kg would be suitable based on manufacturer’s recommendation (0.015 ml/kg) and clinical experiences with canine liver and spleen imaging in our facility [19,20,21]. For continuous infusion of microbubble contrast agent, based on preliminary studies (unpublished data), a dose 5 times of the bolus injection dose that was reported to be safe [17] was used. The experiments using bolus injection and continuous infusion were carried out on separate days for each dog in order to eliminate residual effects of microbubble contrast agent in the organs of interest or blood circulation.
For bolus injection, a single bolus of contrast agent was administered by hand through a 21 G butterfly catheter attached to a 22 G intravenous catheter placed in the cephalic vein, flushed by 3 ml of heparinized saline. For continuous infusion model, a single infusion of contrast agent diluted to 5 ml of saline was administered using a syringe pump (Top-5300, Top, Tokyo, Japan) at a rate of 5 ml/min. The contrast agent was diluted and gently shaken immediately before infusion. The injections were given in a standardized manner by the same person throughout the study. The timer on the US machine was manually started when the milky-white contrast agent entered the intravenous catheter.
Scanning was performed without sedation to exclude anesthetic influence on CEUS. The animal was positioned on left lateral recumbency, and the US probe was placed longitudinally between 2 ribs to image the transverse view of the right pancreatic limb and proximal portion of the descending duodenum. Scanning was done continuously for 5 min after bolus injection and at the start of continuous infusion of microbubble contrast agent.
For quantitative analysis, US images were analyzed using an off-line image analysis (ImageJ, US National Institutes of Health, Bethesda, MD, U.S.A.). In this system, the gray-scale level ranged from 0 to 255 mean pixel value (MPV). For bolus injection, one image per sec for the first 60 sec was analyzed. For continuous infusion, one image per sec for the first 120 sec followed by one image every 10 sec interval until 300 sec from start of infusion was analyzed. Tissue intensity was observed and evaluated for each of the region of interest (ROI) typically containing 300–600 pixels [26] placed in the pancreatic parenchyma and duodenal mucosa for bolus injection and continuous infusion (Fig. 1D). A time intensity curve (TIC) was created for each injection model. The wash-in as reflected by time to initial upslope (TTU) and peak time (Tp); peak intensity (PI) and the wash-out as reflected by time to wash-out (TTW) were measured in all ROIs (Fig. 2). TTU and TTW were defined as the time when the gray-scale level increased to and decreased to 30% of PI. All data were expressed as median and range.
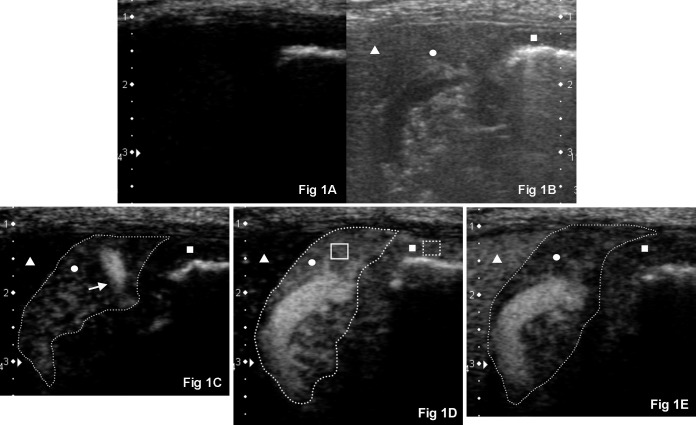
Fig. 1.
CEUS images before and after bolus injection. Right intercostal approach imaging the transverse view of the normal right pancreatic limb (circle, outlined by a dashed line) and proximal descending duodenal mucosa (square) in a representative adult dog (dorsal is to the left, ventral is to the right and medial is to the bottom). (A) CEUS at 0 sec. Baseline tissue echo components of the pancreatic parenchyma and duodenal mucosa were minimal in the CEUS mode. (B) Corresponding twin view B-mode image of (A). (C) CrPDA (white arrow) was more enhanced compared to the pancreatic parenchyma at 8 sec after bolus injection of contrast agent. Duodenal mucosa was still unenhanced. (D) Both the pancreatic parenchyma and duodenum reached its PI (shown here 12 sec after bolus injection of contrast agent). The pancreas is well delineated from the unenhanced neighboring liver (triangle). Region of interests (ROIs) are manually placed in the pancreatic parenchyma (solid box) and duodenal mucosa (dashed box) to measure the tissue intensity. (E) Contrast washing out of the pancreatic parenchyma and duodenal mucosa at 20 sec.
