ABSTRACT
A novel hematology analyzer for small animal medicine, ProCyte Dx, was developed from combination of the fluorescence laser flow cytometry and laminar flow impedance technologies, and its accuracy was evaluated by comparing with the conventional impedance-based hematology analyzer, pocH-100iV Diff, or microscopic manual cell counting methods with staining blood smears in the canine blood. Blood samples of 59 dogs were hematologically analyzed and compared by Pearson’s correlation coefficients. Analyses between the two analyzers showed excellent correlation in RBC (r=0.998), HGB (r=0.999), HCT (r=0.998), MCV (r=0.994), MCH (r=0.974), MCHC (r=0.906), WBC (r=0.998) and PLT (r=0.993). Analyses between ProCyte Dx and microscopic manual counting results showed excellent correlation in neutrophils (r=0.920), lymphocytes (r=0.913) and reticulocyte percentages (r=0.924), good correlation in eosinophils (r=0.815) and reticulocyte numbers (r=0.850) and fair correlation in monocytes (r=0.770). The present study indicates that ProCyte Dx is acceptably accurate and can be a powerful tool for canine clinical medicine.
Keywords: canine, complete blood count, flow cytometry, hemogram
A complete blood count (CBC) is a routine clinical examination in veterinary medical practice. CBC includes erythrocyte indices, leukogram and platelet counts, as well as reticulocyte counts, especially in anemic patients [1, 5]. A high demand is placed on the hematology analyzer to produce rapid and accurate CBC which is essential for correct diagnosis, treatment and follow-up of animal patients with a variety of disorders. Some veterinary hematology analyzers have replaced time-intensive manual counting methods with automated instruments to respond increasing demand for CBC. The conventional instruments used were based on the impedance technology enabling fast and accurate enumeration of blood cell numbers [3, 9], but not permitting detailed demarcation of cell morphology to produce leukogram and evaluation of reticulocytes.
Advances in laser-based technology provide powerful instruments to evaluate leukocytes and reticulocytes in human clinical medicine [4, 6]. Popularization of the laser-based hematology analyzers contributes to rapid determination of clinical condition in human patients. However, such analyzers were uncommon in veterinary medicine. In the present study, a new hematology analyzer, ProCyte Dx (IDEXX Laboratories, Westbrook, MA, U.S.A.), was basically developed from combination of the fluorescence laser flow cytometry and laminar flow impedance technologies, and its accuracy was evaluated by comparing with the conventional impedance-based hematology analyzer, pocH-100iV Diff (Sysmex Corporation, Kobe, Japan) [3, 9], or manual cell counting methods in the canine blood.
Fresh blood samples were collected from 59 dogs and analyzed on 2 in-clinic hematology analyzers, ProCyte Dx (IDEXX Laboratories) and pocH-100iV Diff (Sysmex Corporation) [3, 9]. All samples were received in the Department of Veterinary Internal Medicine, the University of Tokyo as 10 clinically normal dogs and a part of the diagnostic work-up of diseased patients at the Veterinary Medical Center in the University of Tokyo. Patients with acute leukemia were excluded. Venous blood samples were collected in tubes containing ethylenediamine-tetraacetic acid (EDTA) as anticoagulants and subjected to both analyzers within 10 min of blood collection.
Blood smears were prepared within 10 min of blood collection and stained with modified Wright’s-Giemsa’s stain. On the staining procedure, smears were fixed with methanol for 3 to 5 min and then stained for 15 min with the modified solution consisting of phosphate buffer pH6.4, Wright’s solution and Giemsa’s solution in the ratio of 10, 1 and 1, respectively. Smears were microscopically evaluated for quality and cell distribution, and leukogram was generated from 200-cell differential counts by 3 experienced veterinary hematologists. The mean of the percentage values was calculated for each cell type. Both band and segmented neutrophils were included in the total neutrophil count, since these cells were undifferentiated separately by ProCyte Dx.
