ABSTRACT
>This study surveyed the Toxoplasma (T.) gondii infection prevalence in the Korean rabbit population. Rabbits (n=142) were obtained from two breeding farms in the Gongju area, Chungnam Province, and in the Kochang area, Junbuk Province, Korea. Of 142 sera samples analyzed by enzyme-linked immunosorbent assay (ELISA), 15 (10.6%) exhibited T. gondii-specific IgG antibodies, and 1 (0.7%) rabbit harbored T. gondii-specific IgM. Female rabbits (9/84; 10.7%) had a similar T. gondii prevalence to males (6/58; 10.3%). When stratified by age, rabbits aged >1 year had a similar prevalence of T. gondii infection (7/66; 10.6%) to rabbits aged <1 year (8/76; 10.5%). Immunoblotting detected 6 major antigenic bands corresponding to T. gondii-positive sera at 20, 28, 30, 35, 63 and 77 kDa. Nested polymerase chain reaction (PCR) of whole-blood samples detected the T. gondii B1 gene in 23 rabbits (16.2%). All PCR-positive samples corresponded to partial T. gondii B1 gene sequences with 99% homology to a T. gondii sequence deposited in GenBank (accession number EU340874). Female rabbits (13/84; 15.5%) harbored a similar prevalence of T. gondii DNA to males (10/58; 17.2%). Rabbits aged >1 year had a similar prevalence (12/66; 18.2%) of T. gondii infection to rabbits aged <1 year (11/76; 14.5%). No statistically significant differences were observed regarding the prevalences of infection according to sex or age using molecular or serological tests. This study is the first survey using serological tests and nested PCR to analyze the T. gondii prevalence in rabbits in Korea.
Keywords: ELISA, immunoblotting, nested PCR, rabbit, Toxoplasma gondii
Toxoplasma gondii is an obligate intracellular parasite that infects all warm-blooded vertebrates [14, 15]. It is generally known that cats are a major contributor to T. gondii transmission via fecal contamination of soil, food and water, because they can excrete millions of oocysts over a period of 1–2 weeks [4]. Although the prevalence of T. gondii infection in many kinds of animals has been described in many reports in the literature, there have been relatively few reports of the prevalence of T. gondii infection in the rabbit.
Rabbits are potential reservoirs for T. gondii transmission, and the detection of T. gondii from rabbits is a public health concern, as human consumption of rabbit meat continues to increase [15]. High titers of anti-Toxoplasma antibodies have been reported among rabbit hunters and workers at rabbit farms, and rabbits have been suggested to be a potential reservoir of Toxoplasma infection in humans [3, 13]. Fetal toxoplasmosis was found in three domestic rabbits in the U.S.A., and the most striking lesions in these rabbits were necrotic foci in the spleen and liver associated with massive presence of multiplying T. gondii tachyzoites [5]. In Korea, rabbits are raised on farms, and some rabbits are sold as food, while others are distributed to pet shops. Also, in Korea, reports of T. gondii infection in dogs, cats, cattle and pigs and in the human population have been continuously published [10]. However, little is known regarding the prevalence of anti-T. gondii antibodies and PCR analysis among rabbits in Korea. The purpose of this study was to survey T. gondii infection among breeding farm rabbits in Korea using ELISA, immunoblotting and nested PCR.
MATERIALS AND METHODS
Animals: One hundred forty-two rabbits (84 females, 58 males; age, 3 months–3 years; weight, 2.5–4 kg) were obtained from two collective breeding farms, one of which was located in the Gongju area of Chungnam Province in central Korea (100 rabbits), and one of which was located in the Kochang area of Junbuk Province in southwestern Korea (42 rabbits). All animals were raised communally and were asymptomatic for Toxoplasma infection. The rabbit breeds were as follows: crossbreed (n=34), Flemish giant (n=28), chinchilla (n=42) and New Zealand white (n=38). All rabbits were vaccinated for viral hemorrhagic disease. Whole blood samples were collected from auricular veins and were centrifuged to obtain sera for serological tests or combined with EDTA for nested polymerase chain reaction (PCR). All blood samples were stored at −80°C prior to use. This study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals as approved by Chungnam National University (No. CNU-00283).