Fig. 2.
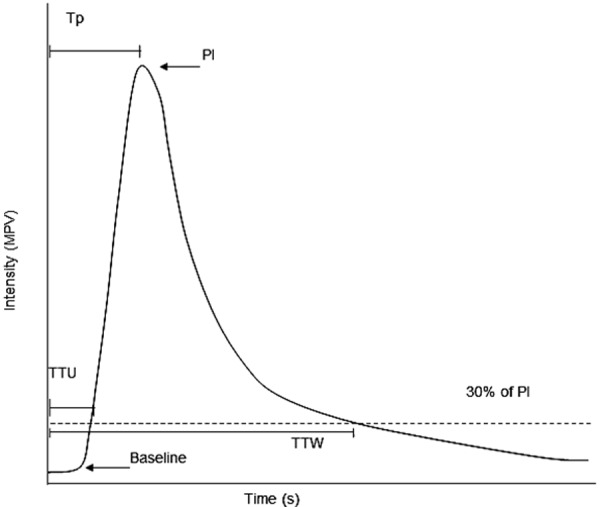
Representative TIC showing measured parameters. Baseline intensity remained unchanged until time to initial upslope (TTU) when intensity first reached 30% of peak intensity (PI). Peak time (Tp) was the time measured from contrast injection to PI. Time to wash-out (TTW) was measured from contrast injection to when intensity dropped to 30% of PI.
Normality of the data was tested using the Shapiro Wilk test. Because the data were not normally distributed, the Wilcoxon signed rank test was used to test for significant differences between pancreas and duodenum during bolus injection and continuous infusion, between pancreas during bolus injection and continuous infusion and between duodenum during bolus injection and continuous infusion for the four measured parameters. For all analyses, values of P<0.05 were considered statistically significant. Statistical analyses were performed with a statistical analysis program (JMP 8, SAS Institute Inc., Cary, NC, U.S.A.).
RESULTS
Pancreatic images from all eight dogs were included for analysis after bolus injection and continuous infusion. Images of the duodenum from one dog were excluded from the continuous infusion group due to inability to image both organs simultaneously in one field throughout the imaging period of 5 min. No adverse effects, such as dyspnea or anaphylaxis, were noticed in any dogs during or after bolus injection or continuous infusion of microbubble contrast agent.
Before contrast agent administration, the baseline tissue echo components of the pancreatic parenchyma and duodenal mucosa were minimal in the pulse subtraction CEUS mode. Some echogenic components were observed due to fat and gastrointestinal gas (Fig. 1A). After bolus injection, the cranial pancreaticoduodenal artery (CrPDA) was enhanced earliest (Fig. 1C). Soon after, enhancement of the pancreatic parenchyma was seen followed almost simultaneously by the duodenal mucosa (Fig. 3). The pancreatic parenchyma reached an intense peak several seconds later, slightly before or at the same time as the duodenal mucosa. The pancreas was more enhanced than the duodenum and was clearly delineated in the CEUS mode (Fig. 1D). Contrast effect of the pancreas and duodenum decreased sharply followed by a gradual loss of enhancement (Fig. 1E). The cranial pancreaticoduodenal vein could still be seen at this time. Subjectively, the parenchyma of the pancreas and duodenum cannot be seen clearly when the intensity dropped to around 30 MPV.
Fig. 3.
