ABSTRACT
An 8-year-old male mongrel dog that had undergone renal transplantation was presented 25 days later with an acute cough, anorexia and exercise intolerance. During the investigation, neutrophilic leukocytosis was noted, and thoracic radiographs revealed caudal lung lobe infiltration. While being treated with two broad-spectrum antibiotics, clinical signs worsened. Pneumonia due to infection with multidrug-resistant (MDR) Pseudomonas (P.) aeruginosa, sensitive only to imipenem and amikacin, was confirmed by bacteria isolation. After treatment with imipenem-cilastatin without reducing the immunosuppressant dose, clinical signs completely resolved. During the 2-year follow-up period, no recurrence was observed. To the best of authors’ knowledge, this is the first report of pneumonia caused by MDR P. aeruginosa in a renal recipient dog and successful management of this disease.
Keywords: canine, immunosuppressants, pneumonia, Pseudomonas, renal transplantation
Infectious pneumonia is a severe complication at the early stage of kidney transplantation and is associated with significant mortality in recipients not only in human clinics but also in veterinary medicine [11, 12, 22]. However, no standard guidelines for managing infectious complications after transplantation have been set in veterinary medicine. Potent immunosuppressants given to prevent host rejection could increase opportunities for microorganism infection and lead to difficulties with treatment [6]. Previous reports in humans have suggested that significantly reducing immunosuppressant doses are extremely important to treat bacterial pneumonia in kidney transplant recipients [19]. Unlike human and feline renal transplant patients, canine recipients require highly aggressive immunosuppressive therapy, because of the intense host response these animals mount against the graft [9]. A previous veterinary report has shown that the death of canine patients can occur due to graft rejection when immunosuppressive drug doses are decreased to control infectious pneumonia [11]. Therefore, it is difficult for veterinarians to decide whether the doses of immunosuppressant reagents should be reduced to treat an infection in a transplant recipient. In this report, we describe a case of pneumonia due to infection with MDR P. aeruginosa in a kidney recipient dog during the high-risk period of acute allorejection and the successful treatment of this case without reducing the immunosuppressive medication dose.
An 8-year-old, 6.3 kg, male mongrel dog was referred to Kangwon National University Veterinary Hospital for treatment of end-stage chronic kidney disease. The dog underwent heterotrophic renal transplantation in the iliac fossa with a kidney from a healthy dog leukocyte antigen cross-matched littermate donor. To assess whether a littermate dog is compatible as a kidney donor, blood typing, erythrocyte cross-match and complement-dependent cytotoxic cross-match tests were performed [14]. The transplant recipient also underwent bilateral native nephrectomy and had been given 20 mg/kg microemulsified cyclosporine (Implanta, PO, q24h; Hanmi, Seoul, Korea), 1 mg/kg predinisolone (Solondo, PO, q24h; Yuhanmedica, Ochang, Korea) and 5 mg/kg azathioprine (Immuthera, PO, q48h; Celltrion, Incheon, Korea) as immunosuppressive therapy 2 days prior to transplantation according to a previously described protocol [14]. The cyclosporine trough concentration was maintained approximately 400–700 ng/ml for the first 6 months after surgery, and then the dosage was reduced to maintain a trough concentration of 250–350 ng/ml as measured by high-performed liquid chromatography [3]. Prednisolone dose was gradually reduced 1 month after surgery and discontinued after 3 months [14]. We administered 22 mg/kg cefazolin (Cefazolin, IV, q12h; Chong Kun Dang Pharm, Seoul, Korea) for 5 days after surgery as prophylaxis. The dog showed good postoperative recovery. Laboratory evaluation results including complete blood count (CBC), serum biochemistry, urinalysis and ultrasonography were also normal 18 days after transplantation.