Blood smears were prepared within 10 min of blood collection and supravitally stained with new methylene blue for 15 min [5]. Manual reticulocyte percentages were microscopically determined by calculating the percentage of reticulocytes in 1,000 erythrocytes. Manual reticulocyte numbers were calculated from the manual percentages and RBC counted by ProCyte Dx. The mean of the percentage values observed by 3 experienced veterinary hematologists was calculated.
On ProCyte Dx and pocH-100iV Diff, hemoglobin (Hb) is measured with a cyanide-free spectophotometric method, and an impedance-based method is used for erythrocyte (red blood cell, RBC) count, hematocrit (HCT), leukocyte (white blood cell, WBC) count and platelet (PLT) count in canine blood. Mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) are calculated automatically from the results.
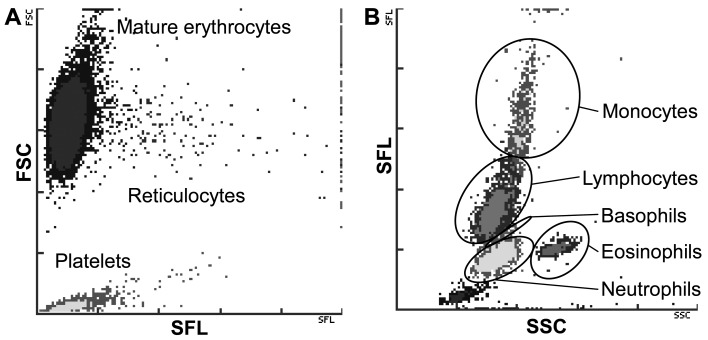
On ProCyte Dx, an optical method using fluorescence flow cytometry and a semiconductor laser is used for reticulocytes and leukogram. After blood cells are stained with polymethylene which is a fluorescent dye staining nucleic acids in ProCyte Dx, flow cytometry can measure reticulocytes by detecting forward scatter (FSC) and side fluorescence (SFL) of the cells (Fig. 1A) and leukogram to differentiate leukocytes into neutrophils, eosinophils, basophils, monocytes and lymphocytes by detecting SFL and side scatter (SSC) of the cells (Fig. 1B).
Fig. 1.
Demonstration of typical red blood cell/platelet (A) and white blood cell (B) dot plots generated by ProCyte Dx. Superior separation of cell population with the combination of both standard flow cytometry and fluorescent-enhanced optical analyses are shown. Forward scatter (FSC), side scatter (SSC) and side fluorescence (SFL) indicate the cellular size, complexity/granularity and nucleic acid content, respectively.
Pearson’s correlation coefficients (r) were used to compare ProCyte Dx and pocH-100iV Diff or manual results. Linear regression was generated for all parameters. All analyses were performed by using Microsoft Excel 2003 software (Microsoft Corp., Redmond, WA, U.S.A.). Based on an objective classification system for evaluation employed in previous studies [2, 3, 8, 9], correlation was characterized with modification as excellent (r>0.90), good (r=0.80 to 0.90), fair (r=0.60 to<0.80) or poor (r<0.60).
The operation of both two in-clinic hematology analyzers was generally simple and easy to follow a guide on the installed display. Hematological data were generated around 2 min of sample collection on both analyzers. The analyzers functioned well throughout the study, and required maintenance procedures were minimal. Correlation of results obtained by 3 microscopic observers was excellent for both leukogram and reticulocyte percentages.
Results for CBC including RBC indices, WBC and PLT in 59 dogs were compared between ProCyte Dx and pocH-100iV Diff. Analyses of Pearson’s correlation coefficients showed excellent correlation in all analytes (RBC, r=0.998; HGB, r=0.999; HCT, r=0.998; MCV, r=0.994; MCH, r=0.974; MCHC, r=0.906; WBC, r=0.998; PLT, r=0.993) between the two analyzers (Table 1 and Fig. 2). Analyses of linear regression showed satisfactory identity of best fit in all analytes (RBC, y=0.951x+0.0; HGB, y=1.005 × −0.2; HCT, y=0.973x+0.4; MCV, y=1.103 × −4.1; MCH, y=1.017x+0.4; MCHC, y=0.960x+1.4; WBC, y=0.951x+168.6; PLT, y=0.865x+2.5) between the two analyzers (Fig. 2).