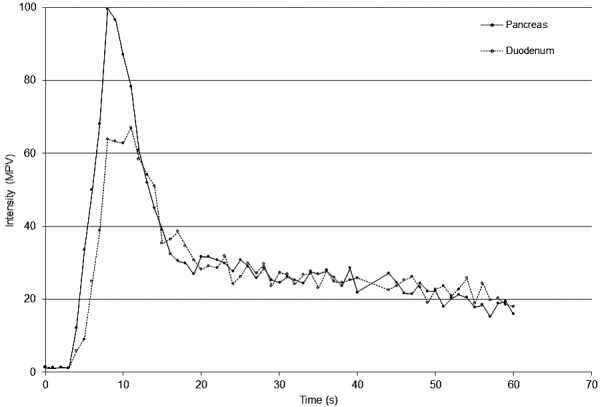
TIC showing the mean pixel intensity in the pancreatic parenchyma (solid line, ●, n=8) and duodenal mucosa (dashed line, ○, n=8) 60 sec after bolus injection of contrast agent. Notice the fast wash-in to sharp peak, followed by fast and then gradual decline in tissue intensity. MPV=Mean pixel value.
The continuous infusion method produced a slightly different enhancement profile (Fig. 4). Initial enhancement of the CrPDA and pancreatic parenchyma was delayed compared to bolus injection. Initial enhancement of the duodenal mucosa was slightly slower compared to the pancreatic parenchyma. Image enhancement of the pancreatic parenchyma and duodenal mucosa was more gradual until its peak enhancement (Fig. 5A); thereafter, the pancreas was continuously enhanced (Fig. 5B). This period of enhancement lasted longer than the period of contrast agent infusion (1 min). Subjectively, the enhancement of the pancreas and duodenum was similar at PI, but was less homogenously enhanced when compared to bolus injection. Thereafter, a gradual loss of enhancement was observed (Fig. 5C).
Fig. 4.
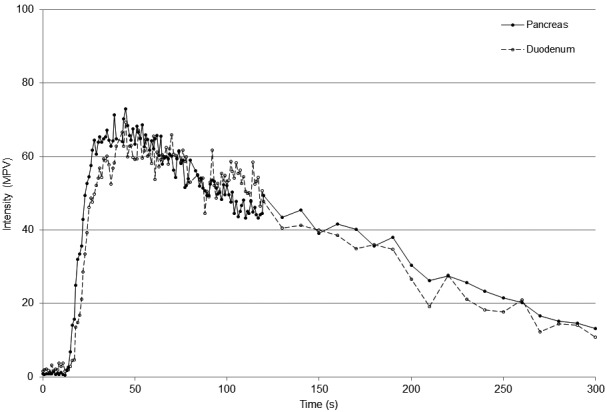
TIC showing the mean pixel intensity in the pancreatic parenchyma (solid line, ● , n=8) and duodenal mucosa (dashed line, ○, n=7) 300 sec after continuous infusion of contrast agent. Notice a more gradual wash-in to peak followed by a long plateau and slow wash-out of tissue intensity. MPV=Mean pixel value.
Fig. 5.
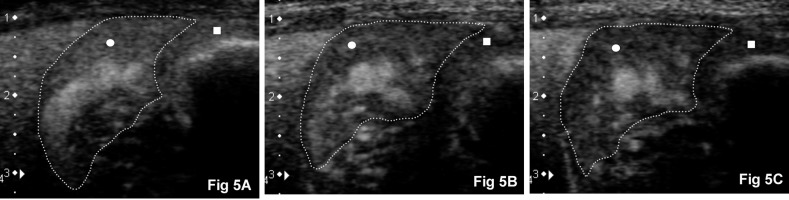
CEUS images after continuous infusion. Right pancreatic limb (circle, outlined by a dashed line) and duodenal mucosa (square) in the same representative adult dog as Fig. 1 (dorsal is to the left, ventral is to the right and medial is to the bottom). (A) Peak intensity of the pancreatic parenchyma at 43 sec. (B) Contrast enhancement of the pancreatic parenchyma persisted at 108 sec with (C) gradual wash-out as seen here at 172 sec.
