ABSTRACT
Regional cases of bovine ephemeral fever (BEF) were documented previously in Turkey. Previous cases were confirmed in South-East Turkey with low cow mortality. Recent BEF-suspected outbreaks with high mortality were documented in many regions of Turkey in 2012. The aim of study was the epidemiological examination of the outbreak and molecular characterization of the viruses detected from the outbreak. For this reason, blood samples were collected from BEF-suspected outbreak regions. From the results of RT-PCR, high rate of BEF-suspected samples (48/60 or 80%) was found positive for BEF virus (BEFV) RNA. The nucleotide sequences of the G1 region of G gene of BEFV in the current study during the 2012 outbreak were grouped into cluster II of BEFV. It was suggested that BEFV may be spread out to other neighbor countries in the future years.
Keywords: bovine ephemeral fever, bovine ephemeral fever virus, outbreak, RT-PCR
Bovine ephemeral fever (BEF) is an important viral disease of cattle causing significant economical losses including dramatic decreases in milk production, abortion in late-pregnant cows, loss of condition, temporary infertility in bulls and rarely deaths. The symptoms of diseases were reported in dairy cattle and may last for a few days. Hence, the illness is more commonly known as a 3-day sickness [5]. BEF virus (BEFV), an arbovirus and a member of the genus Ephemerovirus of family Rhabdoviridae, causes the diseases. The virus has RNA genome with a single stranded and negative sense [3]. The virus is transmitted among cattle via various species of mosquitoes. As a result, the disease exhibits a distinct increasing in warmer seasons [11, 12].
Despite the low mortality rate of the illness, the high morbidity rate and significant economic loss prompt even countries that are considered as free of BEF to closely pursue epidemics and take strict preventive actions [8].
The disease can be diagnosed by epidemiological observations and clinical symptoms [14]. However, confirmatory diagnose requires isolation of virus from the infected cattle or detection of specific BEF virus antibodies in sera, both during the acute phase of the disease and 2 weeks afterwards. These methods are time consuming and labor demanding. In addition, a method for virus detection does not always produce reliable results, and false negatives are not rare [8]. Reverse transcriptase-polymerase chain reaction (RT-PCR) has been successfully used for detection of BEFV during the period of fever [10].
In recent years, BEF cases were documented in Turkey [1]. The previous cases were reported in the South-East region of Turkey where there is a border with Syria, Iraq and Iran [1]. However, BEF-suspected outbreaks with high mortality were documented in many regions of Turkey in 2012. The aim of the study was epidemiological and molecular characterization of the virus detected from the latest outbreak.
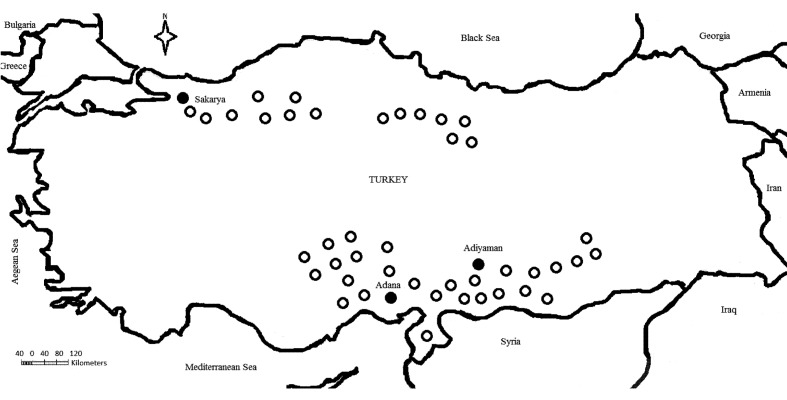
From the official and non-official reports, BEF suspected cases were announced in many provinces of Southern, Eastern and Central Anatolia, Black Sea and the Marmara Regions of Turkey during the summer of 2012. However, the samples of this study were collected from one province (Sakarya; 40°45’0”N, 30°35’0”E) of the Marmara Region and two provinces (Adana; 37°0’6”N, 35°19’44”E and Adiyaman; 37°45’51”N, 38°16’34”E) of Southern Anatolia (Fig. 1). Fifty-six blood and 35 serum samples were taken from 56 cattle (10 cattle from 2 farms in Sakarya, 20 cattle from 4 farms in Adiyaman and 26 cattle from 6 farms in Adana) from 12 farms where clinical symptoms of BEF and high fever (41–42ºC) were reported in sampling time. In addition to BEF-suspected samples, 20 serum samples were taken from cattle from 2 farms with no BEF history in Adiyaman province. Upon visiting the BEF-suspected regions, multiple farms with a number of suspected animals (dairy cattle of Holstein and Simmental breeds) were detected. The air temperature was between 38–40 degrees Celsius at the sampling time from the BEF-suspected regions.