ELISA for T. gondii infection: Toxoplasma lysate antigen (TLA) was prepared from T. gondii tachyzoites (RH strain). Briefly, tachyzoites were obtained from BALB/c mice that had been infected intraperitoneally with T. gondii 4 days earlier. Tachyzoites were washed several times with PBS and were homogenized by 5 sonications on ice for 30 sec each. Homogenates were centrifuged at 15,000 × g at 4°C, and supernatants were retained. The protein concentrations of the lysates were measured using a protein assay kit (Bio-Rad, Richmond, VA, U.S.A.), and lysates were stored at −70°C. ELISA was performed using a previously reported method [12]. Plates were read at 490 nm using an automatic ELISA plate reader (Sunrise, Techan, Salzburg, Austria). This cutoff value was calculated by method of Kimbita et al. [9]. Briefly, the mean optical densities of negative samples plus three standard deviations. ELISA results were considered positive for T. gondii infection when optical density values >0.39 were obtained.
Immunoblotting assay for T. gondii infection: Immunoblotting was performed using the modified method of Quan et al. [12]. Briefly, TLA was boiled with sample buffer at 100°C for 5 min, and antigens were resolved through 12% acrylamide gels (Bio-Rad). Antigens were electro-transferred to nitrocellulose membranes (Millipore, Billerica, MA, U.S.A.) at a constant voltage of 100 V for 1 hr at 4°C. Membranes were blocked overnight with 5% skim milk in PBS at 4°C. Each serum sample was diluted 1:100 in 1% BSA/PBS and was incubated with nitrocellulose strips for 2 hr at room temperature. After 3 washes with PBST, the strips were incubated for 2 hr in HRP-conjugated anti-rabbit IgG (Abcam, Cambridge, MA, U.S.A.) diluted 1:5,000 in 1% BSA/PBS at room temperature. After 3 washes, the strips were incubated with 4-chloro-1-naphthol solution for 1 hr at room temperature.
Nested PCR of T. gondii and sequencing analysis: Genomic DNA was extracted from whole blood isolates using a PrimePrep Genomic DNA Isolation Kit (GeNet Bio, Daejeon, Korea). Samples were stored at 4°C until analysis by nested PCR. The following two PCR primer pairs were used to amplify the B1 gene [7]: S1 (5’-TGTTCTGTCCTATCGCAACG-3’) and AS1 (5’-ACGGATGCAGTTCCTTTCTG-3’), which amplify a 580-bp fragment, and S2 (5’- TCTTCCCAGACGTGGATTTC-3’) and AS2 (5’-CTCGACAATACGCTGCTTGA-3’), which amplify a 530-bp fragment. Nested PCR was performed using a previously reported method [1]. PCR products were resolved through an ethidium bromide-stained 2% agarose gel in TAE buffer, and amplicons were visualized under UV light. Upon confirmation of the band of interest (530 bp), PCR products were purified using a Gene All Elution kit (GenAll, Seoul, Korea) according to the manufacturer’s instructions.
The purified PCR products were sequenced by Cosmo Genetech (Seoul, Korea). The Lasergene sequence analysis software package (DNASTAR, Madison, WI, U.S.A.) was used to edit DNA sequences. Sequence alignment was conducted using ClustalV.
Statistical analysis: Statistical analyses of T. gondii prevalence among age and gender groups were performed using a Chi-squared (χ2) test in Predictive Analytics Software (PASW®) Statistics 18 (Release 18.0 standard version, SPSS Inc., Chicago, IL, U.S.A.). A P-value <0.05 was considered statistically significant.
RESULTS
ELISA to detect T. gondii infection: Fifteen sera samples were positive for IgG antibodies against T. gondii, and one sample was positive for T. gondii-specific IgM antibodies. Variable optical densities were measured among the samples (Table 1).
Table 1. The prevalence of T. gondii infection in rabbits by ELISA (IgG antibody) assay.
| Gongju area (Chungnam Province) |
Kochang area (Junbuk Province) |
Total |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| NE | NP | PR (%) | NE | NP | PR (%) | NE | NP | PR (%) | |
| Sex | |||||||||
| Females | 59 | 6 | 10.2 | 25 | 3 | 12 | 84 | 9 | 10.7 |
| Males | 41 | 3 | 7.3 | 17 | 3 | 17.6 | 58 | 6 | 10.3 |
| Age (years) | |||||||||
| <1 | 53 | 4 | 7.5 | 23 | 4 | 17.4 | 76 | 8 | 10.5 |
| >1 | 47 | 5 | 10.6 | 19 | 2 | 10.5 | 66 | 7 | 10.6 |
| Total | 100 | 9 | 9 | 42 | 6 | 14.3 | 142 | 15 | 10.6 |
NE, numbers of examined; NP, numbers of positives; PR, positive rate.