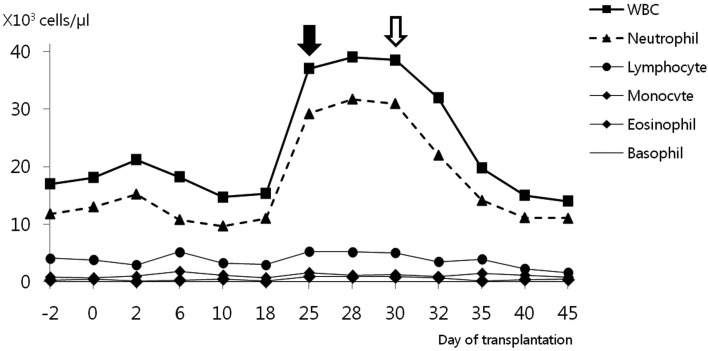
Twenty-five days after surgery, the dog was readmitted to the hospital due to acute onset anorexia, frequent coughing and exercise intolerance. Physical examination revealed a body condition score of 4/9, mild exertional dyspnea, increased respiratory rate (36 breaths/min) and fever (39.8°C). Slight crackling lung sounds were also heard during inspiration, but a cardiac murmur was not detected. Laboratory examination including serum chemistry analysis, blood gas analysis, pulse oxymetry, urine examination, excretory urography and ultrasonography did not produce any atypical findings. Lung lobe infiltration and air bronchogram signs with a generalized increase in lung opacity in both the left and right side caudal lobes were viewed on ventrodorsal and right lateral thoracic radiographs (Fig. 1a and 1b). CBC test results (Fig. 2) were indicative of neutrophilic leukocytosis (37.0 × 103/µl, reference range 4 to 15.5 × 103/µl) with a mild left shift (band neutrophils 1.0 × 103/µl, reference range 0 to 0.3 × 103/µl). Results of a canine distemper polymerase chain reaction (PCR) test (Neodin Vetlab, Seoul, Korea) and heartworm antigen kit test (SNAP® Heartworm RT Test, IDEXX Laboratories Inc., Westbrook, ME, U.S.A.) were negative (data not shown). There was no clinical evidence of renal graft failure or rejection based on the results of CBC, serum chemistry and urinalysis.
Fig. 1.
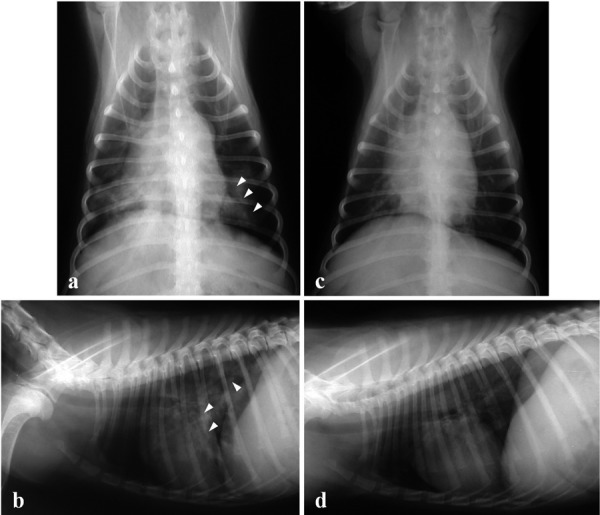
Thoracic radiographs of a dog with pneumonia after renal transplantation. Ventrodorsal (a) and right lateral thoracic radiographs (b) were taken 25 days after surgery. Infiltration in the caudal lung lobe and air bronchogram signs (white arrowheads) were observed. Ventrodorsal (c) and right lateral thoracic radiographs (d) were taken 10 days after imipenem-cilastatin administration. Abnormal lung infiltration had disappeared.
Fig. 2.
White blood cell (WBC) count of a dog with pneumonia during the post-transplantation period. The black arrow indicates the day clinical symptoms of respiratory infection were observed. The white arrow indicates the first day of imipenem-cilastatin administration.
We made a provisional diagnosis of bacterial pneumonia based on radiography, CBC and physical examination findings. Empirical treatments were started with a combination of two broad-spectrum antibiotics [10 mg/kg enrofloxacine (Baytril, SQ, q24h; Bayer Korea, Seoul, Korea) and 22 mg/kg ampicillin-sulbactam (Ucillin, IV, q12h; Dongkoo, Seoul, Korea)], fluids, nebulizer treatment combined with gentamycin (Gentamycin sulfate, Kukje, Seongnam, Korea) and coupage for 5 days until the final diagnosis was made. We continued immunosuppressive therapy without any dosage reduction due to concerns about the high risk for acute rejection during the early stage of kidney allograft.