Table 1. Hematological results obtained from 59 canine blood samples and correlation between results of ProCyte Dx and pocH-100iV Diff.
| Analyte (Unit) | ProCyte Dx |
pocH-100iV Diff |
r | ||
|---|---|---|---|---|---|
| Range | Mean (SD) | Range | Mean (SD) | ||
| RBC (×106/μl) | 1.18 – 8.53 | 5.74 (1.50) | 1.24 – 9.06 | 6.05 (1.58) | 0.998 |
| HGB (g/dl) | 2.8 – 17.8 | 12.7 (3.3) | 3.0 – 18.3 | 12.9 (3.3) | 0.999 |
| HCT (%) | 9.1 – 50.0 | 36.4 (8.9) | 9.0 – 51.4 | 37.1 (9.2) | 0.998 |
| MCV (fl) | 45.8 – 77.1 | 64.1 (5.7) | 45.3 – 72.8 | 61.8 (5.2) | 0.994 |
| MCH (pg) | 13.5 – 25.5 | 22.2 (2.0) | 13.0 – 24.4 | 21.4 (1.9) | 0.974 |
| MCHC (g/dl) | 27.7 – 39.5 | 34.7 (2.0) | 27.4 – 38.8 | 34.7 (1.9) | 0.906 |
| WBC (×103/μl) | 0.6 – 36.5 | 13.2 (8.9) | 0.5 – 37.8 | 13.9 (9.3) | 0.998 |
| PLT (×103/μl) | 2 – 932 | 388 (214) | 17 – 1,156 | 446 (245) | 0.993 |
Fig. 2.
Correlation of RBC, HGB, HCT, MCV, MCH, MCHC, WBC and PLT between results of ProCyte Dx (vertical axis) and pocH-100iV Diff (horizontal axis) in blood samples from 59 dogs using linear regression analyses. The diagonal line represents the regression equation.
Results for leukogram were compared between ProCyte Dx and the microscopic manual differentials. Results of basophils were statistically uncomparable, because basophils were unobserved in all of the microscopic blood smears. Analyses of Pearson’s correlation coefficients and linear regression showed excellent correlation in neutrophils (r=0.920, y=0.757x+17.9), lymphocytes (r=0.913, y=0.730x+9.0), good correlation in eosinophils (r=0.815, y=0.597x+1.2) and fair correlation in monocytes (r=0.770, y=0.492x+3.0) between ProCyte Dx and microscopic manual differentials (Table 2 and Fig. 3).
Table 2. Results of leukogram and reticulocyte counts obtained from 59 canine blood samples and correlation between results of ProCyte Dx and microscopic manual counts.
| Analyte (Unit) | ProCyte Dx |
Microscopic manual count |
r | ||
|---|---|---|---|---|---|
| Range | Mean (SD) | Range | Mean (SD) | ||
| Neutrophils (%) | 18.0 – 88.6 | 68.4 (14.1) | 6.0 – 90.5 | 63.5 (18.7) | 0.920 |
| Lymphocytes (%) | 6.8 – 53.5 | 19.9 (9.6) | 1.0 – 65.0 | 14.7 (12.1) | 0.913 |
| Monocytes (%) | 1.8 – 42.4 | 8.6 (6.7) | 2.5 – 50.0 | 9.4 (7.3) | 0.770 |
| Eosinophils (%) | 0 – 10.7 | 3.1 (2.7) | 0 – 12.0 | 3.3 (3.3) | 0.815 |
| Reticulocytes (%) | 0.04 – 7.28 | 1.08 (1.29) | 0.05 – 6.53 | 1.18 (1.23) | 0.924 |
| Reticulocytes (×103/μl) | 2.5 – 245.9 | 54.8 (48.8) | 3.0 – 204.9 | 61.1 (50.6) | 0.850 |
Fig. 3.