The measured parameters for bolus injection and continuous infusion are summarized (Table 1). Median contrast enhancement durations (TTW–TTU) of the pancreas and duodenum were prolonged by continuous infusion from 11 sec (range, 10 to 23 sec) and 16 sec (range, 3 to 43 sec) at bolus injection to 205 sec (range, 170 to 264 sec, P<0.01) and 193 sec (range, 169 to 216 sec, P<0.05), respectively. Median PI of the pancreas at bolus injection (100.9 MPV; range, 80.2 to 124.3 MPV) was significantly (P<0.01) greater than median PI of the duodenum at bolus injection (86.5 MPV; range, 36.6 to 120.2 MPV) and median PI of the pancreas at continuous infusion (77.6 MPV; range, 58.2 to 99.5 MPV, P<0.05). Contrast enhancement of the pancreatic parenchyma was subjectively adequate with continuous infusion (Fig. 5A–C).
Table 1. Results (median, range) of the characteristic parameters of the time intensity curve measured after bolus injection and continuous infusion in the normal pancreas and duodenum of healthy dogs.
| Bolus injection |
Continuous infusion |
|||
|---|---|---|---|---|
| Pancreas | Duodenum | Pancreas | Duodenum | |
| Time to initial upslope (sec) | 6.0 (4.0–8.0) | 7.5 (4.0–9.0) | 20.0 (16.0–25.0)b, c) | 22.0 (18.0–26.0)d) |
| Peak time (sec) | 8.5 (8.0–10.0) | 10.5 (6.0–12.0) | 33.0 (19.0–52.0)c) | 52.0 (25.0–66.0)d) |
| Peak intensity (MPV e)) | 100.9 (80.2–124.3)a) | 86.5 (36.6–120.2) | 77.6 (58.2–99.5)d) | 70.2 (60.9–90.3) |
| Time to wash-out (sec) | 17.0 (15.0–30.0) | 23.5 (10.0–49.0) | 225.0 (190.0–280.0)c) | 215.0 (169.0–216.0)d) |
a) Significant (P<0.01) difference versus duodenum during bolus injection. b) Significant (P<0.05) difference versus duodenum during continuous infusion. c) Significant (P<0.01) difference versus bolus injection for corresponding organ. d) Significant (P<0.05) difference versus bolus injection for corresponding organ. e) MPV, mean pixel value.
DISCUSSION
In our study, after microbubble contrast agent administration as bolus or continuous infusion, the enhanced pancreas was clearly delineated from surrounding organs enabling good visualization. For bolus injection, the pancreas and duodenum had a similar early, intense and uniform enhancement, as could be expected in organs receiving all its blood and therefore microbubbles directly from the arterial supply [6]. The enhancement was followed by a sharp peak and then a fast initial decline; followed by a gradual washout. The patterns of enhancement of these organs were similar to another study on the canine pancreas and duodenum [11]. For continuous infusion, the enhancement of the pancreas and duodenum was initially delayed and was more gradual until it reached its peak and then plateaued. This was followed by a slow wash-out. The order at which pancreas and duodenum were enhanced did not change with bolus injection or continuous infusion.
Perfusion parameters TTU, Tp and TTW were significantly prolonged in the pancreas and duodenum using continuous infusion method when compared to bolus injection. This result can be expected, because in continuous infusion, microbubbles were infused steadily and continuously over a minute in comparison to the quick bolus injection. Reasonably, it took a longer time for the enhancement of the pancreas and duodenum to be appreciable subjectively, thus a longer TTU and Tp. The contrast enhancement was prolonged, because of this continuous inflow of microbubbles into the ROI leading to slow wash-out as shown by prolonged TTW. This effect was beneficial, because the duration of diagnostically useful enhancement for the pancreas and duodenum was prolonged to 18 and 12 times, respectively when compared to bolus injection, even though the continuous infusion dose used was only 5 times the bolus injection dose. Preliminary studies performed with lower dose (3 times of bolus dose) showed poor enhancement subjectively and were inadequate for objective analysis.