Nine of the 84 female rabbits (10.7%) harbored T. gondii-positive sera compared with 6 of the 58 males (10.3%). Among animals aged >1 year, 7 of 66 (10.6%) were T. gondii positive, versus 8 of 76 (10.5%) in the <1-year-old group. There were no statistically significant differences regarding the prevalences of infection according to sex or age. In the Gongju area of Chungnam Province, of 100 sera samples analyzed by ELISA, 9 (9.0%) exhibited T. gondii-specific IgG antibodies, and 1 (1.0%) contained T. gondii-specific IgM. Female rabbits (6/59; 10.2%) had a similar T. gondii prevalence to males (3/41; 7.3%). Rabbits aged >1 year had a similar prevalence of T. gondii infection (5/47; 10.6%) to rabbits aged <1 year (4/53; 7.5%). No statistically significant differences were detected regarding the prevalences of infection according to sex or age. In the Kochang area of Junbuk Province, of 42 sera samples analyzed by ELISA, 6 (14.3%) exhibited T. gondii-specific IgG antibodies. Female rabbits (3/25; 12.0%) had a similar prevalence to males (3/17; 17.6%). Rabbits aged >1 year had a similar prevalence of T. gondii infection (2/19; 10.5%) to rabbits aged <1 year (4/23; 17.4%). No significant differences were observed regarding the prevalences of infection according to sex or age. In the ELISA assay, the prevalence of T. gondii infection according to rabbit breeds was as follows: 14.3% (4/28) in the Flemish giant, 14.2% (6/42) in the chinchilla, 8.8% (3/34) in the crossbreed and 5.3% (2/38) in the New Zealand white.
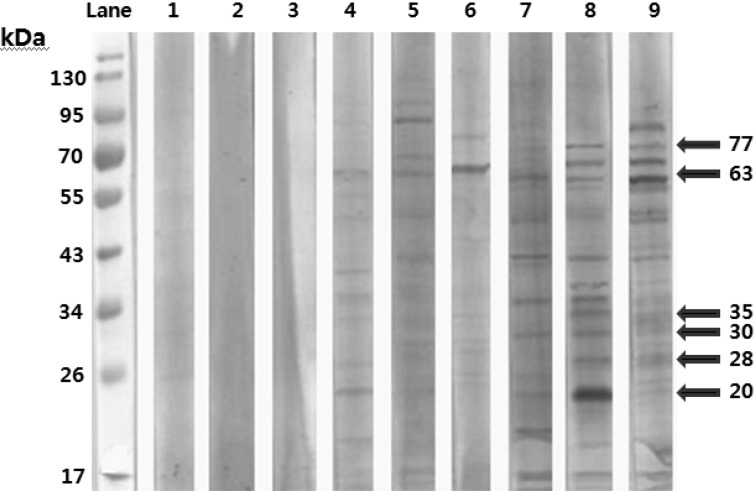
Immunoblotting assay to detect T. gondii infection: Immunoblotting was performed in three T. gondii-negative samples and six T. gondii-positive samples by ELISA. The positive samples corresponded to antigenic bands at 20, 28, 30, 35, 63 and 77 kDa (Fig. 1).
Fig. 1.
Antigenic bands of T. gondii-positive samples in rabbits (lanes 1, 2 and 3 are negative samples by ELISA; lanes 4, 5, 6, 7, 8 and 9 are positive samples by ELISA) by immunoblotting.
Nested PCR of T. gondii and sequencing analysis: Of 142 DNA samples, 23 (16.2%) were positive for the T. gondii B1 gene as detected by nested PCR (Table 2). A similar prevalence of female rabbits (13/84, 15.5%) harbored the B1 gene compared with male rabbits (10/58, 17.2%). When the population was stratified by age, rabbits aged >1 year (12/66, 18.2%) exhibited a similar T. gondii-positive prevalence to rabbits aged <1 year (11/76, 14.5%). We detected no statistically significant differences regarding the prevalences of infection according to sex or age.