Fig. 1.
Bovine ephemeral fever (BEF) outbreaks in Turkey, 2012. ○; BEF-suspected regions and ●; the provinces where the samples were collected for testing.
Detections of the specific IgG antibodies to the BEFV from the serum samples were performed using blocking ELISA kits by following the procedures recommended by the manufacturers (EMAI, Camden NSW, Australia).
The RNAs from the total blood samples were extracted with RNA extraction kit (ZR RNA MiniPrepTM) as described by the manufacturer (Zymo Research, Orange, CA, U.S.A.). The RNA pellets were resuspended in 25 µl of the water with diethylpyrocarbonate (DEPC). Reverse transcriptase-polymerase chain reaction (RT-PCR) was performed as described elsewhere [8]. For detection limiting of RT-PCR, 10-fold dilutions from 10–6.25 tissue culture infectious dose50 (TCID50) ml−1 in VERO cells of the virus isolated from 2008 cases were used and then tested by RT-PCR. To overcome both false positivity and false negativity, we included negative and positive controls in each experiment.
The randomly selected three RT-PCR positive products were purified using QIAquick gel purification kit (Qiagen, Hilden, Germany) and sequenced in both directions with the forward and reverse primers. The gene sequences were aligned using ClustalW program and then compared to the sequences of BEFV obtained from GenBank database.
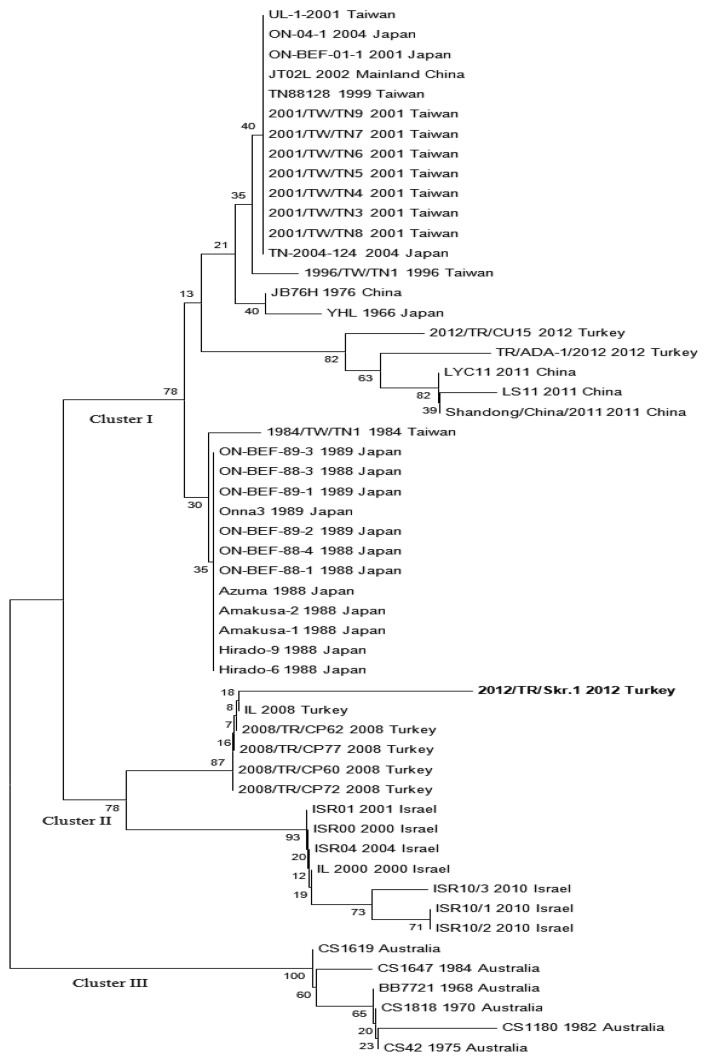
The phylogenetic analysis of nucleotide sequences of the G1 region of G gene of BEFV amplified by RT-PCR in this study was performed with comparing of the sequences of the same gene region of BEFVs obtained from GenBank database using the neighbor-joining method with MEGA 5 software (Fig. 2).