The pancreatic parenchyma was less homogenously enhanced during continuous infusion when compared to bolus injection. This could be explained by the lesser amount of microbubbles at PI due to the dilution of microbubbles during continuous infusion in contrast to bolus injection where all microbubbles injected intravenously perfuse the ROI almost simultaneously in high concentration. Lower PI was significantly evident in the pancreas; however, imaging of the pancreas was subjectively adequate. Using continuous infusion, a reduction of 23% in the PI of the pancreatic parenchyma was comparable to 16% reduction in a previous continuous infusion CEUS study of the liver [22] in which enhancement was deemed adequate despite that reduction.
In human medicine, CEUS of the pancreas is applied for identification of pancreatic tumors, such as pancreatic ductal adenocarcinoma [13] and neuroendocrine tumor [5], based on its vascularization. In severe acute pancreatitis, areas of necrosis are delineated as hypoechoic areas due to lack of vascularization [24]. However, with bolus injection of microbubble contrast agent, enhancement of the pancreas is brief due to the absence of venous blood supply unlike the portal blood supply for the liver [6]. The same limitation was seen in our study with the bolus injection method as the pancreas and duodenum were enhanced for only a brief period.
A statistical difference was noted for TTU of the pancreas and duodenum for continuous infusion, although this time difference was small. More clinically observable was the prolonged Tp of the duodenum when compared to the pancreas in continuous infusion, but not in bolus injection. This could be explained by the vascular supply of these organs. The pancreatic blood supply originates from the celiac artery through the splenic and hepatic arteries. The splenic artery is the primary blood supply to the left limb of the pancreas. The hepatic artery terminates as the CrPDA, which enters the body of the pancreas and courses through the proximal portion of the right limb of the pancreas. Branches from the CrPDA exit the pancreatic tissue and supply the closely associated duodenum [3]. In bolus injection, because of the fast speed of microbubbles washing in, this order of vascular supply was not appreciable. However, because the buildup of microbubbles to PI was slower in continuous infusion, the order of this vascular supply became appreciable, although not statistically significant. The CrPDA courses through and supplies the distal portion of the pancreatic right limb. The cranial and caudal pancreaticoduodenal arteries anastomose within the right limb of the pancreas [3]. The right pancreatic limb was analyzed in this study along with the neighboring proximal descending duodenum. Further studies will need to be conducted on the left limb and body of the pancreas due to the tripartite blood supply of the pancreas and the distal portion of the descending duodenum.
The obvious advantage of bolus injection is that it is easy to administer, less cumbersome, more repeatable [30], gives good tissue to tumor contrast and is the conventional method employed in characterizing nodular lesions [9, 12, 16, 20, 21, 27, 29]. However, contrast enhancement is too rapid and brief in the pancreas [6]. Continuous infusion method provides a gradual increase in tissue intensity and longer period of tissue enhancement [22], however, necessitating a higher dose in our study to achieve adequate imaging. Extra equipment (syringe pump) and personnel were also needed. The continuous infusion method may therefore be potentially useful in detecting differences in pancreatic perfusion in diffuse pancreatic disease that may otherwise be too subtle for detection using bolus injection method. The prolonged enhancement of the pancreas may also allow screening of the pancreas for hypovascular areas, such as necrosis. For other applications, such as characterization of nodular lesions, bolus injection should be applied so that the lesion can be studied dynamically in comparison with surrounding normal parenchyma. However, because no lesions were included into this study, further research is needed to determine when the bolus or continuous infusion method is preferable.
Wash-in time can be taken as the time from contrast agent injection when a certain percentage of increase in baseline tissue intensity is observed [11]. However, as our baseline tissue intensity was set to a minimum (nearing zero), this method was not feasible. Another method of determining wash-in time is when tissue intensity reaches a certain percentage of PI [13]. In our study, TTU and TTW were defined as time when the gray-scale level increased and decreased to 30% of PI. This percentage resulted in tissue intensity values of above 20 MPV for both bolus and continuous infusion methods. In the gray-scale, an increase of more than 20 units is generally needed for visual recognition [27].