Table 2. The prevalence of T. gondii infection in rabbits by nested PCR assay.
| Gongju area (Chungnam Province) |
Kochang area (Junbuk Province) |
Total |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| NE | NP | PR (%) | NE | NE | PR (%) | NE | NE | PR (%) | |
| Sex | |||||||||
| Females | 59 | 9 | 15.3 | 25 | 4 | 16 | 84 | 13 | 15.5 |
| Males | 41 | 5 | 12.2 | 17 | 5 | 29.4 | 58 | 10 | 17.2 |
| Age (years) | |||||||||
| <1 | 53 | 6 | 11.3 | 23 | 5 | 21.7 | 76 | 11 | 14.5 |
| >1 | 47 | 8 | 17 | 19 | 4 | 21.1 | 66 | 12 | 18.2 |
| Total | 100 | 14 | 14 | 42 | 9 | 21.4 | 142 | 23 | 16.2 |
NE, numbers of examined; NP, numbers of positives; PR, positive rate.
In the Gongju area of Chungnam Province, of 100 DNA samples analyzed by nested PCR, 14 (14.0%) were positive for the T. gondii B1 gene (Table 2). A total of 9 of 59 (15.3%) female rabbits were positive for the B1 gene versus 5 of 41 (12.2%) male rabbits. When the population was stratified by age, rabbits aged >1 year (8/47, 17.0%) had a similar T. gondii-positive prevalence to rabbits aged <1 year (6/53, 11.3%). No statistically significant differences were observed regarding the prevalences of infection according to sex or age.
In the Kochang area of Junbuk Province, of 42 DNA samples analyzed by nested PCR, 9 (21.4%) were positive for the T. gondii B1 gene (Table 2). Then, 4 of 25 (16.0%) female rabbits were positive for the B1 gene versus 5 of 17 (29.4%) male rabbits. Rabbits aged >1 year (4/19, 21.1%) had a similar T. gondii-positive prevalence to rabbits aged <1 year (5/23, 21.7%). No significant differences were observed regarding the prevalences of infection according to sex or age. In nested-PCR analysis, the prevalence of T. gondii infection according to rabbit breeds was as follows: 21.4% (6/28) in the Flemish giant, 21.4% (9/42) in the chinchilla, 11.8% (4/34) in the crossbreed and 10.5% (4/38) in the New Zealand white.
PCR amplicons corresponding to T. gondii-positive samples were sequenced. All PCR-positive samples corresponded to partial T. gondii B1 gene sequences with 99% homology to a T. gondii sequence deposited in GenBank (accession number EU340874). In the present study, we deposited data for 16 partial sequences that showed 99% homology to each other and to the corresponding B1 gene of T. gondii (accession numbers KF038116, KF038117, KF038118, KF038119, KF038120, KF038121, KF038122, KF038123, KF038124, KF038125, KF038126, KF038127, KF038128, KF038129, KF413760 and KF143761).
DISCUSSION
The rabbit population in Korea has increased recently with more people keeping rabbits as pets. We examined rabbits housed at two breeding farms in a mountainous region in the Gongju area of Chungnam Province and the Kochang area of Junbuk Province. Rabbits were reared outdoors, so they could potentially contact stray cats living in the area. It has been reported that the main factor affecting the prevalence of T. gondii infection is contact with T. gondii-shedding cats [2]. Almeria et al. [2] reported that rabbits could be infected by T. gondii after ingesting food or water contaminated with T. gondii oocysts that had been excreted by felids. The rabbits examined in the present study may have consumed food or water contaminated with the feces of wild cats harboring T. gondii oocysts. However, no rabbits in the study population displayed clinical symptoms of toxoplasmosis (e.g., diarrhea, weight loss), and all rabbits were observably healthy. Using ELISA, Figueroa-Castillo et al. [6] detected serum antibodies to T. gondii in rabbits at three farms in Mexico. The farm with higher rearing standards carried a T. gondii seroprevalence of 18.7%, whereas the farm with mid-level standards and a family-managed farm carried seroprevalences of 39.7 and 33.3%, respectively. In the present study, T. gondii-specific IgG antibodies were detected in 10.6% of rabbits surveyed from 2 family-managed farms. These results are lower than those reported by Figueroa-Castillo et al. [6]. We observed no significant differences regarding the prevalences of T. gondii infection when rabbits were stratified by sex or age, consistent with a report by Almeria et al. [2]. The presence of IgG antibodies in blood samples suggests chronic infection with T. gondii.