A microorganism culture test was performed with tracheal wash fluid, blood and urine from the recipient dog as well as blood and urine from the donor animal. To obtain a tracheal wash specimen, the canine was anesthetized with 5 mg/kg propofol (Provive, Myungmoon, Seoul, Korea). We subsequently inserted an endotracheal tube with a rubber catheter into the trachea, pushed in 5 ml of warm saline and immediately withdrew the liquid to collect a sample. Bacterial pneumonia caused by P. aeruginosa was confirmed by a microorganism isolation test carried out according to a previous protocol [18]. Test results revealed that the bacterial infection was localized in the respiratory tract. Bacterial cultures of urine and blood from both the recipient and donor were both aerobically and anaerobically negative. Despite administration of broad-spectrum antibiotics (enrofloxacine and ampicillin-sulbactam) for 5 days, clinical symptoms of the recipient dog did not improve.
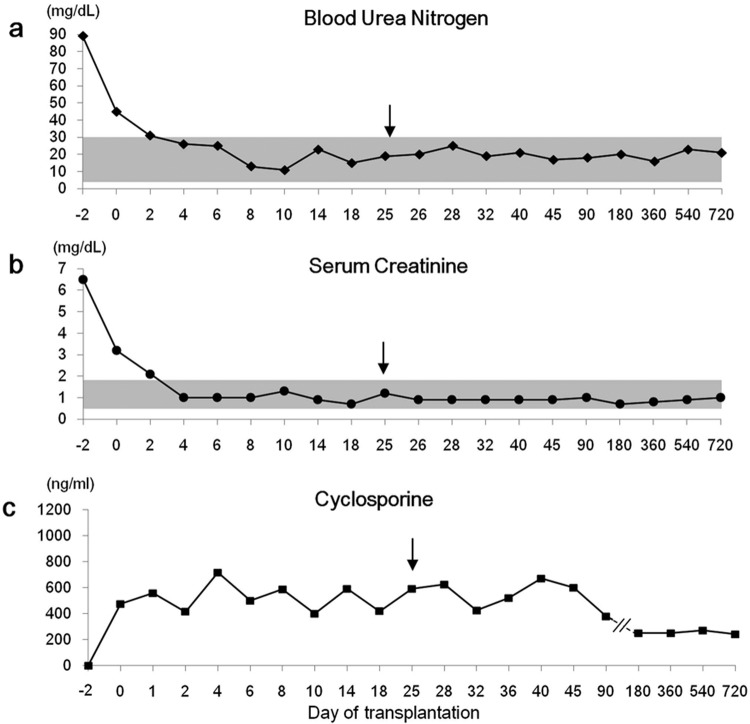
The P. aeruginosa strain isolated from the tracheal washing was sensitive to only imipenem and amikacin, had intermediate susceptibility to gentamycine and polymixinB; and was resistant to ceftazidime, piperacillin and ciprofloxacine. The patient was started on 5 mg/kg imipenem-cilastatin (Imipenem-cilastatin, IV over 30 min, q8h; Yungjin, Goyang, Korea). After 10 days of treatment, the cough and nasal discharge had markedly diminished, and neutrophil counts were in the normal range (Fig. 2). Lung lesions observed on thoracic radiographs had clearly improved. (Fig. 1c and 1d). Blood urea nitrogen (17–25 mg/dl, reference range 5–30 mg/dl) and serum creatinine (0.9–1.3 mg/dl, reference range 0.7–1.8 mg/dl) concentrations were maintained within the reference range during the entire post-transplantation period (Fig. 3a and 3b). Blood cyclosporine levels did not show any marked changes during the course of anti-pneumonia therapy (Fig. 3c). The antibiotic therapy was continued for 1 week, after all symptoms had subsided to prevent recurrent pneumonia. No complications or disease recurrence was observed during the 2-year follow-up period.
Fig. 3.