Previous CEUS studies performed in veterinary medicine employed the usage of sedatives or general anesthesia [10, 11, 26]. In our study, no sedatives were used to eliminate its confounding effects on patterns of contrast enhancement. However, without sedatives, imaging was affected by greater motion and respiratory artifact. These movements can affect the TIC, because of difficulty in maintaining a similar ROI throughout the entire imaging period. Other than that, because of the placement of US beam parallel to the ribs, excessive movements might cause acoustic shadowing effects from the ribs resulting in compromised images with lowered tissue intensity or that cannot be analyzed. Therefore, great care was taken to place ROIs manually at similar locations whenever possible and large blood vessels were avoided. Applying these precautions resulted in a more homogenous TIC; however, large variation was seen in PI both in bolus injection and continuous infusion. Looking at individual data, all dogs had higher PI in pancreas compared to duodenum, except for one dog receiving continuous infusion. The different PI could be attributed to individual differences and perfusion status of an individual animal at any one time. Therefore, the duodenum could be used as an internal control when investigating CEUS pattern of the pancreas. However, it is important to remember that diseases of the pancreas, such as pancreatitis, may affect the surrounding organs including the duodenum [18].
The present results indicate that CEUS using bolus injection and continuous infusion can be used in dogs to image the pancreas and duodenum. However, bolus injection provides brief window for pancreatic imaging. Prolonged imaging with lowered pancreatic enhancement is afforded by continuous infusion method. The obtained baseline data using bolus injection and continuous infusion may serve as a reference in future assessment of canine pancreatic diseases.
REFERENCES
- 1.Albrecht T., Urbank A., Mahler M., Bauer A., Dore C. J., Blomley M. J., Cosgrove D. O., Schlief R.1998. Prolongation and optimization of Doppler enhancement with a microbubble US contrast agent by using continuous infusion: preliminary experience. Radiology 207: 339–347 [DOI] [PubMed] [Google Scholar]
- 2.An L., Li W., Yao K. C., Liu R., Lv F., Tang J., Zhang S.2011. Assessment of contrast-enhanced ultrasonography in diagnosis and preoperative localization of insulinoma. Eur. J. Radiol. 80: 675–680. doi: 10.1016/j.ejrad.2010.09.014 [DOI] [PubMed] [Google Scholar]
- 3.Cornell K., Fischer J.2002. Surgery of the exocrine pancreas. pp.752–753. In: Textbook of Small Animal Surgery, 3rd ed. (Slatter, D. ed.), WB Saunders, Philadelphia. [Google Scholar]
- 4.Correas J. M., Bridal L., Lesavre A., Méjean A., Claudon M., Hélénon O.2001. Ultrasound contrast agents: properties, principles of action, tolerance, and artifacts. Eur. Radiol. 11: 1316–1328. doi: 10.1007/s003300100940 [DOI] [PubMed] [Google Scholar]
- 5.D’Onofrio M., Mansueto G., Vasori S., Falconi M., Procacci C.2003. Contrast-enhanced ultrasonographic detection of small pancreatic insulinoma. J. Ultrasound Med. 22: 413–417 [DOI] [PubMed] [Google Scholar]
- 6.D’Onofrio M., Zamboni G., Faccioli N., Capelli P., Pozzi Mucelli R.2007. Ultrasonography of the pancreas. 4. Contrast-enhanced imaging. Abdom. Imaging 32: 171–181. doi: 10.1007/s00261-006-9010-6 [DOI] [PubMed] [Google Scholar]