Quan et al. [12] reported that IgM antibodies increase from day 4 post experimental infection with T. gondii to day 14 post infection and then decreased on day 16 post infection. In contrast, these researchers found that IgG antibodies increased from day 10 post infection to the end of the experiment. In the present study, a rabbit was identified by ELISA as harboring both IgM and IgG antibodies recognizing T. gondii. This result suggests an overlap of increasing IgM and IgG antibodies. This same rabbit was positive for T. gondii DNA by nested PCR. Of 142 rabbits in the study population, only this rabbit was shown to be T. gondii-positive by both ELISA and nested PCR. We also performed immunoblotting experiments on the T. gondii infected rabbits and detected major antigenic bands corresponding to 20, 28, 30, 35, 63 and 77 kDa. The results of our immunoblotting analyses were similar to those of described by Quan et al. [12].
Ishikawa et al. [8] reported a case of cervical toxoplasmosis transmitted from a rabbit to a man. The B1 genomic sequence is the primary molecular target for diagnosing toxoplasmosis [9], and several primer sets have been designed against various regions on the gene [11]. The primer set S1-AS1/S2-AS2 has been used for differentiation of T. gondii strains, and these primers are sufficiently sensitive and specific to be used as a diagnostic tool for cerebral toxoplasmosis [7].
Nested PCR amplified the T. gondii B1 gene in 23 of the 142 rabbits (16.2%). DNA samples derived from whole blood. No significant differences were observed in the prevalence of infection according to sex or age, consistent with the serological results. The presence of T. gondii DNA in whole blood indicates parasitemia, most likely related to acute T. gondii infection.
In the present study, there was a higher T. gondii prevalence by nested PCR (16.2%) than by ELISA (10.6%). We suggest that the nested PCR-positive/ELISA-negative rabbits might have harbored prepatent or latent infections with T. gondii, because nested PCR is an extremely sensitive method. All PCR-positive samples corresponded to partial T. gondii B1 gene sequences with 99% homology to a T. gondii sequence deposited in GenBank (accession number EU340874).
We deposited data for 16 of 23 partial sequences that showed 99% homology to each other and to the corresponding B1 gene of T. gondii (accession numbers KF038116, KF038117, KF038118, KF038119, KF038120, KF038121, KF038122, KF038123, KF038124, KF038125, KF038126, KF038127, KF038128, KF038129, KF413760 and KF143761). In the present study, the rabbit breeds included the crossbreed (n=34), Flemish giant (n=28), chinchilla (n=42) and New Zealand white (n=38). Higher prevalence of T. gondii infection according to rabbit breed was found for the Flemish giant and chinchilla than the crossbreed and New Zealand white in the ELISA and nested PCR assay.
This study is the first survey to use serological tests and nested PCR to assess T. gondii prevalence in rabbits in Korea. A further study is needed to characterize the infective pathways from wild cats to rabbits and from rabbits to humans.
Acknowledgment
This work was supported by funding (4847-302-210-13, 2012) from the Korea National Institute of Health, Korea Centers for Disease Control and Prevention.