Fig. 2.
The phylogenetic tree constructed by the neighbor-joining method with Kimura two-parameter model distances using the MEGA5 program. The branching pattern was evaluated by bootstrap test analysis with 1,000 replicates. The phylogenenetic tree was performed with comparing of the nucleotide sequences of the same gene region of BEF viruses obtained from GenBank database of nucleotide sequences of the G1 region of G gene of BEFV (Sequence ID; KC337118 or 2012/TR/Skr.1) amplified by RT-PCR in this study.
The BEF suspected cases were reported by Official Veterinarians in many regions (Southern, Eastern, Western and Central Anatolia, Black Sea and Marmara Regions of Turkey) in the summer of 2012 (Fig. 1). First cases were noticed from the provinces (Adana, Şanliurfa and Hatay) adjacent to the Middle East in June, 2012. Then, the same cases were reported from provinces in many regions of Turkey during the other months of 2012 summer. The cases were reported until the end of September by animal owner and veterinarians. Animal owners reported that the disease was detected in many animals (mean 35% morbidity) in the farms, and many of the animals recovered within 3 to 4 days. But, mortality was reported from about 15–20% of the ill animals. The most obvious clinical signs reported from mortality were dyspnea and shallow respiration, and abomasal and rumen tympani were detected from BEF suspected cases by official veterinarians. In ELISA, the specific IgG antibodies to the BEFV were not detected from any of the serum samples. In the RT-PCR carried out with 10-fold dilutions of the virus isolated from the 2008 outbreak, detection limiting of RT-PCR was 0.3 TCID50 /reaction. At the end of RT-PCR of the clinical samples, expected size (520 base pair) band was obtained (data not shown). According to RT-PCR results, 48 (85.71%) out of 56 BEF suspected cows were positive, and others (14.28%) were negative for BEFV RNA. The application of RT-PCR on samples collected from this study detected BEFV genome in all of the farms.
Partial G gene of BEFV amplified by RT-PCR in this study was deposited in GenBank under sequence ID; KC337118. Sequence analysis of randomly selected three samples revealed 99% homology. Furthermore, the sequences of the samples were similar between 91% and 99% with the sequences of the same gene region of BEF viruses obtained from GenBank database. The phylogenetic results including the sequences of viruses obtained in this outbreak were shown to be 99% similar with the same region of BEF viruses (GenBank accession numbers; between GQ229451 and GQ229454) isolated from the 2008 outbreak in Turkey (Fig. 2).
It has been known that BEF has caused epidemics for years in Asia, Africa and subtropical parts of Australia [1, 2, 3, 4, 5, 6, 7, 11, 12, 13, 14, 15]. The recent evidence suggests that new endemic cases of the disease were reported from countries, such as Iran and Pakistan in the epidemic zone [9]. The foremost important reason for this increase is the spread of virus via wind and flies [1]. Climate is another important factor affecting the incidence of the virus due to its significant effects on vector population [12, 13].
In this study, the presence of BEFV RNA was detected in many of the suspected animals with BEFV. Failure of determination of amplification product in remaining suspected animals might be explained by the presence of virus in the blood stream for a shorter period of time.