- 7.Dominique P.2008. Pancreas. pp. 319–338. In: Atlas of Small Animal Ultrasonography (Dominique, P. and d’Anjou, M. A. eds.), Blackwell Publishing, Oxford. [Google Scholar]
- 8.Haers H., Daminet S., Smets P. M., Duchateau L., Aresu L., Saunders J. H.2013. Use of quantitative contrast-enhanced ultrasonography to detect diffuse renal changes in Beagles with iatrogenic hypercortisolism. Am. J. Vet. Res. 74: 70–77. doi: 10.2460/ajvr.74.1.70 [DOI] [PubMed] [Google Scholar]
- 9.Ivancic M., Long F., Seiler G. S.2009. Contrast harmonic ultrasonography of splenic masses and associated liver nodules in dogs. J. Am. Vet. Med. Assoc. 234: 88–94. doi: 10.2460/javma.234.1.88 [DOI] [PubMed] [Google Scholar]
- 10.Jimenez D. A., O’Brien R. T., Wallace J. D., Klocke E.2011. Intraoperative contrast-enhanced ultrasonography of normal canine jejunum. Vet. Radiol. Ultrasound 52: 196–200. doi: 10.1111/j.1740-8261.2010.01767.x [DOI] [PubMed] [Google Scholar]
- 11.Johnson-Neitman J. L., O’Brien R. T., Wallace J. D.2012. Quantitative perfusion analysis of the pancreas and duodenum in healthy dogs by use of contrast-enhanced ultrasonography. Am. J. Vet. Res. 73: 385–392. doi: 10.2460/ajvr.73.3.385 [DOI] [PubMed] [Google Scholar]
- 12.Kanemoto H., Ohno K., Nakashima K., Takahashi M., Fujino Y., Nishimura R., Tsujimoto H.2009. Characterization of canine focal liver lesions with contrast-enhanced ultrasound using a novel contrast agent-sonazoid. Vet. Radiol. Ultrasound 50: 188–194. doi: 10.1111/j.1740-8261.2009.01515.x [DOI] [PubMed] [Google Scholar]
- 13.Kersting S., Konopke R., Kersting F., Volk A., Distler M., Bergert H., Saeger H. D., Grutzmann R., Bunk A.2009. Quantitative perfusion analysis of transabdominal contrast-enhanced ultrasonography of pancreatic masses and carcinomas. Gastroenterology 137: 1903–1911. doi: 10.1053/j.gastro.2009.08.049 [DOI] [PubMed] [Google Scholar]
- 14.Konig H. E., Sautet J., Liebich H.G.2004. Digestive system. pp. 340–342. In: Veterinary Anatomy of Domestic Mammals: Textbook and Colour Atlas, 2nd ed. (Konig, H. E. and Liebich, H-G. eds.), Stuttgart: Schattauer GmbH, Germany. [Google Scholar]
- 15.Kuntz-Hehner S., Goenechea J., Pohl C., Schlosser T., Veltmann C., Lentz C., Lohmaier S., Ehlgen A., Omran H., Becher H., Tiemann K.2001. Continuous-infusion contrast-enhanced US: in vitro studies of infusion techniques with different contrast agents. Radiology 220: 647–654. doi: 10.1148/radiol.2203001628 [DOI] [PubMed] [Google Scholar]
- 16.Kutara K., Asano K., Kito A., Teshima K., Kato Y., Sasaki Y., Edamura K., Shibuya H., Sato T., Hasegawa A., Tanaka S.2006. Contrast harmonic imaging of canine hepatic tumors. J. Vet. Med. Sci. 68: 433–438. doi: 10.1292/jvms.68.433 [DOI] [PubMed] [Google Scholar]
- 17.Landmark K. E., Johansen P. W., Johnson J. A., Johansen B., Uran S., Skotland T.2008. Pharmacokinetics of perfluorobutane following intravenous bolus injection and continuous infusion of sonazoid in healthy volunteers and in patients with reduced pulmonary diffusing capacity. Ultrasound Med. Biol. 34: 494–501. doi: 10.1016/j.ultrasmedbio.2007.09.019 [DOI] [PubMed] [Google Scholar]