REFERENCES
- 1.Alfonso Y., Fraga J., Jimenez N., Fonseca C., Dorta-Contreras A. J., Cox R., Capo V., Bandera F., Pomier O., Ginorio D.2009. Detection of Toxoplasma gondii in cerebrospinal fluid from AIDS patients by nested PCR and rapid identification of type I allele at B1 gene by RFLP analysis. Exp. Parasitol. 122: 203–207. doi: 10.1016/j.exppara.2009.03.009 [DOI] [PubMed] [Google Scholar]
- 2.Almeria S., Calvete C., Pages A., Gauss C., Dubey J. P.2004. Factors affecting the seroprevalence of Toxoplasma gondii infection in wild rabbits (Oryctolagus cuniculus) from Spain. Vet. Parasitol. 123: 265–270. doi: 10.1016/j.vetpar.2004.06.010 [DOI] [PubMed] [Google Scholar]
- 3.Beverley J. K. A., Beattie C. P., Roseman C.1954. Human Toxoplasma infection. J. Hyg. 52: 37–46. doi: 10.1017/S0022172400027236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubey J. P.1994. Toxoplasmosis. J. Am. Vet. Med. Assoc. 205: 1593–1598 [PubMed] [Google Scholar]
- 5.Dubey J. P., Brown C. A., Carpenter J. L., Moore J. J.1992. Fatal toxoplasmosis in domestic rabbits in the USA. Vet. Parasitol. 44: 305–309. doi: 10.1016/0304-4017(92)90127-U [DOI] [PubMed] [Google Scholar]
- 6.Figueroa-Castillo J. A., Duarte-Rosas V., Juarez-Acevedo M., Luna-Pasten H., Correa D.2006. Prevalence of Toxoplasma gondii antibodies in rabbits (Oryctolagus cuniculus) from Mexico. J. Parasitol. 92: 394–395. doi: 10.1645/GE-663R.1 [DOI] [PubMed] [Google Scholar]
- 7.Grigg M. E., Boothoroyd J. C.2001. Rapid identification of virulent type I strain of the protozoan pathogen Toxoplasma gondii by PCR-restriction fragment length polymorphism analysis at the B1 gene. J. Clin. Microbiol. 39: 398–400. doi: 10.1128/JCM.39.1.398-400.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishikawa T., Nishino H., Ohara M., Shimosato T., Nanba K.1990. The identification of a rabbit-transmitted cervical toxoplasmosis mimicking malignant lymphoma. Am. J. Clin. Pathol. 94: 107–110 [DOI] [PubMed] [Google Scholar]
- 9.Kimbita E. N., Xuan X., Huang X., Miyazawa T., Fukumoto S., Mishima M., Suzuki H., Sugimoto C., Nagasawa H., Fujisaki K., Suzuki N., Mikami T., Igarashi I.2001. Serodiagnosis of Toxoplasma gondii infection in cats by enzyme-linked immunosorbent assay using recombinant SAG1. Vet. Parasitol. 102: 35–44. doi: 10.1016/S0304-4017(01)00522-2 [DOI] [PubMed] [Google Scholar]
- 10.Lee J. Y., Lee S. E., Lee E. G., Song K. H.2008. Nested PCR-based detection of Toxoplasma gondii in German shepherd dogs and stray cats in South Korea. Res. Vet. Sci. 85: 125–127. doi: 10.1016/j.rvsc.2007.09.006 [DOI] [PubMed] [Google Scholar]
- 11.Pujol-Rique M., Derouin F., Garcia-Quintanilla A., Valls M. E., Miro J. M., Jimenez de Anta M. T.1999. Design of a one tube hemi-nested PCR for the detection of Toxoplasma gondii and comparison of three DNA purification methods. J. Med. Microbiol. 48: 857–862. doi: 10.1099/00222615-48-9-857 [DOI] [PubMed] [Google Scholar]
- 12.Quan J. H., Hassan H. A., Cha G. H., Shin D. W., Lee Y. H.2009. Antigenemia and specific IgM and IgG antibody response in rabbits infected with Toxoplasma gondii. Korean J. Parasitol. 47: 409–412. doi: 10.3347/kjp.2009.47.4.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sroka J.2001. Seroepidemiology of toxoplasmosis in the Lublin region. Ann. Agric. Environ. Med. 8: 25–31 [PubMed] [Google Scholar]
- 14.Sugi T., Kato K., Kobayasi K., Kurokawa H., Takemae H., Gong H., Recuenco F. C., Iwanaga T., Horimoto T., Akashi H.2011. 1NM-PP1 treatment of mice infected with Toxoplasma gondii. J. Vet. Med. Sci. 73: 1377–1379. doi: 10.1292/jvms.11-0085 [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y., Zhang H., Cao J., Gong H., Zhou J.2013. Isolation and genotyping of Toxoplasma gondii from domestic rabbits in China to reveal the prevalence of type III strains. Vet. Parasitol. 193: 270–276. doi: 10.1016/j.vetpar.2012.11.031 [DOI] [PubMed] [Google Scholar]