Correlation of leukocyte differential and reticulocyte results between ProCyte Dx (vertical axis) and microscopic manual counts (horizontal axis) in blood samples from 59 dogs using linear regression analyses. The diagonal line represents the regression equation.
Results for reticulocyte percentages and numbers were compared between ProCyte Dx and the microscopic manual count on new methylene blue staining preparations. Analyses of Pearson’s correlation coefficients and linear regression showed excellent correlation in reticulocyte percentages (r=0.924, y=1.025x+0.0) and good correlation in reticulocyte numbers (r=0.850, y=0.860x+6.4) between ProCyte Dx and the microscopic manual result (Table 2 and Fig. 3).
Fluorescence laser flow cytometry is essential methodology for immunological research, and the technology is widely applied to clinical hematology in human medicine [4, 6]. However, the flow cytometric technology has been scarcely ever applied to veterinary clinical hematology. Most of commercially available in-clinic CBC analyzers have been conventionally made up of the impedance technology in small animal clinical medicine [3, 9]. General advantages of the flow cytometric technology over the impedance-based technology in clinical hematology are accurate differentials of blood cells and an accurate measurement of reticulocytes. ProCyte Dx is a novel in-clinic analyzer developed in combination with fluorescence laser flow cytometry and laminar flow impedance technologies for veterinary clinical hematology, and its accuracy in dogs has been evaluated in the present study.
Analyses of Pearson’s correlation coefficients and linear regression for results of RBC, HGB, HCT, MCV, MCH, MCHC, WBC and PLT in 59 dogs showed excellent correlation between ProCyte Dx and pocH-100iV Diff (Table 1 and Fig. 2). Since pocH-100iV Diff is a globally validated and wide use hematology analyzer [3, 9], the correlated analytes of ProCyte Dx are sufficient to obtain reliable data in dogs.
Analyses for leukogram showed excellent correlation in neutrophils and lymphocytes, good correlation in eosinophils and fair correlation in monocytes between results of ProCyte Dx and the microscopic manual differential (Table 2 and Fig. 3). Similar analyses on leukogram were previously reported in LaserCyte (IDEXX Laboratories) which was made up of laser flow cytometry [8]. Making a comparison between the present and previous studies, all analytes in leukogram on ProCyte Dx could provide better results than those on LaserCyte (neutrophils, r=0.55; lymphocytes, r=0.76; monocytes, r=-0.05; eosinophils, r=0.60) in canine blood [8]. Although ProCyte Dx is thought to be a better analyzer, careful observation of blood preparations should be required, especially in cases with hematological abnormality, since leukogram by ProCyte Dx provides excellent results as the present study but may not be perfect, and morphological abnormality of blood cells cannot be recognized by the data.
Analyses for reticulocytes showed excellent correlation in the percentages and good correlation in the numbers between results of ProCyte Dx and the microscopic manual counts (Table 2 and Fig. 3). Similar analyses on reticulocyte percentages were previously reported in XT-2000iV (Sysmex Corporation) and ADVIA 2120 (Siemens Medical Solution Diagnostics, Eschborn, Germany) which were made up of laser flow cytometry [2, 7]. Making a comparison between the present and previous studies, reticulocyte percentages on ProCyte Dx could provide better results than those on XT-2000iV (r=0.90 or r=0.63) and ADVIA 2120 (r=0.53) [2, 7] in canine blood. Thus, ProCyte Dx is supposed to be the best in-clinic analyzer for evaluation of canine reticulocytes at present. Conventional evaluation of reticulocytes requires taking time and cumbersome procedures, such as supravital staining and microscopic counts in thousands of erythrocytes [5]. ProCyte Dx can contribute to saving time and labor for veterinary clinicians, nurses and technicians.