- 18.Moon M. L., Biller D. S., Armbrust L. J.2003. Ultrasonographic appearance and etiology of corrugated small intestine. Vet. Radiol. Ultrasound 44: 199–203. doi: 10.1111/j.1740-8261.2003.tb01271.x [DOI] [PubMed] [Google Scholar]
- 19.Nakamura K., Sasaki N., Yoshikawa M., Ohta H., Hwang S. J., Mimura T., Yamasaki M., Takiguchi M.2009. Quantitative contrast-enhanced ultrasonography of canine spleen. Vet. Radiol. Ultrasound 50: 104–108. doi: 10.1111/j.1740-8261.2008.01499.x [DOI] [PubMed] [Google Scholar]
- 20.Nakamura K., Takagi S., Sasaki N., Bandula Kumara W. R., Murakami M., Ohta H., Yamasaki M., Takiguchi M.2010. Contrast-enhanced ultrasonography for characterization of canine focal liver lesions. Vet. Radiol. Ultrasound 51: 79–85. doi: 10.1111/j.1740-8261.2009.01627.x [DOI] [PubMed] [Google Scholar]
- 21.Nakamura K., Sasaki N., Murakami M., Bandula Kumara W. R., Ohta H., Yamasaki M., Takagi S., Osaki T., Takiguchi M.2010. Contrast-enhanced ultrasonography for characterization of focal splenic lesions in dogs. J. Vet. Intern. Med. 24: 1290–1297. doi: 10.1111/j.1939-1676.2010.0609.x [DOI] [PubMed] [Google Scholar]
- 22.Okada M., Hoffmann C. W., Wolf K. J., Albrecht T.2005. Bolus versus continuous infusion of microbubble contrast agent for liver US: initial experience. Radiology 237: 1063–1067. doi: 10.1148/radiol.2373041619 [DOI] [PubMed] [Google Scholar]
- 23.Pey P., Vignoli M., Haers H., Duchateau L., Rossi F., Saunders J. H.2011. Contrast-enhanced ultrasonography of the normal canine adrenal gland. Vet. Radiol. Ultrasound 52: 560–567. doi: 10.1111/j.1740-8261.2011.01823.x [DOI] [PubMed] [Google Scholar]
- 24.Ripollés T., Martínez M. J., López E., Castelló I., Delgado F.2010. Contrast-enhanced ultrasound in the staging of acute pancreatitis. Eur. Radiol. 20: 2518–2523. doi: 10.1007/s00330-010-1824-5 [DOI] [PubMed] [Google Scholar]
- 25.Sontum P. C.2008. Physicochemical characteristics of Sonazoid, a new contrast agent for ultrasound imaging. Ultrasound Med. Biol. 34: 824–833. doi: 10.1016/j.ultrasmedbio.2007.11.006 [DOI] [PubMed] [Google Scholar]
- 26.Waller K. R., O’Brien R. T., Zagzebski J. A.2007. Quantitative contrast ultrasound analysis of renal perfusion in normal dogs. Vet. Radiol. Ultrasound 48: 373–377. doi: 10.1111/j.1740-8261.2007.00259.x [DOI] [PubMed] [Google Scholar]
- 27.Watanabe R., Matsumura M., Chen C. J., Kaneda Y., Ishihara M., Fujimaki M.2003. Gray-scale liver enhancement with Sonazoid (NC100100), a novel ultrasound contrast agent; detection of hepatic tumors in a rabbit model. Biol. Pharm. Bull. 26: 1272–1277. doi: 10.1248/bpb.26.1272 [DOI] [PubMed] [Google Scholar]
- 28.Wei K., Jayaweera A. R., Firoozan S., Linka A., Skyba D. M., Kaul S.1998. Basis for detection of stenosis using venous administration of microbubbles during myocardial contrast echocardiography: bolus or continuous infusion? J. Am. Coll. Cardiol. 32: 252–260. doi: 10.1016/S0735-1097(98)00212-5 [DOI] [PubMed] [Google Scholar]
- 29.Xu H. X.2009. Contrast-enhanced ultrasound: The evolving applications. World J. Radiol. 1: 15–24. doi: 10.4329/wjr.v1.i1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziegler L. E., O’Brien R. T., Waller K. R., Zagzebski J. A.2003. Quantitative contrast harmonic ultrasound imaging of normal canine liver. Vet. Radiol. Ultrasound 44: 451–454. doi: 10.1111/j.1740-8261.2003.tb00484.x [DOI] [PubMed] [Google Scholar]