There is no vaccination against BEFV in Turkey. Therefore, detection of specific antibody for BEFV indicates the presence of the infection in the region. For this reason, samples were collected from both BEFV suspected (n=35) and non-suspected cattle (n=20) to obtain general conviction of the existence of BEFV. From the results of ELISA, the serum samples collected from BEF suspected or non- suspected cases during 2012 outbreak had not shown specific IgG to BEFV. In previous years, regional cases were reported during summer with 2 or 4 years intervals. Both the ELISA results of this study and previous reports show that BEFV is not enzootic in Turkey, and introductions of BEFV from endemic countries may be causes of the repeated outbreaks in Turkey.
Although the actual vectors for BEFV (virus isolation from mosquitoes) have not been definitively identified in Turkey, RT-PCR results clearly showed existing BEFV in Turkey. Further investigations are needed to define genomes of the BEFV in mosquito species present in various areas from Turkey. From the results of phylogenetic analysis of BEFV isolates based on comparison of G gene sequences, BEFV was grouped into 3 clusters [15]. Moreover, the phylogenetic results showed that the clusters of BEF viruses were closely related with their source of origin [1, 15]. The BEF viruses isolated from the 2008 cases were grouped into cluster II together with Israel isolates of BEFV. In this study, the G1 region of BEFV G gene was analyzed. The analysis indicated that the BEF viruses of the 2012 outbreak in Turkey in this study were also into cluster II (Fig. 2). Moreover, the sequences of G1 obtained during the 2012 outbreak were similar between 95% and 99% with the same region of G gene of BEFV Israel isolates. From the results of this study, it was speculated that the same BEF viruses might be responsible for BEF cases within the geographic regions including Turkey and Israel for a long time. However, the nucleotide sequences (Accession numbers; KC470312 and KC155353) of BEF viruses submitted to GenBank by 2 different researcher groups from Turkey were into the cluster I according to our phylogenetic analysis (Fig. 2). In a phylogenetic analysis of BEFV sequences, other researchers and our study from Turkey were into cluster I and II clusters, respectively (Fig. 2). This indicated that there is simultaneous circulation of clusters I and II of BEFV in Turkey. We think that two or more outbreaks may be caused by different BEFV strains separately occurred in Turkey in 2012.
The presence of BEF in Turkey was determined previously [1, 15]. The previous BEF cases were detected from the Southern Anatolia Regions of Turkey close to the Middle East. Moreover, it should be emphasized that the regions have a common border with Syria and Iran where BEF was reported previously [1]. However, the cases of the 2012 BEF outbreak were detected from many regions of Turkey. For example, Sakarya province has a distance of approximately 1,000 km from the Southern Anatolia Region. In comparison with the distance between these two epidemic regions (Southern Anatolia and Sakarya province), it is quite obvious that Sakarya province is not very far (about 400 km) from the Balkan countries. Therefore, due to the convenient geographical location of Turkey, it will be possible that the disease may be spread out to Europe countries, such as Greece and Bulgaria, in the future years, if effective control measures are not taken. The results of the present study may prompt other countries, in some parts of the neighbors of Turkey, to cooperate in prevention from BEF.
The mortality rate (15–20%) in the outbreak of 2012 was higher than that reported in 2008, according to the official reports obtained from the registered farms. We have no data concerning causes of effect in wide geographical areas and higher mortality of 2012 BEF outbreak than 2008 BEF cases. Therefore, for a detailed description of virulence of the virus and the pathogenesis and epidemiology of the cases in this outbreak, we are planning future studies.