Blood urea nitrogen (BUN) as well as serum creatinine and cyclosporine trough concentrations was maintained within the reference range during the post-operation period. An arrow indicates the day on which acute onset of clinical signs of pneumonia occurred. The shaded area indicates the reference range.
Reports of renal transplantation in canines have been sporadically published in veterinary literature [11]. Most of these studies have focused on the effectiveness of immunosuppression protocols, surgical techniques and the survival of the animal recipients [4, 9, 17]. Although some reports have briefly described successful recovery from bacterial complications that were not due to MDR strains, others have presented cases in which the infections did not respond well to treatment with antimicrobials [9, 11]. Infection with multidrug-resistant (MDR) Pseudomonas (P.) aeruginosa in kidney transplant recipients leads to life-threatening complication in humans [16]. This condition is difficult to manage due to the limited susceptibility of MDR P. aeruginosa to antibiotics [7, 15]. To the best of our knowledge, several case reports on this topic have been published in human medicine, but no report has appeared in veterinary medical literature and no standard guidelines for diagnosis or treatment have been established for animal recipients [15, 16, 20, 23].
We performed a tracheal wash to obtain a sample to identify the best antibiotics for the bacteria isolated from the trachea. Cultivation of sputum or blood is not specific and sensitive method to distinguish real pathogen. On the other hand, transbronchial or open lung biopsy is effective for diagnosis, but is associated with high morbidity [19]. In our protocol, the recovery of a patient from anesthesia and tracheal wash procedure was relatively fast, and no complication has occurred. Tracheal wash would be a necessary protocol for early diagnosis of pulmonary infection in the canine transplant patients.
The strong immunosuppressants that are administered to prevent host rejection can increase the risk of bacterial infection, because these reagents impair cell-mediated immunity while reducing T cell and immunoglobulin function. Moreover, neutrophils and alveolar macrophages also become unresponsive to chemoattractants and their phagocytotic activities decrease [5]. In the case presented in this report, we did not reduce the immunosuppressant drug dosage, because sufficient immunosupression is necessary to prevent organ rejection during the early stage of transplantation and reducing the medication dosage could lead to irreversible acute organ rejection and eventual failure.
It is unclear how MDR P. aeruginosa pneumonia occurred. We speculate that the MDR P. aeruginosa infection in this case was opportunistic or acquired in the hospital. P. aeruginosa is an opportunistic human and animal pathogen that can be isolated from the ear canal, skin, nasal cavity and mouth of healthy dogs [18]. This bacterial strain is the second most common (17%) cause of nosocomial pneumonia in human clinics [7]. However, no surveillance data are available for veterinary patients to determine which microorganism is the most common cause of pulmonary infections.
P. aeruginosa is notorious for its MDR characteristics and causes concern among clinicians, because it can induce a life-threatening disease in immunocompromised patients [18]. In the present case, we used erofloxacin and ampicillin-sulbactam for the initial treatment protocol. These antibiotics are recommended for ameliorating severe and community-acquired cases of pneumonia [10, 21]. However, the reagents we administered were ineffective against the MDR P. aeruginosa infection in this case. P. aeruginosa is often resistant to ampicillin, cephalosporin, chloramphenicol and sulphonamides [1]. The traditionally preferred antibiotics include ciprofloxacine, ceftazidime, aminoglycosides and carbapenem [2, 7, 18]. In human medicine, new anti-pseudomonal antibiotics including doripenem, ceftobiprole and sitafloxacine have been dispensed due to the increased resistance rate of P. aeruginosa against traditionally preferred antibiotics [7]. A more recent study reported that the resistance rates of P. aeruginosa isolated from dogs and cats against enrofloxacine, ciprofloxacine and cefotaxime were 31.5, 20.5 and 17.8%, respectively [8]. Another report showed that large numbers of P. aeruginosa isolated from canine otitis externae are resistant to enrofloxacine (51.9%) and gentamycin (43.3%) [13].