In the present study, a novel in-clinic hematological analyzer, ProCyte Dx, was developed, and its accuracy was evaluated in dogs. The present results indicate that the analyzer is considered quite acceptable for routine hematology analysis in dogs. ProCyte Dx has great advantages over conventional hematological analyzers and can be a powerful tool for all kinds of veterinary practitioners as well as canine patients. Further studies on other animal species and each of various disorders will require to expand validated availability of ProCyte Dx.
ACKNOWLEDGMENTS
The authors appreciate the instrumental, technical and analytical supports of Sysmex Corporation (Kobe, Japan) and IDEXX laboratories Japan branch. The Sysmex and IDEXX instruments used in the present study were made available as a free loan by Sysmex Corporation and IDEXX Laboratories. The authors thank veterinary practitioners of internal medicine in Veterinary Medical Center, the University of Tokyo for helpful contribution to obtain hematological data of canine patients.
REFERENCES
- 1.Barger A. M. 2003. The complete blood cell count: a powerful diagnostic tool. Vet. Clin. North Am. Small Anim. Pract. 33: 1207–1222. doi: 10.1016/S0195-5616(03)00100-1 [DOI] [PubMed] [Google Scholar]
- 2.Bauer N., Nakagawa J., Dunker C., Failing K., Moritz A. 2012. Evaluation of the automated hematology analyzer Sysmex XT-2000iV compared to the ADVIA (R) 2120 for its use in dogs, cats, and horses. Part II: Accuracy of leukocyte differential and reticulocyte count, impact of anticoagulant and sample aging. J. Vet. Diagn. Invest. 24: 74–89. doi: 10.1177/1040638711436243 [DOI] [PubMed] [Google Scholar]
- 3.Bauer N. B., Nakagawa J., Dunker C., Failing K., Moritz A. 2012. Evaluation of the impedance analyzer PocH-100iV Diff for analysis of canine and feline blood. Vet. Clin. Pathol. 41: 194–206. doi: 10.1111/j.1939-165X.2012.00405.x [DOI] [PubMed] [Google Scholar]
- 4.Cherian S., Levin G., Lo W. Y., Mauck M., Kuhn D., Lee C., Wood B. L. 2010. Evaluation of an 8-color flow cytometric reference method for white blood cell differential enumeration. Cytometry B Clin. Cytom. 78: 319–328 [DOI] [PubMed] [Google Scholar]
- 5.Cowgill E. S., Neel J. A., Grindem C. B. 2003. Clinical application of reticulocyte counts in dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 33: 1223–1244. doi: 10.1016/S0195-5616(03)00099-8 [DOI] [PubMed] [Google Scholar]
- 6.Lacombe F., Lacoste L., Vial J. P., Briais A., Reiffers J., Boisseau M. R., Bernard P. 1999. Automated reticulocyte counting and immature reticulocyte fraction measurement. Comparison of ABX PENTRA 120 Retic, Sysmex R-2000, flow cytometry, and manual counts. Am. J. Clin. Pathol. 112: 677–686 [DOI] [PubMed] [Google Scholar]
- 7.Lilliehook I., Tvedten H. 2009. Validation of the Sysmex XT-2000iV hematology system for dogs, cats, and horses. I. Erythrocytes, platelets, and total leukocyte counts. Vet. Clin. Pathol. 38: 163–174. doi: 10.1111/j.1939-165X.2009.00125.x [DOI] [PubMed] [Google Scholar]
- 8.Papasouliotis K., Cue S., Crawford E., Pinches M., Dumont M., Burley K. 2006. Comparison of white blood cell differential percentages determined by the in-house LaserCyte hematology analyzer and a manual method. Vet. Clin. Pathol. 35: 295–302. doi: 10.1111/j.1939-165X.2006.tb00134.x [DOI] [PubMed] [Google Scholar]
- 9.Riond B., Weissenbacher S., Hofmann-Lehmann R., Lutz H. 2011. Performance evaluation of the Sysmex pocH-100iV Diff hematology analyzer for analysis of canine, feline, equine, and bovine blood. Vet. Clin. Pathol. 40: 484–495 [DOI] [PubMed] [Google Scholar]