REFERENCES
- 1.Aziz-Boaron O., Klausner Z., Hasoksuz M., Shenkar J., Gafni O., Gelman B., David D., Klement E. 2012. Circulation of bovine ephemeral fever in the Middle East–strong evidence for transmission by winds and animal transport. Vet. Microbiol. 158: 300–307. doi: 10.1016/j.vetmic.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 2.Daniels P. W., Sendow I., Soleha E., Sukarsih, Hunt N. T., Bahri S. 1995. Australian-Indonesian collaboration in veterinary arbovirology–a review. Vet. Microbiol. 46: 151–174. doi: 10.1016/0378-1135(95)00080-T [DOI] [PubMed] [Google Scholar]
- 3.Della-Porta A. J., Brown F. 1979. The physico-chemical characterization of bovine ephemeral fever virus as a member of the family Rhabdoviridae. J. Gen. Virol. 44: 99–112. doi: 10.1099/0022-1317-44-1-99 [DOI] [PubMed] [Google Scholar]
- 4.Farag M. A., Al-Sukayran A., Mazloum K. S., Al-Bukomy A. M. 1998. Epizootics of bovine ephemeral fever on dairy farms in Saudi Arabia. Rev. Sci. Tech. 17: 713–722 [DOI] [PubMed] [Google Scholar]
- 5.Fenner F. J., Gibbs E. P., Murphy F. A., Rott R., Studdert M. J., White D. O. 1993. Bovine ephemeral fever. pp. 504–555. In: Veterinary Virology, 2nd ed., Academic Press, San Diego. [Google Scholar]
- 6.Kato T., Aizawa M., Takayoshi K., Kokuba T., Yanase T., Shirafuji H., Tsuda T., Yamakawa M. 2009. Phylogenetic relationships of the G gene sequence of bovine ephemeral fever virus isolated in Japan, Taiwan and Australia. Vet. Microbiol. 137: 217–223. doi: 10.1016/j.vetmic.2009.01.021 [DOI] [PubMed] [Google Scholar]
- 7.Liao Y. K., Inaba Y., Li N. J., Chain C. Y., Lee S. L., Liou P. P. 1998. Epidemiology of bovine ephemeral fever virus infection in Taiwan. Microbiol. Res. 153: 289–295. doi: 10.1016/S0944-5013(98)80014-1 [DOI] [PubMed] [Google Scholar]
- 8.Nandi S., Negi B. S. 1999. Bovine ephemeral fever: a review. Comp. Immunol. Microbiol. Infect. Dis. 22: 81–91. doi: 10.1016/S0147-9571(98)00027-7 [DOI] [PubMed] [Google Scholar]
- 9.Roya S. 2008. Survey on serological diagnosis of bovine ephemoral fever (BEF) by IR-BK and Vero cell line in Southern Provinces of Iran by ın vitro method. p. 267. In: The 15th Congress of the Federation of Asian Veterinary Assocation (FAVA) and FAVA/OIE Joint Symposium on Emerging Diseases Bangkok and Thailand.
- 10.Stram Y., Kuznetzova L., Levin A., Yadin H., Rubinstein-Giuni M. 2005. A real-time RT-quantative (q) PCR for the detection of bovine ephemeral fever virus. J. Virol. Methods 130: 1–6. doi: 10.1016/j.jviromet.2005.05.024 [DOI] [PubMed] [Google Scholar]
- 11.Venter G. J., Hamblin C., Paweska J. T. 2003. Determination of the oral susceptibility of South African livestock-associated biting midges, Culicoides species, to bovine ephemeral fever virus. Med. Vet. Entomol. 17: 133–137. doi: 10.1046/j.1365-2915.2003.00414.x [DOI] [PubMed] [Google Scholar]
- 12.Yeruham I., Braverman Y., Yadin H., Van Ham M., Chai D., Tiomkin D., Frank D. 2002. Epidemiological investigations of outbreaks of bovine ephemeral fever in Israel. Vet. Rec. 151: 117–121. doi: 10.1136/vr.151.4.117 [DOI] [PubMed] [Google Scholar]
- 13.Yeruham I., Van Ham M., Bar D., Yadin H., Tiomkin D. 2003. Economic aspects of the 1999 outbreak of bovine ephemeral fever in dairy cattle herds in the Jordan Valley in Israel. Vet. Rec. 153: 180–182. doi: 10.1136/vr.153.6.180 [DOI] [PubMed] [Google Scholar]
- 14.Young P. L., Spradbrow P. B. 1990. Clinical response of cattle to experimental infection with bovine ephemeral fever virus. Vet. Rec. 126: 86–88 [PubMed] [Google Scholar]
- 15.Zheng F., Qiu C. 2012. Phylogenetic relationships of the glycoprotein gene of bovine ephemeral fever virus isolated from mainland China, Taiwan, Japan, Turkey, Israel and Australia. Virol. J. 9: 268–275. doi: 10.1186/1743-422X-9-268 [DOI] [PMC free article] [PubMed] [Google Scholar]