In the present study, we showed that tracheal washing was a safe and effective approach for making an early diagnosis of pneumonia during the initial stage of renal transplantation in canines. We demonstrated that localized pneumonia caused by MDR P. aeruginosa infection after renal transplantation in a dog could be treated using appropriate antibiotics according to the results of an antibiotic susceptibility test. We suggest that small animal clinicians do not need to consider reducing the dosage of immunosuppressants to treat MDR P. aeruginosa pneumonia during the early stage of renal transplantation in canines. However, effective management protocols for systemic MDR P. aeruginosa-associated complications, such as bacteremia, neutropenia and vascular dehiscence, in canine renal transplant patients should be described in future studies. Our case report provides beneficial data for establishing standardized diagnostic and therapeutic guidelines to manage MDR bacterial pneumonia in canine renal transplant patients.
ACKNOWLEDGMENTS
This work was supported by a grant from the Next-Generation BioGreen 21 program (No.PJ009627), Rural Development Administration, Republic of Korea. This work was also supported by the Korea Research Foundation Grant funded by the Korean Government (KRF-2010-0025387).
REFERENCES
- 1.Aires J. R., Köhler T., Nikaido H., Plésiat P. 1999. Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob. Agents Chemother. 43: 2624–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alamartine E., Sabido O., Berthoux F. 1994. In-vitro effects of cyclosporin A, FK506, 6-mercaptopurine, and prednisolone on lymphokine-activated killer cells. Nephrol. Dial. Transplant. 9: 1456–1461 [PubMed] [Google Scholar]
- 3.Andert S. E., Grimm M., Schreiner W., Müller M. 1994. Therapeutic whole-blood levels of cyclosporin A in heart and lung transplantation. Clin. Chim. Acta 224: 159–166. doi: 10.1016/0009-8981(94)90182-1 [DOI] [PubMed] [Google Scholar]
- 4.Bernsteen L., Gregory C. R., Kyles A. E., Griffey S. M., Patz J. 2003. Microemulsified cyclosporine-based immunosuppression for the prevention of acute renal allograft rejection in unrelated dogs: preliminary experimental study. Vet. Surg. 32: 213–219. doi: 10.1053/jvet.2003.50027 [DOI] [PubMed] [Google Scholar]
- 5.Donnelly J. P. 1995. Bacterial complications of transplantation: diagnosis and treatment. J. Antimicrob. Chemother. 36: 59–72. doi: 10.1093/jac/36.suppl_B.59 [DOI] [PubMed] [Google Scholar]
- 6.Durrbach A., Francois H., Beaudreuil S., Jacquet A., Charpentier B. 2010. Advances in immunosuppression for renal transplantation. Nat. Rev. Nephrol. 6: 160–167. doi: 10.1038/nrneph.2009.233 [DOI] [PubMed] [Google Scholar]
- 7.El Solh A. A., Alhajhusain A. 2009. Update on the treatment of Pseudomonas aeruginosa pneumonia. J. Antimicrob. Chemother. 64: 229–238. doi: 10.1093/jac/dkp201 [DOI] [PubMed] [Google Scholar]
- 8.Harada K., Arima S., Niina A., Kataoka Y., Takahashi T. 2012. Characterization of Pseudomonas aeruginosa isolates from dogs and cats in Japan: current status of antimicrobial resistance and prevailing resistance mechanisms. Microbiol. Immunol. 56: 123–127. doi: 10.1111/j.1348-0421.2011.00416.x [DOI] [PubMed] [Google Scholar]
- 9.Hopper K., Mehl M. L., Kass P. H., Kyles A., Gregory C. R. 2012. Outcome after renal transplantation in 26 dogs. Vet. Surg. 41: 316–327 [DOI] [PubMed] [Google Scholar]
- 10.Marinella M. A. 2004. Community-acquired pneumonia due to Pasteurella multocida. Respir. Care 49: 1528–1529 [PubMed] [Google Scholar]
- 11.Mathews K. A., Holmberg D., Miller C. 2000. Kidney transplantation in dogs with naturally occurring end-stage renal disease. J. Am. Anim. Hosp. Assoc. 36: 294–301 [DOI] [PubMed] [Google Scholar]
- 12.Mathews K. A., Holmberg D. L., Johnston K., Miller C. M., Binnington A. G., Maxie G., Atilola M., Smith G. 1994. Renal allograft survival in outbred mongrel dogs using rabbit anti-dog thymocyte serum in combination with immunosuppressive drug therapy with or without donor bone marrow. Vet. Surg. 23: 347–357. doi: 10.1111/j.1532-950X.1994.tb00494.x [DOI] [PubMed] [Google Scholar]
- 13.Mekić S., Matanović K., Šeol B. 2011. Antimicrobial susceptibility of Pseudomonas aeruginosa isolates from dogs with otitis externa. Vet. Rec. 169: 125. doi: 10.1136/vr.d2393 [DOI] [PubMed] [Google Scholar]
- 14.Nam H. S., McAnulty J. F., Kwak H. H., Yoon B. I., Hyun C., Kim W. H., Woo H. M. 2008. Gingival overgrowth in dogs associated with clinically relevant cyclosporine blood levels: observations in a canine renal transplantation model. Vet. Surg. 37: 247–253. doi: 10.1111/j.1532-950X.2008.00373.x [DOI] [PubMed] [Google Scholar]
- 15.Nasim A., Baqi S., Akhtar S. 2012. Pseudomonas aeruginosa endocarditis in renal transplant recipients. Transpl. Infect. Dis. 14: 180–183. doi: 10.1111/j.1399-3062.2011.00667.x [DOI] [PubMed] [Google Scholar]
- 16.Orlando G., Di Cocco P., Gravante G., D’Angelo M., Famulari A., Pisani F. 2009. Fatal hemorrhage in two renal graft recipients with multi-drug resistant Pseudomonas aeruginosa infection. Transpl. Infect. Dis. 11: 442–447. doi: 10.1111/j.1399-3062.2009.00412.x [DOI] [PubMed] [Google Scholar]
- 17.Phillips H., Aronson L. R. 2012. Use of end-to-side arterial and venous anastomosis techniques for renal transplantation in two dogs. J. Am. Vet. Med. Assoc. 240: 298–303. doi: 10.2460/javma.240.3.298 [DOI] [PubMed] [Google Scholar]
- 18.Šeol B., Naglić T., Madić J., Bedeković M. 2002. In vitro antimicrobial susceptibility of 183 Pseudomonas aeruginosa strains isolated from dogs to selected antipseudomonal agents. J. Vet. Med. B Infect. Dis. Vet. Public Health 49: 188–192. doi: 10.1046/j.1439-0450.2002.00548.x [DOI] [PubMed] [Google Scholar]
- 19.Sileri P., Pursell K. J., Coady N. T., Giacomoni A., Berliti S., Tzoracoleftherakis E., Testa G., Benedetti E. 2002. A standardized protocol for the treatment of severe pneumonia in kidney transplant recipients. Clin. Transplant. 16: 450–454. doi: 10.1034/j.1399-0012.2002.02079.x [DOI] [PubMed] [Google Scholar]
- 20.Simkins J., Muggia V. 2012. Favorable outcome in a renal transplant recipient with donor-derived infection due to multidrug-resistant Pseudomonas aeruginosa. Transpl. Infect. Dis. 14: 292–295. doi: 10.1111/j.1399-3062.2011.00674.x [DOI] [PubMed] [Google Scholar]
- 21.Tilley L. P., Smith F. W. 2004. The 5-Minute Veterinary Consult, 3rd ed., Lippincott Williams & Wilkins, Philadelphia. [Google Scholar]
- 22.Tilney N. L., Strom T. B., Vineyard G. C., Merrill J. P. 1978. Factors contributing to the declining mortality rate in renal transplantation. N. Engl. J. Med. 299: 1321–1325. doi: 10.1056/NEJM197812142992401 [DOI] [PubMed] [Google Scholar]
- 23.Zeglen S., Wojarski J., Wozniak-Grygiel E., Siola M., Jastrzebski D., Kucewicz-Czech E., Zembala M. 2009. Frequency of Pseudomonas aeruginosa colonizations/infections in lung transplant recipients. Transplant. Proc. 41: 3222–3224. doi: 10.1016/j.transproceed.2009.07.063 [DOI] [PubMed] [Google Scholar]